Abstract
While nitrogen (N) amendment is known to affect the stability of ecological communities, whether this effect is scale-dependent remains an open question. By conducting a field experiment in a temperate grassland, we found that both plant richness and temporal stability of community biomass increased with spatial scale, but N enrichment reduced richness and stability at the two scales considered. Reduced local-scale stability under N enrichment arose from N-induced reduction in population stability, which was partly attributable to the decline in local species richness, as well as reduction in asynchronous local population dynamics across species. Importantly, N enrichment did not alter spatial asynchrony among local communities, which provided similar spatial insurance effects at the larger scale, regardless of N enrichment levels. These results suggest that spatial variability among local communities, in addition to local diversity, may help stabilise ecosystems at larger spatial scales even in the face of anthropogenic environmental changes.
Keywords: Aboveground biomass, asynchrony, biodiversity, metacommunity, nitrogen deposition, spatial heterogeneity, spatial insurance, spatial scale, species richness, steppe
Introduction
Many terrestrial ecosystems are limited by the availability of reactive nitrogen (LeBauer & Treseder 2008). Accordingly, adding nitrogen (N) through either fertilisation or atmospheric deposition to these ecosystems tends to increase their primary productivity (Smith et al. 1999; LeBauer & Treseder 2008; Zhang et al. 2015). As this positive effect of N addition on plant productivity is desirable in most agriculture settings, global N fertiliser production and consumption have increased tremendously during the past century, which, along with other anthropogenic activities (mainly fossil fuel consumption), has resulted in the doubling in the magnitude of global nitrogen cycles during this period (Fowler et al. 2013). However, increased N deposition to natural plant communities may carry other, often unwanted, consequences, including the loss of plant diversity (Stevens et al. 2004; Bobbink et al. 2010; Payne et al. 2017) and the reduction in community stability (Yang et al. 2012; Hautier et al. 2014; Zhang et al. 2016a).
Nitrogen enrichment has been recognised as one of the major threats to global biodiversity (Payne et al. 2017). Field experiments with N addition (e.g. Clark & Tilman 2008; Zhang et al. 2014), as well as surveys of natural communities across atmospheric N deposition gradients (Stevens et al. 2004) and through time (Duprè et al. 2010), have independently found that increased N inputs often result in local plant diversity loss. This result arose mainly because in N limited systems acid- and ammonium-loving species with tall stature and clonal growth tend to benefit from N enrichment (Dickson et al. 2014; Zhang et al. 2014), driving some of the acid, ammonium and light sensitive species, which are disadvantaged under N-enriched conditions, to extinction (Bobbink et al. 2010). While much research on the N enrichment effect on plant diversity has focused on the local scale diversity (i.e. alpha diversity), several recent investigations have recognised similar negative effects on plant diversity at larger spatial scales (i.e. gamma diversity) (Chalcraft et al. 2008; Lan et al. 2015). Given that reactive N from both atmospheric deposition and fertilisation is often added across broad areas, it is essential to evaluate the generality of these findings in various systems.
Besides biodiversity loss, increased N input into an ecosystem may also result in reduced temporal stability of its functions (Yang et al. 2012; Hautier et al. 2014; Zhang et al. 2016a). Several mutually non-exclusive mechanisms could contribute to this pattern. For example N enrichment relieves plants from nitrogen limitation and allows them to more strongly respond to the fluctuation in other limiting resources (Grman et al. 2010; Xu et al. 2015), thereby reducing the temporal stability of both populations (Yang et al. 2012; Xu et al. 2015) and whole communities (Hautier et al. 2014; Zhang et al. 2016a). Moreover, N-induced loss of community stability may also be linked to the often-observed N-induced loss of plant species richness, which could lead to a decline in population stability as well as a decline in the degree of asynchronous dynamics among species (Yang et al. 2012; Hautier et al. 2015; Zhang et al. 2016a). Alternatively, N enrichment may also reduce species asynchrony (e.g. via increasing the relative abundance of species with similar temporal niches) and population stability (e.g. via the destabilisation of plant–herbivore interactions; Rosenzweig 1971), without changing species richness (Grman et al. 2010; Xu et al. 2015). In addition, when communities are dominated by a small number of species, reduction in the stability of dominant species in response to N input could also translate into loss of community stability (Xu et al. 2015). While the number of investigations of N enrichment effects on community stability is rapidly accumulating, these studies have invariably focused on stability at the local scale (i.e. alpha stability). It remains unknown whether community stability at larger spatial scales (i.e. gamma stability) would exhibit similar responses to N enrichment as alpha stability.
Investigations of N enrichment effects on gamma stability are facilitated by adopting a hierarchical perspective on community stability (Wang & Loreau 2014; Wang et al. 2017), which allows the partitioning of gamma stability into alpha stability and beta variability (i.e. spatial asynchrony among local communities) (Wang & Loreau 2014, 2016). Adopting this perspective, one can ask how N enrichment influences gamma stability through its effects on alpha stability and spatial asynchrony. On the one hand, the commonly observed negative impacts of N enrichment on alpha stability (see the previous paragraph) may translate into reduced gamma stability, especially in systems where alpha stability contributes dominantly to gamma stability, as reported by recent empirical studies (Wilcox et al. 2017; Polley & Wilsey 2018). On the other hand, there is uncertainty on how N enrichment influences spatial asynchrony among local communities. N enrichment may reduce environmental heterogeneity in N availability among local communities (Western 2001; Fraterrigo et al. 2005), and the resulting similar environmental conditions are thus likely to drive similar community dynamics across localities (Western 2001; Wesche et al. 2012), resulting in smaller spatial asynchrony. Furthermore, lower environmental heterogeneity among localities may also cause local communities to be increasingly dominated by species best suited for the prevailing environmental conditions, resulting in smaller beta diversity (Passy & Blanchet 2007; Donohue et al. 2009; Wang & Loreau 2016), and in turn, smaller spatial asynchrony (Fukami et al. 2001; France & Duffy 2006; Wang & Loreau 2014, 2016). Alternatively, N enrichment could increase both beta diversity and spatial asynchrony among local communities, as increased productivity following N enrichment may promote the importance of stochastic processes in structuring ecological communities and increase the incidence of alternative community states among localities (Chase 2010). The relative importance of these different mechanisms would determine how spatial asynchrony and gamma stability vary with N enrichment.
To examine the effects of N enrichment on gamma stability and their associated mechanisms, we conducted a field N addition experiment in a temperate grassland in northern China. The temperate grassland has received relatively low levels (i.e. < 1.0 g N m−2 year−1) of atmospheric N deposition (Zhao et al. 2017) and, as a result, has not yet experienced a significant loss of plant species (Bai et al. 2004). This relatively low atmospheric N deposition background is ideally suited to assess N effects on ecological stability. We show that although N fertilisation reduced gamma stability, it did not alter spatial asynchrony among local communities, which provided spatial insurance (i.e. spatio-temporal effects arising from asynchronous dynamics of communities linked by dispersal; Loreau et al. 2003) for our study communities at the larger spatial scale.
