Abstract
Background
Data on the pharmacological treatment of trichotillomania are limited. Milk thistle has antioxidant properties, and showed promise in trichotillomania in a prior case report. The goal of the current study was to determine the efficacy and tolerability of silymarin in children and adults with trichotillomania.
Methods
20 individuals (19 [95.0%] women; 16 adults; mean age = 27.9 [11.5] years) with trichotillomania entered a 12-week, double-blind, placebo-controlled cross-over study (6 weeks of milk thistle and 6 weeks of placebo with a one-week wash-out in between). Dosing of milk thistle ranged from 150mg bid to 300mg bid. Subjects were assessed with the NIMH Trichotillomania Severity Scale (primary outcome), the Massachusetts General Hospital Hair Pulling Scale, Clinical Global Impression scale, and measures of depression, anxiety, and psychosocial functioning. Outcomes were examined using linear mixed models with a random intercept for subject; and t-tests.
Results
There was no statistically significant treatment type-by-time interactions for the main outcome measure but significant effects were seen for secondary measures (for example time spent pulling per day for the past week). From baseline to week 6 there was a significant decrease in CGI severity for the milk thistle group but not in the placebo group.
Conclusions
This trial failed to show that milk thistle was more effective than placebo on the main outcome measure, but milk thistle did demonstrate significant improvements on select secondary outcome measures. These findings may shed light on important neurochemical targets worthy of future investigation.
Trial Registration
ClinicalTrials.gov identifier: NCT02473913
Keywords: trichotillomania, silymarin, milk thistle, treatment, natural supplement
Background
Trichotillomania is a potentially disabling, under-recognized condition in which individuals repeatedly pull out their hair, leading to noticeable hair loss. Psychosocial problems are common in trichotillomania and often include significantly reduced quality of life, reduced work productivity, and impaired psychosocial functioning.1–3 Although there is no FDA-approved treatment for trichotillomania, some evidence in adults suggests that N-acetylcysteine and olanzapine may help reduce hair pulling urges and behavior, but findings in support of both options for use with adults and children are limited and have not been replicated.4–6 Given the lack of clearly effective pharmacological treatments for trichotillomania, additional treatment options are needed.
A few years ago we reported a case of a young woman with trichotillomania who responded to 150mg twice a day of milk thistle.7 Silymarin is a complex extract from the milk thistle plant and contains a number of components- notably the flavonolignans (e.g. silybin A, silybin B) that are purported to have therapeutic activity such as antioxidant and immune modulating properties. Milk thistle extracts have been used for centuries for medicinal purposes including hepatoprotective effects. One basis for the possible use of milk thistle in trichotillomania is that oxidative stress has been associated with neurodegenerative processes and a range of psychological disorders, including body focused repetitive behavior disorders (BFRBs).8 Milk thistle extracts may have potential in treating trichotillomania because silymarin seems to inhibit the formation of free radicals and nitric oxide9, while silibin appears to attenuate inflammatory responses and increases glutathione levels.10 In a pilot study, we recently found that low glutathione levels correlated with worse inhibitory control in trichotillomania, which may implicate glutathione in its pathophysiology.8 Milk thistle appears sufficient to induce peripheral antioxidant properties, and even central nervous system effects in rodents,10 but whether and to what extent it exerts neurobiological affects in humans remains unclear. An additional and non-mutually exclusive hypothesis suggests that monoaminergic transmission may play an important role in the possible pathophysiology of compulsive/habitual behaviors such as BFRBs via its ability to modulate many cognitive functions including memory, attention, task switching, and response inhibition.11 Thus, any agent capable of increasing frontal cortical levels of key neurotransmitters could improve the executive functioning deficits associated with compulsive/habitual behaviors. In vitro, milk thistle was found to inhibit glial cell monoamine oxidase (MAO) activity,12 which if reflected in vivo in the central nervous system would be expected to enhance monoaminergic transmission in such a fashion. In preclinical work, repeated methamphetamine dosing led to memory impairment and prefrontal dopamine reduction; and these cognitive and neurochemical effects were diminished by administering a milk thistle derivative.13 Again, if reflected in humans, this may indicate that milk thistle could strengthen cortical dopaminergic transmission and associated top-down cognitive abilities in situations of sub-optimal functioning. Given its potential efficacy for reducing symptoms of BFRBs, the present study assessed the tolerability and efficacy of milk thistle in the treatment of children and adults with trichotillomania using a double-blind, placebo-controlled cross-over design. We hypothesized that milk thistle would reduce compulsive hair pulling in individuals with trichotillomania to a greater extent than placebo.
