Abstract
Retigabine, currently used as antiepileptic drug, has a wide range of potential medical uses. Administration of the drug in patients can lead to QT interval prolongation in the electrocardiogram and to cardiac arrhythmias in rare cases. This suggests that the drug may perturb the electrical properties of the heart, and the underlying mechanisms were investigated here.
Effects of retigabine on currents through human cardiac ion channels, heterologously expressed in tsA-201 cells, were studied in whole-cell patch-clamp experiments. In addition, the drug's impact on the cardiac action potential was tested. This was done using ventricular cardiomyocytes isolated from Langendorff-perfused guinea pig hearts and cardiomyocytes derived from human induced pluripotent stem cells. Further, to unravel potential indirect effects of retigabine on the heart which might involve the autonomic nervous system, membrane potential and noradrenaline release from sympathetic ganglionic neurons were measured in the absence and presence of the drug. Retigabine significantly inhibited currents through hKv11.1 potassium, hNav1.5 sodium, as well as hCav1.2 calcium channels, but only in supra-therapeutic concentrations. In a similar concentration range, the drug shortened the action potential in both guinea pig and human cardiomyocytes. Therapeutic concentrations of retigabine, on the other hand, were sufficient to inhibit the activity of sympathetic ganglionic neurons.
We conclude that retigabine-induced QT interval prolongation, and the reported cases of cardiac arrhythmias after application of the drug in a typical daily dose range, cannot be explained by a direct modulatory effect on cardiac ion channels. They are rather mediated by indirect actions at the level of the autonomic nervous system.
Keywords: Cardiac arrhythmia, Cardiomyocytes, Ion channels, QT interval, Retigabine, Sympathetic ganglionic neurons
1. Introduction
Prolongation of the QT interval in the electrocardiogram (ECG) by therapeutic drugs and associated cardiac arrhythmias are a frequently encountered clinical problem. This also applies to antiepileptic drug therapy (recently reviewed in (Feldman and Gidal, 2013)).
Retigabine is an anticonvulsant approved for the adjunctive treatment of “partial onset seizures”. Its main mode of action as an opener of neural Kv7 potassium channels (Kv7.2–7.5; KCNQ2–5) at low micromolar concentrations (half maximal effective concentrations, EC50 values between 1 and 5 μM) (Gunthorpe et al., 2012) is different from all other presently used antiepileptic drugs. Besides positive allosteric modulation of Kv7 channels, somewhat higher concentrations of retigabine also affect the GABA-ergic system (Gunthorpe et al., 2012). Thus, retigabine facilitates the functions of GABAA receptors (Rundfeldt and Netzer, 2000; Otto et al., 2002) in a subtype selective manner (Treven et al., 2015). This latter effect may also contribute to retigabine's anticonvulsant activity. In addition to retigabine's current application for the treatment of partial onset seizures, recent studies have implied that the drug could potentially also be used for a series of other medical applications ranging from treating other epilepsy forms (Stafstrom et al., 2011) to amyotrophic lateral sclerosis (Wainger et al., 2014), depression (Friedman et al., 2016), chronic pain (Brown and Passmore, 2009), and alcohol addiction (Knapp et al., 2014).
In contrast to its potentiating modulatory effects on neuronal Kv7 potassium channels at low micromolar concentrations, retigabine does not activate the Kv7 channel isoform Kv7.1 (KCNQ1), which is primarily expressed in the heart. Currents through Kv7.1 channels are rather inhibited by retigabine, but for this effect high drug concentrations in a supra-therapeutic range are required (concentration generating 50% current inhibition, IC50 ~ 100 μM) (Gunthorpe et al., 2012; Tatulian et al., 2001). Kv7.1 channels are major determinants of cardiac repolarization, and their modulation by drugs is a well-known source of arrhythmia generation (Moss and Kass, 2005; Towart et al., 2009). Therefore, the lack of retigabine action on Kv7.1 channels at therapeutic concentrations is considered beneficial with regard to the cardiovascular safety profile of the drug.
In spite of the fact that Kv7.1 channels are not significantly affected by retigabine, the application of this drug in a typical daily dose range between 600 and 1200 mg (Gunthorpe et al., 2012; Fattore and Perucca, 2011) has been associated with rare cases of cardiac arrhythmia. For example, in an abuse-potential study, one subject developed asystole, and another subject ventricular tachycardia after single doses of 900 mg retigabine (Potiga. Prescribing information: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022345s000lbl.pdf) (Fattore and Perucca, 2011). The causes for the occurrence of these cardiac adverse events after retigabine intake remained unknown. Moreover, even though several clinical studies failed to reveal significant ECG abnormalities after retigabine administration to patients (Porter et al., 2007; Brodie et al., 2010; French et al., 2011; Gil-Nagel et al., 2012), a slight but consistent prolongation of the QT interval in the ECG has been demonstrated in a cardiac safety study in healthy subjects treated with retigabine (1200 mg/day) (Trobalt. Summary of product characteristics: https://www.medicines.org.uk/emc/medicine/24527) (Fattore and Perucca, 2011; Barrese et al., 2010). Accordingly, retigabine application in patients with partial onset seizures induced QT prolongation in some cases, especially during co-medication with the antiepileptic drug lamotrigine (Lerche et al., 2015). Since drug-induced QT interval prolongation can be associated with potentially lethal “torsades de pointes” arrhythmias, this issue has become a major safety concern during drug development, and has even resulted in the withdrawal of various drugs from the market (e.g. (Redfern et al., 2003; Vandenberg et al., 2001)). In the case of retigabine, the drug's potential QT prolonging action also impacts its clinical administration: caution is recommended when retigabine is used in patients taking other medications known to prolong the QT interval as well, and in patients at high risk for cardiac arrhythmia (Fattore and Perucca, 2011; Barrese et al., 2010; Splinter, 2013).
QT interval prolongation by drugs may be generated directly via modulation of cardiac ion channels (Redfern et al., 2003), or in an indirect manner by interference with the autonomic nervous system (Diedrich et al., 2002). The mechanism by which retigabine can induce QT interval prolongation has remained elusive. Here, in order to gain mechanistic insights, we first tested the effects of the drug on human cardiac voltage-gated ion channels with major impact on electrical impulse propagation in the heart. Thereafter, we studied retigabine's effects on the cardiac action potential (AP), and finally the drug's impact on the activity of neurons from sympathetic ganglia, which are involved in the regulation of the heart's electrical properties.
2. Materials and methods
The study complies with the University Animal Welfare Committee rules. All our procedures were conducted according to the European Community Council Directive for Care and Use of Laboratory Animals.
2.1. Culture and transfection of tsA-201 cells
tsA-201 cells (American Type Culture Collection (ATCC, Manassas, VA, USA)) were cultured and transfected using the procedures and plasmids described in detail in our earlier work (Koenig et al., 2013). For hCav1.2 calcium channel expression, 77-pcDNA3 (Soldatov et al., 2000) was used in this work with the permission of Dr. Nikolai M. Soldatov (Humgenex, Inc., Maryland, USA).
2.2. Isolation of adult guinea pig ventricular cardiomyocytes
Ventricular cardiomyocytes from adult female Dunkin-Hartley guinea pigs (200–400 g) were acutely isolated by using a Langendorff setup following the procedures previously described (Koenig et al., 2011).
2.3. Human induced pluripotent stem cell-derived cardiomyocytes
Human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes were purchased from Cellectis (hiPS-CM, Sweden). After thawing, the cells were propagated in CM culture medium (Cellectis) for up to 14 days, according to the manufacturer's protocols. The medium was changed every third day.