Material and Methods
Study site
The field experiment was conducted in a temperate steppe (43°32′51″N, 116°40′23″E), located in the Xilin River Basin, Inner Mongolia, China. Mean (1985–2014) annual precipitation was 350.5 mm, with 72.0% falling during the growing season (i.e. from May to August). Mean annual temperature was 1.0 °C, ranging from −21.1 °C in January to 19.8 °C in July. The soil is classified as Calcic-Orthic Aridisol in the U.S. soil classification system. Four C3 perennial grasses, Stipa grandis, Leymus chinensis, Achnatherum sibiricum and Agropyon cristatum, accounted for the majority of the peak plant community aboveground biomass (Zhang et al. 2015). No fertilisers were applied to the study area prior to this experiment.
Experimental design
The experiment, which covers an area of 7-ha, was established in September 2008 within a 50-ha relatively flat natural grassland that had been fenced to exclude large animal grazing since 1999. A complete randomised block design, with 10 blocks, was used. N, in the form of NH4NO3, was evenly added to the plots at nine different rates (0, 1, 2, 3, 5, 10, 15, 20 and 50 g N m−2 year−1) and two frequencies (2 times year−1 vs. monthly). NH4NO3 was dissolved in purified water (less than 1 mm annually) for even spraying. In addition to these treatments, we also established a control treatment in which neither N nor water was added. Hence, there were 19 experimental treatments (9 rates × 2 frequencies of N addition + 1 control). Each experimental plot was 8 × 8 m2. Within each block, all 19 treatments were randomised to plots, which were separated by 1 m walkways. Each block was 45 × 70 m2, with 2 m walkways between blocks. The average spatial distance between plots within a treatment was c. 140 m. More details about the experimental design can be found in Zhang et al. (2014). This experiment was initially designed to assess how the frequency and rate of N addition affect grassland community structure and ecosystem functioning (Zhang et al. 2014, 2015, 2016a,b, 2017), and is used here to explore how increased N deposition influences ecosystem stability across spatial scales.
Plant sampling
Aboveground net primary productivity (ANPP) of plant communities was estimated annually from peak aboveground plant biomass, which is a reasonable approximation for ANPP in this region where all aboveground plant tissues die during the winter season (Zhang et al. 2018). Aboveground biomass was assessed every year from 2009 to 2013 between 10 and 15 August using a 0.5 × 2 m2 strip, which was randomly placed in each 8 × 8 m2 experimental plot (Zhang et al. 2015). The aboveground components of all living vascular plants were cut, sorted to species, oven-dried at 65 °C for at least 48-h to a constant weight, and weighed. Species richness was recorded for the same strip as aboveground biomass.
Alpha, gamma and beta diversity
Following recent theoretical work (Wang & Loreau 2014, 2016) and previous empirical studies (e.g. Chalcraft et al. 2008; Wilcox et al. 2017), we defined alpha (α) diversity as the species richness of a 1-m2 strip (small scale), and gamma (γ) diversity as the total species richness of the 10 1-m2 strips (large scale) in the same treatment. We calculated beta (β) diversity through multiplicative (βm) as well as additive (βa) partitioning of gamma diversity, following Whittaker (1972) and Lande (1996) respectively. The use of these classic metrics allowed direct comparison of our results to theoretical predictions (Wang & Loreau 2014, 2016), which were also based on multiplicative and additive partitions of gamma diversity. In addition, we also calculated two most frequently used beta dissimilarity indices: the presence/absence-based Jaccard dissimilarity index (βJ), and the abundance-based Bray–Curtis dissimilarity index (βBC). The consideration of these dissimilarity metrics allowed us to compare our results to previous work on scale-dependent diversity responses to N enrichment (e.g. Chalcraft et al. 2008), which generally calculated beta diversity as dissimilarity metrics.
Population, alpha and gamma stability
Following previous work (e.g. Tilman 1999), we calculated population temporal stability of each species as where μi and σi are the interannual mean and standard deviation of ANPP of species i in a 1-m2 strip over the 5 years (2009–2013) respectively. Population stability was then averaged across all species within each strip. Likewise, we calculated community temporal stability as where μT and σT are the interannual mean and standard deviation of community ANPP over the 5 years respectively. Alpha (local-scale) stability was defined as the temporal stability of community ANPP of a 1-m2 strip, and gamma stability defined as the temporal stability of total ANPP of the 10 1-m2 strips in the same treatment. Following Wang & Loreau (2014) and Wilcox et al. (2017), we also calculated biomass-weighted population and alpha stability. Biomass-weighted and unweighted measures of stability exhibited similar trends along the N addition gradient (Fig. S1), and there were strong correlations between biomass-weighted and unweighted population stability measures (F1,17 = 92.7, P < 0.0001; R2 = 0.85) and between biomass-weighted and unweighted alpha stability measures (F1,17 = 23047.9, P < 0.0001; R2 = 0.99). As the majority of stability studies have only considered unweighted stability measures, we focus on these measures here for easier comparison.
Species asynchrony within local communities
For each 1-m2 strip, the degree of asynchrony in the population dynamics of constituent species of its local community was quantified as where is the temporal variance of community ANPP, and σi is the standard deviation of ANPP of species i in the N-species community (Loreau & de Mazancourt 2008). Species asynchrony values range from 0 (perfect synchrony) to 1 (perfect asynchrony).
Spatial asynchrony among local communities
Spatial asynchrony of communities among the 10 1-m2 strips in the same treatment was calculated as (Wang & Loreau 2014; Wilcox et al. 2017), where wij is the temporal covariance of ANPP between local communities i and j, and wii is the temporal variance of ANPP of local community i. Spatial asynchrony of populations was calculated in the same way, using temporal variance of populations within strips and covariance of populations between strips. Spatial asynchrony values range from 0 (perfect spatial synchrony) to 1 (perfect spatial asynchrony).
Statistical analysis
Population, alpha and gamma stability values were logarithm transformed to meet the normality requirement for data analyses. For the smaller-scale (1-m2) analyses, we averaged alpha diversity and stability values across the 10 replicate local communities, and averaged beta dissimilarity values across all local community pairs, at each N addition rate and frequency combination. Averaging allowed the calculation of βm and βa as well as facilitated comparison with previous theoretical (Wang & Loreau 2016) and empirical (Wilcox et al. 2017) studies. Two-way analysis of covariance (ancova) was used to assess the effects of N addition rate and spatial scale on plant diversity (alpha and gamma diversity) and community stability (alpha and gamma stability) with N addition rate as the continuous variable, and spatial scale as the categorical variable. Linear and (where appropriate) quadratic regressions were used to study the relationships between diversity and stability variables and N addition rate. We also performed nonmetric multidimensional scaling (NMDS), based on βBC, to visualise how plant community structure changed in response to N amendment.