Methods
Participants
Men and women aged 12 to 65 years with a primary DSM-5 diagnosis of trichotillomania were recruited by newspaper advertisements and referrals from local clinicians. Children and adults were both included because current available evidence suggests that trichotillomania presents similarly in children and adults, and successful treatments for both children and adults are lacking. DSM diagnosis was confirmed using a validated clinical interview (Minnesota Impulse Disorders Inventory, MIDI14). Exclusion criteria included: 1) unstable medical illness; 2) history of significant neurological disorder; 3) current pregnancy or lactation, or inadequate contraception in women of childbearing potential; 4) any thoughts of suicide (as determined by the Columbia Suicide Severity Rating Scale); 5) lifetime history of bipolar disorder, dementia, or any psychotic disorder; 6) past three months substance use or alcohol use disorder; 7) previous treatment with milk thistle; and 8) current participation in any other clinical trial.
Participants who were currently taking psychotropic medications were allowed into the study as long as the dose of medication had been stable for three months prior to study inclusion and there were no plans to modify the dose during the study duration. Similarly, participants attending individual or group psychotherapy were allowed to participate if attendance had been ongoing weekly for at least three months prior to study entry. Participants who changed doses of medication or started therapy, based on their self-report, were discontinued from the study (changes in treatment were assessed at each study visit and no one was withdrawn due to this reason). Participants were compensated the equivalent of $20 per visit paid in the form of a check at the end of the study.
The institutional review board for the University of Chicago approved the study, the informed consent, and in the case of children the parental consent and child assent. Prior to participation, the primary investigator and/or trained study personnel discussed potential risks of the study and alternative treatments with participants prior to obtaining informed consent. After receiving a complete description of the study, participants provided written informed consent. This study was carried out in accordance with the Declaration of Helsinki. Data were collected between September 1, 2016 and May 17, 2018. The study was registered at ClinicalTrials.gov (identifier: NCT02473913).
Study Design
Following baseline assessment, eligible participants were randomly assigned in a double-blind fashion to receive EITHER (a) 6-week milk thistle, followed by one-week washout, followed by 6-week placebo; OR (b) 6-week placebo, followed by one-week washout, followed by 6-week milk thistle. Order of drug-placebo was fully counterbalanced across the study participants and all blinding and assignment was conducted using a randomization code provided by a pharmacy independent of the research team. The study used the Jarrow brand of milk thistle, and the manufacturer provided a certificate of analysis to our investigational pharmacy.
During the time on active medication, all eligible study participants were started on milk thistle 150mg BID for two weeks and then increased to 300mg po bid for the remaining 4 weeks. Dose range selection was based on safety and efficacy data from case reports using milk thistle in obsessive compulsive spectrum disorders.7 Participants who were not compliant with their use of study medication (i.e. failing to take medication for three or more consecutive days) were discontinued from the study. Participants were asked at each visit about the number of doses missed per week.
Assessments
Demographics and clinical features of trichotillomania were assessed with a semi-structured interview. Race/ethnicity was defined by the study participants and was included to learn more about this variable in trichotillomania. Psychiatric comorbidity was assessed using the Mini-International Neuropsychiatric Interview.15 A complete medical history and general physical examination were also obtained for each participant.