2.4. Electrophysiological recordings
The whole cell patch clamp technique was used to record ionic currents from tsA-201 cells expressing human cardiac ion channels (24–48 h after transfection), and from ventricular cardiomyocytes isolated from guinea pig hearts up to 10 h after preparation. The measurements were performed at room temperature (22 ± 1.5 °C) using an Axoclamp 200B patch clamp amplifier (Axon Instruments, Union City, CA). The recording pipettes were formed from aluminosilicate glass (A120-77-10; Science Products, Hofheim, Germany) with a P-97 horizontal puller (Sutter Instruments, Novato, CA), and had resistances between 0.8 and 2 MΩ when filled with the respective pipette solutions. Data acquisition was carried out with the pClamp 6.0 software (Axon Instruments) through a 12-bit A-D/D-A interface (Digidata 1200; Axon Instruments). Data were low-pass filtered with 1–10 kHz (− 3 dB) and digitised at 10–100 kHz. Data analyses were performed using Clampfit 10.2 (Axon Instruments) and GraphPad Prism 5.01 (San Diego, USA) software. Rapid exchange of external solutions was performed with a DAD-8-VC superfusion system (ALA Scientific Instruments, Westbury, NY, USA). Experimental details and the compositions of the solutions used for the hKv11.1 (hERG) potassium, hNav1.5 sodium, and hCav1.2 barium current recordings are described elsewhere (Koenig et al., 2013). For the drug application experiments retigabine was obtained from Alomone Labs (Jerusalem, Israel).
Action potentials (APs) were recorded from guinea pig ventricular cardiomyocytes and from ventricular-like hiPSC-derived cardiomyocytes in the current-clamp mode of the whole cell patch clamp technique at room temperature (22 ± 1 °C) as in (Koenig et al., 2013) and (Rubi et al., 2016). APs were elicited at 1 Hz by rectangular current pulses of 4 ms duration at 125% threshold level. Human cardiomyocytes were classified as ventricular-like when their APs showed a distinct “shoulder” (plateau or flat repolarization phase) prior to a final steep phase of repolarization as in our previous work (Rubi et al., 2016). The composition of the experimental solutions is given in (Koenig et al., 2013). This former study from our group also contains experiments to validate the AP recording procedure: the externally applied hERG channel blocker E-4031 considerably prolonged the AP, and the L-type calcium channel blocker isradipine reduced AP duration (see (Koenig et al., 2013)).
2.5. Experiments on sympathetic ganglionic neurons
In order to obtain primary cultures of sympathetic ganglionic neurons as described before (Salzer et al., 2014), superior cervical ganglia (SCG) were dissected from 2- to 6-day-old Sprague-Dawley rat pups which had been killed by decapitation after CO2 asphyxia in accordance with the rules of the university animal welfare committee. Ganglia were then cut into three to four pieces and incubated in collagenase (1.5 mg ml−1; Sigma) and dispase (3.0 mg ml−1; Boehringer Mannheim) for 20 min at 36 °C. After removal of these enzymes, the tissue was further digested by trypsin (0.25% for 15 min at 36 °C; %; Worthington), dissociated by trituration, and resuspended in Dulbecco's modified Eagle's medium (Gibco) containing 2.2 g l−1 glucose, 10 mg l−1 insulin, 25,000 IU l−1 penicillin and 25 mg l−1 streptomycin (Gibco), 50 mg l−1 nerve growth factor (Gibco), and 5% fetal calf serum (Gibco). Finally, cells were seeded either onto 5 mm plastic discs for superfusion experiments or onto 35 mm culture dishes (Nunc) for electrophysiological experiments, both coated with rat tail collagen (Biomedical Technologies Inc., Stoughton, MA, USA). Cultures were kept in a humidified 5% CO2 atmosphere at 36 °C for 4–8 days. On day 1 after plating, the medium was completely exchanged, and after 4–5 days, the medium was exchanged again and the serum was omitted.
The membrane potential was measured at room temperature (20–24 °C) on the somata of single SCG neurons using the perforated-patch modification of the patch-clamp technique as described previously (Salzer et al., 2014). Patch pipettes were front-filled with a solution consisting of (in mM) K2SO4, 75; KCl, 55; MgCl2, 8; and HEPES, 10, adjusted to pH 7.3 with KOH. Then, electrodes were backfilled with the same solution containing 200 μg/ml amphotericin B or 50 μg/ml gramicidin D (in 0.8% DMSO), which yielded tip resistances of 1–3 MΩ. The bathing solution consisted of (in mM) NaCl, 140; KCl, 3.0; CaCl2, 2.5; MgCl2, 2.0; glucose, 20; and HEPES, 10, adjusted to pH 7.4 with NaOH. With this bath solution, the liquid junction potential was − 8 mV and was corrected for during experimentation. Drugs were applied via a DAD12 drug application device (Adams & List, Westbury, NY).
Release of noradrenaline was assessed as described before (Boehm, 1994; Salzer et al., 2014). Culture discs as described above were incubated in 0.05 μM [3H]noradrenaline (specific activity 52.0 Ci mmol−1) in culture medium supplemented with 1 mM ascorbic acid at 36 °C for 1 h. After labelling, culture discs were transferred to small chambers and superfused with a buffer containing (mM): NaCl (120), KCl (6.0), CaCl2 (2.0), MgCl2 (2.0), glucose (20), Hepes (10), fumaric acid (0.5), sodium pyruvate (5.0) and ascorbic acid (0.57), adjusted to pH 7.4 with NaOH. Superfusion was performed at 25 °C at a rate of about 1.0 ml min−1. Collection of 4-min superfusate fractions was started after a 60 min washout period to remove excess radioactivity. To investigate noradrenaline release evoked by the endogenous ganglionic transmitter, [3H] overflow was induced by the inclusion of acetylcholine (ACh) (10 μM) in the superfusion buffer for one min, unless indicated otherwise. For comparison, tritium overflow was also elicited by the application of sixty monophasic rectangular electrical pulses (0.5 ms, 60 mA, 50 V cm−1) at 1 Hz. This electrical field stimulation leads to the generation of APs within the axons of the sympathetic neurons and thereby triggers noradrenaline release independently of ganglionic ACh receptors. Retigabine was added to the buffer after 50 min of superfusion (i.e. 10 min prior to the start of sample collection). The buffer then remained unchanged until the end of experiments. The radioactivity remaining in the cells after the completion of experiments was extracted by immersion of the discs in 2% (v/v) perchloric acid followed by sonication. Radioactivity in extracts and collected fractions was determined by liquid scintillation counting (Packard Tri-Carb 2100 TR). Radioactivity released in response to electrical field stimulation from rat sympathetic neurons after labelling with tritiated noradrenaline under conditions similar to those of the present study had previously been shown to consist predominantly of the authentic transmitter and to contain only small amounts (≤15%) of metabolites (Schwartz and Malik, 1993). Hence, the outflow of tritium measured in this study was assumed to reflect the release of noradrenaline and not that of metabolites.
The spontaneous (unstimulated) rate of [3H] outflow was obtained by expressing the radioactivity of a collected fraction as a percentage of the total radioactivity in the cultures at the beginning of the corresponding collection period. Stimulation-evoked tritium overflow was calculated as the difference between the total [3H] outflow during and after stimulation and the estimated basal outflow, which was assumed to decline linearly throughout experiments. Therefore, basal outflow during periods of stimulation was assumed to equate to the arithmetic mean of the samples preceding and those following stimulation, respectively. The difference between the total and the estimated basal outflow was expressed as a percentage of the total radioactivity in the cultures at the beginning of the respective stimulation (% of total radioactivity; S %). The amount of stimulation-evoked tritium release may vary considerably between different SCG preparations. Therefore, tritium overflow in the presence of retigabine was compared with that obtained within the same SCG preparation in its absence. To directly compare the effects of retigabine on different types of stimulation-evoked overflow, the values obtained in its presence were expressed as percentage of those obtained in its absence within the same preparation.
