Abstract
Background
Systemic levels of interleukin (IL)-7 at antiretroviral therapy (ART) initiation have previously been shown to be predictive of HIV-linked paradoxical cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS). We therefore explored IL-7/IL-7 receptor (IL-7/IL-7R) signaling pathway dysfunction, with related alterations in immune function, as a mechanism underlying C-IRIS.
Method
HIV-infected patients with cryptococcal meningitis (CM) who experienced C-IRIS (n=27) were compared to CD4+ T-cell count-matched counterparts without C-IRIS (n=27), following antifungal therapy and pre-ART initiation. Flow cytometry was used to assess T-cell and monocyte phenotypes and functions.
Results
Proportions of IL-7R+ CD4+ or CD8+ T-cells correlated positively with CD4+ T-cell counts and proportions of central memory and naïve CD4+ and CD8+ T-cells pre-ART (all r>0.50 and p<0.05), however the former negatively correlated with CD4+ T-cell counts fold-increase on ART in non-C-IRIS but not C-IRIS patients. Higher frequencies of activated monocytes (CD14+CD86+ or CD14+HLA-DR+; p≤0.038) were also observed in C-IRIS compared to non-C-IRIS patients and those who failed to clear cryptococci from cerebrospinal fluid pre-ART had higher levels of activated monocytes (CD14+HLA-DR+, p=0.017) compared to those who cleared. In multivariate regression, CD14+HLA-DR+ monocytes were independently associated with C-IRIS (HR=1.055 [1.013-1.098]; p=0.009).
Conclusion
In contrast to non-C-IRIS patients, C-IRIS patients displayed a lack of association between proportions of IL-7R+ T-cells and several markers of T-cell homeostasis. They also exhibited higher monocyte activation linked to CSF cryptococcal culture positivity pre-ART. These data suggest a role for IL-7/IL-7R signaling pathway dysregulation in the pathogenesis of C-IRIS, possibly linked to monocyte activation and residual pathogen burden pre-ART.
Keywords: AIDS-induced cryptococcal meningitis, Cryptococcosis-associated Immune Reconstitution Inflammatory Syndrome, T-cells, Monocytes, IL-7/IL-7 Receptor Signaling Pathway, Antigen-specific responses and Immune activation
Introduction
Cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS) is a common clinical complication in some patients with cryptococcal meningitis (CM), usually within six months of initiating combination antiretroviral therapy (cART) 1–4. C-IRIS presents clinically as a neurological deterioration (ND) during immune reconstitution after cART initiation. Mortality rates vary widely but may exceed 50% 1,5–8. C-IRIS is thought to be caused by a paucity of ‘protective’ immune responses, resulting in cryptococcal antigen accumulation pre-cART; followed by a storm of pro-inflammatory responses as the immune system recovers 1,6,9,10. Severely immunocompromised patients who initiate cART with low CD4+ T-cell counts are at high risk for C-IRIS 1,2. Paradoxical C-IRIS is the commonest form of C-IRIS, and usually presents as a ND in individuals with known CM before initiating cART 1,4,6,10,11. Understanding the immunopathogenesis of C-IRIS may facilitate targeted interventions.
We recently demonstrated that plasma but not cerebrospinal fluid (CSF) levels of interleukin(IL)-5 and IL-7 pre-initiation of cART were predictive of C-IRIS 12. High IL-5 levels may reflect a generalized type 2 T-helper cell (Th2) environment that impairs the clearance of cryptococci, which is primarily dependent on Th1 responses in the absence of HIV infection 6,9,10. Conversely, IL-7 is a hematopoietic cytokine that is involved in T-cell survival, proliferation, and differentiation. IL-7 may also play a role in T-cell activation and responses as immune cell subsets repopulate upon cART initiation and encounter residual cryptococcal antigens 12–15. Levels of circulating IL-7 are thought to be primarily regulated by the rate at which the cytokine is consumed by T-cells 13,16, which in turn may depend on the expression and function of IL-7R on T-cells. Defects in the IL-7R signaling pathway are associated with lymphopenia, with other cell lineages largely unaffected 17,18. We therefore hypothesized that high plasma IL-7 levels in HIV patients with CM who developed C-IRIS reflects an impairment of the IL-7/IL-7 receptor (IL-7R/CD127) signaling pathway in T-cells in general, or in Cryptococcus-specific T-cells, following cART initiation. Defects in the IL-7/IL-7R signaling pathway may lead to aberrant T-cell responses to cryptococcal antigens as an underlying factor in the immunopathogenesis of C-IRIS. Alternatively, high IL-7 in C-IRIS patients may be just an indicator of generalized immune activation. It has also been shown that polymorphisms in the IL-7Rα gene are associated with faster CD4+ T-cell recovery after initiation of cART [28]; and gene variations in IL-7Rα have been shown to affect IL-7R expression on CD4+ T-cells in HIV-infected individuals [29]. Moreover, reduced IL-7R expression on T-cells and increased plasma sCD127 have been demonstrated in late-presenting HIV-infected individuals [30]. Additionally, evidence from a large body of literature indicates that activation of innate immune responses could play a major role in the pathogenesis of C-IRIS 6,9,12,19.