Structural equation modelling (SEM), which allows the assessment of hypothesised causal relationships between variables (Grace et al. 2015), was used to identify significant pathways through which N enrichment influences gamma stability. We first constructed an initial structural equation model considering all plausible pathways (see Fig. S2). Data were fitted to the model using the maximum likelihood estimation method. Adequacy of the model was determined using a χ2 test, root mean square errors of approximation (RMSEA) and the Akaike Information Criteria (AIC). Adequate model fits are indicated by a non-significant χ2 test (P > 0.05), low RMSEA (< 0.08; Browne & Cudeck 1992) and lower AIC. We obtained the final SEM, which yielded a non-significant χ2 test and the lowest AIC, by simplifying the initial SEM via eliminating non-significant pathways.
NMDS was performed using the ‘vegan’ package (Oksanen et al. 2018) in R 3.5.1 (R Core Team 2018). AMOS 22.0 (Amos Development Co., Greene, Maine, USA) was used for the SEM analysis. All other statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
Results
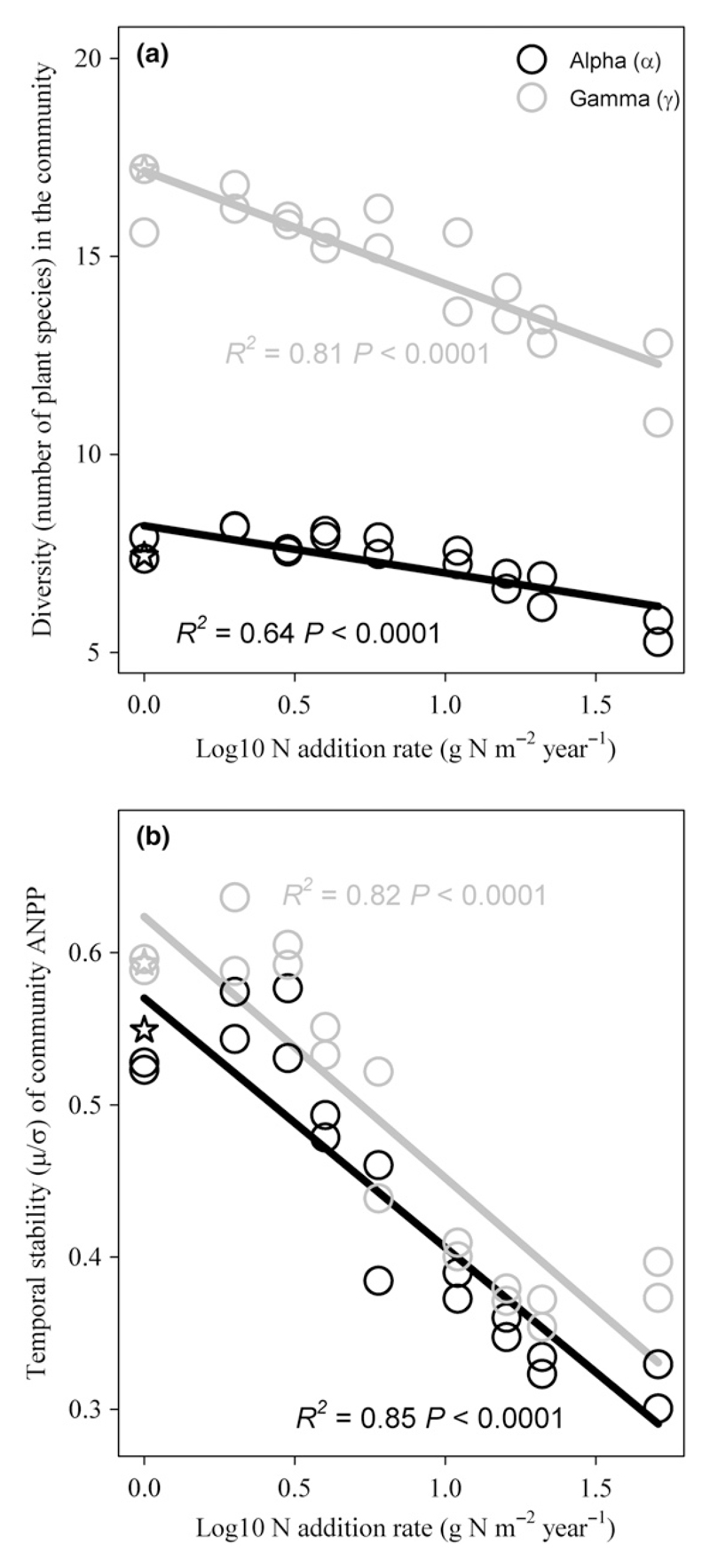
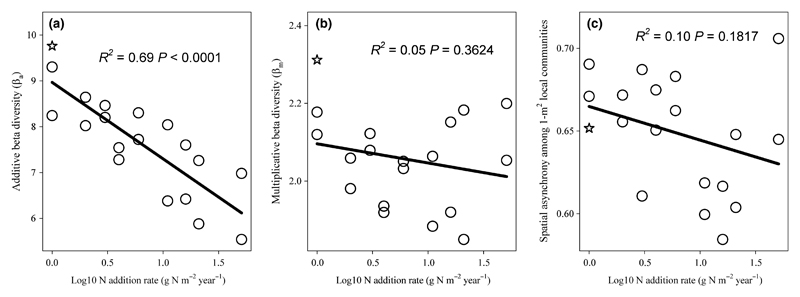
As expected, ancova revealed that gamma diversity was significantly greater than alpha diversity (Fig. 1a; F1,35 = 899.1, P < 0.0001). N addition significantly reduced both alpha (Fig. 1a; F1,17 = 29.7, P < 0.0001; slope = −1.20 ± 0.22) and gamma diversity (Fig. 1a; F1,17 = 74.3, P < 0.0001; slope = −2.86 ± 0.33). The negative effect of N addition, however, was stronger for gamma diversity, as indicated by a significant N × spatial scale interaction term in the ancova (F1,34 = 17.6, P = 0.0002). N addition significantly reduced βa (Fig. 2a; F1,17 = 37.0, P < 0.0001), but did not alter βm (Fig. 2b; F1,17 = 0.9, P = 0.3624). N addition also did not alter beta dissimilarity, calculated as either βJ or βBC (Fig. S3; P > 0.3650). Correspondingly, NMDS did not detect appreciable change in plant community structure across the N gradient (Fig. S4).