2.6. Statistical analyses
Data always represent means ± SD. Statistical comparisons of data before and after retigabine application at different drug concentrations were performed by one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test using GraphPad Prism 5.01 (San Diego, USA) software. In case only two groups had to be compared, a paired two-tailed Student's t-test was used. A p-value < 0.05 was considered significantly different. For the experiments to assess concentration dependences, the group sizes (= number of experiments (n) performed at different drug concentrations) are displayed in brackets in the respective figures. Data normalization was employed to control for unwanted sources of variation.
3. Results
3.1. Effects of retigabine on cardiac voltage-gated ion channels
(Moss and Kass, 2005) defined three cardiac ion channels with major influence on the cardiac action potential (AP). These are Kv7.1 and Kv11.1 (hERG) potassium channels, as well as Nav1.5 sodium channels. Kv7.1 is the only one among these channels for which retigabine effects have been described before. The drug has been found to be a weak inhibitor of Kv7.1 channels (IC50 ~ 100 μM) (Gunthorpe et al., 2012; Tatulian et al., 2001).
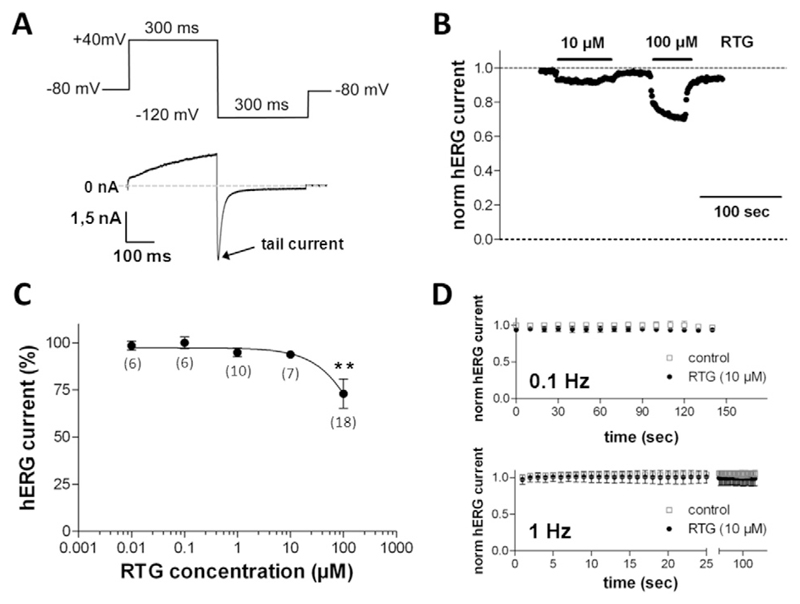
In the present study, we first tested the effects of retigabine on human Kv11.1 channels. These were heterologously expressed in tsA201 cells. We found that external application of up to 1 μM retigabine did not affect the currents through hKv11.1 channels. At higher concentrations (≥ 10 μM), the drug reduced hKv11.1 currents (Fig. 1B), but a considerable inhibition was achieved at 100 μM retigabine only. The inhibitory effect was reversible after wash out of the drug (Fig. 1B). A summary of an analysis of a series of such experiments as exemplarily displayed in Fig. 1B is presented in Fig. 1C. As it can be deduced, a statistically significant hKv11.1 current reduction only occurred at the highest retigabine concentration tested, 100 μM. At this concentration the drug generated a reduction of the current amplitude to approximately 70% of the current under drug-free control conditions.
Fig. 1.
hKv11.1 (hERG) channel inhibition by retigabine. (A) Pulse protocol to elicit hERG currents during a drug application experiment, which was applied at a frequency of 1 Hz. (B) Retigabine (RTG), at the indicated concentrations, was externally applied until steady-state block was reached, and consequently washed out. The tail current peaks (see A) normalized to the drug-free control condition at the beginning of the experiment were plotted against time. (C) Concentration–response curve for the reduction of hERG tail currents by RTG (100% represents the current amplitude in the absence of RTG, data point not shown). Data points represent means ± SD (n values are given in brackets). The solid line represents part of a sigmoidal fit to the data points with a Hill equation. ** indicates a significant difference vs. the drug-free control (p < 0.01, Dunnett's post hoc test). (D) hERG current amplitudes during 150 s of repetitive pulsing with low (0.1 Hz) and high (1 Hz) frequency under drug-free control conditions (empty squares, n = 7 at 0.1 Hz and 4 at 1 Hz) and in the presence of 10 μM RTG (filled circles, n = 7 at 0.1 Hz and 5 at 1 Hz). The pulse protocol was started 2 min after beginning of superfusion with bath solution or bath solution containing RTG. hERG tail current amplitudes were normalized to the drug-free control condition (at 0 s).
Many drugs enhance their inhibition of hKv11.1 channels if the cell membrane is depolarized frequently. This phenomenon is known as frequency-dependent block. To test if retigabine might inhibit hKv11.1 channels in a frequency-dependent manner, we first applied 10 μM of the drug for 2 min to allow for basal block without pulsing. This was then followed by repetitive pulsing at low (0.1 Hz) and high (1 Hz) frequencies, respectively, for a prolonged period of time (up to 150 s). The hKv11.1 current amplitudes normalized to drug-free control conditions are shown in Fig. 1D. It can be observed that, at both frequencies, and in the absence or presence of retigabine, repetitive pulsing did not generate significant current reduction. Thus, the drug did not cause a considerable frequency-dependent hKv11.1 channel block.
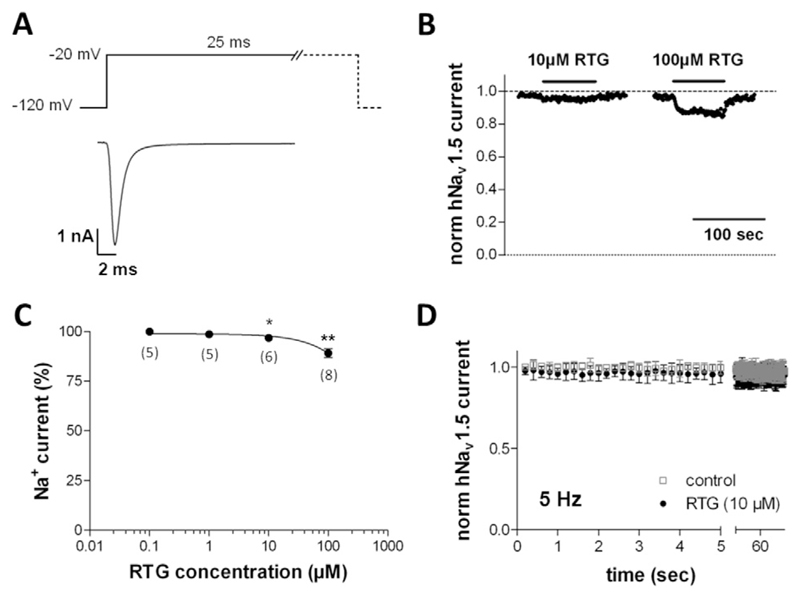
Fig. 2 describes the effects of retigabine on human Nav1.5 sodium channels heterologously expressed in tsA-201 cells. As described for hKv11.1 above, retigabine did not affect the currents through hNav1.5 channels at concentrations up to 1 μM. At higher concentrations (≥ 10 μM), the drug reversibly reduced hNav1.5 currents (Fig. 2B). Although only a small effect, the current reduction by 10 μM retigabine turned out to be statistically significant when compared with drug-free control conditions (Fig. 2C). 100 μM retigabine reduced the current amplitude to approximately 90% of the control value.