We hypothesized that the IL-7/IL-7R pathway may be differentially regulated in C-IRIS versus non-C-IRIS patients. We therefore sought to determine if IL-7R expression on T-cells negatively correlates with IL-7 levels, and whether IL-7R expression was independently associated with C-IRIS or T-cell numbers pre-cART and their recovery following cART initiation in either C-IRIS or non-C-IRIS patients. We then assessed whether T-cell and monocyte activation profiles pre-cART could distinguish between subsequent non-C-IRIS and C-IRIS conditions. Finally, previous work has demonstrated that cryptococcal mannoprotein (CMP)-specific CD4+ T-cell responses pre-cART may differ between non-C-IRIS and C-IRIS patients 19,20. There is also evidence that cryptococcal strain variation can impact immune responses, and therefore potentially influence C-IRIS risk 21. However, the magnitude of immune response to autologous cryptococcal strains and how this may impact on clinical outcomes following cART initiation is largely unknown. We therefore investigated whether cryptococcal-specific T-cell responses differed between C-IRIS and non-C-IRIS patients using autologous cryptococcal antigens (ACA) isolated from cryptococci infecting each study participant versus mannoproteins derived from cap67 strain (CMP) as the recall antigens.
Materials and methods
Study participants
This retrospective CD4+ T-cell count-matched case-control study was performed in participants enrolled into a larger study of 130 HIV-infected, cART-naive patients (mean age=18years, between 33-40.5 years) who experienced their first episode of CM in Durban, South Africa. Participants were consecutively enrolled into a longitudinal cohort study between August 2009 and March 2011 1. Fifty-four patients with CM were included in this sub-study. CM was diagnosed by CSF India ink and/or by cryptococcal antigen (CrAg) positivity (Cryptococcal Latex Agglutination System; Meridian Bioscience Inc, Cincinnati, Ohio, USA). Patients who experienced probable or possible C-IRIS while on cART (n=27) were compared to CD4+ T-cell count-matched counterparts without C-IRIS in a 1:1 ratio. All study subjects were diagnosed with paradoxical C-IRIS, with all other forms of IRIS excluded from the study. All parameters of interest including IL-7 and cellular markers by flow cytometry were measured following antifungal treatment but prior to antiretroviral therapy commencement, and at time of C-IRIS event in the C-IRIS group. Demographic and clinical characteristics of participants are provided in Table S2 and detailed clinical findings have been presented elsewhere 1.
Cytokine measurements
Plasma samples were collected following antifungal treatment and pre-cART initiation, and at the C-IRIS event and frozen at -80°C until use. IL-7 levels were quantified in a 17-plex-high-sensitivity Luminex kit on a Bio-Plex® 200 system (Bio-Rad, California, USA) as described in detail elsewhere 12.
Preparation of cryptococcal antigens
Two sets of cryptococcal antigen preparations were used in the studies. CMP derived from a capsular strain cap67 (ATCC #52817) were purified from culture supernatants using Con A sepharose affinity chromatography as described 22. CMP have been shown to stimulate lymphoproliferative responses from cryptococcosis patients, including those with AIDS and immune reconstitution 19. CMP was stored in lyophilized aliquots at -80°C until use. ACA were prepared by growing each patient-specific Cryptococcus strain and then performing an alkaline extraction, as described 23. ACA include a broad array of antigens, including mannoproteins. The pellet was suspended in 300 μl of 20 mM Tris-buffered saline (TBS) containing a protease inhibitor cocktail of serine, cysteine and metallo-proteases inhibitors (Roche Diagnostic, Boston, USA). Protein concentration of the preparations was determined using the bicinchoninic acid. Cells were stimulated using a final concentration of 10 μg protein/mL.
Flow cytometry
Cryopreserved PBMCs were thawed in RPMI 1640 (Sigma-Aldrich, Johannesburg, South Africa) supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin, 100 g/mL streptomycin sulfate, and 1.7 mM sodium glutamate. After 2 hours of resting at 37°C in a 5% CO2 incubator, half a million viable cells were aliquoted in a volume of 200 μL per well in a 96-well plate. Cells were washed in phosphate-buffered saline (PBS) and then incubated with fixable near infra-red (NIR) staining dye on APC-Cy7 (BioLegend Inc., California, USA) for dead cell exclusion for 30 minutes. Cells were then stained with the following antibodies, all from BD Biosciences (California, USA) unless otherwise indicated: anti-CXCR3 (clone FUN-1) -BV421, anti-CD27 (clone M-T271) (BioLegend) -BV510, anti-CD45RA (clone 5H9) -quantum dot (Qdot)605 (Invitrogen, California, USA), anti-CD4 (clone SK3) -BV711, anti-CD127 (clone M-A251) -BV786, anti-CD8 (clone MAb11) -Alexa F488, anti-PD-1 (clone RPA-T8) -PerCP-Cy5.5, anti-CD25 (clone SK7) -PE, anti-CD3- (clone 5344.111) -PE-CF594, anti-CCR6 (clone G46-6) PE-Cy7 and anti-CCR7 (clone 150503) -Alexa F 700. Cells were then washed and fixed (Perm/fix Medium A, Invitrogen).
Separately, one million viable cells/well were stimulated with staphylococcus enterotoxin B (SEB) and lipopolysaccharide (LPS) (both from Sigma-Aldrich) as positive control at a concentration of 1 μg/mL each for 4.5 hours (to avoid downregulation of the CD14 molecule by LPS), CMP/ACA at a concentration of 10 μg/mL each for 18 hours in 5% CO2 at 37°C. Unstimulated negative control (NC) and fluorescence minus one (FMO) control wells were also added. Co-stimulatory antibodies, CD28 and CD49d (1 μg/ml each; BD Biosciences) were added to each well. Brefeldin A (BioLegend) was also added to each well after 1 hour of incubation. Cells were surface stained with: anti-CD86 (clone FUN-1) -Brilliant violet (BV)421, anti-CD38 (clone HIT2) -BV510, anti-CD14 (clone M5E2) -BV605, anti-CD134 (clone ACT35) -BV650, anti-CD4 (clone SK3) -BV711, anti-CD8 (clone M-A251) -BV786, anti-PD-1-PerCP-Cy5.5 (clone RPA-T8), anti-CD25 (clone SK7) -phycoerythrin (PE), anti-CD16 (clone 3G8) -PE-Cy5, anti-HLA-DR (clone G46-6) PE-Cy7 and anti-CD3 (clone SK7) -Alexa F 700. Subsequently, PBMCs were washed, fixed (Perm/fix medium A, Invitrogen), permeabilized (Perm/fix Medium B, Invitrogen) and intracellularly stained with anti-TNF-α (clone MAb11) -Alexa F488, anti-IL-2 (clone 5344.111) -PE-CF594 and anti-IFN-γ (clone 4S.B3) -Alexa F647. Cells were acquired on a BD LSRFortessa.