Figure 1.
Nitrogen enrichment reduced species diversity and community stability at both local and large scales. (a) Both alpha (i.e. species richness in the 1-m2 local community) and gamma diversity (i.e. species richness in the 10 1-m2 communities of the same experimental treatment) declined with increasing N addition rate. (b) Both alpha (i.e. community stability at the 1-m2 scale) and gamma stability (i.e. community stability at the 10-m2 aggregated large scale) declined with increasing N addition rate. For alpha diversity/stability, the value of each of the two points at each N addition level is the average of the 10 local communities at each N addition frequency; for gamma diversity/stability, the value of each of the two points at each N addition level is for the 10-m2 aggregated community at each N addition frequency. The open star symbol indicates data from the control (neither N nor water was added). Black and grey symbols are data from the 1-m2 and the 10-m2 scales respectively; solid lines are corresponding regression lines.
Figure 2.
Effects of N enrichment on beta diversity and variability. (a) N addition significantly reduced additive beta diversity (βa), but not (b) multiplicative beta diversity (βm) or (c) beta variability (i.e. spatial asynchrony among local communities). The value of each of the two points at each N addition level is the average of beta diversity at each N addition frequency. The open star symbol indicates data from the control.
ancova also revealed that gamma stability was significantly greater than alpha stability (Fig. 1b; F1,35 = 12.6, P = 0.0011). N addition significantly reduced both alpha (Fig. 1b; F1,17 = 96.4, P < 0.0001; slope = −0.16 ± 0.02) and gamma stability (Fig. 1b; F1,17 = 78.4, P < 0.0001; slope = −0.17 ± 0.02). This negative N addition effect did not differ between alpha and gamma stability (F1,34 = 0.1, P = 0.7639). N addition, however, did not alter spatial asynchrony among local communities (Fig. 2c; linear regression: R2 = 0.1024, P = 0.1817; quadratic regression: R2 = 0.2371; P = 0.1140).
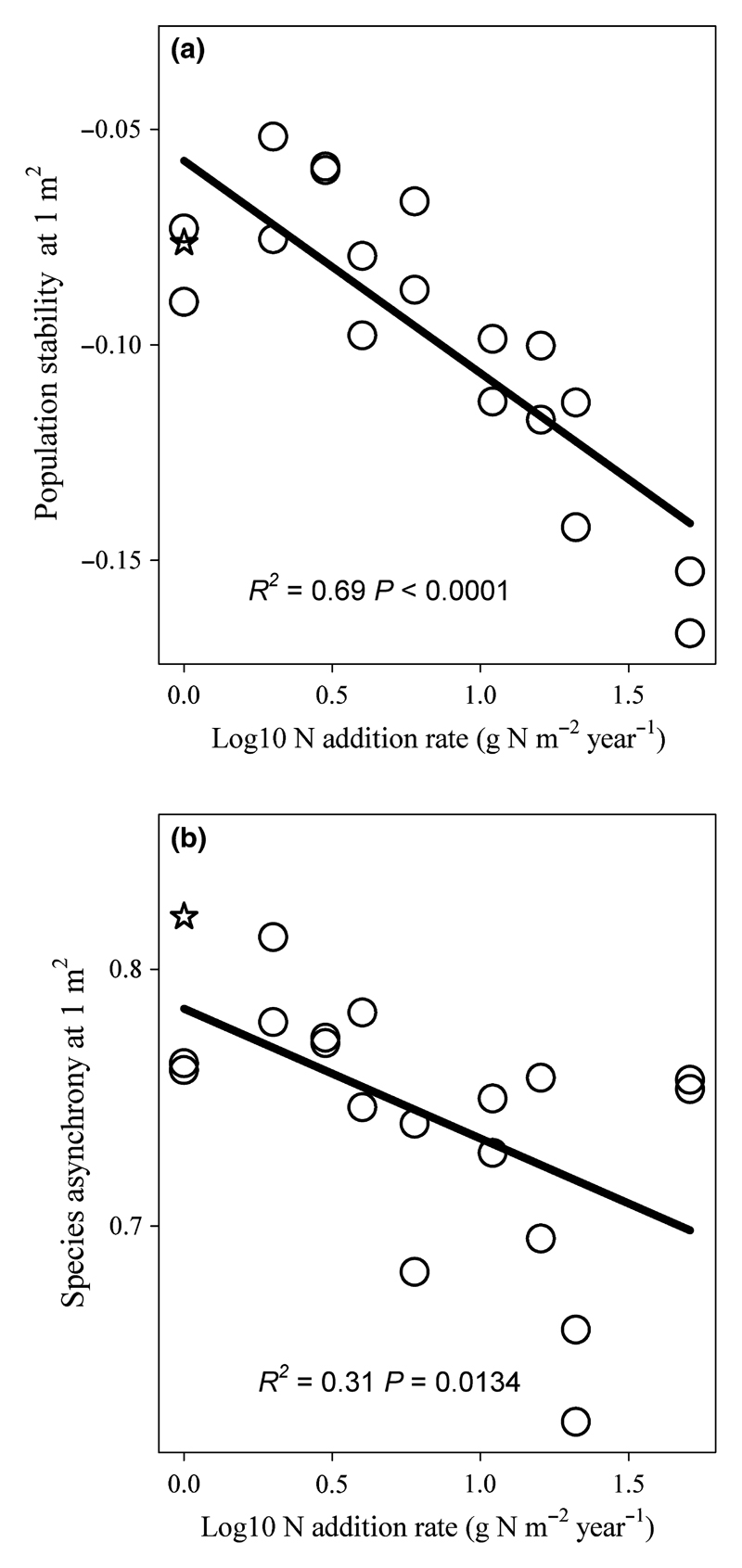
N addition significantly reduced population stability (Fig. 3a; F1,17 = 38.2, P < 0.0001) and species asynchrony within the 1-m2 local communities (Fig. 3b; F1,17 = 7.6, P = 0.0134). N addition also decreased spatial asynchrony among local communities of seven species, including six perennial species (L. chinensis, Cleistogenes squarrosa, Koeleria cristata, Poa subfastigiate, Festuca dahurica and Allium bidentatum), and one annual species (Artemisia scoparia), but did not alter spatial asynchrony of other species (Table S1).
Figure 3.
Effects of N enrichment on population stability and species asynchrony at the local (1-m2) scale. (a) Population stability within the 1-m2 local community declined with N addition rate. (b) Species asynchrony within the 1-m2 local community declined with N addition rate. The value of each of the two points at each N addition level is the average of the 10 local communities at each N addition frequency. The open star symbol indicates data from the control.