Fig. 2.
hNav1.5 sodium channel blockade by retigabine. (A) Typical current through hNav1.5 channels expressed in a tsA-201 cell elicited by the pulse protocol given on top. (B) Retigabine (RTG) was externally applied during 1 Hz pulsing (see A) until steady-state block was reached, and consequently washed out. The normalized current peaks were plotted against time. (C) hNav1.5 current inhibition by various concentrations of RTG (100% represents the current amplitude in the absence of RTG, data point not shown). * and ** indicate a significant difference vs. the drug-free control (Dunnett's post hoc test, p < 0.05 and 0.01, respectively; n values are given in brackets). (D) hNav1.5 current amplitudes during repetitive high frequency (5 Hz) pulsing under drug-free control conditions (empty squares, n = 5) and in the presence of 10 μM RTG (filled circles, n = 5). The pulse protocol under experimental conditions was started 2 min after beginning of superfusion with bath solution containing the drug. hNav1.5 current amplitudes were normalized to the drug-free control condition (at 0 s).
As a next step we tested if hNav1.5 channels were inhibited by retigabine in a frequency-dependent manner. Therefore, either under control conditions or 2 min after initiating the external application of 10 μM retigabine, repetitive high frequency pulsing at 5 Hz was started (Fig. 2D). Both in the absence and presence of retigabine, repetitive 5 Hz pulsing did not generate considerable current reduction. Therefore, the drug does not seem to block hNav1.5 channels in a frequency-dependent manner.
3.2. Effects of retigabine on the cardiac AP
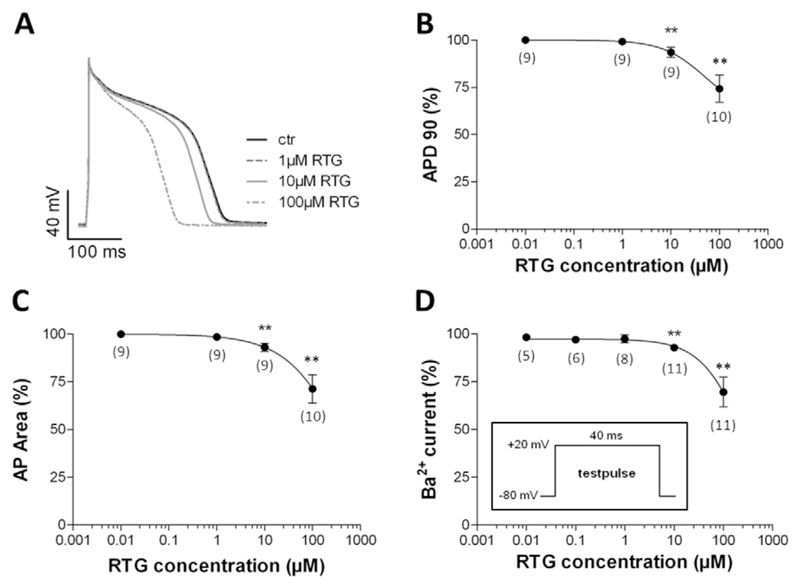
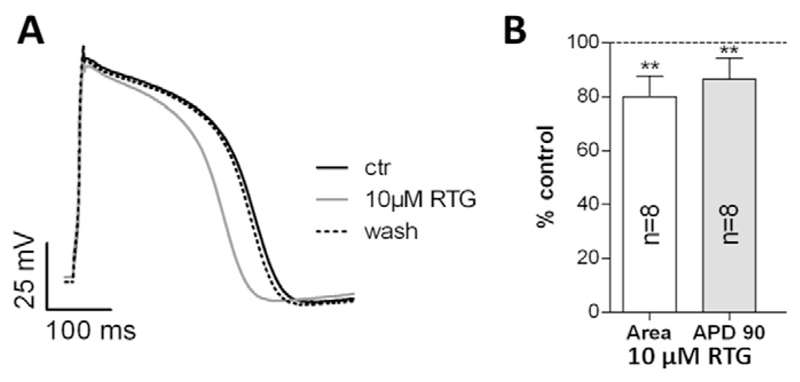
After having tested the effects of retigabine on relevant cardiac ion channels, we studied the drug's impact on the cardiac AP as the latter reflects the concerted action of a multitude of different types of ion channels, which, in theory, could all be affected by retigabine. A further reason to perform AP studies was the well-known direct correlation between cardiac ventricular AP duration and the QT interval in the ECG (Redfern et al., 2003). Thus, the retigabine-induced QT prolongation (Fattore and Perucca, 2011; Barrese et al., 2010) is expected to be paralleled by a broadening of the AP in ventricular cardiomyocytes. For these studies, we used cardiomyocytes isolated from the ventricles of hearts from adult guinea pigs. This cell type is considered a suitable model system to study potential drug effects on the human cardiac AP (e.g. (Davie et al., 2004; Yao et al., 2008)). Fig. 3 shows that externally applied retigabine at concentrations up to 1 μM did not affect the AP in guinea pig ventricular cardiomyocytes. At higher concentrations (≥ 10 μM), the drug unexpectedly shortened the duration of the AP (Fig. 3A). This effect was fully reversible after wash out of the drug (data not shown). Two different types of AP analyses (i, AP duration at 90% repolarization, APD90, Fig. 3B and ii, area under the AP, AP Area, Fig. 3C) revealed that its duration was significantly shortened in the presence of 10 μM retigabine when compared to drug-free control conditions. The highest drug concentration tested, 100 μM, generated a pronounced AP shortening.
Fig. 3.
Effects of retigabine on the action potential (AP) in guinea pig ventricular cardiomyocytes. During drug application and wash out experiments, APs were elicited at a frequency of 1 Hz. (A) AP under control conditions (ctr), and each time after steady-state was reached during superfusion with bath solution containing 1, 10 and 100 μM retigabine (RTG). The RTG effect on the AP was completely reversible after wash out (data not shown). (B) Concentration-dependence of the RTG effect on AP duration at 90% repolarization (APD90). (C) Concentration dependence of the RTG effect on the area under the AP (AP Area). (D) Barium current inhibition by various concentrations of RTG in guinea pig cardiomyocytes. The pulse protocol to elicit the currents during the sequence of drug application and wash out was applied at 0.5 Hz and is shown in the inset. ** indicates a significant difference vs. the drug-free control (Dunnett's post hoc test, p < 0.01; n values are given in brackets). 100% represents APD90 (B), AP Area (C), and current amplitude (D) each time in the absence of RTG (data points not shown).
As AP shortening by retigabine (≥ 10 μM) in guinea pig cardiomyocytes was an unexpected result in light of the drug's potential QT interval prolonging effect in humans (Fattore and Perucca, 2011; Barrese et al., 2010), we further searched for the potential underlying mechanism. Accordingly, we studied the effects of the drug on L-type calcium channels again in guinea pig cardiomyocytes: an inhibition of these channels is known to reduce the AP duration. Fig. 3D shows that retigabine did reduce the currents through L-type calcium channels in guinea pig cardiomyocytes at concentrations ≥ 10 μM. The concentration dependence of this retigabine action was very similar compared to the drug's effect on AP duration (compare with Fig. 3B and C). This suggested a causal relation between calcium channel inhibition and AP shortening by retigabine in guinea pig cardiomyocytes.
