Abstract
Therapeutic options in atherosclerosis have largely been limited to the control of risk factors, such as hypercholesterolemia, hypertension, or diabetes. However, atherosclerosis is a chronic inflammatory disease in which dyslipidemia and inflammation are equally involved in disease pathogenesis. Moreover, abundant epidemiological and experimental evidence point to an important modulatory role of innate and adaptive immunity in atherogenesis, providing novel therapeutic targets for this disease. Indeed, there is now accumulating data in animal models demonstrating the potential for immunotherapeutic approaches to treat atherosclerosis. These include both general and antigen-specific ways of modulating immune functions, and they show great promise for the development of alternative and/or adjuvant therapies for atherosclerosis.
Keywords: Atherosclerosis, Immunomodulation, Oxidized LDL, Oxidized phospholipids, Immunization, Tolerance, Antibodies, Adjuvants, Peptide
Introduction
Clearly, hypercholesterolemia is a key pathogenic factor in the initiation and progression of atherosclerotic lesions [1]. Nevertheless, there is now ample evidence that certain aspects of innate and adaptive immunity play an important role in atherogenesis [2,3]. Importantly, the recruitment of monocyte/macrophages is a rate limiting step in atherosclerosis and macrophages are not only hallmark cells of atherosclerotic lesions but of innate immunity as well. Thus, innate immune functions are central in the pathogenesis of atherosclerosis. On the other hand, adaptive immune functions are not obligatory for disease development, as atherosclerosis-prone mice that lack both functional B- and T-cells still develop atherosclerotic lesions [4–7]. However, lesion formation in these mice was found to be significantly decreased compared to immunocompetent apoE–/– and LDLR–/– mice, indicating an overall pro-atherogenic role for adaptive immunity; though protective properties of adaptive immunity are clearly present as well. Importantly, when plasma cholesterol levels were sufficiently high in these mice, the differences between lymphocyte deficient and immunocompetent mice disappeared. Thus, under certain conditions adaptive immune functions can have a profound impact. However, when the atherogenic pressure of hypercholesterolemia is sufficiently high, adaptive immune functions may no longer be active. Nevertheless, modulation of certain immune functions in mouse models of atherosclerosis through genetic and other approaches have indicated a critical role for different components of the immune system, and these data were instrumental in the identification of targets for potential therapeutic interventions.
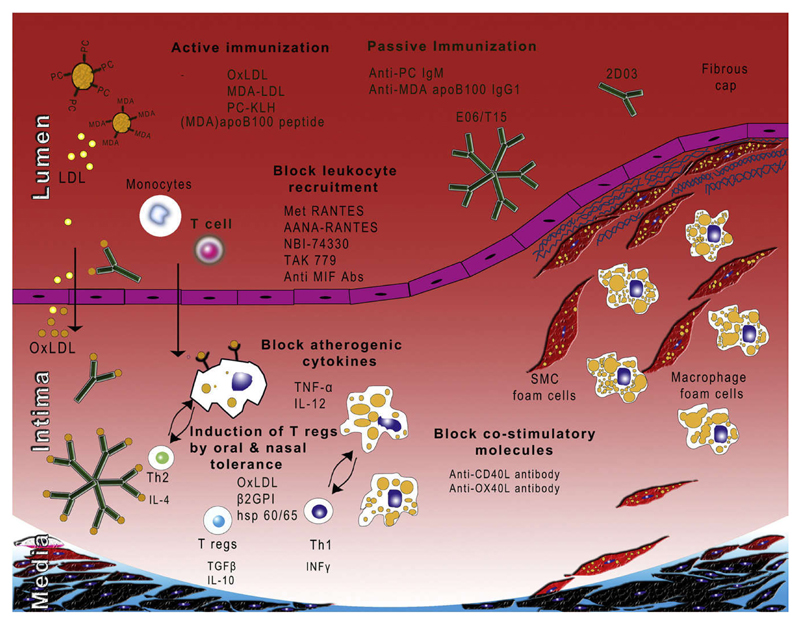
Although immunotherapeutic approaches may also include immune based strategies to interfere with lipoprotein metabolism and vascular cell function, this review will focus on the modulation of specific immune responses relevant to atherogenesis at various levels (Fig. 1). Specifically, the effectiveness of general immune modulation by interference with cytokine and chemokine signalling and T-cell activation will be discussed, as well as antigen-specific approaches including active vaccination and tolerization, and passive immunization strategies.
Figure 1. Immunotherapeutic strategies for atherosclerosis.
Interference with cytokine and chemokine signalling
In analogy to other chronic inflammatory diseases, certain cytokines have been demonstrated to modulate the development of atherosclerotic lesions. In addition, the chemotactic recruitment of monocyte/macrophages is a hallmark of atherosclerotic lesion formation.Thus, a number of pro- and anti-atherogenic cytokines and chemokines have been identified as potential therapeutic targets. Although the administration of specific agents that provide functional blockage of immune signals may be inappropriate for a disease such as atherosclerosis, certain clinical settings or specific agents may favour their chronic use in spite of the risk of chronically impairing host defense functions. Table 1 lists selected agents that show promise in protecting from atherosclerosis by targeting cytokine and chemokine signalling.
