Abstract
Circulating tumor cells (CTCs) are the source of metastases, but only an infinitesimal fraction of them eventually succeed in colonizing a distant organ. New results show that CD44-dependent aggregation in the circulation provides CTCs with cancer stem cell-like characteristics, suggesting an explanation for the low metastatic efficiency of CTCs, but also avenues for therapeutic intervention.
Whether entering the circulation directly through the venous system in the primary tumor, or by traveling via the lymphatic system, most metastases develop from cells that eventually enter the secondary organ via arterial circulation. At some point, cancer clones that form metastases thus exist as CTCs. Some data, mostly from model systems, suggest that neoplastic cells may leave primary tumors already at an early stage of cancer development (1). Genetic evidence from multiple different tumor types has demonstrated, however, that metastases are typically formed by advanced cancers with few, if any, new driver mutations acquired at the latest stages of cancer progression (2,3). This suggests that the qualities needed for successful metastatic colonization are in most cases exclusive to CTCs from advanced cancers, and that any possible early leavers are unlikely to form clinically relevant secondary tumors. The vast majority of CTCs, even from the most aggressive clones, perish before being able to colonize distant organs (3), but the molecular mechanisms that determine the fate of individual CTCs have remained poorly understood.
The presence of CTCs is associated with poor clinical outcome in several cancers (4). Patients with metastatic breast cancer may at a given time point have millions of tumor cells in their circulation, but the variation between individuals is large, with many patients not having any detectable CTCs in the standard 5-10ml of blood processed (5). The typically small volume of blood sampled complicates the interpretation of CTC data, and novel approaches based on apheresis or in vivo CTC collecting devices (6) are being developed to increase the yield of CTCs from patients. Due to technical challenges, experimental analysis of CTCs is still difficult and consequently their biology is poorly characterized, although some interesting results are starting to emerge. For example, recent studies have reported that CTCs may exist in clusters, which are derived from either collectively invasive groups of cells or by passive shedding of the primary tumor into the vasculature (7,8). A commonly detected feature of CTC clusters seems to be that even though they are rare, they are significantly more metastatic than individual CTCs.
In this issue, Liu and colleagues (9) use breast cancer PDX models, cell lines and clinical data sets to study the mechanisms of breast cancer progression and CTC biology (Figure 1). Their initial observations were made by immunohistochemical analysis of human tissue sections from cancer patients, in which they detected both CTCs and CTC clusters. The authors followed up on these findings by analyzing CTCs directly from patient blood as well as in animal models in which cancer cells labelled with fluorescent markers unequivocally confirmed that different cancer clones could form polyclonal metastasis. Interestingly, intravital multiphoton microscopic imaging revealed that, in contrast to previous reports (7,8), CTC clusters were not derived from collectively migrating groups of cells. Rather, individual tumor cells aggregated into clusters within the vasculature. Follow-up experiments using differentially timed intravenous inoculation of cancer cells confirmed the results. Collectively these data show that spatially and temporally proximal intravasation events can lead to the formation of CTC clusters even in areas with rapid blood flow.
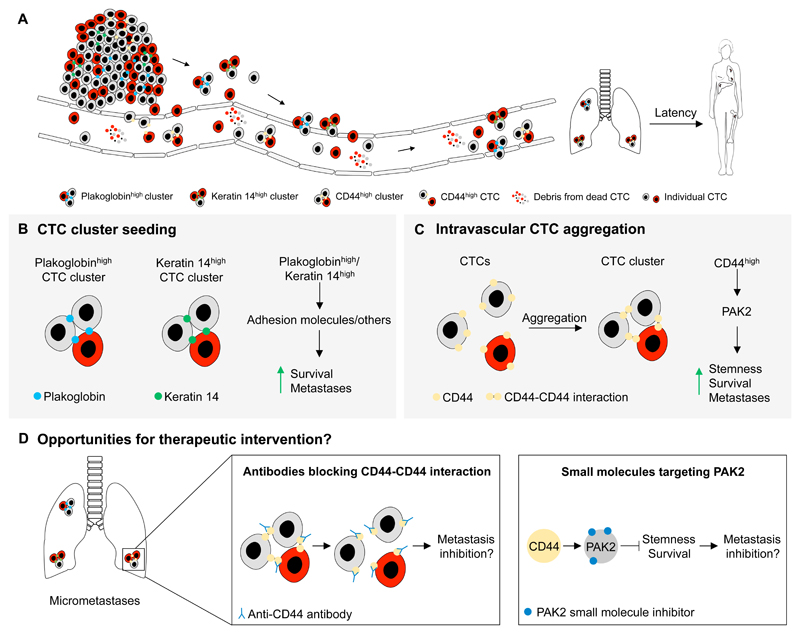
Figure 1. Circulating tumor cell clusters display enhanced metastatic fitness.