At baseline and at every two-week visit, participants were assessed with several measures of severity and symptom change. Investigators assessed trichotillomania symptoms using the clinician-administered NIMH Trichotillomania Severity Scale (NIMH-TSS),16 the primary outcome measure for the study. The NIMH scale is a valid and reliable 5-item, clinician-administered scale that rates hair pulling symptoms during the past week. The items assess pulling frequency (both on the previous day and during the past week), urge intensity, urge resistance, subjective distress, and interference with daily activities.
Other measures included: the valid and reliable 7-item self-report Massachusetts General Hospital Hair Pulling Scale (MGH-HPS)17; the clinician-administered Clinical Global Impression - Severity and Improvement (CGI)18; the Sheehan Disability Scale (SDS)19, a three-item, reliable and valid self-report assessment of psychosocial functioning; the Hamilton Anxiety Rating Scale (HAM-A)20, a reliable, valid, clinician-administered, 14-item scale that provides an overall measure of global anxiety; and the Hamilton Depression Rating Scale (HAM-D)21, a valid, reliable, clinician-administered rating scale assessing severity of depressive symptoms consisting of 17-items.
Safety assessments were conducted at each visit and included evaluations of sitting blood pressure, heart rate, and weight. Adverse effects were documented, including time of onset, time of resolution (if applicable), severity, action taken, and outcome. The investigator recorded use of concomitant medications in terms of daily dosage, start and stop dates, and reason for use.
Data Analysis
Descriptive summaries were presented in the total sample and in adults alone (since the majority of participants were adults). Outcomes were also summarized at all study timepoints, and baseline levels were statistically compared by study condition using linear mixed models with a random intercept for subject. Changes within-patient from baseline to week 6 were assessed using paired t-tests within the placebo study condition and milk thistle study condition separately. Differences in the changes from baseline to week 6 between study conditions were also assessed using paired t-tests. Missing data were not imputed. Because this was a pilot study, and in view of the sample size, a significance level of 0.05 was used. All analyses were conducted in SAS v9.4 and R.
Results
Participant Characteristics