Table 1. Cytokines and chemokines.
| Target | Treatment | Immunological/metabolic effect | Effect on atherosclerosis | Experimental model | Diet | Reference |
|---|---|---|---|---|---|---|
| TNF α | Chimeric sTNF-RI/Fc (pellets) | Combined inhibition of TNF-α and LT-α, tendency of ↑TC | 75% ↓ in lesion size, ↑ in CD3+ stained area in the plaques | Male apoE−/− mice | Normal chow diet | Branen et al., 2004 [64] |
| IL-12 | Immunization with IL-12–PADRE complex | Blocking endogenous IL-12 function by induction of anti IL-12 Abs (IgG1, IgG2a), ↓ serum IFN-γ | 69% ↓ in intima area, 67% ↓ intima/media ratio, 58% ↓ in the amount of stenosis, 4-fold ↑ in SMCs, 3-fold ↑ in collagen content, ↓ IFN-γ+ cells | Female LDLR−/− mice (bilateral CA collar) | Western type diet | Hauer et al., 2005 [65] |
| CCL5/RANTES receptor | Met RANTES (RANTES receptor antagonist) | Limits monocyte/macrophage and T-cell chemotaxis and T-cell activation by blocking RANTES signalling | 43% ↓ aortic root, 58% ↓ thoraco-abdominal aorta, 43% ↓ Mac-1+, 83% ↓ CD4+, ↑ SMCs and collagen, ↓ MMP-9 expression, ↓ CCR5 and CCR2 mRNA expression | Male LDLR−/− mice | High chelesterol diet | Veillard et al., 2004 [66] |
| MIF | Anti-MIF monoclonal antibody | Neutralization of MIF, ↑ serum IL-2, IL-4, IL-6, IL-10 and TNFα | ↓ Mac-1+ cells (26% vs. 52%), ↓ Mac-2+ foam cells, ↑ αSMA (44% vs. 23%), ↑ neointimal collagen type I (28% vs. 16%) | Female apoE−/−, (wire induced injury in left CA) | Atherogenic diet | Schober et al., 2004 [67] |
| MIF | Anti-MIF monoclonal antibody | Neutralization of MIF, ↓ serum MIF, ↓ serum IL-6, ↓ fibrinogen | Small non-significant ↓ in aortic plaque, ↓ mRNA expression of ICAM-1, MMP-2, TNF and IL-12, ↓ protein expression of CD40L, phospho-c-Jun and C/EBPβ | apoE−/− mice | Normal chow diet | Burger-Kentischer et al., 2006 [68] |
| MIF | Anti-MIF monoclonal antibody | Neutralization of MIF, blockage MIF/CXCR2 interaction | Regression of pre-existing atherosclerotic plaques (aortic root), ↓ macrophages and CD3+ T-cells | apoE−/− mice | Atherogenic diet | Bernhagen et al., 2007 [69] |
| CCR5 and CXCR3 | HIV entry inhibitor, TAK-779 (CCR5 and CXCR3 antagonist) | Antagonism of CCR5 and CXCR3, CD4+ T-cells (↓ in blood and lymph nodes; ↑ in spleen), CD8+ T-cells (↓ in blood and lymph nodes), ↑ mRNA of CCR5, CCR2, MCP-1, IL-12, IL-4 in spleen | 68% ↓ carotid lesion size, 49% ↓ intima-media ratio, 56% ↓ intima lumen ratio, 95% ↓ CD3+ T-cells, 98% ↓ INF-γ+ area | Female LDLR−/− mice (CA collar) | Western type diet | Van Wanrooij et al., 2005 [70] |
| CXCR3 | NBI-74330 (CXCR3 antagonist) | Antagonism of CXCR3, 64% ↓ cell numbers in draining lymph nodes (with ↑ of CD4+CD25high, ↑ CD4+CD62Lhigh) | ↓ Lesion size (27% ↓ in aortic root, 53% ↓ in aorta), ↑ TGF-β+ area, ↑ mRNA expression of Foxp3,CD25, CTLA-4 (in carotid artery) | Female LDLR−/− mice | Western type diet | Van Wanrooij et al., 2008 [71] |
| RANTES | Inhibitor of endogenous RANTES [44MNA47]-RANTES | Inhibition of RANTES, ↓ leukocyte rolling and arrest in mesenteric, vessels, ↓ secretion of IFNγ and TNFα, ↓ mRNA expression of TIM-3 (splenocytes) | ↓ Lesion size in established atheroscierotic plaques (thoraco-abdominal aorta and aortic root), ↓ CD4+ cells, ↓ macrophages, ↓ MMP-9, ↑ SMCs, ↑ collagen, ↓ mRNA of CCR2, CCR5 and CCL2/MCP-1 | Male LDLR−/− mice | High chelesterol diet | Braunersreuther et al., 2008 [72] |
Abbreviations: TC, total cholesterol; CA, carotid artery; TNF α, tumor necrosis factor alpha; LT-α, lymphotoxin alpha; Chimeric TNF-RI/Fc, recombinant murine TNF-RI fused with Fc fragment of human IgG; IL, interleukin; SMC, smooth muscle cells; IFN-γ, interferon gamma; MIF, migration inhibitory factor; αSMA,alpha smooth muscle actin; Mac-1, macrophage antigen 1; Mac-2, macrophage antigen 2; ICAM-1, Inter-Cellular Adhesion Molecule 1; MMP2, matrix metallopeptidase 2; CD40L, CD40 ligand; CXCR, CXC chemokine receptor; CCR, chemokine (C–C motif) receptor; MCP1, monocyte chemotactic protein-1(CCL2), Tim-3, Th1-specific cell surface transmembrane protein; RANTES(CCL5),chemokine (C–C motif) ligand 5; LDLR, low-density lipoprotein (LDL) receptor; ApoE, apolipoprotein E.
General immune modulation
The fact that certain adaptive immune responses have been found to modulate atherogenesis, suggested the possibility for therapeutic interventions by interfering with specific effector functions of the immune system. While some immune responses such asTh1- and in particular IFNγ-mediated responses have been found to promote lesion formation, others such as B-cell associated responses have been suggested to be protective [3]. Thus, the general interference with immune functions bears the risk that such strategies may potentially inhibit protective effects to the same extent as pro-atherogenic ones. Nevertheless, approaches targeting T-cell mediated immune responses have been successful in murine atherosclerosis, and may qualify as useful therapies for short term interventions in certain conditions.