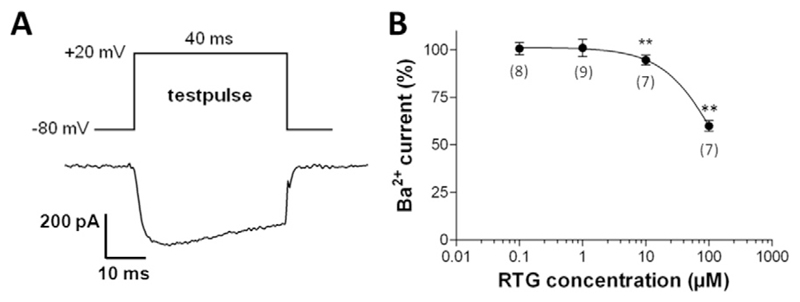
In the next set of experiments we tested the effects of retigabine on human Cav1.2 L-type calcium channels heterologously expressed in tsA-201 cells. Fig. 4 shows that retigabine significantly reduced the currents through hCav1.2 calcium channels at concentrations ≥ 10 μM. The extent and concentration dependence of this drug effect (Fig. 4B) was similar to that on L-type calcium channels in guinea pig cardiomyocytes (compare with Fig. 3D).
Fig. 4.
hCav1.2 channel inhibition by retigabine. (A) Pulse protocol to elicit barium currents in tsA-201 cells expressing hCav1.2 channels applied at 0.5 Hz. (B) Barium current inhibition by various concentrations of retigabine (RTG) (100% represents the current amplitude in the absence of RTG, data point not shown). ** indicates a significant difference vs. the drug-free control (Dunnett's post hoc test, p < 0.01; n values are given in brackets).
Considering the potential QT interval prolonging effect of retigabine in humans (Fattore and Perucca, 2011; Barrese et al., 2010), and our opposing result of retigabine-induced AP shortening in guinea pig cardiomyocytes, it seemed reasonable to additionally study the drug's effect on the human cardiac AP. Thereby we wanted to exclude potential misinterpretations due to species differences. For these studies ventricular-like cardiomyocytes derived from hiPSCs were used. In parallel with the results obtained in guinea pig cardiomyocytes (Fig. 3), 10 μM externally applied retigabine significantly shortened the AP in human cardiomyocytes when compared to drug-free control conditions (Fig. 5A and B). The AP shortening caused by retigabine in human cardiomyocytes was also reversible after wash out of the drug (see Fig. 5A).
Fig. 5.
Effects of retigabine on the AP in hiPSC-derived cardiomyocytes. During drug application and wash out experiments, APs were elicited at a frequency of 1 Hz. (A) AP under control conditions (ctr), after steady-state was reached during superfusion with bath solution containing 10 μM retigabine (RTG), and after wash out. (B) AP durations (AP Area, left column; APD90, right column) after steady-state was reached during superfusion with bath solution containing 10 μM RTG. The drug-free control condition represents 100%. ** indicates a significant difference vs. the drug-free control (p < 0.01, paired two-tailed Student's t-test).
Taken together, retigabine concentrations ≥ 10 μM retigabine shortened the cardiac AP.
3.3. Effects of retigabine on the activity of neurons from sympathetic ganglia
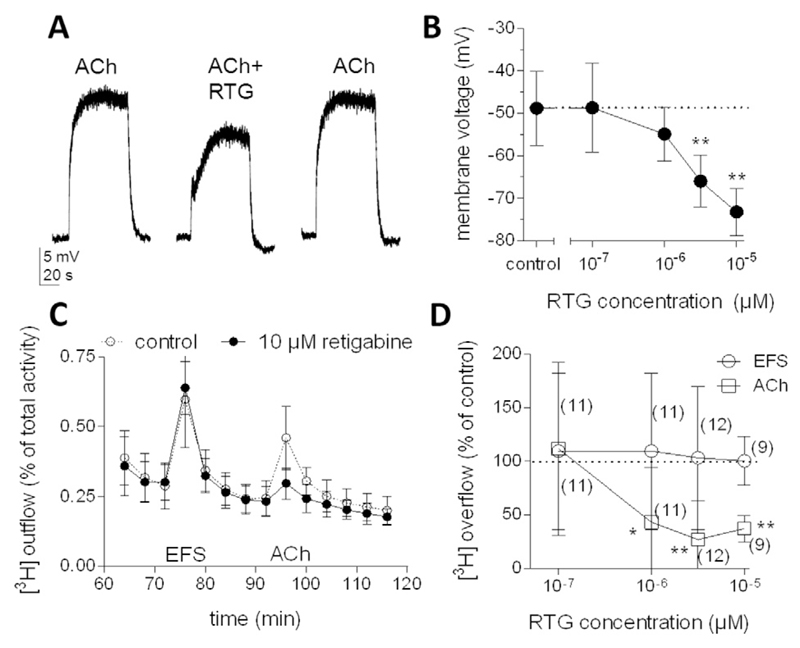
Having established that retigabine affected cardiac ion channels and the cardiac AP at high micromolar concentrations only, we investigated the drug's action on sympathetic ganglionic neurons to reveal whether lower concentrations might interfere with some mechanisms involved in the control of the heart via the sympathetic nervous system. However, the function of an entire neuron in sympathetic ganglia, from the input through somatodendritic receptors way down to the output at axon terminals which release noradrenaline, cannot be studied in one tissue preparation. Therefore, primary cultures of dissociated sympathetic ganglia were used instead. After dissociation, the neurons extend new axons which form presynaptic varicosities that display all morphological and functional features of sympathetic nerve terminals physiologically situated within target organs such as the heart (Boehm and Kubista, 2002). We first focused on the response of SCG neurons to acetylcholine (ACh), the major ganglionic transmitter, by measuring the membrane potential at neuronal somata. The average resting membrane potential as determined in 7 neurons was − 73.3 ± 2.4 mV. Application of 10 μM ACh caused rapid depolarization to about − 50 mV (Fig. 6A), and the addition of increasing concentrations of retigabine counteracted these ACh-evoked depolarizations in a concentration-dependent manner (Fig. 6B).
Fig. 6.
Effects of retigabine on membrane potential and noradrenaline release from sympathetic ganglionic neurons. (A) Sample traces of depolarizations evoked by 10 μM acetylcholine (ACh) applied either alone or together with 1 μM retigabine (RTG). (B) Membrane voltage values achieved by 10 μM ACh applied either alone or together with the indicated concentrations of retigabine (RTG); means ± SD (n = 7 for each data point). ** indicates significant differences vs. the drug-free control (Dunnett's post hoc test, p < 0.01). (C and D) Cultures were labelled with [3H]noradrenaline and superfused. Subsequent to a 60 min washout period, 4 min fractions of superfusate were collected. Tritium overflow was evoked by electrical field stimulation (EFS; 60 pulses, 1 Hz) starting at 72 min of superfusion and by the application of 10 μM ACh for 1 min starting at 92 min of superfusion. From minute 50 of superfusion onward, the buffer contained either the indicated concentrations of or no retigabine. (C) Time course of fractional [3H] outflow in the absence or presence of 10 μM retigabine obtained in one experiment (carried out in triplicates, means ± SD). (D) Concentration dependence of the effects of retigabine on [3H]noradrenaline release induced by EFS or 10 μM ACh. Tritium overflow in the presence of retigabine is depicted as a percentage of the overflow in its absence (% of control); means ± SD (n values are given in brackets). *and ** indicate significant differences vs the drug-free control (Dunnett's post hoc test, p < 0.05 and < 0.01, respectively).