Total of twenty participants were included in the study. Of these, 14 (70%) had complete data and 2 (10%) were only missing baseline data for their second study condition (1 for placebo and 1 for milk thistle). Lastly, 20% (4 participants) dropped out/had missing data at each subsequent visit. Two of the dropouts were randomized to milk thistle first, and the other 2 were randomized to placebo first. Thus all missing data were missing at random with respected to treatment assignment. All 20 participants had baseline data and at least one follow-up visit for at least one of the outcomes examined in at least one of the study conditions. Demographic summaries are presented in Table 1 for all participants and adults only.
Table 1. Demographic Summaries.
| All Participants (n=20) |
Adults Only (n = 16) |
|
|---|---|---|
| Randomization | ||
| Milk thistle first | 8 (40.0%) | 6 (37.5%) |
| Placebo first | 12 (60.0%) | 10 (62.5%) |
| Age (Years) - Mean (SD) | 27.9 (11.5) | 31.3 (10.3) |
| Age at Onset (Years) – Mean (SD), missing n=1 | 12.4 (4.6) | 13.0 (5.0) |
| Average minutes of pulling per day – Mean (SD) | 94.5 (113.6) | 85.9 (91.2) |
| Sex – n (%) | ||
| Female | 19 (95.0%) | 15 (93.7%) |
| Education – n (%) | ||
| Currently in high school | 4 (20.0%) | 0 (0.0%) |
| Graduated high school/GED | 2 (10.0%) | 2 (12.5%) |
| Some college | 4 (20.0%) | 4 (25.0%) |
| College graduate | 3 (15.0%) | 3 (18.8%) |
| Post graduate (College +) | 7 (35.0%) | 7 (43.8%) |
| Marital Status – n (%) | ||
| Single | 16 (80.0%) | 12 (75.0%) |
| Married | 4 (20.0%) | 4 (25.0%) |
| Occupation – n (%) | ||
| Student | 8 (40.0%) | 5 (31.3%) |
| Student and employed | 3 (15.0%) | 2 (12.5%) |
| Work full-time | 5 (25.0%) | 5 (31.3%) |
| Work part-time | 2 (10.0%) | 2 (12.5%) |
| Unemployed | 2 (10.0%) | 2 (12.5%) |
| Current Psychotropic Medication – n (%) | ||
| Yes | 7 (35.0%) | 4 (25.0%) |
| Current ADHD – n (%) | ||
| Yes | 5 (25.0%) | 3 (18.8%) |
| Current Depression – n (%) | ||
| Yes | 7 (35.0%) | 4 (25.0%) |
| Current Anxiety – n (%) | ||
| Yes | 6 (30.0%) | 3 (18.8%) |
| Current Nail Biting – n (%) | ||
| Yes | 5 (25.0%) | 4 (25.0%) |
| Current Skin-Picking – n (%) | ||
| Yes | 12 (60.0%) | 8 (50.0%) |
Efficacy Results
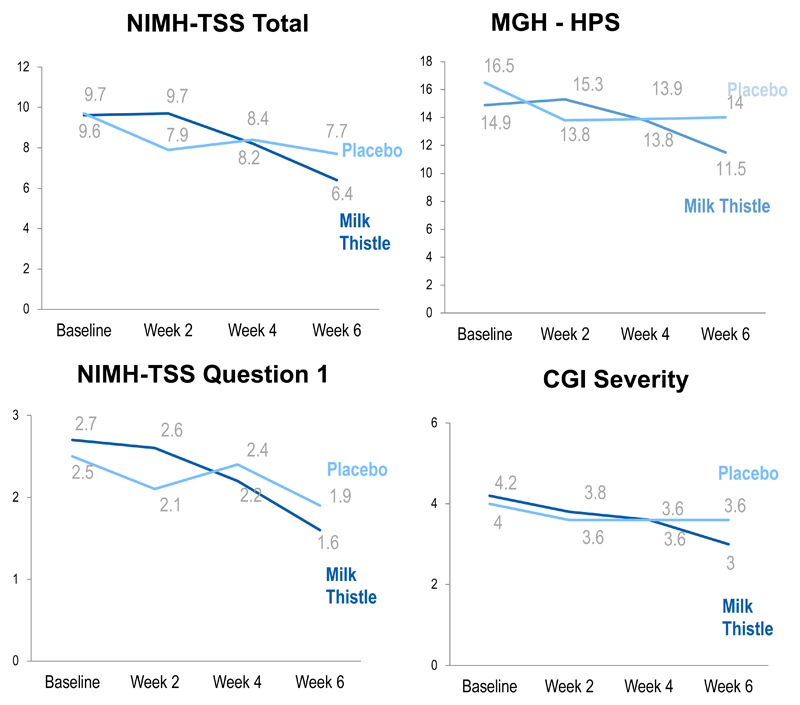
As indicated in Table 2 (see also Figure 1), there was no statistically significant treatment type-by-time interactions for the main outcome measure (NIMH-TSS) in linear mixed modeling, but significant effects were seen for secondary measures such as the MGH-HPS and question #1 of the NIMH-TSS scale (time spent pulling per day for the past week). From baseline to week 6 there was a significant decrease in CGI severity for the milk thistle group (p=0.004). The CGI-Improvement Scale score showed that 41.2% of participants assigned to milk thistle were much or very much improved compared with 31.3% of the placebo group. Both milk thistle and placebo showed significant improvement in psychosocial functioning (reflected by the Sheehan Disability Scale).