Data analysis
Data were analyzed using the FlowJo version 10.01 (TreeStar Inc., Oregon, USA). Gating schemes are illustrated in the gating strategy figures. All parameters measured were based on FMO controls. For intracellular cytokine staining, the background was considered as the frequency of cells producing cytokines in the absence of antigenic stimulation (unstimulated NC) and was subtracted for each sample 24.
Statistical analysis
The Mann-Whitney test was used to compare proportions of cells expressing a particular marker and intracellular cytokine responses pre-cART in C-IRIS individuals versus controls for both T-cells and monocytes. The Wilcoxon signed ranks test was used to compare baseline (pre-cART initiation) percentage of cells expressing a marker and intracellular cytokine responses versus the same parameters at the C-IRIS-event (post-cART). To determine the predictors of C-IRIS in multivariable analysis, Cox proportional hazards regression analysis was performed using all parameters with a p-value ≤0.1 in the univariate analysis. All statistical analyses were performed using GraphPad Prism v7.1 (La Jolla, California, USA) and Stata v13.0 (StataCorp, College Station, Texas, USA). Statistical significance was defined at p<0.05.
Ethics Statement
Written informed consent was obtained from participants or next-of-kin (if the participant was not competent). Review boards at the University of KwaZulu-Natal, Monash University, and the University of Western Australia granted ethics approval.
Results
Pre-cART CD4+ T-cell counts correlated with proportions of IL-7R+ CD4+ T-cells in non-C-IRIS patients only but not with plasma IL-7 levels in either group
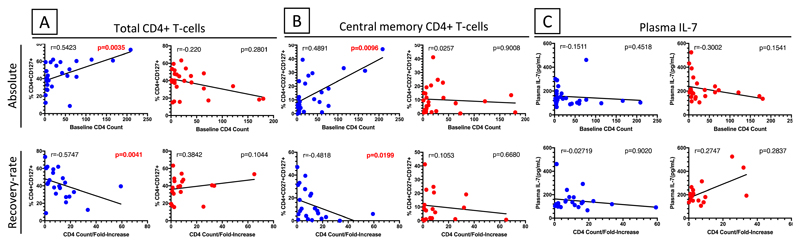
Demographic and clinical characteristics of the cohort are presented in Table S2 and detailed clinical findings have been reported elsewhere 1,12. T-cell subsets were defined by CD45RA and CD27 expression such that naïve T-cells (Nv) were defined as CD27+CD45RA+; central memory (Cm) as CD27+CD45RA-; effector memory (Em) as CD27-CD45RA-; and terminal effector (Ef) as CD27-CD45RA+, as described 25,26. The proportions of these cell subsets expressing IL-7R were measured ex-vivo (Fig. S1). There was no correlation between proportions of T-cells expressing IL-7R and plasma IL-7 levels pre-cART in both non-C-IRIS and C-IRIS patients (Fig. S2) as would have been expected from previous studies 27,28. We next explored whether there was an association between percentage of IL-7R+ CD4+ T-cells or plasma IL-7 levels pre-cART and pre-cART CD4+ T-cell count or fold-increase in CD4+ T-cell count following cART initiation according to C-IRIS outcome as the latter two have been reported to be predictors of C-IRIS 1. Interestingly, in non-C-IRIS but not C-IRIS patients, pre-cART CD4+ T-cell counts positively correlated with the proportion of IL-7R+ total CD4+ T-cells (r=0.5423, p=0.0035) (Fig. 1A) and central memory CD4+ T-cells (r=0.4891, p=0.0096) (Fig. 1B). In contrast, fold-increase in CD4+ T-cell counts from pre-cART to 24 weeks of ART negatively correlated with pre-cART IL-7R+ total CD4+ T-cells (r=-0.5747, p=0.0041) (Fig. 1A) and central memory CD4+ T-cells (r=-0.4818, p=0.0199) (Fig. 1B). Pre-cART, CD4+ T-cell counts did not correlate with plasma IL-7 levels in either the non-C-IRIS or C-IRIS group (Fig. 1C). Collectively, these data suggest differential regulation of the IL-7/IL-7R signaling pathway in C-IRIS versus non-C-IRIS individuals.
Fig. 1. Correlations between pre-cART (baseline) proportions of IL-7R+ CD4+ T cells or plasma IL-7 levels and pre-cART CD4+ T cell counts or fold-increase in CD4+ T cell counts after ART in non-C-IRIS and C-IRIS patients.
Proportions of (A) IL-7R+ total CD4+ T cells (CD4+CD127+) and (B) central memory CD4+ T cells (CD4+CD27+CD127+) positively correlated with pre-cART CD4+ T cell counts but negatively correlated with fold-increase in CD4+ T cell counts after ART in non-C-IRIS patients (blue), but not C-IRIS patients (red). (C) There were no correlations between pre-cART plasma IL-7 levels and pre-cART CD4+ T cell counts or fold-increase in CD4+ T cell counts after ART in either study group. An analysis using IL-7R expression median fluorescence intensity (MFI) showed a similar trend.