(A) Cancer cells can leave the primary tumor either as individual cells or as clusters, entering the circulation directly or via the lymphatic system. Spatially and temporally proximal intravasation events can lead to cluster formation also within the vasculature. Most CTCs are eliminated and do not form clinically significant metastases. However, both pre-formed and de novo CTC clusters have a survival advantage over individual CTCs and they display enhanced cancer stem cell phenotypes, consequently leading to more efficient metastasis formation in secondary organs. (B) CTC clusters with high expression of either plakoglobin or keratin 14 can originate from collective migration or passive shedding of the primary tumor. These factors and their downstream effectors increase survival and metastatic potential. (C) Individual migrating tumor cells can aggregate in the blood forming CTC clusters in a CD44-dependent manner. CD44-CD44 homophilic interactions stabilize these CTC clusters, leading to the activation of PAK2-signaling. This improves stemness, survival and metastatic growth of CTC clusters. (D) Preventing CTC cluster formation or targeting CTC clusters in circulation is challenging due to the narrow window of therapeutic opportunity. If CD44-dependent signaling supports cancer cell clusters also at the early stages of metastatic colonization, targeting CD44-CD44 interaction with antibodies or PAK2 activation with small molecule inhibitors could reduce the viability of disseminated CTC clusters and consequently help reduce the likelihood of clinical metastases in the adjuvant setting.
In line with previous observations, Liu and colleagues found that CTC clusters were more potent in forming distant metastases when compared to individual CTCs. This led the authors to ask whether CTC clusters were associated with cancer stem cell-like properties. The authors found that in addition to being capable of more efficient tumor sphere formation in vitro, CTC clusters also expressed CD44, a cell surface transmembrane glycoprotein that has been used to isolate breast cancer stem cells in previous studies. Mammary fat pad implants and tail-vein infusion experiments of clustered PDX cells showed that cancer cell clusters had higher tumor-initiating and metastatic growth potential when compared to individual cancer cells. To interrogate the functional importance of CD44 in tumor cell aggregation and lung colonization, the authors compared cell aggregation and spontaneous lung metastasis potential after orthotopic implantation or tail-vein inoculation of CD44+ and CD44- tumor cells sorted from PDXs. Only CD44+ tumor cells formed large polyclonal aggregates and had higher tumorigenic and metastatic capacity. Moreover, inhibition of CD44 with siRNAs or CRISPR/Cas9 decreased cluster formation and reduced tumor growth and lung colonization, independently of the CD44 splice variant inhibited. These experiments convincingly show that CD44 mediates cancer cell aggregation, leading to increased tumorigenic fitness in different contexts, a result which is in line with previous evidence linking CD44 expression with increased metastatic capacity of CTCs (5). The connection between CTC aggregation and cancer stem cell functions may also partially explain why the majority of CTCs from even the most aggressive cancer clones fail to form distant metatases.
Liu and colleagues went on to demonstrate that CD44 elicits its pro-aggregation effects through homophilic CD44-CD44 interaction between neighboring cancer cells. Furthermore, using mass spectrometry, the authors identified PAK2, a serine/threonine kinase, as one of the most differentially expressed proteins after CTC aggregation. Further mechanistic dissection showed that PAK2 protein levels were significantly reduced in CD44 knockdown cells and inactivation of PAK2 impaired tumor cell aggregation and lung colonization, phenocopying the effects of CD44 inhibition. A direct interaction between CD44 and PAK2 was also detected by co-immunoprecipitation experiments, and both proteins were shown to co-localize in the plasma membrane of aggregated cells. Collectively, these results led the authors to propose that CD44-CD44 signaling in CTC clusters enhances PAK2 activation, which in turn supports cancer cell stemness and metastatic fitness, but the precise mechanisms through which CD44-CD44 interaction leads to PAK2 activation and the proposed downstream effects remain open for further studies. Finally, in clinical data sets high levels of CD44 and PAK2 mRNA as well as the presence of CTC clusters were associated with poor patient outcome.