Anti-CD3 antibodies
Anti-CD3 antibodies act as potent immunosuppressive agents by modulating the CD3/T-cell receptor (TCR) complex. They have been used in patients to prevent short term allograft rejection and were also shown to induce long-term remission of autoimmune diabetes in nonobese diabetic (NOD) mice [8]. Administration of nonmitogenic anti-CD3 antibodies in LDLR–/– mice fed a high cholesterol diet reduced the development of atherosclerotic lesions but also slowed the progression of established lesions. Treatment markedly boosted the production of TGFβ and increased the expression of Foxp3 mRNA in lymph nodes and spleen cells, suggesting the induction of a regulatory immune response though the number of CD4+CD25+ Tregs was not increased after the treatment [9]. Of great relevance is the fact that these profound effects were observed even though only a short term treatment regimen was used.
Co-stimulatory signals
In analogy, co-stimulatory signals of T-cell activation also present suitable targets for immunological interventions. For example, the co-stimulatory molecules CD40 and CD40L have been shown to play a central role in atherosclerosis progression. CD40 and CD40L are co-expressed on numerous cell types within atherosclerotic lesions, including endothelial cells, macrophages, smooth muscle cells and T-cells. Treatment of LDLR–/– mice with an anti-CD40L antibody and fed a high cholesterol diet for 12 weeks significantly reduced atherosclerotic lesion formation. Furthermore, fewer macrophages and lymphocytes were found in lesions of treated mice [10]. Importantly, while anti-CD40L treatment of LDLR–/– mice with established atherosclerosis did not reduce the size of atherosclerotic lesion, it triggered a change in lesion composition to a more stable plaque phenotype with reduced content of lipids and macrophages and a relative increase in collagen content and smooth muscle cells [11]. Similar results were obtained by Lutgens et al. in apoE–/– mice, in which anti-CD40L treatment did not show an effect on lesion size but did alter the morphology of lesions towards more lipid-poor, collagen-rich stable plaques [12]. Thus, interference with co-stimulatory signals has also the important potential to alter plaque phenotypes towards more stable less vulnerable and rupture-prone ones. Another example are the co-stimulatory molecules OX40 and OX40L of the TNF/TNFR family, which are expressed on activated CD4+ and CD8+ T-cells and APCs and endothelial cells, respectively [13]. In a genomic association study OX40L was identified as a putative pro-atherogenic gene within a locus on chromosome 1, which is associated with aggravated atherosclerosis [14]. Indeed, OX40L–/– mice were found to show smaller atherosclerotic lesion size and overexpression of OX40L led to a larger atherosclerotic lesion compared to controls [15]. Consistent with this, treatment of LDLR–/– mice with anti-OX40L antibody induced a more than 50% decrease in atherosclerotic lesion formation [16].
Antigen-specific immune modulation
Several potential antigens have been suggested to trigger immune responses that modulate atherosclerotic lesion formation [2,3,17,18]. These include bacterial and viral antigens, such as Chlamydia pneumonia, CMV, as well as (altered) self antigens such as, heat shock protein 60 (including cross-reactive microbial mHSP65 and cHSP60), β2gpI and oxidized LDL. Immune responses to most of these antigens have been found to modulate disease progressions to different degrees. Therefore, current immunological interventions to prevent atherogenesis aim at dampening pro-atherogenic responses or boosting protective ones (Table 2).
Table 2. Oral, active and passive immunizations.
| Target | Treatment | Immunological/metabolic effect | Effect on atherosclerosis | Experimental model | Diet | Reference |
|---|---|---|---|---|---|---|
| HSP65 | Oral administration of HSP65 | Induction of tolerance, 4-fold ↑ production of IL-4 by lymph node cells in HSP65-fed and immunized mice | 61% ↓ in fatty streak formation in aortic origin | Female LDLR–/– mice |
Western type diet |
Harats et al., 2002 [56] |
| HSP65 | Oral (O) or nasal (N) admin stration of HSP65 | Induction of tolerance (↓ proliferation; IFN-γ production and ↑ in IL-10 production by lymph node cells (N) to HSP65), ↓ anti-HSP65 IgG and ↑ IgG1 |
↓ Lesion size in aortic arch (O,N), ↓ macrophages and CD4+ T-cells(N), ↓ IFN-γ and ↑ IL-10 expression (N), co-localization of IL-10 with macrophages and SMCs | Female LDLR–/– mice |
High cholesterol diet |
Maron et al., 2002 [57] |
| HSP60 | Oral administration of HSP60, HSP60* (253–268) | Induction of tolerance (↓ splenocytes proliferation to HSP60), ↑ TGF-β and IL-10 by mesenteric lymph node cells, ↑ CD4+CD25+Foxp3+ cells in blood, spleen and mesenterlc lymph nodes |