Proportions of IL-7R+ total and central memory CD8+ T-cells were lower in C-IRIS patients compared to non-C-IRIS controls pre-cART, but increased at the time of C-IRIS
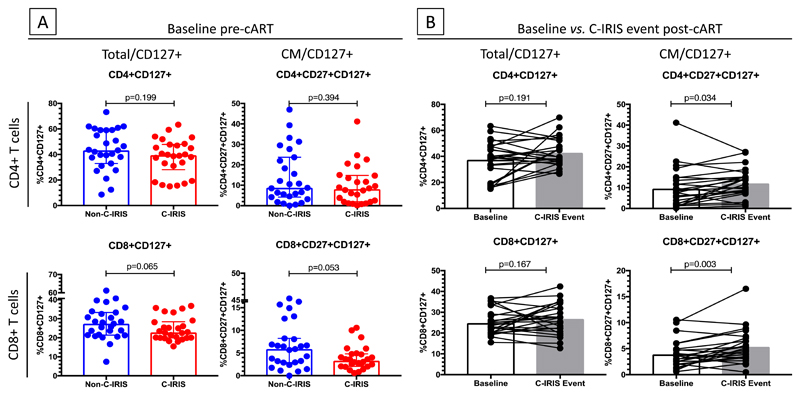
We further compared pre-cART proportions of IL-7R+ T-cells in C-IRIS vs. non-C-IRIS patients to determine if proportions of IL-7R+ cells could distinguish patients who subsequently experienced C-IRIS from those who did not (Fig. 2). There were no significant differences in percentage of either total or central memory IL-7R+ CD4+ T-cells (p=0.199 and p=0.394, respectively) or any other CD4+ T-cell subsets (data not shown). However, a trend was noted of lower percentages of IL-7R+ total CD8+ T-cells and central memory CD8+ T-cells in C-IRIS compared to non-C-IRIS individuals (p=0.065 and p=0.053 respectively) (Fig. 2A). In C-IRIS patients, IL-7R+ central memory CD4+ and CD8+ T-cells increased between baseline and time of C-IRIS (p=0.034 and 0.003, respectively; Fig. 2B).
Fig. 2. Comparison of proportions of IL-7R+ CD4+ and CD8+ T cells pre-cART in non-C-IRIS and C-IRIS patients and change in proportions of each cell type from pre-cART to C-IRIS in C-IRIS patients.
(A) Pre-cART, proportions of IL-7R+ total (CD4+CD127+) and central memory (CD4+CD27+CD127+) CD4+ T cells did not differ between non-C-IRIS and C-IRIS patients but there was a trend towards higher IL-7R+ total (CD8+CD127+) and central memory (CD8+CD27+CD127+) CD8+ T cells in non-C-IRIS patients (p=0.065 and 0.053, respectively). (B) Proportions of total and central memory CD8+ T-cells, but not CD4+ T cells, were higher at C-IRIS event compared with baseline. Analysis using IL-7R expression median fluorescence intensity (MFI) showed a similar trend.
Proportions of IL-7+ CD4+ T-cells pre-cART correlated with pre-cART proportions of central memory and naïve CD4+ and CD8+ T-cells in non-C-IRIS patients and fold-increase in central memory and naïve CD4+ T-cells at C-IRIS event
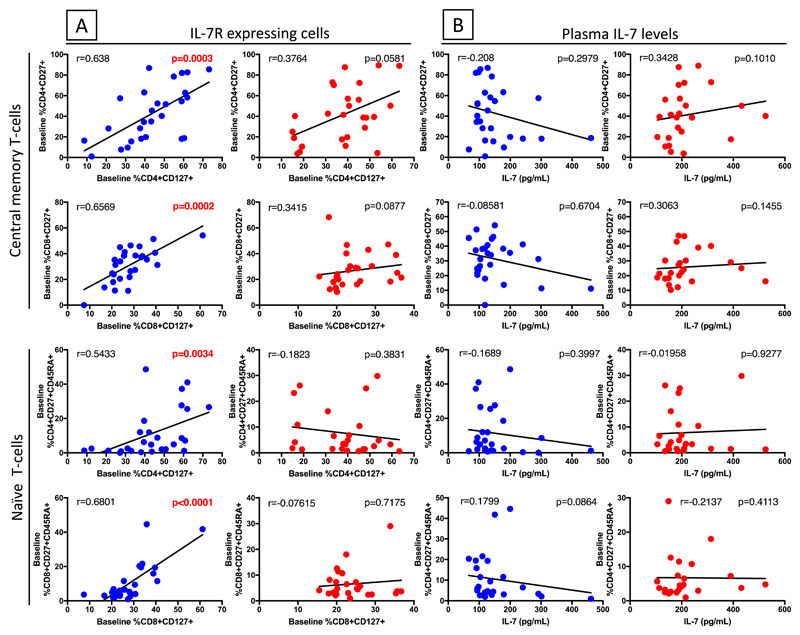
Since IL-7 and IL-7R are involved in the production and homeostatic maintenance of T-cells, we correlated proportions of T-cells expressing IL-7R or plasma IL-7 levels pre-cART with pre-cART percentages of central memory (CD27+CD45RA-) and naïve (CD27+CD45RA+) T-cells in both non-C-IRIS and C-IRIS patients and with fold-increase in these cell populations from pre-cART to time of C-IRIS in C-IRIS patients. Similar to the observation for CD4+ T-cell counts (Fig. 1), in non-C-IRIS but not C-IRIS patients the pre-cART proportions of central memory (CD4+CD27+CD45RA-) and naïve (CD4+CD27+CD45RA+) CD4+ T-cells showed highly significant positive correlations with proportions of IL-7R+ CD4+ T-cells (r=0.638, p=0.0003 and r=0.5433, p=0.0034 respectively) (Fig. 3A). The percentage of IL-7R+ CD8+ T-cells also significantly correlated with the percentages of central memory (CD8+CD27+CD45RA-) and naïve (CD8+CD27+CD45RA+) CD8+ T-cells (r=0.6569, p=0.0002 and r=0.6801, p<0.0001 respectively) but only in non-C-IRIS patients (Fig. 3A). In contrast, pre-cART proportions of none of these T-cell subsets correlated with plasma IL-7 levels (Fig. 3B). These data further suggest functional differences in the IL-7/IL-7R receptor signaling pathway of T-cells in C-IRIS versus non-C-IRIS patients and suggest that the high IL-7 plasma levels observed in C-IRIS patients may be a marker of decreased IL-7 utilization by a dysfunctional IL-7/IL-7R pathway.