To reveal whether this attenuation of ACh-induced depolarizations at the somatodendritic region of sympathetic neurons, which reflect their input, might be sufficient to also affect their output, we assayed noradrenaline release (Fig. 6C and D). The basic mechanisms of noradrenaline release in such cultures are the same as those described for sympathetic nerve terminals located within target organs (Boehm and Kubista, 2002).
Tritium overflow from cultures previously loaded with [3]H noradrenaline and challenged by 10 μM ACh was reduced by retigabine in a concentration-dependent manner (Fig. 6D). To reveal whether this effect was specific for the stimulation by ACh, noradrenaline release was also triggered by electrical field stimulation, which elicits APs in the post-ganglionic axons. Electrically evoked noradrenaline release was not altered by retigabine at concentrations up to 10 μM (Fig. 6C and D). Thus, retigabine did not affect some general mechanism involved in AP-dependent release of noradrenaline from sympathetic ganglionic neurons.
Together, these experiments suggest that low micromolar concentrations of retigabine specifically impede the excitation of postganglionic sympathetic neurons by the endogenous ganglionic transmitter ACh.
4. Discussion and conclusions
Retigabine provides anticonvulsant activity by modulating Kv7.2 to Kv7.5 channels which are expressed in the nervous system. Cardiac Kv7.1 channels, in contrast, remain unaffected by therapeutic concentrations of this drug. Nevertheless, retigabine has been reported to cause QT interval prolongation in the ECG (Trobalt. Summary of product characteristics: https://www.medicines.org.uk/emc/medicine/24527) (Fattore and Perucca, 2011; Barrese et al., 2010; Lerche et al., 2015) and cardiac arrhythmias in rare cases (Potiga. Prescribing information: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022345s000lbl.pdf) (Fattore and Perucca, 2011). However, the underlying mechanisms have remained elusive. Therefore, the present study investigated the effects of retigabine on human cardiac voltage-gated ion channels that are of utmost importance for electrical impulse propagation in the heart: hKv11.1 potassium, hNav1.5 sodium, and hCav1.2 calcium channels. Modulation of one or more of these ion channels by drugs in their therapeutic concentrations may cause serious cardiac adverse events (Hoffman and Rosen, 1981; Rosen and Wit, 1987). We found that retigabine significantly inhibited currents through hKv11.1 potassium, hNav1.5 sodium, as well as hCav1.2 calcium channels at concentrations ≥ 10 μM. For none of these ionic currents, however, 100 μM retigabine sufficed to generate 50% inhibition. A similar concentration dependence was also observed for AP shortening by retigabine in guinea pig cardiomyocytes. Previously, sub-millimolar concentrations of retigabine had been reported to block hKv7.1 potassium channels (IC50 ~ 100 μM) (Gunthorpe et al., 2012), other important determinants of the heart's electrical properties (Moss and Kass, 2005; Towart et al., 2009).
Retigabine concentrations in whole blood samples of humans after a single oral dose of 100 mg reached a maximum concentration (Cmax) of 387 ng/ml (Ferron et al., 2002). Since the pharmacokinetics of retigabine is linearly dose proportional (Ferron et al., 2002), a dose of 1200 mg retigabine will yield a calculated Cmax of 4644 ng/ml, which corresponds to 15.3 μM. Taking into account an estimated 80% plasma protein binding of retigabine (Hermann et al., 2003a; Barrese et al., 2010; Fattore and Perucca, 2011), the free Cmax value after intake of 1200 mg can amount to 3.1 μM. Such plasma levels after oral administration of retigabine to humans have been reported in several studies (Hermann et al., 2003a; Otto et al., 2002; Ferron et al., 2003). As therapeutic doses of retigabine are limited to a maximum of 1200 mg/day, free plasma concentrations of the drug will hardly exceed 3 μM. Therefore, the inhibition of cardiac ion channels by retigabine with IC50 values of ≥ 100 μM as described above is unlikely to considerably impair the electrical properties of the heart. Accordingly, after intake of therapeutic doses direct pro-arrhythmic actions of retigabine at the level of cardiomyocytes can be excluded.
Nevertheless, in case of intoxication due to retigabine overdosing the drug's inhibitory effects on cardiac ion channels may become relevant. Based on the current data, free retigabine plasma concentrations of 10 μM or above will induce a significant block of various cardiac ion channel types and may thereby cause arrhythmias. In this context, it should be noted that abnormally high and potentially dangerous free plasma concentrations of retigabine may also arise in patients in the case of impairment of drug metabolism and elimination. Retigabine is primarily metabolized in the liver by N-acetylation and even more so by N-glucuronidation, both followed by renal clearance (Hempel et al., 1999; Weisenberg and Wong, 2011). Accordingly, retigabine elimination is reduced in subjects with severe hepatic impairment and in individuals with moderate or severe renal insufficiency (Bialer et al., 2010; Weisenberg and Wong, 2011). Clearance of the drug is also reduced in elderly patients most likely due to an age-related decline in renal function (Bialer et al., 2010), and in African American compared with Caucasian subjects, which is consistent with ethnic differences in N-glucuronidation (Ferron et al., 2002). Finally, drug-drug interactions may lead to exceedingly high retigabine plasma concentrations. For instance, in the presence of the antiepileptic drug lamotrigine, the rate of retigabine clearance is decreased (Hermann et al., 2003b). Interestingly, retigabine-induced QT interval prolongation in patients with partial onset seizures was observed especially during co-medication with lamotrigine (Lerche et al., 2015).
Even within a therapeutic range of doses and in the absence of pharmacokinetic disturbances as mentioned above, retigabine has been found to cause QT prolongation (Trobalt. Summary of product characteristics: https://www.medicines.org.uk/emc/medicine/24527) (Fattore and Perucca, 2011; Barrese et al., 2010). Although this effect was not apparent in several clinical trials of retigabine (Porter et al., 2007; Brodie et al., 2010; French et al., 2011; Gil-Nagel et al., 2012), its potential occurrence impacts the drug's clinical usefulness, as caution is warranted when retigabine is used in patients taking medications known to also prolong the QT interval, and in patients at high risk for cardiac arrhythmia (Fattore and Perucca, 2011; Barrese et al., 2010; Splinter, 2013). The cellular correlate of a prolonged QT interval in the ECG is AP prolongation in ventricular cardiomyocytes (Redfern et al., 2003; Sanguinetti and Tristani-Firouzi, 2006). Drug-induced AP prolongation at the cardiomyocyte level (and consequently QT prolongation) can be generated either by inhibition of outward currents through potassium channels, which determine the repolarization phase of the AP (most prominently hKv11.1 and hKv7.1 channels) (Vandenberg et al., 2001), or by enhancement of the activity of depolarizing currents through hNav1.5 sodium or hCav1.2 calcium channels (Moss and Kass, 2005). Since therapeutic concentrations of retigabine did neither prolong APs nor exert relevant effects on any of the aforementioned ion channels, we conclude that the drug's QT prolonging effect cannot be explained by direct modulatory effects on cardiomyocytes. Quite unexpectedly, in higher concentrations (≥ 10 μM), retigabine shortened rather than prolonged cardiomyocyte APs, which predictably leads to QT interval shortening. To the best of our knowledge, QT shortening after retigabine administration has never been observed, but rather the opposite effect. This suggests that retigabine can induce QT interval prolongation by an alternative mechanism that overweighs the consequences of AP shortening in cardiomyocytes. One obvious target for retigabine is the autonomic nervous system that fine tunes the cardiac rhythm.