↓ Lesion size in aortic orgin (HSP60–27% ↓) and in carotid artery (HSP60–81%, HSP60*–83%), ↓ intima/lumen ratio (HSP60–69% and HSP60*–74%), ↑ mRNA expression of CTLA-4, CD25, Foxp3 | Male LDLR–/– mice (+CA collar) |
Western type diet |
van Puijvelde et al., 2007 [58] |
| HSP65 | Nasal administration of HSP65 | Induction of tolerance (↓ splenocytes proliferation to HSP65), 7-fold ↑ in IL-10 production by T-cells, ↓ serum lipids (TC, HDL-C, LDL-C) |
↓ Lesion size in aorta (from 63% to 13%) | Male NZW rabbits | High cholesterol diet |
Xiong et al., 2009 [63] |
| β2-GPI | Oral administration of human and bovine β2-GPI | Induction of tolerance, ↓ lymph node proliferation to β2GPI and reactivity to OxLDL, ↑ of IL-4 and ↑ IL-10 production in lymph node cells | Suppression of early atherosclerotic lesions (45% ↓ with human β2GPI, 57% ↓ with bovine β2GPI) | Female LDLR–/– mice |
Western type diet |
George et al., 2004 [59] |
| OxLDL | Oral administration of human oxidized LDL | Induction of tolerance, ↓ CD4+CD25+Foxp3+ T-cells in spleen and mesenteric lymph nodes, 6-fold ↑ of TGF-β in lymph node cells after re-stimulation with OxLDL | ↓ Initiation (30% ↓ aortic root, 71% ↓ carotid artery) and progression (45% ↓ aortic root), ↑ mRNA expression of CD25, Foxp3 and CTLA | LDLR–/– mice (+CA collar) |
Western type diet |
Van puijvelde et al., 2006 [60] |
| MDA-LDL | Immunization with homologous MDA-LDL | Induction of anti-MDA-LDL antibodies (IgG) | ↓ In lesion size (entire aorta: 48% vs. 68%, arch and thoracic aorta: 57% vs. 79%, abdominal aorta: 36% vs. 52%) | Male and female LDLR–/– WHHL rabbits | Normal chow diet |
Palinski et al., 1995 [27] |
| LDL and OxLDL | Immunization with homologous LDL and CuOx-LDL | Induction of antibodies against OxLDL (IgG) | ↓ Lesion size in proximal aorta 74% (LDL) and 48% (CuOx-LDL) | Male NZW rabbits | High cholesterol diet |
Ameli et al., 1996 [28] |
| MDA-LDL | Immunization with homologous MDA-LDL | Induction of anti-MDA-LDL antibodies (IgG) | 53% ↓ Lesion size (aortic origin) | Female apoE–/– mice | Normal chow diet |
George et al., 1998 [30] |
| LDL and OxLDL | Immunization with homologous LDL and CuOx-LDL | Induction of antibodies against OxLDL (IgG) | ↓ Neointimal area (58% ↓ with OxLDL, 19% ↓ with LDL) | Male NZW rabbits (femoral artery balloon injury) | High cholesterol diet |
Nilsson et al., 1998 [29] |
| LDL and MDA-LDL | Immunization with MDA-LDL | ↑ Anti-MDA-LDL IgG (↑ of IgG1 and IgG2a Abs) and ↑ IgG to other oxidative neoepitopes, ↑ IgM (to CuOx-LDL and 4HNE-LDL) | ↓ Lesion size aortic origin (46% ↓ with MDA-LDL and 37% ↓ with LDL) | Male LDLR–/– mice | High cholesterol diet |
Freigang et al., 1998 [31] |
| Plaque homogenates and MDA-LDL | Immunization with homologous plaque homogenates and MDA-LDL | ↑ Anti-MDA-LDL IgG and anti-cardiolipin IgG and association with ↓ serum cholesterol, activation of lymph node cells with MDA-LDL | 39% ↓ Lesion size (homogenate) 46% ↓ (MDA-LDL) in aortic origin, ↓ CD8+ cells | Male apoE–/– mice | Western diet. | Zhou et al., 2001 [32] |
| PC epitopes | Immunization with heat inactivated PC-containing pneumococci | Induction of PC-specific Abs, molecular mimicry between S. pneumoniae and OxLDL, ↑ anti-OxLDL IgM (T15id+ IgM), ↑ OxLDL-specific T15id+ IgM secreting cells (spleen and bone marrow) | 22% ↓ Lesion size (aortic rorigin) | Male LDLR–/– mice | High cholesterol diet |
Binder et al., 2003 [41] |
| MDA-LDL | Immunization with homologous MDA-LDL | ↑ Anti-MDA-LDL Abs (IgM, IgG1 >>IgG2a), ↑ plasma IL-5, ↑ secretion of MDA-LDL specific Th2 cytokines, ↑ T15id+ IgM, ↑ plasma IgM/apoB immune complexes | 25% ↓ Lesion size (aortic origin) | Female LDLR–/– mice | High chlesterol diet |
Binder et al., 2004 [34] |
| MDA-LDL | Immunization with homologous MDA-LDL | ↑ Anti-MDA-LDL IgG (IgM, IgG2b> IgG1>IgG2a Ab titer), lower IgM, IgG2a, IgG2b titer in CD4–/–apoE–/– vs. apoE–/– | 39% ↓ Lesion size (aortic origin) for CD4–/–apoE–/– and 23% ↓ lesion size for apoE–/– | Female CD4–/– apoE–/– and apoE–/– mice | Normal chow diet |
Zhou et al., 2005 [33] |
| PC-KLH | Immunization with PC-KLH | ↑ Anti-PC and anti-OxLDL Abs (IgM and IgG), ↑ splenic mature B -cells | ↓ 40% Lesion size (aortic origin), ↓ expression of MHCII, presence of T15d+Abs, B-cellclusters | Female apoE–/– mice | Normal chow diet |
Caligiuri et al., 2007 [42] |
| MDA-modified apoB100 peptides | Immunization with different native and MDA-modified apoB100 peptides | ↑ IgG Abs against native and MDA-modified apoB100 peptides | 60% ↓ lesion size in descending aorta, ↑ collagen in subvalvular plaques (P143, P210), no ↓ in lesion size (5 peptide mixture) | Male apoE–/– mice | Normal chow diet |
Fredrikson et al., 2003 [44] |