Fig. 3. Correlations of IL-7R+ CD4+ and CD8+ T cells or plasma IL-7 levels pre-cART with naïve and central memory CD4+ and CD8+ T-cells pre-cART in non-C-IRIS (blue) and C-IRIS (red) patients.
(A) Pre-cART, proportions of naïve and central memory CD4+ and CD8+ T-cells correlated with IL-7R+ CD4+ and CD8+ T cells in non-C-IRIS, but not C-IRIS, patients. (B) Plasma IL-7 levels did not correlate with proportions of naïve or central memory CD4+ or CD8+ T-cells in either patient group. Analysis using IL-7R expression median fluorescence intensity (MFI) showed a similar trend.
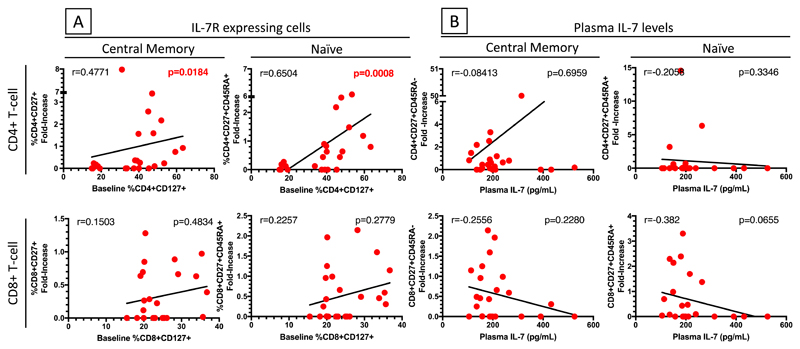
We further assessed the association between proportion of IL-7R+ T-cells pre-cART and fold-increase in central memory and naïve T-cell subsets from pre-cART to the time of C-IRIS event in C-IRIS patients. The fold increase in central memory and naïve CD4+ T-cells positively correlated with the proportion of IL-7R+ CD4+ T-cells pre-cART (r=0.4771, p=0.0184 and r=0.6504, p=0.008, respectively) (Fig. 4A). However, similar correlation was not observed for the CD8+ T-cell compartment. In contrast, no correlations between fold-increase of these T-cell subsets and plasma IL-7 levels were observed (Fig. 4B), suggesting that in C-IRIS patients, increases in naïve and central memory CD4+ T-cell subsets are dependent on the proportion of CD4+ T-cells expressing IL-7R (CD4+CD127+) but not the amount of plasma IL-7.
Fig. 4. Correlations of IL-7R+ CD4+ and CD8+ T cells or plasma IL-7 levels pre-cART with fold-increase in proportions of central memory and naïve CD4+ and CD8+ T cells at C-IRIS event in C-IRIS patients.
(A) Fold increase in proportions of central memory and naïve CD4+, but not CD8+, T-cells at C-IRIS event correlated with proportions of IL-7R+ CD4+ T cells pre-cART. (B) Plasma IL-7 levels pre-cART did not correlate with fold-increase in central memory or naïve CD4+ or CD8+ T-cells at C-IRIS event. Analyses using IL-7R expression median fluorescence intensity (MFI) showed a similar trend.
Development of C-IRIS was associated with higher monocyte activation and CSF cryptococcal culture positivity pre-cART
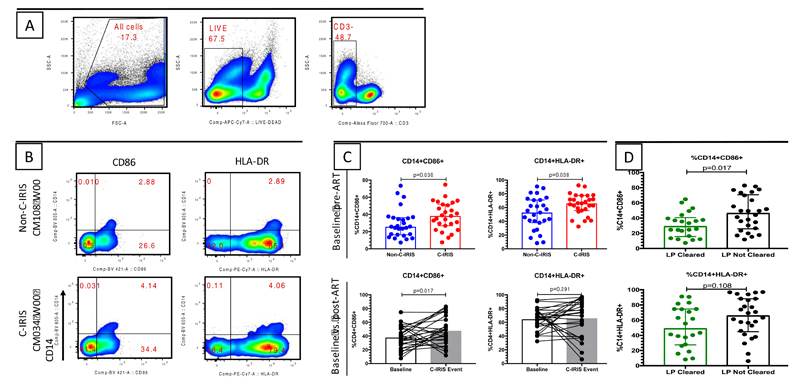
We further sought to investigate the immunopathogenesis of C-IRIS by assessing T-cell and monocyte (CD14+ cells) activation. CD4+ and CD8+ T-cell activation was defined by expression of both CD38 and HLA-DR, while exhaustion was assessed by PD-1 expression. We observed comparable percentages of activated T-cells between the C-IRIS and non-C-IRIS patients at pre-cART (Fig. S3A). These percentages did not change significantly at the time of C-IRIS event for those who developed C-IRIS (Fig. S3B). Interestingly, when monocyte activation was assessed by CD86 or HLA-DR expression on CD14+ cells as illustrated by the gating strategy (Fig. 5A) and representative flow plot (Fig. 5B), C-IRIS individuals had significantly higher activation than non-C-IRIS controls pre-cART (p=0.036 and p=0.038 respectively) (Fig. 5C). Furthermore, at the time of C-IRIS event, CD14+CD86+ cells were more frequent than pre-cART in C-IRIS patients (p=0.017) (Fig. 5C).