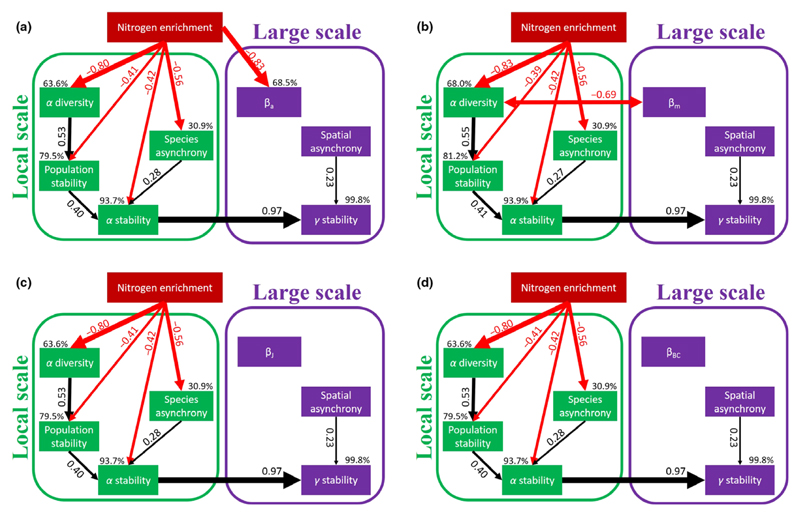
When beta diversity was measured as βa, the SEM showed that N enrichment had a negative effect on gamma diversity via reduced alpha diversity and reduced beta diversity (Fig. 4a). When beta diversity was measured as βm or dissimilarity metrics (βJ and βBC), however, the SEMs found that N enrichment reduced gamma diversity mainly through reducing alpha diversity, without altering beta diversity (Fig. 4b–d). Nevertheless, in all SEMs N enrichment had a negative effect on gamma stability through reduced alpha stability, without altering spatial asynchrony among local communities. In all SEMs both reduced population stability and species asynchrony within local communities to the decline in alpha stability under N enrichment. However, none of the beta diversity metrics was significantly associated with spatial asynchrony.
Figure 4.
The results of the final structural equation modelling (SEM) showing the pathways through which nitrogen enrichment influences gamma stability. The final SEM, (a) based on the additive beta diversity (βa) (χ2 = 13.534, P = 0.759; root mean square error of approximation (RMSEA) = 0.000; the Akaike Information Criteria (AIC) = 49.534), (b) based on the multiplicative beta diversity (βm) (χ2 = 14.526, P = 0.694; RMSEA = 0.000; AIC = 50.526), (c) based on the presence/absence-based index (Jaccard dissimilarity, βJ) (χ2 = 13.998, P = 0.784; RMSEA = 0.000; AIC = 47.998) and (d) based on the abundance-based beta dissimilarity (Bray-Curtis dissimilarity index, βBC) (χ2 = 15.889, P = 0.665; RMSEA = 0.000; AIC = 49.889). Note that RMSEA = 0.000 indicates a perfectly specified model constrained by the fact that alpha stability and spatial asynchrony completely determine gamma stability. Black and red arrows indicate significant (i.e. P < 0.05) positive and negative pathways respectively. Numbers adjacent to the arrows are standardised path coefficients, and the width of the arrows indicates the strength of the relationship. Percentages next to endogenous variables indicate the variance explained by the model (R2).
Discussion
To our knowledge, our study is the first to examine the scale dependence of ecosystem stability responses to N enrichment. By examining diversity and stability patterns at two different spatial scales, we found that N enrichment lowered alpha diversity, which contributed to lower alpha stability, and in turn, lower gamma stability. However, we also found that spatial asynchrony among local communities was unaffected by nitrogen enrichment. Importantly, the lack of response of spatial asynchrony means that nitrogen enrichment did not alter the stabilising role of spatial asynchrony (i.e. spatial insurance effects; Loreau et al. 2003; Wang & Loreau 2014, 2016) for the studied ecosystems at the larger spatial scale. Our study thus provides rare empirical evidence that spatial asynchrony among localities may help stabilise ecosystem dynamics at larger spatial scales, even in the face of anthropogenic environmental changes.
In line with previous studies (Chalcraft et al. 2008; Lan et al. 2015), N enrichment significantly reduced both alpha and gamma diversity in our experiment. However, the scale dependence of diversity responses in our experiment differs from that reported by other studies. For example Lan et al. (2015) reported that N-induced plant diversity loss was alleviated in larger plots in a Chinese temperate grassland. Our study, however, found that gamma diversity suffered greater loss from N enrichment than alpha diversity (Fig. 1a). This pattern arose as N enrichment caused beta diversity (specifically βa) to decline in our experiment (see next paragraph). Presumably, the opposite—that beta diversity increased with N enrichment—occurred in the experiment of Lan et al. (2015). Elucidating the mechanisms driving beta diversity responses to N enrichment therefore holds the key to understanding the variation in the scale dependence of N-induced diversity change.
We found that N addition reduced βa, but did not change βm or beta dissimilarity (i.e. βJ and βBC). This result supports the idea that different beta diversity metrics emphasises different aspects of structural differences among communities and, therefore, their trends may not necessarily agree with each other (Anderson et al. 2011). In particular, additive beta diversity (βa) accounts for information on joint absences of species (i.e. the extinction of the same species in the communities being compared), whereas multiplicative beta diversity (βm) and beta dissimilarity (βJ and βBC) do not consider joint absences (Anderson et al. 2011). Therefore, the fact that additive beta diversity (βa), but not the other beta diversity measures, declined with N enrichment suggests that the observed βa responses to N enrichment were largely driven by joint absences of species that became increasingly frequent with more N addition. The lack of beta dissimilarity response to N addition, however, contrasts with those of previous studies reporting that N enrichment could either increase (Chalcraft et al. 2008; Houseman et al. 2008; Chase 2010) or decrease (Inouye & Tilman 1995; Chalcraft et al. 2008; Donohue et al. 2009; Conradi et al. 2017) beta dissimilarity. Whereas the positive effect of N enrichment on beta dissimilarity has been frequently attributed to greater stochasticity in community assembly leading to alternative community states in more productive environments (Steiner & Leibold 2004; Chase 2010), the negative effect of N enrichment on beta dissimilarity has often been explained by reduced abiotic heterogeneity among localities selecting for more similar local communities (Chalcraft et al. 2008; Donohue et al. 2009). Note, however, that these mechanisms have not been investigated directly and their linkage to the observed beta dissimilarity responses to N enrichment remains largely conjectural. Our study was also unable to uncover the exact mechanisms underlying the observed lack of N enrichment effect on beta dissimilarity. Nevertheless, our finding could presumably be caused by the lack of treatment effect on both stochastic assembly and soil heterogeneity, or by increased stochasticity counteracting the effect of reduced soil heterogeneity under higher N input. Future research should strive to understand mechanisms associated with nutrient fertilisation effect on beta dissimilarity, which would significantly improve our understanding of scale-dependent diversity changes.