| MDA-modified apoB100 peptides | Immunization with MDA-modified apoB100 peptides (P45 and P74, control P240) | 50-fold ↑ anti-peptide IgG1 (P45, P74) | ↓ Lesion size (48% ↓ with P45, 31% ↓ with P74), ↓ macrophage content | apoE–/– mice | Fredrikson et al., 2005 [45] | |
| MDA-modified apoB100 peptides | Immunization with apoB100 peptides (P45 and P210) | ↑ Anti-LDL and anti-OxLDL IgM in P45, similar trend with P210, no change in anti-MDA-LDL IgG or IgM Abs | ↓ Lesion size (66% ↓ with P45, 59% ↓ with P210) | Male LDLR–/–/human apo B-100 mice | Fredrikson et al., 2008 [46] | |
| PC epitopes | Murine T15id+ anti-PC IgM | ↑ Anti-PC IgM titers | 43% ↓ vein graft lesion size, ↓ neointimal thickness, ↓ inflammatory cell content, no effect on established atherosclerosis in aortic origin | Male apoE–/– mice (vein graft model) | Western type diet |
Faria-Neto et al., 2006 [47] |
| MDA-modified apoB100 peptide | Recombinant human anti-MDA-apoB100 peptide IgG1 (IEl-E3) | ↑ Human anti-MDA -modified apoB100 IgG1 titers | 41% ↓ lesion size (descending aorta), ↓ macrophages, ↓ in IEl-E3 immunoreactivity in plaque | Male apoE–/– mice | High cholesterol diet |
Schiopu et al., 2004 [48] |
| MDA-modified apoB100 peptide | Recombinant human anti-MDA-apoB100 peptide IgG1 (IEl-E3 and 2D03) | ↑ Human anti-MDA -modified apoB100 IgG1 titers | Regression of established plaques (50% – 2D03, 36% – IEl-E3), ↓ macrophages, ↑ ABCA-1 expression | Male Apobec-1–/–/LDLR–/– mice | High cholesterol and normal chow diet |
Schiopu et al., 2007 [49] |
| MDA-modified apoB100 peptides | Recombinant human anti-MDA-apoB100 peptide IgG1 (2D03) | ↑ Human anti-MDA -modified apoB100 IgG1 titers, ↓ plasma levels of oxidized LDL | 95% ↓ injury-induced atherosclerosis, no influence on neointima, | LDLR–/–/human apoB100+/+ (CA collar) | Normal chow diet |
Ström et al., 2007 [73] |
Abbreviations: HSP, heat shock protein; NZW, New Zealand White; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HSP60*, HSP60 peptide (256–268); CD4+CD25+Foxp3+ cell, regulatory T-cells; β2-GPI, β2-glycoprotein I; Ox LDL, Oxidized LDL; TGF-β, Transforming growth factor beta; MDA-LDL, malondialdehyde-modified LDL; WHHL, watanabe heritable hyperlipidemic; CuOx-LDL, copper-oxidized LDL; 4HNE-LDL, 4-hydroxynonenal modified LDL; VCAM-1, vascular cell adhesion molecule-1; ICs, immune complexes; PC, phosphorylcholine; KLH, keyhole limpet hemocyanin; MHC, major histocompatibility complex.
Active immunization
When LDL is trapped in the artery wall it undergoes progressive oxidative changes that lead to the generation of pro-inflammatory oxidized lipid moieties on OxLDL, which is taken up by macrophage scavenger receptors leading to foam-cell formation [1]. OxLDL also contains multiple “oxidation-specific” epitopes that are recognized by specific immune responses. For example, when the most abundant phospholipid of LDL phosphatidylcholine (PC), which contains an oxidation-prone sn-2 polyunsaturated fatty acid, undergoes oxidation, highly reactive breakdown products such as malondialdehyde (MDA), 4-hydroxynonenal (4-HNE) and the remaining “core-aldehyde”, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphatidylcholine (POVPC) are generated [19]. These aldehydes form covalent adducts with amino-groups of proteins and lipids and are recognized by specific antibodies in a hapten-specific manner. An array of innate pattern recognition receptors recognize these and many other oxidation-specific epitopes as PAMPs [20]. Moreover, both IgG and IgM antibodies to models of OxLDL (e.g. MDA-modified LDL and copper-oxidized LDL) are present in lesions and plasma of humans and animal models of atherosclerosis, respectively [21,22]. In atherosclerosisprone apoE–/– or cholesterol-fed LDLR–/– mice IgM antibodies to OxLDL are increased and dominate the humoral immune response to oxidation-specific epitopes. In humans many but not all epidemiological studies have demonstrated an association of OxLDL-specific autoantibody titers with disease progression or clinical events (reviewed in [23]). More recently, emerging data suggest an inverse correlation of OxLDL-specific IgM but not IgG titers with CVD [24,25], suggesting a protective role for IgM antibodies. There is, however, too limited data available to fully support a pro- or anti-atherogenic role for endogenous anti-OxLDL antibodies in humans, and epidemiological studies need to further address potential differences between anti-OxLDL responses of different Ig subclasses. In addition, T-cells with specificity for OxLDL have been documented in atherosclerotic lesions, and OxLDL-specific Th1 cells are thought to be responsible for the local production of IFNγ in atherosclerotic lesions [26]. Thus, OxLDL-specific immune responses have likely a complex and dual role in atherosclerosis.