Fig. 5. Activated monocytes were higher in C-IRIS than non-C-IRIS patients and in patients who had positive CSF cultures for Cryptococci pre-cART.
(A) Gating strategy. (B) Representative flow cytometry gating strategy for defining activated (CD86+ or HLA-DR+) monocytes (CD14+). CM108 did not experience C-IRIS while CM034 did. (C) Proportions of activated monocytes were higher in C-IRIS patients compared to non-C-IRIS patients pre-cART, and CD86+ monocytes increased further at the time of C-IRIS event. (D) Proportions of activated monocytes (CD14+HLA-DR+) were higher in patients who had positive CSF cultures for Cryptococci pre-cART. Comparison between non-C-IRIS vs. C-IRIS was undertaken using the Mann-Whitney test.
Since studies have suggested that activation of innate immune responses in IRIS conditions may be largely caused by opportunistic infection antigen load 11,29, we further investigated if monocyte activation correlated with CSF cryptococcal antigen burden. We observed that monocyte activation (CD14+CD86+) was associated with CSF cryptococcal positivity pre-cART (p=0.017), and CD14+HLA-DR+ monocytes were also higher in those individuals who failed to clear cryptococci from CSF pre-cART, although in the latter case the difference was not statistically significant (p=0.108) (Fig. 5D).
We also explored whether the presumptive dysregulation of the IL-7/IL-7R signaling pathway in T-cells of patients who developed C-IRIS might affect Cryptococcus-specific T-cell responses by measuring responses to ACA, derived from the Cryptococcus cultured from each patient’s CSF, and to CMP from the cap67 strain of Cryptococcus (Fig. S4). Pre-cART, SEB-activated and ACA- and CMP-specific IFN-γ, IL-2 and TNF-α T-cell responses of C-IRIS individuals tended to be higher, albeit not statistically significant, than in non-C-IRIS patients (Fig. S4A and Fig. S4C). At the time of C-IRIS, cases showed a decrease in the frequency of SEB-activated (p=0.056), ACA-specific (p=0.046) and CMP-specific (p=0.005) CD4+ T-cell IFN-γ responses compared to pre-cART (Fig. S4B). Similarly, significant decreases of ACA-specific CD8+ T-cell IL-2 responses (p=0.008) and CMP-specific IFN-γ responses (p=0.022) were observed at C-IRIS event (Fig. S4D). We therefore did not find that ACA- or CMP-specific T-cell responses were lower pre-cART or rose after cART in C-IRIS patients.
Both plasma IL-7 levels and proportion of activated monocytes were predictive of C-IRIS in a multivariable regression analysis
Finally, we assessed the risk of C-IRIS occurrence in a ten-parameter multivariable Cox proportional-hazards regression analysis where all pre-cART correlates from univariate analysis with a p-value <0.1 were considered relevant (Table S1). High plasma IL-7 levels remained predictive of C-IRIS (HR=5.7 [95% CI, 1.70-19.3]; p=0.005). In addition, the proportion of activated monocytes (CD14+HLA-DR+) (HR=1.06 [95% CI, 1.01-1.10]; p=0.009) was also predictive of C-IRIS. There was also a non-significant trend for low proportions of IL-7R+ CD8+ T-cells as a predictor of C-IRIS (HR=0.836 [95% CI, 0.694-1.007], p=0.059).
Discussion
In this study of C-IRIS cases and non-C-IRIS controls matched for CD4+ T-cell counts, several findings provided evidence that dysregulation of the IL-7/IL-7R signaling pathway in T-cells might contribute to the immunopathogenesis of C-IRIS and explain our previous observation that a high plasma IL-7 level is predictive of C-IRIS 30,31. In non-C-IRIS patients, there was a positive correlation between proportion of total and central memory CD4+ T-cells expressing IL-7R and CD4+ T-cell count pre-cART, however, no such correlation was observed in C-IRIS patients. Similarly, there was also a positive correlation between proportion of IL-7R+ T-cells and the proportion of naïve and central memory CD4+ and CD8+ T-cells but only in non-C-IRIS patients. In contrast, the proportion of IL-7R+ total and central memory CD4+ T-cells pre-cART negatively correlated with fold-increase in CD4+ T-cell counts in non-C-IRIS but not C-IRIS patients. These findings suggest that IL-7R may play a role in the homeostasis of T-cells pre-cART initiation in non-C-IRIS patients but not C-IRIS patients. However, in C-IRIS patients, we demonstrated a positive correlation between the proportion of IL-7R+ CD4+ T-cells pre-cART and the fold increase in central memory and naïve CD4+ T-cells at the time of C-IRIS, which was much earlier than the time at which fold-increase in CD4+ T-cell counts was examined. Interestingly, plasma IL-7 levels did not correlate with pre-cART CD4+ T-cell counts or proportions of IL-7+ central memory and naïve CD4+ T-cells, nor with fold-increase in any of these cell populations in any patient group at any time point. On assessing immune activation, we observed that pre-cART monocyte activation, linked to CSF cryptococcal culture positivity, was associated with C-IRIS. In contrast, we did not observe a relationship with T-cell activation, and baseline Cryptococcus-specific T-cell responses were similar in non-C-IRIS and C-IRIS individuals. In a multivariable regression analysis, IL-7 levels and the proportion of activated monocytes remained predictive of C-IRIS.
While acknowledging that we have not assessed the IL7/IL-7R signaling pathway directly, to our knowledge, our study is the first to implicate dysfunction of the IL7/IL-7R signaling pathway in the immunopathogenesis of C-IRIS, although plasma IL-7 levels have previously been implicated 6,12.