Table 2. Summaries of primary and secondary measures at each visit.
| All Patients | Adults Only | |||
|---|---|---|---|---|
| Milk Thistle | Placebo | Milk Thistle | Placebo | |
| NIMH Trichotillomania Severity Scale | ||||
| Baseline (n=17/17/14/15)1 | 9.6 (5.6) | 9.7 (4.5) | 9.0 (5.8) | 10.1 (4.7) |
| Week 2 (n=18/18/15/15) | 9.7 (5.3) | 7.9 (4.5) | 9.7 (5.6) | 8.0 (4.7) |
| Week 4 (n=18/18/15/15) | 8.2 (5.7) | 8.4 (5.3) | 9.1 (5.8) | 8.4 (5.5) |
| Week 6 (n=17/16/14/13) | 6.4 (5.5) | 7.7 (5.0) | 7.4 (5.4) | 8.4 (5.2) |
| P-value (BL to Week 6 change)2 | 0.054 | 0.088 | 0.230 | 0.110 |
| NIMH Trichotillomania Severity Scale –Question 1 – | ||||
| Baseline (n=19/17/15/15) | 2.7 (1.8) | 2.5 (1.4) | 2.5 (1.8) | 2.7 (1.4) |
| Week 2 (n=18/18/15/15) | 2.6 (1.6) | 2.1 (1.6) | 2.4 (1.6) | 2.3 (1.7) |
| Week 4 (n=18/18/15/15) | 2.2 (1.5) | 2.4 (1.6) | 2.3 (1.6) | 2.3 (1.6) |
| Week 6 (n=17/16/14/13) | 1.6 (1.4) | 1.9 (1.6) | 1.8 (1.4) | 2.2 (1.7) |
| P-value (BL to Week 6 change) | 0.039 | 0.144 | 0.131 | 0.145 |
| Massachusetts General Hospital Hair Pulling Scale –Total Score – | ||||
| Baseline (n=19/17/15/15) | 14.9 (5.7) | 16.5 (4.8) | 15.0 (6.0) | 16.3 (5.0) |
| Week 2 (n=18/18/15/15) | 15.3 (6.4) | 13.8 (5.5) | 16.1 (6.6) | 14.1 (5.7) |
| Week 4 (n=18/18/15/15) | 13.8 (7.6) | 13.9 (5.2) | 15.1 (7.1) | 14.0 (5.6) |
| Week 6 (n=17/16/14/13) | 11.5 (6.8) | 14.0 (5.3) | 12.5 (6.3) | 14.3 (5.4) |
| P-value (BL to Week 6 change) | 0.020 | 0.127 | 0.066 | 0.169 |
| CGI Severity - | ||||
| Baseline (n=17/17/14/15) | 4.2 (1.0) | 4.0 (0.6) | 4.1 (1.1) | 4.1 (0.6) |
| Week 2 (n=18/18/15/15) | 3.8 (1.2) | 3.6 (1.0) | 3.9 (1.1) | 3.7 (1.0) |
| Week 4 (n=18/18/15/15) | 3.6 (1.3) | 3.6 (1.0) | 3.8 (1.3) | 3.6 (1.1) |
| Week 6 (n=17/16/14/13) | 3.0 (1.4) | 3.6 (0.9) | 3.3 (1.3) | 3.8 (0.8) |
| P-value (BL to Week 6 change) | 0.004 | 0.173 | 0.006 | 0.175 |
| CGI Improvement – n (%) Much/Very Much Improved | ||||
| Week 2 (n=18/18/15/15) | 4 (22.2%) | 4(22.2%) | 1 (6.7%) | 3 (20.0%) |
| Week 4 (n=18/18/15/15) | 6 (33.3%) | 5(27.8%) | 4 (26.7%) | 5 (33.3%) |
| Week 6 (n=17/16/14/13) | 7 (41.2%) | 5(31.3%) | 5 (35.7%) | 4 (30.8%) |
| Sheehan Disability Scale - | ||||
| Baseline (n=18/17/15/15) | 9.7 (9.1) | 10.5 (5.4) | 8.7 (9.5) | 10.7 (5.4) |
| Week 2 (n=18/18/15/15) | 8.3 (8.3) | 8.1 (8.3) | 8.9 (9.0) | 8.4 (8.8) |
| Week 4 (n=18/18/15/15) | 6.8 (8.1) | 7.1 (7.6) | 7.6 (8.5) | 7.3 (8.3) |
| Week 6 (n=17/16/14/13) | 5.3 (7.8) | 6.0 (6.8) | 5.8 (8.4) | 6.4 (7.3) |
| P-value (BL to Week 6 change) | 0.027 | 0.004 | 0.056 | 0.008 |
| Hamilton Anxiety Scale - | ||||
| Baseline (n=17/17/14/15) | 5.9 (5.6) | 5.1 (4.5) | 6.1 (6.1) | 4.1 (3.9) |
| Week 2 (n=18/18/15/15) | 4.6 (4.3) | 4.7 (3.6) | 4.8 (4.7) | 4.7 (3.8) |
| Week 4 (n=18/18/15/15) | 4.5 (5.1) | 5.1 (3.4) | 4.8 (5.5) | 4.5 (3.2) |
| Week 6 (n=17/16/14/13) | 2.8 (3.5) | 3.3 (3.1) | 2.7 (3.7) | 3.1 (3.5) |
| P-value (BL to Week 6 change) | 0.125 | 0.100 | 0.183 | 0.342 |