Homologous oxidized LDL
In a seminal study the laboratory of Dr. Witztum made the surprising observation that immunization of LDLR deficient rabbits with homologous MDA-LDL suspended in complete Freund's adjuvant (CFA) and subsequent booster immunizations of antigen in incomplete Freund's adjuvant (IFA) at monthly intervals for 6 months markedly reduced the extent of atherosclerosis compared to the controls. All MDA-LDL immunized animals developed high and specific anti-MDA-LDL IgG titers and this demonstrated the potential for immunological modulation of the atherogenic process, though the mechanism by which immunization led to a protective effect remained unclear [27]. Similarly, Nilsson et al. performed an immunization study in which NZW rabbits received only two injections of either native LDL or copper-oxidized LDL and demonstrated a significant reduction of lesion formation in LDL-immunized rabbits after 16 weeks of cholesterol-feeding [28]. Likely LDL immunization resulted in the oxidative modification of the native LDL, as after 16 weeks of diet IgG titers to OxLDL were significantly increased by twofold and similar to the titers achieved with OxLDL immunization. Using an analogous protocol the same authors showed a protective effect of copper-oxidized LDL immunization on neointima formation in a balloon injury model of NZW rabbits [29]. These original studies in rabbit models paved the way for a number of subsequent studies that further corroborated the protective effect of OxLDL immunization in mouse models of atherosclerosis providing increasing insight into potential mechanisms. Indeed, immunization with homologous MDA-LDL emulsified in CFA/IFA has been shown to decrease atherosclerotic lesion formation in both apoE–/– and LDLR–/– mice [30,31]. Remarkably, the protective effect in LDLR–/– mice was found despite extremely high plasma cholesterol levels of around 1400 mg/dl. In the same study the authors demonstrate the induction of robust T-cell dependent IgG1 and IgG2a antibodies to MDA-LDL, but also IgGs to other epitopes of OxLDL, including CuOx-LDL, oxidized cardiolipin, oxidized cholesterol, oxidized cholesterol linoleate. Moreover, IgM titers to some of these also continuously increased in response to immunization with MDA-LDL. Interestingly, the authors also reported a protective effect of immunization with native LDL, which was associated with a minimal but significant increase of IgG titers to oxidation-specific epitopes [31]. These data suggested that atheroprotection may not only be due to the induction of high titered antibodies. Zhou et al. also confirmed the protective effect of immunization with MDA-LDL, which they found associated with a robust induction of anti-MDA-LDL IgG. Moreover, they could document increased activation of CD69+CD4+ and CD8+ T-cells in lymph nodes from immunized mice after in vitro incubation with MDA-LDL [32]. However, in a later study the authors demonstrated that the protective effect of MDA-LDL immunization does not depend on the presence of CD4+ T-cells, as immunization was still protective in CD4–/–apoE–/– mice; although the importance of CD4+ T-cells for atherogenesis was demonstrated by the fact that CD4–/–apoE–/– developed significantly less lesions than apoE–/–-mice. Anti-MDA-LDL antibodies were significantly elevated in MDA-LDL immunized apoE–/– and CD4–/–apoE–/– mice as compared with PBS-immunized ones. However, antibodies of the IgM, IgG2a, and 2b subclasses were significantly lower in CD4–/–apoE–/– mice than in apoE–/– animals. Therefore, the protective effect of immunization may primarily involve other mechanisms than the induction of CD4+ T-cells and these effects seem to target atherogenic events that do not involve CD4+ T-cells [33]. To obtain better mechanistic insights into the protective effect of immunization, we analyzed in more detail the immune response induced by immunization with MDA-LDL. Immunization with MDA-LDL, led to the preferential induction of IL-5 secreting T-cells associated with a rise of Th2-cytokine dependent IgG1 titers but also IgM titers to MDA-LDL. Moreover, plasma levels of IL-5 were also found significantly increased in MDA-LDL immunized LDLR–/– mice. Importantly, immunization with MDA-LDL was also found to increase the levels of the atheroprotective natural IgM T15/EO6 (see below), which specifically recognize the phosphocholine group of oxidized phospholipids, in an IL-5 dependent manner.Thus, MDA-LDL immunization induces a specific Th2-type response that is dominated by IL-5, which provides non-cognate help for the expansion of natural IgM Abs by B-1 cells [34]. Importantly, we have recently demonstrated that a large percentage of B-1-cell derived natural IgM have specificity for different oxidation-specific epitopes, and such natural IgM may mediate important house-keeping functions in defending against the impact of accumulating oxidation-specific epitopes, as it is the case in atherosclerosis [35,36]. However, the absolute role of IL-5 or IgM in the protective effect of this immunization still needs to be established. Similarly, the in vivo role of IgG antibodies with specificity for OxLDL has not been established. Unlike IgM, IgG are recognized by specific Fcγ-receptors and in vitro studies have shown that IgG-containing OxLDL-immune complexes promote lipid accumulation and pro-inflammatory responses in macrophages [37]. Thus, OxLDL-specific IgG antibodies (or certain subclasses) may well have proatherogenic effects. Additional protective mechanisms of these immunizations may also include the induction of regulatory immune responses. Though, no evidence has been provided in this regard so far.
Phosphocholine (PC)
An important issue regarding the design of atheroprotective vaccinesis the identification of relevant epitopes. OxLDL is a complex and large particle that contains numerous lipid-peroxidation products that are recognized as oxidation-specific epitopes. Phosphocholine (PC) of oxidized phospholipids such as POVPC of OxLDL is the best studied one of them. This is largely due to the characterization of the monoclonal IgM antibody EO6, which was cloned from the spleens of cholesterol-fed apoE–/– mice and subsequently found to be a germline encoded natural IgM and identical to the prototypic B-1-cell clone T15. T15 is specific for PC linked to cell wall polysaccharides of pathogens such as S. pneumoniae and provides optimal protection against pneumococcal infections in mice. Thus, the same natural antibody binds to PC of OxPL as well as to PC of the CPS of S. pneumoniae. Based on this molecular mimicry, we immunized cholesterol-fed LDLR–/– mice with heat inactivated PC-containing pneumococci that led to the induction of high titers of IgM antibodies against OxLDL. In fact, the rise in anti-OxLDL IgM was conferred primarily by a near monoclonal expansion IgM antibodies with the T15 idiotype and significantly decreased lesion formation. Consistent with the thymus independent (Tl-2) character of the response, there was only a minimal induction of TI IgG3, but not of T-cell dependent (TD) IgG titers to OxLDL. Thus, the anti-PC IgM EO6/T15 that binds OxLDL mediate atheroprotection, and likely mediate this effect by neutralizing pro-inflammatory effects of OxPL on endothelial cells [38] and macrophages [39] and/or by preventing foam-cell formation [40]. Indeed, plasma of immunized mice had an enhanced ability to inhibit the binding of OxLDL by macrophages in vitro [41]. In a subsequent study, the impact of PC-based immunizations was tested in apoE–/– mice that were immunized with PC-KLH using CpG as adjuvant. This approach led to more than 40% reduction in lesion format on compared to mice immunized with KLH alone or with PBS. In contrast to the previous study using pneumococcal extracts, both IgM and IgG titers to PC were increased with this immunization protocol, though the contribution of the induced IgG titers in the protective effect is unclear [42]. While IgM antibodies provide profound neutralizing effects, IgG Abs may – depending on the induced isotype – provide further modulatory effects.