We found that N enrichment had a similar negative effect on alpha and gamma stability. The N-induced loss in alpha stability is not surprising and accords with findings of many previous studies that examined local-scale stability responses to N input (e.g. Grman et al. 2010; Yang et al. 2012; Hautier et al. 2014; Xu et al. 2015; Zhang et al. 2016a). The SEMs identified important pathways through which N enrichment reduced alpha stability (Fig. 4), including the N-induced loss in species asynchrony (Xu et al. 2015; Zhang et al. 2016a), and the loss of population stability due, partly, to reduced alpha diversity (Romanuk et al. 2006; Yang et al. 2012). Moreover, consistent with previous studies reporting that alpha stability largely contributed to gamma stability in naturally (Wilcox et al. 2017) and experimentally (Polley & Wilsey 2018) assembled herbaceous communities, we also found that alpha stability more strongly influenced gamma stability than beta variability (Fig. 4). These results suggest that preserving local diversity, which is often positively associated with alpha stability (Jiang & Pu 2009), may carry the additional benefit of safeguarding regional communities against large fluctuations (Loreau et al. 2003).
Consistent with theory (Wang & Loreau 2014, 2016), we found that gamma stability was significantly greater than alpha stability at each level of N addition, reflecting the presence of spatial asynchronous dynamics among local communities (i.e. beta variability). Spatial asynchrony, in principle, can arise from different local community dynamics due to the presence of different species (which exhibit different population dynamics) and different environmental conditions (which may cause even the same species to exhibit different population dynamics) among localities (Wang & Loreau 2014, 2016), as well as demographical stochasticity. Substantial small-scale (at the metre scale) heterogeneity for both soil nutrients and plant communities is known to exist at our study site (Zhou et al. 2008), which likely contributed to the observed spatial asynchrony. Moreover and probably more importantly, we found that spatial asynchrony among local communities was unaffected by N enrichment. Beta diversity is often assumed to be an important driver of spatial asynchrony, such that increasing dissimilarity in the structure of local communities is predicted to result in greater differences in their dynamics (Wang & Loreau 2014, 2016). However, we found that spatial asynchrony among localities was unrelated to beta diversity and dissimilarity (Fig. 4), which is at odds with this prediction. Likewise, Wilcox et al. (2017) also found non-significant associations between the two in a global-scale analysis of 62 herbaceous communities. In our study, both the lack of the response of spatial asynchrony among local communities to N amendment and its weak relationship with beta diversity and dissimilarity may be driven by strong community-level responses to temporal variation in precipitation and population-level demographic stochasticity. Plant communities in our study area are known to be primarily limited by water availability, with growing season precipitation largely determining community biomass production in the region (Bai et al. 2004). It is likely that N addition, or variation in species composition and abundance among local communities (see the paragraph on beta diversity), did little to alter the general trend of local community biomass responses to temporal variation in precipitation (Zhang et al. 2018). This is probably related to the fact that the same species that dominated in the control (i.e. perennial grasses including S. grandis, L. chinensis, A. sibiricum and A. cristatum), characterised by tall stature and clonal growth, remained dominant under N addition (Table S1; see also Zhang et al. 2015), resulting in little change in overall plant community structure across the N gradient (Fig. S4). At the population level, strong demographic stochasticity, known to influence ecosystem stability (Loreau & de Mazancourt 2013; de Mazancourt et al. 2013), may also diminish the responses of community biomass production to N amendment and variation in community structure. This latter hypothesis is supported by the fact that populations of many plant species (i.e. 86% of the species found in our experimental plots) in our experiment fluctuated asynchronously among local communities, and that for most species, the degree of their population asynchrony across localities was insensitive to N amendment (Table S1). Regardless of the mechanisms, the lack of response of beta variability to N enrichment suggests that spatial insurance effects (Loreau et al. 2003) were robust to substantial changes in soil N availability and played a consistent role in stabilising the studied ecosystem at the larger spatial scale.
Two caveats of our study merit discussion here. First, our study was conducted in a relatively small area (c. 7-ha) at a single site, inviting the question of whether our findings can be generalised to larger spatial scales. For our study, it is worth noting that we operationally defined gamma diversity as all the species that were present in our experimental plots within a given N treatment. This definition follows Tuomisto’s (2010) suggestion that gamma diversity can be measured at various spatial scales, a practice that has been adopted by many previous studies (e.g. Chalcraft et al. 2008; Wilcox et al. 2017). As alpha and gamma diversity differed between N treatments in our experiment, we were able to use our experiment to examine how N addition influences stability across spatial scales. Previous work has shown that the maximum range of spatial autocorrelation of both plant life forms and soil nutrients is ca. 2 m in our study area (Zhou et al. 2008). Therefore, our experimental area, although relatively small (7-ha), cannot be considered homogeneous. Thus, it is likely that our findings could be generalised to other spatial scales, at least for our study grassland. Consistent with this idea, when we repeated our analyses at the 5-m2 scale, we found essentially the same results as for the 10-m2 scale (Fig. S5). Nevertheless, we acknowledge that the robustness of our results needs to be assessed by directly studying metacommunities across large spatial scales. Second, our experiment used a randomised block design, such that local communities belonging to the same treatments do not cluster together, which leads to the question whether spatial configurations of the plots would influence their diversity. All our experimental plots can be, in fact, considered being linked by dispersal, given the relative small experimental area. However, although all species can potentially colonise our experimental plots, not all species can successfully establish in these plots, especially at higher N levels, where the rate of loss of resident species is higher and the rate of successful colonisation of non-resident species is lower (Zhang et al. 2016b). Indeed, when considering all experimental plots together, we found that their species composition was significantly influenced by N treatment differences (Mantel test: r = 0.04, P = 0.0004), but not by their spatial differences (Mantel test: r = 0.01, P = 0.1854), indicating the small role of among-plot dispersal in shaping the local communities. An alternative approach to studying N influences on stability across scales would be to survey communities along a natural atmospheric N deposition and/or soil N gradient, which would allow plots with similar N levels to cluster together as components of a natural metacommunity. However, the findings of these studies, which are observational, would be inevitably subject to alternative interpretations.