| Hamilton Depression Scale - | ||||
| Baseline (n=17/17/14/15) | 6.6 (5.8) | 5.5 (4.4) | 5.6 (5.4) | 4.9 (4.3) |
| Week 2 (n=18/18/15/15) | 4.1 (3.6) | 4.5 (4.6) | 4.3 (3.9) | 4.1 (4.1) |
| Week 4 (n=18/18/15/15) | 4.8 (5.9) | 4.4 (3.6) | 4.9 (6.3) | 3.3 (2.7) |
| Week 6 (n=18/16/14/13) | 2.8 (3.2) | 2.6 (2.8) | 2.7 (3.6) | 2.1 (2.8) |
| P-value (BL to Week 6 change) | 0.054 | 0.012 | 0.230 | 0.047 |
All values are Mean (SD) unless otherwise noted
The number of participants with complete data is indicated for each group for each measure at each time point in the following notation: n= All patients: placebo condition/milk thistle condition/adults only: placebo condition/milk thistle condition.
p-values corresponds to the mean within-person change from baseline to week 6 within each study condition.
Figure 1. Mean outcome measure scores over the course of treatment.
In terms of efficacy, most of the improvement seemed to have occurred in the first 6 weeks of the first treatment for both groups (milk thistle and placebo). The milk thistle group appeared to have a little more improvement in the first 6 weeks compared to placebo (a regression model using only the first 6 weeks of data confirmed this - results for that regression model are not shown in the results document). Even though the milk thistle group showed improvement in the first 6 weeks, during the second 6 weeks, very little change happened regardless of treatment group.
Safety and Tolerability
Four participants reported adverse events while assigned placebo (four reports of nausea/upset stomach/bloating, one report of dry mouth, and one of diarrhea); and five participants reported adverse events while receiving milk thistle (four reports of nausea/upset stomach/bloating, one report of insomnia, and one report of headache). The few adverse events were exclusively of mild intensity. Mean values in HAM-D and HAM-A scores remained at low levels throughout the study during both phases (placebo significantly reduced depressive symptoms but the scores were low and the range restricted). No subject reported any suicidal thoughts during the study and vital signs remained within normal limits throughout the study.
Discussion
This randomized, double-blind, cross-over clinical trial indicates that milk thistle may be more effective than placebo in reducing the hair pulling symptoms of trichotillomania in children and adults based on select secondary outcome measures, but the benefits of the supplement may be short-lived. The study hypothesis that milk thistle would reduce trichotillomania symptom severity was only partially supported by the data, but these findings may offer some insight into important neurochemical targets worthy of future investigation. Milk thistle was well tolerated, with limited side effects (comparable to placebo here), and is available over-the-counter and so could potentially be a useful treatment for this disorder.