Our finding that baseline CD4+ T-cell counts strongly correlated with proportions of T-cells expressing IL-7R (but not baseline plasma IL-7 levels) in non-C-IRIS individuals but not in C-IRIS individuals strongly suggests a functional difference in the IL-7/IL-7R signaling pathway of T-cells in non-C-IRIS and C-IRIS patients. It was previously demonstrated that cART initiation rescued IL-7R expression on T-cells in HIV infection 32, and deficiencies in the IL-7R expression have been shown to result in severe lymphopenia 17,18. The significant correlations of CD4+ T-cell counts and proportions of central memory and naïve T-cells with IL-7R expression in non-C-IRIS patients may indicate that the cellular machinery required for T-cell homeostasis before cART initiation remains intact whereas this may not be the case in C-IRIS patients. Overall, our data are consistent with the current theory that a low CD4+ T-cell count is an underlying factor in C-IRIS immunopathogenesis. The data further suggest defects in IL-7R-dependent T-cell proliferation and maturation in C-IRIS but not non-C-IRIS HIV-1-infected patients with CM. Previously observed differences in plasma IL-7 levels between C-IRIS and non-C-IRIS patients may therefore reflect a dysfunction in IL-7R expression or in the signaling pathway.
Consistent with a recent study of TB-IRIS, there was no significant difference in T-cell activation or exhaustion between non-C-IRIS and C-IRIS patients pre-cART 33. In contrast, others have suggested that CD8+ T-cell activation might be relevant in some instances of IRIS 34,35. Interestingly, we observed that monocyte activation was associated with C-IRIS, in line with previous data implicating aberrant innate immune responses by neutrophils and monocytes in C-IRIS 36,37. High monocyte activation was associated with CSF cryptococcal culture positivity pre-cART, suggesting that persisting antigens following antifungal therapy may induce a proinflammatory antigen presenting cell bias, consistent with high plasma IL-6 levels observed in C-IRIS following cART commencement 9,12,33. Our data suggests that the monocyte activation observed in C-IRIS may be due to high fungal load, which has already been shown to be a major risk factor for C-IRIS 1,6,38. Considering that there may be interactions between components of the immune system, we performed a multivariable regression analysis of relevant components from the univariate analysis. Plasma IL-7 levels and monocyte activation remained strongly associated with C-IRIS.
In conclusion, these data provide evidence of a functional defect in the IL-7/IL-7R signaling pathway in HIV/CM coinfection that may contribute to the immunopathogenesis of C-IRIS. Specifically, the proportion of CD4+ or CD8+ T-cells expressing IL-7R but not plasma IL-7 levels correlated with CD4+ T-cell counts and proportions of central memory and naïve T-cells in non-C-IRIS but not C-IRIS individuals, suggesting IL-7/IL-7R signaling pathway dysregulation in the C-IRIS cases. These data also demonstrate that monocyte activation, linked to CSF cryptococcal culture positivity pre-cART, is a risk factor for developing C-IRIS. The cause of the presumed IL-7/IL-7R signaling pathway dysregulation in C-IRIS individuals deserves further investigation.
Supplementary Material
Acknowledgments
We acknowledge the patients and their families, and the staff at HIV Pathogenesis Programme, King Edward VIII Hospital, and members of the Ndung’u laboratory at AHRI in Durban, South Africa. We also acknowledge members of the Levitz laboratory at University of Massachusetts Medical School, Worcester, Massachusetts, United States of America. The initial flow cytometry staining panel was designed by Andrew Lim from the Department of Clinical Immunology, Royal Perth Hospital and PathWest Laboratory Medicine, Perth 6000, Australia.
Source of funding
Financial support granted by REACH Initiative (Research and Education in HIV/AIDS for Resource Poor Countries) (MAF) and Pfizer Neuroscience Grant (NS052.10 – CCC). CCC is supported by the Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship APP1092160. MAF was supported by NHMRC grant 510448. SRL is a NHMRC practitioner fellow APP1042654. TN was supported in by grants from the Howard Hughes Medical Institute and by the South African Department of Science and Technology/National Research Foundation Research Chairs Initiative. Additional support for this study was provided by the Victor Daitz Foundation. This work was also supported in part through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative [grant # DEL-15-006]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [grant # 107752/Z/15/Z] and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Chang CC, Dorasamy AA, Gosnell BI, et al. Clinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndrome. AIDS. 2013;27(13):2089–2099. doi: 10.1097/QAD.0b013e3283614a8d. [DOI] [PubMed] [Google Scholar]
- 2.Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10(11):791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King MD, Perlino CA, Cinnamon J, Jernigan JA. Paradoxical recurrent meningitis following therapy of cryptococcal meningitis: an immune reconstitution syndrome after initiation of highly active antiretroviral therapy. Int J STD AIDS. 2002;13(10):724–726. doi: 10.1258/095646202760326516. [DOI] [PubMed] [Google Scholar]
- 4.Walker NF, Scriven J, Meintjes G, Wilkinson RJ. Immune reconstitution inflammatory syndrome in HIV-infected patients. HIV AIDS (Auckl) 2015;7:49–64. doi: 10.2147/HIV.S42328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(3):424–433. doi: 10.1093/cid/cir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS medicine. 2010;7(12) doi: 10.1371/journal.pmed.1000384. e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Cunha Colombo ER, Mora DJ, Silva-Vergara ML. Immune reconstitution inflammatory syndrome (IRIS) associated with Cryptococcus neoformans infection in AIDS patients. Mycoses. 2011;54(4):e178–182. doi: 10.1111/j.1439-0507.2010.01870.x. [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7(6):395–401. doi: 10.1016/S1473-3099(07)70085-3. [DOI] [PubMed] [Google Scholar]