By exploring the scale dependence of diversity and stability responses to N enrichment in a temperate grassland, we found that N-induced local diversity loss contributed to the loss of alpha stability, and in turn, the loss of gamma stability, whereas spatial asynchrony among local communities was robust to increased N input, providing consistent spatial insurance effects irrespective of the levels of N enrichment. These results suggest effective means to preserve ecosystem stability across spatial scales. First, protecting local biodiversity may help maintain community stability at both local and large spatial scales, providing the additional rationale for protecting biodiversity within small habitats. Second, preserving asynchrony among local communities (e.g. through protecting different habitats/communities across space) in the face of anthropogenic environmental changes helps maintain community stability at larger spatial scales. We hope that our study will stimulate the analyses of the existing data from a considerable number of multi-year experiments simulating anthropogenic environmental changes, in an effort to evaluate the generality of our results.
Supplementary Material
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Acknowledgements
We thank Guojin Feng for assistance on data analysis. This study was supported by the International Postdoctoral Exchange Fellowship Program (20170070) of China to Y.Z., the TULIP Laboratory of Excellence (ANR-10-LABX-41) and by the BIOSTASES Advanced Grant and the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 666971) to M.L., the National Key R&D program of China (2016YFC0500202) to N.H., the National Key R&D program of China (2016YFC0500700) and National Natural Science Foundation of China (NSFC; 31430016) to X.H. and US National Science Foundation (DEB-1342754) to L.J. and NSFC (31361123001).
Footnotes
Authors’ Contributions
Y.Z., X.H. and L.J. designed the research. Y.Z. performed the experiment. Y.Z. and J.F. analysed the data. Y.Z. and L.J. wrote the first draft of the manuscript. All authors contributed substantially to data interpretation and manuscript writing. Y.Z. and J.F. contributed equally to this work.
Data Accessibility
The data supporting the results are deposited to the Dryad Digital Repository (https://doi.org/doi:10.5061/dryad.m3g5jm6).
Conflict of Interest
The authors have no competing interests.
References
- Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, et al. Navigating the multiple meanings of b diversity: a roadmap for the practicing ecologist. Ecol Lett. 2011;14:19–28. doi: 10.1111/j.1461-0248.2010.01552.x. [DOI] [PubMed] [Google Scholar]
- Bai YF, Han XG, Wu JG, Chen ZZ, Li LH. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature. 2004;431:181–184. doi: 10.1038/nature02850. [DOI] [PubMed] [Google Scholar]
- Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl. 2010;20:30–59. doi: 10.1890/08-1140.1. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociol Methods Res. 1992;21:230–258. [Google Scholar]
- Chalcraft DR, Cox SB, Clark C, Cleland EE, Suding KN, Weiher E, et al. Scale-dependent responses of plant biodiversity to nitrogen enrichment. Ecology. 2008;89:2165–2171. doi: 10.1890/07-0971.1. [DOI] [PubMed] [Google Scholar]
- Chase JM. Stochastic community assembly causes higher biodiversity in more productive environments. Science. 2010;328:1388–1391. doi: 10.1126/science.1187820. [DOI] [PubMed] [Google Scholar]
- Clark CM, Tilman D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature. 2008;451:712–715. doi: 10.1038/nature06503. [DOI] [PubMed] [Google Scholar]
- Conradi T, Temperton VM, Kollmann J. Resource availability determines the importance of niche-based versus stochastic community assembly in grasslands. Oikos. 2017;126:1134–1141. [Google Scholar]
- Dickson TL, Mittelbach GG, Reynolds HL, Gross KL. Height and clonality traits determine plant community responses to fertilization. Ecology. 2014;95:2443–2452. [Google Scholar]
- Donohue I, Jackson AL, Pusch MT, Irvine K. Nutrient enrichment homogenizes lake benthic assemblages at local and regional scales. Ecology. 2009;90:3470–3477. doi: 10.1890/09-0415.1. [DOI] [PubMed] [Google Scholar]
- Duprè C, Stevens CJ, Ranke T, Bleeker A, Peppler-Lisbach C, Gowing DJG, et al. Changes in species richness and composition in European acidic grasslands over the past 70 years: the contribution of cumulative atmospheric nitrogen deposition. Global Change Biol. 2010;16:344–357. [Google Scholar]
- Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, et al. The global nitrogen cycle in the twenty-first century. Philos Trans R Soc B-Biol Sci. 2013;368 doi: 10.1098/rstb.2013.0164. 20130164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France KE, Duffy JE. Diversity and dispersal interactively affect predictability of ecosystem function. Nature. 2006;441:1139–1143. doi: 10.1038/nature04729. [DOI] [PubMed] [Google Scholar]
- Fraterrigo JM, Turner MG, Pearson SM, Dixon P. Effects of past land use on spatial heterogeneity of soil nutrients in southern Appalachian forests. Ecol Monogr. 2005;75:215–230. [Google Scholar]
- Fukami T, Naeem S, Wardle DA. On similarity among local communities in biodiversity experiments. Oikos. 2001;95:340–348. [Google Scholar]
- Grace JB, Scheiner SM, Schoolmaster DR., Jr . Structural equation modeling: building and evaluating causal models. In: Fox GA, Negrete-Yankelevich S, Sosa VJ, editors. Ecological Statistics: Contemporary Theory and Application. Oxford University Press; Oxford, UK: 2015. pp. 168–199. [Google Scholar]
- Grman E, Lau JA, Schoolmaster DR, Gross KL. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol Lett. 2010;13:1400–1410. doi: 10.1111/j.1461-0248.2010.01533.x. [DOI] [PubMed] [Google Scholar]
- Hautier Y, Seabloom EW, Borer ET, Adler PB, Harpole WS, Hillebrand H, et al. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature. 2014;508:521–525. doi: 10.1038/nature13014. [DOI] [PubMed] [Google Scholar]
- Hautier Y, Tilman D, Isbell F, Seabloom EW, Borer ET, Reich PB. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science. 2015;348:336–340. doi: 10.1126/science.aaa1788. [DOI] [PubMed] [Google Scholar]
- Houseman GR, Mittelbach GG, Reynolds HL, Gross KL. Perturbations alter community convergence, divergence, and formation of multiple community states. Ecology. 2008;89:2172–2180. doi: 10.1890/07-1228.1. [DOI] [PubMed] [Google Scholar]
- Inouye RS, Tilman D. Convergence and divergence of old-field vegetation after 11 yr of nitrogen addition. Ecology. 1995;76:1872–1887. [Google Scholar]
- Jiang L, Pu ZC. Different effects of species diversity on temporal stability in single-trophic and multitrophic communities. Am Nat. 2009;174:651–659. doi: 10.1086/605961. [DOI] [PubMed] [Google Scholar]