Given the small study size, the cross-over design, and the positive results only on some secondary measures, multiple questions arise from this study. First, the effects of milk thistle seemed pronounced only during the first phase (6 weeks) of the study. Why would milk thistle not be more effective in the second phase? There are several possible explanations. It is conceivable that the improvement seen in the placebo group during the first phase, which was significant on several measures (consistent as well with a notable placebo effect in treatment studies of trichotillomania22 and consistent with a positive expectancy bias in many people regarding health supplements23), created a floor effect and milk thistle provided little additional benefit after placebo. Alternatively, for those who did not benefit from placebo, some people may have had a negative expectancy bias for the study as a whole, possibly due to the use of a nutritional supplement, which may be regarded as weaker or less effective than a prescription pharmaceutical option, which made any treatment less effective.
One possible conclusion from this study is that a certain subset of individuals with trichotillomania may respond preferentially to treatment with milk thistle. Recent research on trichotillomania has suggested that it may be a heterogeneous disorder, which could result in different responses to milk thistle based on distinct characteristics. This possibility has found some support from previous clinical trials in trichotillomania which included neurocognitive assessments of impulsivity to attempt to differentiate the participant population.24 Conceptualizing trichotillomania in this manner may suggest that different circuits could give rise to symptoms matching the profile for trichotillomania, but would require different pharmacological interventions. The current study sample size was simply too small to make such sub-analyses possible but future studies may want to examine whether specific neurocognitive, behavioral, or genetic factors support the use of milk thistle for select patients.
Although these results are somewhat provocative, they must be interpreted in light of several limitations of this clinical trial. First, the cross-over design of the study may have complicated our understanding of the effects of milk thistle. The design was used to minimize the need for a larger sample, but in using it, order effects may have occurred given the counterbalance was not equal between groups. Similarly, the sample size was likely too small to explore notable predictors of treatment response. A larger study, powered to perform subgroup analyses, may ultimately allow for greater clarification of whether certain subgroups of trichotillomania subjects would benefit from milk thistle. Third, counter-balancing was performed in batches of 30 pharmacy side, and hence the actual number of people randomized to each condition differed, because the study ended at N=20. Finally, trichotillomania may be a heterogeneous disorder, and a range of subjects was included with varying sites from where participants pulled, severity levels, and associated distress. This variation in the patient population could be indicative of biological variations between participants, and the possible variation in neurobiological underpinnings for different participants could influence whether individuals did or did not respond. Future research will be necessary to clarify this issue and determine whether this factor had an impact on the outcomes of the present study
Overall, the present study weakly supported the use of milk thistle in the treatment of trichotillomania consistent with our initial case series.7 Due to the small sample size, it was not possible to conduct a sufficiently powerful assessment of potential predictors of treatment response. Despite the overall equivocal findings in the present study, it remains possible that milk thistle may be a beneficial treatment options for certain patients with trichotillomania.
Footnotes
Author disclosure information: The authors declare no conflicts of interest.