A number of interesting questions arise from the work by Liu and colleagues. Previous studies have identified the expression of either keratin 14 (8) or plakoglobin (7) as important mediators of cancer cell clustering and enhanced metastatic potential. Whether and how the CD44-PAK2 axis interacts with keratin 14 and/or plakoglobin in support of CTC clustering and metastasis remains unclear, but it could be that the functional output of these pathways converges at some level. Also, the relative contribution and biological implications of collectively migrating cancer cells, passively shedding cancer cell clusters and de novo CTC cluster formation in circulation remains unexplored. For example, are CTC clusters that arise through different routes functionally different? Do they have unique molecular vulnerabilities? Furthermore, circulating tumor cells are thought to suffer from increased oxidative stress, leading to their widespread elimination (10). Do any of these known molecular pathways that support CTC clusters protect CTCs from oxidative stress-induced death?
From a clinical point of view, exploring whether CD44-dependent aggregation supports disseminated cancer cells during metastatic dormancy seems of relevance. Especially in the case of estrogen receptor positive (ER+) breast cancer, metastases can develop years after primary tumor removal. It would therefore be interesting to know whether disseminated cancer cell clusters also rely on the CD44-PAK2 axis for the maintenance of tumor forming capacity. Targeting CTC clusters in circulation or preventing their formation in the first place is likely to be difficult due to the narrow window of opportunity: the time between cancer cells leaving the primary tumor, forming aggregates and reaching a secondary organ is very short. On the other hand, if similar mechanisms support the survival of disseminated cancer cells after they have reached secondary organs, blocking CD44-CD44 interaction with antibodies, targeting PAK2 with a small molecule or inhibiting the downstream effectors of PAK2 could result in therapeutic benefit in the adjuvant setting. Targeting plakoglobin or keratin 14-dependent pathways could also be beneficial in this context.
There is little doubt that understanding the consequences of the cross talk between tumor cells is critical for a comprehensive understanding of cancer progression and metastasis. Innovative approaches such as those used by Liu and colleagues will hopefully therefore continue to uncover the intricate molecular mechanisms that govern the interplay between metastatic cells in transit and beyond. New developments in CTC technologies are likely to facilitate both clinical follow-up of cancer patients and in-depth functional analyses of CTC biology. Already now it seems clear, however, that getting together may synergistically increase the likelihood of successful metastatic colonization by CTCs. The next focus should be in testing whether these developments could be translated into patient benefit.
Acknowledgements
Our research is supported by the Medical Research Council (MC_UU_12022/7).
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Klein CA. Selection and adaptation during metastatic cancer progression. Nature. 2013;501(7467):365–72. doi: 10.1038/nature12628. [DOI] [PubMed] [Google Scholar]
- 2.Patel SA, Vanharanta S. Epigenetic determinants of metastasis. Mol Oncol. 2016 doi: 10.1016/j.molonc.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24(4):410–21. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2018 doi: 10.1038/s41576-018-0071-5. [DOI] [PubMed] [Google Scholar]
- 5.Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31(6):539–44. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 6.Gorges TM, Penkalla N, Schalk T, Joosse SA, Riethdorf S, Tucholski J, et al. Enumeration and Molecular Characterization of Tumor Cells in Lung Cancer Patients Using a Novel In Vivo Device for Capturing Circulating Tumor Cells. Clin Cancer Res. 2016;22(9):2197–206. doi: 10.1158/1078-0432.CCR-15-1416. [DOI] [PubMed] [Google Scholar]
- 7.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–22. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A. 2016;113(7):E854–63. doi: 10.1073/pnas.1508541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Taftaf R, Kawaguchi M, Chang YF, Chen W, Entenberg D, et al. Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov. 2018 doi: 10.1158/2159-8290.CD-18-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527(7577):186–91. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]