The autonomic innervation of the heart affects ventricular repolarization, and thus also the QT interval in the ECG. This is evidenced, for instance, by the finding that patients with pure autonomic failure, who lack a physiological sympathetic tone, exhibit prolonged QTc intervals (heart rate corrected QT interval) (Diedrich et al., 2002). Moreover, several studies reported that drug-induced ganglionic blockade (i.e. autonomic block) results in QT interval prolongation. For example, i.v. application of the ganglionic blocker hexamethonium to monkeys increased the QT interval (Champeroux et al., 2015). Furthermore, autonomic blockade induced by the infusion of propranolol and atropine (Nakagawa et al., 2005) or by trimethaphan administration (Diedrich et al., 2002) led to significant QTc interval prolongation in humans. In line with the latter observation, a case of severe QT prolongation with associated life-threatening torsades de pointes arrhythmias due to trimethaphan intoxication was reported (Hirata et al., 1994). Together, these results indicate that a reduction of the autonomic tone by ganglionic blocking agents leads to QT interval prolongation. The present data obtained in primary cultures of sympathetic ganglia prove that retigabine may also act as ganglionic blocking agent whose actions can also impinge on the release of autonomic neurotransmitters within target organs. The concentrations required for this effect to occur were in the therapeutic range (half maximal inhibition around 1 μM, see Fig. 6) and at least an order of magnitude lower than the drug concentrations required for shortening APs in cardiomyocytes. Accordingly, QT prolongation rather than shortening may be expected after intake of retigabine in standard therapeutic doses. Because drug-induced QT interval prolongation is a risk factor for cardiac arrhythmia generation (Redfern et al., 2003; Vandenberg et al., 2001), the ganglionic blocking action of retigabine may also have accounted for the rare cases of arrhythmias as observed after routine drug administration (Potiga. Prescribing information: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022345s000lbl.pdf) (Fattore and Perucca, 2011).
Taken together, we conclude from the present results that retigabine-induced QT interval prolongation and potentially resulting cardiac arrhythmias cannot be explained by direct modulatory effects of the drug on cardiac ion channels. They are rather mediated by indirect actions at the level of the autonomic nervous system. Clinical studies of retigabine have only reported rare cases of cardiac adverse events, and therefore the drug's cardiac safety is not considered a major problem (Porter et al., 2007; Brodie et al., 2010; French et al., 2011; Gil-Nagel et al., 2012). Nevertheless, the findings of this study have exposed a new concern regarding the interaction of retigabine with other drugs known to prolong the QT interval. Thus, by inhibition of the autonomic tone, retigabine may exaggerate the QT prolonging action of other drugs (Smith et al., 2007), and thereby increase the risk for sudden cardiac death.
Acknowledgements
This work was supported by the Austrian Science Fund FWF (P23060 to K.H.). The funding source had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication. We thank Michael C. Sanguinetti (University of Utah, USA) for providing the plasmid encoding hKv11.1, and Nikolai M. Soldatov (Humgenex, Inc., Maryland, USA) for providing the plasmid encoding hCav1.2.
Abbreviations
- ACh
acetylcholine
- ANOVA
analysis of variance
- AP
action potential
- APD
action potential duration
- ECG
electrocardiogram
- EFS
electrical field stimulation
- hiPSC
human induced pluripotent stem cell
- nAChRs
nicotinic acetylcholine receptors
- RTG
retigabine
- SCG
superior cervical ganglia
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Barrese V, Miceli F, Soldovieri MV, Ambrosino P, Iannotti FA, Cilio MR, Taglialatela M. Neuronal potassium channel openers in the management of epilepsy: role and potential of retigabine. Clin Pharm. 2010;2:225–236. doi: 10.2147/CPAA.S15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X) Epilepsy Res. 2010;92:89–124. doi: 10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Boehm S. Noradrenaline release from rat sympathetic neurons evoked by P2-purinoceptor activation. Naunyn Schmiedeberg's Arch Pharmacol. 1994;350:454–458. doi: 10.1007/BF00173013. [DOI] [PubMed] [Google Scholar]
- Boehm S, Kubista H. Fine tuning of sympathetic transmitter release via ionotropic and metabotropic presynaptic receptors. Pharmacol Rev. 2002;54:43–99. doi: 10.1124/pr.54.1.43. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Lerche H, Gil-Nagel A, Elger C, Hall S, Shin P, Nohria V, Mansbach H. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. 2010;75:1817–1824. doi: 10.1212/WNL.0b013e3181fd6170. [DOI] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champeroux P, Thireau J, Jude S, Laigot-Barbe C, Maurin A, Sola ML, Fowler JS, Richard S, Le Guennec JY. Short-term variability in QT interval and ventricular arrhythmias induced by dofetilide are dependent on high-frequency autonomic oscillations. Br J Pharmacol. 2015;172:2878–2891. doi: 10.1111/bph.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie C, Pierre-Valentin J, Pollard C, Standen N, Mitcheson J, Alexander P, Thong B. Comparative pharmacology of guinea pig cardiac myocyte and cloned hERG (I(Kr)) channel. J Cardiovasc Electrophysiol. 2004;15:1302–1309. doi: 10.1046/j.1540-8167.2004.04099.x. [DOI] [PubMed] [Google Scholar]
- Diedrich A, Jordan J, Shannon JR, Robertson D, Biaggioni I. Modulation of QT interval during autonomic nervous system blockade in humans. Circulation. 2002;106:2238–2243. doi: 10.1161/01.CIR.0000035241.76918.6C. [DOI] [PubMed] [Google Scholar]
- Fattore C, Perucca E. Novel medications for epilepsy. Drugs. 2011;71:2151–2178. doi: 10.2165/11594640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Feldman AE, Gidal BE. QTc prolongation by antiepileptic drugs and the risk of torsade de pointes in patients with epilepsy. Epilepsy Behav. 2013;26:421–426. doi: 10.1016/j.yebeh.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Ferron GM, Paul J, Fruncillo R, Richards L, Knebel N, Getsy J, Troy S. Multiple-dose, linear, dose-proportional pharmacokinetics of retigabine in healthy volunteers. J Clin Pharmacol. 2002;42:175–182. doi: 10.1177/00912700222011210. [DOI] [PubMed] [Google Scholar]
- Ferron GM, Patat A, Parks V, Rolan P, Troy SM. Lack of pharmacokinetic interaction between retigabine and phenobarbitone at steady-state in healthy subjects. Br J Clin Pharmacol. 2003;56:39–45. doi: 10.1046/j.1365-2125.2003.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Abou-Khalil BW, Leroy RF, Yacubian EM, Shin P, Hall S, Mansbach H, Nohria V. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology. 2011;76:1555–1563. doi: 10.1212/WNL.0b013e3182194bd3. [DOI] [PubMed] [Google Scholar]