Native and MDA-modified apoB-100 peptides
During the oxidation of LDL reactive aldehydes such as MDA are generated, which can form covalent and immunoreactive adducts with apoB-100. In addition, during this process apoB100 is also fragmented likely leading to the generation of other neoepitopes. Fredrikson et al. synthesized a library of native and malondialdehyde-modified polypeptides covering the complete apoB-100 sequence and demonstrated autoantibodies against these in human plasma [43]. To investigate the immune response against these peptides, apoE–/– mice were immunized with peptides linked to BSA. Immunization with apoB-100 peptides resulted in reduction of atherosclerosis by about 60% compared to controls given carrier and adjuvant alone. Interestingly, only IgG but not IgM titers against the MDA-peptides increased in immunized mice [44]. In a subsequent study the efficacy of three different MDA-apoB100 peptides (P45, P74, and P240) were tested in apoE–/– mice. Only immunization with P45 had a significant effect on early lesion formation in the descending aorta, while both P45 and P74 decreased the macrophage content in atherosclerotic plaques. Immunization increased IgG1antibodies specific for each peptide more than 50-fold, whereas the levels of specific IgM and IgG2a were only slightly affected. Though plaque INFγ expression was not down-regulated, the increase in IgG1 suggested a predominant Th2 response [45]. As all apoB100 sequences were derived from human apoB100, it was important to test the efficacy of this approach in a mouse model that would exclude potential immune reactions to non-self apoB. Using human apoB-100 transgenic LDLR–/– mice the authors confirmed this protective effect using the peptides P45 and P210, respectively. P45 immunization resulted in significantly reduced lesion formation while the reduction achieved by P210 did not reach significance. Interestingly, in this model the immunization with P45 in particular primarily induced IgM but not IgG antibodies against native LDL and CuOx-LDL [46]. Thus, in summary studies using apoB-peptide vaccines result in atheroprotection, but the exact mechanisms of the protective effect is still elusive. Again, humoral immune responses may be responsible for part of it.
Passive immunization
As most of the active immunization approaches were found to induce high titered antibodies against the respective model antigens, the therapeutic potential of isolated antibodies in atherosclerosis has been tested.
Anti-PC IgM
Most prominently, PC-specific EO6/T15 IgM antibodies are hypothesized to partially mediate the protective effect of OxLDL immunizations. Indeed, four weekly intraperitoneal injections of purified T15-idiotype positive IgM antibodies in apoE–/– mice significantly reduced lesion formation in a carotid artery vein graft derived from the V. cava of homologous mice. However no significant effect was observed on native established aortic lesions in the aortic sinus, likely due to the short duration of therapy with the purified antibody [47]. Nevertheless, this study demonstrated that administration of purified anti-PC IgM has the potential to directly decrease atherosclerotic lesion formation and further supports a protective function of anti-OxLDL IgM antibodies.
Anti-MDA-peptide IgG1
The effectiveness of passive immunizations was also tested using recombinant human IgG1 antibodies specific for MDA-modified apoB-100 peptides, which were identified from single-chain antibody-fragment library that was screened with different peptides. One antibody, termed IEI-E3, indeed showed an protective effect on lesion formation in apoE–/– mice when administered weekly during the last 4 weeks of a 19 week feeding period. The authors also demonstrated that the same antibody had the capacity to increase OxLDL binding and uptake by monocytes in vitro [48]. In a follow-up study using Apobec-1–/–/LDLR–/– mice with established atherosclerosis the authors tested the potential of two human antipeptide antibodies to stimulate regression. After 20 weeks of high fat diet, regression was induced by changing to regular chow diet for a 5-week period. During this time, mice received three weekly injections of two MDA-peptide specific (IEl-E3 or 2D03) or a control IgG1, respectively. Both specific IgG1 promoted the regression significantly, and 2D03 also stimulated the expression of ABCA-1 in the plaques, suggesting that the antibody may mediate induction of cholesterol efflux [49]. Moreover, Goncalves et al. demonstrated the presence of 2D03-reactive epitopes in human plaques [50]. Thus, human anti-MDA-peptide IgG1 have a protective capacity in mouse models of atherosclerosis. As the functional properties of human IgGs are difficult to assess in murine models, interpretation of the exact mechanism by which these antibodies mediate protection is difficult.
Mucosal tolerance
Unlike active immunization approaches that aim at the induction of protective immune responses, tolerization approaches aim at the weakening or suppression of cellular and/or humoral immune response to endogenous antigens. Depending on the dose, time and route of antigen administration, potential effects include the clonal deletion [51] or anergy [52],53] of specific T-cells, or the induction of different types of regulatory T-cells [54]. The latter of which has been successfully employed to attenuate different autoimmune-mediated diseases in experimental models [55]. In murine atherosclerosis mucosal tolerization approaches have also shown success.