- Lan ZC, Jenerette GD, Zhan SX, Li WH, Zheng SX, Bai YF. Testing the scaling effects and mechanisms of N-induced biodiversity loss: evidence from a decade-long grassland experiment. J Ecol. 2015;103:750–760. [Google Scholar]
- Lande R. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos. 1996;76:5–13. [Google Scholar]
- LeBauer DS, Treseder KK. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology. 2008;89:371–379. doi: 10.1890/06-2057.1. [DOI] [PubMed] [Google Scholar]
- Loreau M, de Mazancourt C. Species synchrony and its drivers: neutral and nonneutral community dynamics in fluctuating environments. Am Nat. 2008;172:E48–E66. doi: 10.1086/589746. [DOI] [PubMed] [Google Scholar]
- Loreau M, de Mazancourt C. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol Lett. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- Loreau M, Mouquet N, Gonzalez A. Biodiversity as spatial insurance in heterogeneous landscapes. Proc Natl Acad Sci USA. 2003;100:12765–12770. doi: 10.1073/pnas.2235465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mazancourt C, Isbell F, Larocque A, Berendse F, De Luca E, Grace JB, et al. Predicting ecosystem stability from community composition and biodiversity. Ecol Lett. 2013;16:617–625. doi: 10.1111/ele.12088. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. version 2.5-3. 2018 [Google Scholar]
- Passy SI, Blanchet FG. Algal communities in human-impacted stream ecosystems suffer beta-diversity decline. Divers Distrib. 2007;13:670–679. [Google Scholar]
- Payne RJ, Dise NB, Field CD, Dore AJ, Caporn SJM, Stevens CJ. Nitrogen deposition and plant biodiversity: past, present, and future. Front Ecol Environ. 2017;15:431–436. [Google Scholar]
- Polley HW, Wilsey BJ. Variability in community productivity—mediating effects of vegetation attributes. Funct Ecol. 2018;32:1410–1419. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2018. [Google Scholar]
- Romanuk TN, Vogt RJ, Kolasa J. Nutrient enrichment weakens the stabilizing effect of species richness. Oikos. 2006;114:291–302. [Google Scholar]
- Rosenzweig ML. Paradox of enrichment: destabilization of exploitation ecosystems in ecological time. Science. 1971;171:385–387. doi: 10.1126/science.171.3969.385. [DOI] [PubMed] [Google Scholar]
- Smith VH, Tilman GD, Nekola JC. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut. 1999;100:179–196. doi: 10.1016/s0269-7491(99)00091-3. [DOI] [PubMed] [Google Scholar]
- Steiner CF, Leibold MA. Cyclic assembly trajectories and scale-dependent productivity-diversity relationships. Ecology. 2004;85:107–113. [Google Scholar]
- Stevens CJ, Dise NB, Mountford JO, Gowing DJ. Impact of nitrogen deposition on the species richness of grasslands. Science. 2004;303:1876–1879. doi: 10.1126/science.1094678. [DOI] [PubMed] [Google Scholar]
- Tilman D. The ecological consequences of changes in biodiversity: a search for general principles. Ecology. 1999;80:1455–1474. [Google Scholar]
- Tuomisto H. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography. 2010;33:2–22. [Google Scholar]
- Wang SP, Loreau M. Ecosystem stability in space: α, β and γ variability. Ecol Lett. 2014;17:891–901. doi: 10.1111/ele.12292. [DOI] [PubMed] [Google Scholar]
- Wang SP, Loreau M. Biodiversity and ecosystem stability across scales in metacommunities. Ecol Lett. 2016;19:510–518. doi: 10.1111/ele.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Loreau M, Arnoldi J-F, Fang J, Rahman KA, Tao S, et al. An invariability-area relationship sheds new light on the spatial scaling of ecological stability. Nat Commun. 2017;8 doi: 10.1038/ncomms15211. 15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche K, Krause B, Culmsee H, Leuschner C. Fifty years of change in Central European grassland vegetation: large losses in species richness and animal-pollinated plants. Biol Conserv. 2012;150:76–85. [Google Scholar]
- Western D. Human-modified ecosystems and future evolution. Proc Natl Acad Sci USA. 2001;98:5458–5465. doi: 10.1073/pnas.101093598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker RH. Evolution and measurement of species diversity. Taxon. 1972;21:213–251. [Google Scholar]
- Wilcox KR, Tredennick AT, Koerner SE, Grman E, Hallett LM, Avolio ML, et al. Asynchrony among local communities stabilises ecosystem function of metacommunities. Ecol Lett. 2017;20:1534–1545. doi: 10.1111/ele.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZW, Ren HY, Li M-H, van Ruijven J, Han XG, Wan SQ, et al. Environmental changes drive the temporal stability of semi-arid natural grasslands through altering species asynchrony. J Ecol. 2015;103:1308–1316. [Google Scholar]
- Yang HJ, Jiang L, Li LH, Li A, Wu MY, Wan SQ. Diversity-dependent stability under mowing and nutrient addition: evidence from a 7-year grassland experiment. Ecol Lett. 2012;15:619–626. doi: 10.1111/j.1461-0248.2012.01778.x. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Lü XT, Isbell F, Stevens C, Han X, He NP, et al. Rapid plant species loss at high rates and at low frequency of N addition in temperate steppe. Global Change Biol. 2014;20:3520–3529. doi: 10.1111/gcb.12611. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Feng JC, Isbell F, Lü XT, Han XG. Productivity depends more on the rate than the frequency of N addition in a temperate grassland. Sci Rep. 2015;5 doi: 10.1038/srep12558. 12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Loreau M, Lü XT, He NP, Zhang GM, Han XG. Nitrogen enrichment weakens ecosystem stability through decreased species asynchrony and population stability in a temperate grassland. Global Change Biol. 2016a;22:1445–1455. doi: 10.1111/gcb.13140. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Stevens CJ, Lü XT, He NP, Huang JH, Han XG. Fewer new species colonize at low frequency N addition in a temperate grassland. Funct Ecol. 2016b;30:1247–1256. [Google Scholar]
- Zhang YH, Loreau M, He NP, Zhang G, Han XG. Mowing exacerbates the loss of ecosystem stability under nitrogen enrichment in a temperate grassland. Funct Ecol. 2017;31:1637–1646. doi: 10.1111/1365-2435.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Loreau M, He NP, Wang JB, Pan QM, Bai YF, et al. Climate variability decreases species richness and community stability in a temperate grassland. Oecologia. 2018;188:183–192. doi: 10.1007/s00442-018-4208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhang L, Chen Y, Liu X, Xu W, Pan Y, et al. Atmospheric nitrogen deposition to China: a model analysis on nitrogen budget and critical load exceedance. Atmos Environ. 2017;153:32–40. [Google Scholar]
- Zhou Z, Sun OJ, Luo Z, Jin H, Chen Q, Han X. Variation in small-scale spatial heterogeneity of soil properties and vegetation with different land use in semiarid grassland ecosystem. Plant Soil. 2008;310:103–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.