- Friedman AK, Juarez B, Ku SM, Zhang H, Calizo RC, Walsh JJ, Chaudhury D, Zhang S, Hawkins A, Dietz DM, Murrough JW, et al. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat Commun. 2016;7 doi: 10.1038/ncomms11671. 11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Nagel A, Brodie MJ, Leroy R, Cyr T, Hall S, Castiglia M, Twomey C, VanLandingham K. Safety and efficacy of ezogabine (retigabine) in adults with refractory partial-onset seizures: interim results from two ongoing open-label studies. Epilepsy Res. 2012;102:117–121. doi: 10.1016/j.eplepsyres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012;53:412–424. doi: 10.1111/j.1528-1167.2011.03365.x. [DOI] [PubMed] [Google Scholar]
- Hempel R, Schupke H, McNeilly PJ, Heinecke K, Kronbach C, Grunwald C, Zimmermann G, Griesinger C, Engel J, Kronbach T. Metabolism of retigabine (D-23129), a novel anticonvulsant. Drug Metab Dispos. 1999;27:613–622. [PubMed] [Google Scholar]
- Hermann R, Ferron GM, Erb K, Knebel N, Ruus P, Paul J, Richards L, Cnota HP, Troy S. Effects of age and sex on the disposition of retigabine. Clin Pharmacol Ther. 2003a;73:61–70. doi: 10.1067/mcp.2003.12. [DOI] [PubMed] [Google Scholar]
- Hermann R, Knebel NG, Niebch G, Richards L, Borlak J, Locher M. Pharmaco-kinetic interaction between retigabine and lamotrigine in healthy subjects. Eur J Clin Pharmacol. 2003b;58:795–802. doi: 10.1007/s00228-003-0558-6. [DOI] [PubMed] [Google Scholar]
- Hirata K, Kyushima M, Kawamitsu K, Asato H, Sunagawa H, Uehara H. A case of torsades de pointes probably caused by trimetaphan intoxication. J Cardiol. 1994;24:243–247. [PubMed] [Google Scholar]
- Hoffman BF, Rosen MR. Cellular mechanisms for cardiac arrhythmias. Circ Res. 1981;49:1–15. doi: 10.1161/01.RES.49.1.1. [DOI] [PubMed] [Google Scholar]
- Knapp CM, O'Malley M, Datta S, Ciraulo DA. The Kv7 potassium channel activator retigabine decreases alcohol consumption in rats. Am J Drug Alcohol Abuse. 2014;40:244–250. doi: 10.3109/00952990.2014.892951. [DOI] [PubMed] [Google Scholar]
- Koenig X, Dysek S, Kimbacher S, Mike AK, Cervenka R, Lukacs P, Nagl K, Dang XB, Todt H, Bittner RE, Hilber K. Voltage-gated ion channel dysfunction precedes cardiomyopathy development in the dystrophic heart. PLoS One. 2011;6:e20300. doi: 10.1371/journal.pone.0020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig X, Kovar M, Rubi L, Mike AK, Lukacs P, Gawali VS, Todt H, Hilber K, Sandtner W. Anti-addiction drug ibogaine inhibits voltage-gated ionic currents: a study to assess the drug's cardiac ion channel profile. Toxicol Appl Pharmacol. 2013;273:259–268. doi: 10.1016/j.taap.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche H, Daniluk J, Lotay N, DeRossett S, Edwards S, Brandt C. Efficacy and safety of ezogabine/retigabine as adjunctive therapy to specified single antiepileptic medications in an open-label study of adults with partial-onset seizures. Seizure. 2015;30:93–100. doi: 10.1016/j.seizure.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115:2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Ooie T, Ou B, Ichinose M, Takahashi N, Hara M, Yonemochi H, Saikawa T. Gender differences in autonomic modulation of ventricular repolarization in humans. J Cardiovasc Electrophysiol. 2005;16:278–284. doi: 10.1046/j.1540-8167.2005.40455.x. [DOI] [PubMed] [Google Scholar]
- Otto JF, Kimball MM, Wilcox KS. Effects of the anticonvulsant retigabine on cultured cortical neurons: changes in electroresponsive properties and synaptic transmission. Mol Pharmacol. 2002;61:921–927. doi: 10.1124/mol.61.4.921. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Partiot A, Sachdeo R, Nohria V, Alves WM. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68:1197–1204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/S0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- Rosen MR, Wit AL. Arrhythmogenic actions of antiarrhythmic drugs. Am J Cardiol. 1987;59:10E–18E. doi: 10.1016/0002-9149(87)90196-2. [DOI] [PubMed] [Google Scholar]
- Rubi L, Eckert D, Boehm S, Hilber K, Koenig X. Anti-addiction drug ibogaine prolongs the action potential in human induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc Toxicol. 2016 doi: 10.1007/s12012-016-9366-y. [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundfeldt C, Netzer R. Investigations into the mechanism of action of the new anticonvulsant retigabine. Interaction with GABAergic and glutamatergic neurotransmission and with voltage gated ion channels. Arzneimittelforschung. 2000;50:1063–1070. doi: 10.1055/s-0031-1300346. [DOI] [PubMed] [Google Scholar]
- Salzer I, Gafar H, Gindl V, Mahlknecht P, Drobny H, Boehm S. Excitation of rat sympathetic neurons via M1 muscarinic receptors independently of Kv7 channels. Pflugers Arch. 2014;466:2289–2303. doi: 10.1007/s00424-014-1487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- Schwartz DD, Malik KU. Cyclic AMP modulates but does not mediate the inhibition of [3H]norepinephrine release by activation of alpha-2 adrenergic receptors in cultured rat ganglion cells. Neuroscience. 1993;52:107–113. doi: 10.1016/0306-4522(93)90186-j. [DOI] [PubMed] [Google Scholar]
- Smith AH, Norris KJ, Roden DM, Kannankeril PJ. Autonomic tone attenuates drug-induced QT prolongation. J Cardiovasc Electrophysiol. 2007;18:960–964. doi: 10.1111/j.1540-8167.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- Soldatov NM, Zhenochin S, AlBanna B, Abernethy DR, Morad M. New molecular determinant for inactivation of the human L-type alpha1C Ca2+ channel. J Membr Biol. 2000;177:129–135. doi: 10.1007/s002320001106. [DOI] [PubMed] [Google Scholar]
- Splinter MY. Efficacy of retigabine in adjunctive treatment of partial onset seizures in adults. J Cent Nerv Syst Dis. 2013;5:31–41. doi: 10.4137/JCNSD.S9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Grippon S, Kirkpatrick P. Ezogabine (retigabine) Nat Rev Drug Discov. 2011;10:729–730. doi: 10.1038/nrd3561. [DOI] [PubMed] [Google Scholar]
- Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towart R, Linders JT, Hermans AN, Rohrbacher J, van der Linde HJ, Ercken M, Cik M, Roevens P, Teisman A, Gallacher DJ. Blockade of the I(Ks) potassium channel: an overlooked cardiovascular liability in drug safety screening? J Pharmacol Toxicol Methods. 2009;60:1–10. doi: 10.1016/j.vascn.2009.04.197. [DOI] [PubMed] [Google Scholar]
- Treven M, Koenig X, Assadpour E, Gantumur E, Meyer C, Hilber K, Boehm S, Kubista H. The anticonvulsant retigabine is a subtype selective modulator of GABAA receptors. Epilepsia. 2015;56:647–657. doi: 10.1111/epi.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg JI, Walker BD, Campbell TJ. HERG K+ channels: friend and foe. Trends Pharmacol Sci. 2001;22:240–246. doi: 10.1016/S0165-6147(00)01662-X. [DOI] [PubMed] [Google Scholar]
- Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Han SS, Sandoe J, Perez NP, Williams LA, Lee S, Boulting G, Berry JD, et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg JL, Wong M. Profile of ezogabine (retigabine) and its potential as an adjunctive treatment for patients with partial-onset seizures. Neuropsychiatr Dis Treat. 2011;7:409–414. doi: 10.2147/NDT.S14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Anderson DL, Ross SA, Lang DG, Desai BZ, Cooper DC, Wheelan P, McIntyre MS, Bergquist ML, MacKenzie KI, Becherer JD, et al. Predicting QT prolongation in humans during early drug development using hERG inhibition and an anaesthetized guinea-pig model. Br J Pharmacol. 2008;154:1446–1456. doi: 10.1038/bjp.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]