References
- 1.Diefenbach GJ, Tolin DF, Hannan S, et al. Trichotillomania: impact on psychosocial functioning and quality of life. Behav Res Ther. 2005;43:869–884. doi: 10.1016/j.brat.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Odlaug BL, Kim SW, Grant JE. Quality of life and clinical severity in pathological skin picking and trichotillomania. J Anxiety Disord. 2010;24:823–829. doi: 10.1016/j.janxdis.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Houghton DC, Maas J, Twohig MP, et al. Comorbidity and quality of life in adults with hair pulling disorder. Psychiatry Res. 2016;239:12–19. doi: 10.1016/j.psychres.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66:756–763. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- 5.Van Ameringen M, Mancini C, Patterson B, et al. A randomized, double-blind, placebo-controlled trial of olanzapine in the treatment of trichotillomania. J Clin Psychiatry. 2010;71:1336–1343. doi: 10.4088/JCP.09m05114gre. [DOI] [PubMed] [Google Scholar]
- 6.Rothbart R, Amos T, Siegfried N, et al. Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev. 2013;8 doi: 10.1002/14651858.CD007662.pub2. CD007662. [DOI] [PubMed] [Google Scholar]
- 7.Grant JE, Odlaug BL. Silymarin treatment of obsessive-compulsive spectrum disorders. J Clin Psychopharmacol. 2015;35:340–2. doi: 10.1097/JCP.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 8.Grant JE, Chamberlain SR. A pilot examination of oxidative stress in trichotillomania. Psychiatry Investigation. 2018 doi: 10.30773/pi.2018.09.07.1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu HJ, Brinda BJ, Chavin KD, et al. An assessment of pharmacokinetics and antioxidant activity of free silymarin flavonolignans in healthy volunteers: a dose escalation study. Drug Metab Dispos. 2013;41:1679–85. doi: 10.1124/dmd.113.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song X, Zhou B, Cui L, et al. Silibinin ameliorates Aβ25-35-induced memory deficits in rats by modulating autophagy and attenuating neuroinflammation as well as oxidative stress. Neurochem Res. 2017;42:1073–1083. doi: 10.1007/s11064-016-2141-4. [DOI] [PubMed] [Google Scholar]
- 11.Voon V, Reiter A, Sebold M, et al. Model-Based Control in Dimensional Psychiatry. Biol Psychiatry. 2017;82:391–400. doi: 10.1016/j.biopsych.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Mazzio EA, Harris N, Soliman KF. Food constituents attenuate monoamine oxidase activity and peroxide levels in C6 astrocyte cells. Planta Med. 1998;64:603–6. doi: 10.1055/s-2006-957530. [DOI] [PubMed] [Google Scholar]
- 13.Lu P, Mamiya T, Lu L, et al. Silibinin attenuates cognitive deficits and decreases of dopamine and serotonin induced by repeated methamphetamine treatment. Behav Brain Res. 2010;207:387–393. doi: 10.1016/j.bbr.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain SR, Grant JE. Minnesota Impulse Disorders Interview (MIDI): Validation of a structured diagnostic clinical interview for impulse control disorders in an enriched community sample. Psychiatry Res. 2018;265:279–283. doi: 10.1016/j.psychres.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 16.Swedo SE, Leonard HL, Rapaport JL, et al. A double-blind comparison of clomipramine and desipramine in the treatment of trichotillomania (hair pulling) N Engl J Med. 1989;321:497–501. doi: 10.1056/NEJM198908243210803. [DOI] [PubMed] [Google Scholar]
- 17.Keuthen NJ, O'Sullivan RL, Ricciardi JN, et al. The Massachusetts General Hospital (MGH) Hair Pulling Scale, 1: development and factor analyses. Psychother Psychosom. 1995;64:141–145. doi: 10.1159/000289003. [DOI] [PubMed] [Google Scholar]
- 18.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976. pp. 76–338. [Google Scholar]
- 19.Sheehan DV. The Anxiety Disease. New York, NY: Scribner; 1983. [Google Scholar]
- 20.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant JE, Chamberlain SR, Redden SA, et al. Placebo response in trichotillomania. Int Clin Psychopharmacol. 2017;32:350–355. doi: 10.1097/YIC.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blendon RJ, Benson JM, Botta MD, et al. Users' views of dietary supplements. JAMA Intern Med. 2013;173:74–76. doi: 10.1001/2013.jamainternmed.311. [DOI] [PubMed] [Google Scholar]
- 24.Grant JE, Odlaug BL, Schreiber LR, et al. The opiate antagonist, naltrexone, in the treatment of trichotillomania: results of a double-blind, placebo-controlled study. J Clin Psychopharmacol. 2014;34:134–138. doi: 10.1097/JCP.0000000000000037. [DOI] [PubMed] [Google Scholar]