- 9.Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203(11):1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarvis JN, Meintjes G, Bicanic T, et al. Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog. 2015;11(4) doi: 10.1371/journal.ppat.1004754. e1004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meya DB, Manabe YC, Boulware DR, Janoff EN. The immunopathogenesis of cryptococcal immune reconstitution inflammatory syndrome: understanding a conundrum. Curr Opin Infect Dis. 2016;29(1):10–22. doi: 10.1097/QCO.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akilimali NA, Chang CC, Muema DM, et al. Plasma but not Cerebrospinal Fluid IL-7 and IL-5 Levels Pre-antiretroviral Therapy Commencement Predict Cryptococcosis-Associated Immune Reconstitution Inflammatory Syndrome. Clin Infect Dis. 2017;65(9):1551–1559. doi: 10.1093/cid/cix598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nature reviews Immunology. 2007;7(2):144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 14.McCaughtry TM, Etzensperger R, Alag A, et al. Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J Exp Med. 2012;209(12):2263–2276. doi: 10.1084/jem.20121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia SK, Tygrett LT, Grabstein KH, Waldschmidt TJ. The effect of in vivo IL-7 deprivation on T cell maturation. J Exp Med. 1995;181(4):1399–1409. doi: 10.1084/jem.181.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura MY, Pobezinsky LA, Guinter TI, et al. IL-7 signaling must be intermittent, not continuous, during CD8(+) T cell homeostasis to promote cell survival instead of cell death. Nat Immunol. 2013;14(2):143–151. doi: 10.1038/ni.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patra AK, Avots A, Zahedi RP, et al. An alternative NFAT-activation pathway mediated by IL-7 is critical for early thymocyte development. Nat Immunol. 2013;14(2):127–135. doi: 10.1038/ni.2507. [DOI] [PubMed] [Google Scholar]
- 19.Chang CC, Lim A, Omarjee S, et al. Cryptococcosis-IRIS is associated with lower cryptococcus-specific IFN-gamma responses before antiretroviral therapy but not higher T-cell responses during therapy. J Infect Dis. 2013;208(6):898–906. doi: 10.1093/infdis/jit271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvis JN, Casazza JP, Stone HH, et al. The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. J Infect Dis. 2013;207(12):1817–1828. doi: 10.1093/infdis/jit099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiesner DL, Moskalenko O, Corcoran JM, et al. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. MBio. 2012;3(5) doi: 10.1128/mBio.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansour MK, Schlesinger LS, Levitz SM. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J Immunol. 2002;168(6):2872–2879. doi: 10.4049/jimmunol.168.6.2872. [DOI] [PubMed] [Google Scholar]
- 23.Specht CA, Lee CK, Huang H, et al. Protection against Experimental Cryptococcosis following Vaccination with Glucan Particles Containing Cryptococcus Alkaline Extracts. MBio. 2015;6(6):e01905–01915. doi: 10.1128/mBio.01905-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79(2):167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nature reviews Immunology. 2012;12(3):191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. European journal of immunology. 2013;43(11):2797–2809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 27.Crawley AM, Angel JB. The influence of HIV on CD127 expression and its potential implications for IL-7 therapy. Semin Immunol. 2012;24(3):231–240. doi: 10.1016/j.smim.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Sasson SC, Zaunders JJ, Zanetti G, et al. Increased plasma interleukin-7 level correlates with decreased CD127 and Increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. J Infect Dis. 2006;193(4):505–514. doi: 10.1086/499309. [DOI] [PubMed] [Google Scholar]
- 29.Wiesner DL, Boulware DR. Cryptococcus-Related Immune Reconstitution Inflammatory Syndrome(IRIS): Pathogenesis and Its Clinical Implications. Curr Fungal Infect Rep. 2011;5(4):252–261. doi: 10.1007/s12281-011-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartling HJ, Ryder LP, Ullum H, Odum N, Nielsen SD. Gene variation in IL-7 receptor (IL-7R)alpha affects IL-7R response in CD4+ T cells in HIV-infected individuals. Sci Rep. 2017;7 doi: 10.1038/srep42036. 42036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartling HJ, Thorner LW, Erikstrup C, et al. Polymorphism in interleukin-7 receptor alpha gene is associated with faster CD4(+) T-cell recovery after initiation of combination antiretroviral therapy. AIDS. 2014;28(12):1739–1748. doi: 10.1097/QAD.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 32.Ndhlovu ZM, Kamya P, Mewalal N, et al. Magnitude and Kinetics of CD8+ T Cell Activation during Hyperacute HIV Infection Impact Viral Set Point. Immunity. 2015;43(3):591–604. doi: 10.1016/j.immuni.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravimohan S, Tamuhla N, Nfanyana K, et al. Robust Reconstitution of Tuberculosis-Specific Polyfunctional CD4+ T-Cell Responses and Rising Systemic Interleukin 6 in Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(6):795–803. doi: 10.1093/cid/civ978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aberg JA, Powderly WG. Cryptococcosis and HIV. HIV InSite Knowledge Base Chapter. 2006 [Google Scholar]
- 35.Sudo T, Nishikawa S, Ohno N, et al. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci U S A. 1993;90(19):9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai RP, Nakiwala JK, Meintjes G, Wilkinson RJ. The immunopathogenesis of the HIV tuberculosis immune reconstitution inflammatory syndrome. European journal of immunology. 2013;43(8):1995–2002. doi: 10.1002/eji.201343632. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Blondel G, Mars LT, Liblau RS. Pathogenesis of the immune reconstitution inflammatory syndrome in HIV-infected patients. Curr Opin Infect Dis. 2012;25(3):312–320. doi: 10.1097/QCO.0b013e328352b664. [DOI] [PubMed] [Google Scholar]
- 38.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175(7):4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.