Heat shock protein 60/65
For example, both nasal or oral administration of hsp65 but not of control proteins in LDLR–/– mice have been shown to significantly reduce atherosclerotic lesions induced by atherogenic diet [56,57]. The best indicator for a successful tolerization are the induced changes in endogenous auto-immune responses. For example, Maron et al. documented a successful mucosal tolerization of endogenous anti-hsp60/65 responses as indicated by decreased proliferation of splenocytes in response to hsp65 and decreased anti-hsp65 IgG titers. In this study, nasal administration resulted in decreased lesional macrophage and T-cell content, reduced expression of IFNγ and increased IL-10 expression, as well increased Th2 dependent anti-hsp65 IgG1 titers. However, despite an atheroprotective effect such differences in cellular lesion content of tolerized mice or hsp65-specific immune responses were not seen in the study by Harats et al. In a later study, van Puijvelde etal. carried out a similar tolerization experiment by orally administering hsp60 or a 16aa peptide of hsp60 (253 to 268) to LDLR–/– mice that were subsequently fed an atherogenic diet [58]. These treatments resulted in a 27% reduction in plaque area at the aortic root (hsp60) and a 80% reduction of lesion formation in carotid arteries following collar placement (hsp60 and hsp60 peptide). Moreover, increased Levels of CD4+CD25+Foxp3+ regulatory T-cells in the lymphoid organs and blood were found. Lymph node cells from hsp60 tolerized mice produced increased amounts of IL-10 and TGFβ when stimulated in vitro with hsp60, but no changes of endogenous anti-hsp60 Abs were seen. Thus, hsp60/65 represent an appropriate and effective target for mucosal tolerization. However, evidence on the actual suppression of endogenous and pro-atherogenic responses in atherosclerotic mice using these approaches is limited. In fact, such effects were to a large extent reported only when immune responses were induced by immunization following the tolerization protocol. In analogy to hsp60/65, oral administration of β2-glycoprotein I (β2GPI) in LDLR–/– mice has also been shown to significantly reduce early atherosclerotic lesion formation [59].
Oxidized LDL
Although the induction of immune responses against OxLDL by active immunization strategies mediates atheroprotection, pro-atherogenic responses specific for OxLDL have been suggested to exist as well. For example, transfer of CD4+ T-cells from mice immunized with MDA-LDL induced significantly more atherosclerosis in recipient apoE–/–SCID mice than T-cells from control immunized mice. These responses are hypothesized to be mostly of the Th1 phenotype and IFNγ -secreting Th1 cells are thought to mediate pro-inflammatory effects in lesions. In light of this, Pujvelde et al. employed an oral tolerization strategy using human CuOx-LDL and MDA-LDL as antigens, respectively. Interestingly, only LDLR–/– mice that received CuOx-LDL through oral administration developed significantly less atherosclerosis, while MDA-LDL feeding did not have an effect. Oral administration of CuOx-LDL also led to a significant increase of CD4+CD25+Foxp3+ cells in spleens and mesenteric lymph nodes, and lymph node cells from tolerized but not control mice stimulated in vitro with CuOx-LDL produced high amounts of TGFβ [60]. No effects were observed in antibody titers to CuOx-LDL. Thus, similar to mucosal tolerization approaches with hsp60/65 the induction of Treg populations is associated with the decrease in atherogenesis, though direct immunological suppression of endogenous responses have not been well documented.
Adjuvant effects
Finally, it is noteworthy to mention the atheroprotective effect of simple adjuvant administration, which were originally identified as chance findings of well controlled experiments. Adjuvants are defined as a group of structurally heterogenous compounds that enhance immunogenicity of co-administered antigens, in part by promoting efficient recruitment and function of antigen presenting cells. Indeed, a number of studies have demonstrated that administration of adjuvants alone (i.e. without antigen) in apoE–/– or LDLR–/– mice induces atheroprotection. In these studies, experimental groups of mice received an initial injection of complete Freund's adjuvant (CFA) followed by subsequent boosts with incomplete Freund's adjuvant (IFA) to control for adjuvant effects in various antigen-specific immunization approaches. Surprisingly, these control experiments revealed a robust atheroprotective effect of FA [33,41,61]. Nicoletti et al. then formally tested the effect of various commonly used adjuvant preparations, including CFA/IFA, IFA alone, Alum, and CpG DNA, on lesion formation in ApoE–/– mice [62]. With the exception of CpG, administration of all other adjuvants led to significantly decreased atherosclerosis. The fact that different adjuvant preparations conferred atheroprotection, suggest that general immune activation can lead to the induction of protective immune responses. The exact mechanism of this effect remains unclear. However, it is noteworthy that in some studies reduced plasma cholesterol levels were found associated with administration of Freund's adjuvant. Moreover, some evidence points to the fact that adjuvant administration leads to the activation of protective IgM responses. For example, we showed that CFA/IFA administration in LDLR–/– mice resulted in increased titers of IgM Abs against MDA-LDL and CuOx-LDL, as well as increased levels of circulating IgM-apoB immune complexes [41]. Consistent with these findings, Khallout-Laschet et al. found that both CFA, IFA, but also Alum, induced anti-MDA-LDL IgM titers. They also suggested that the mineral oil component in Freund's adjuvant may be responsible for mediating this atheroprotection [62]. Further mechanistic insight was provided in a study by Zhou et al., who demonstrated that CD4+ T-cells were required for the atheroprotective effect of adjuvant administration, as in CD4 deficient apoE–/– mice the effect of CFA/IFA administration on lesion formation was abolished. In their report, injections of adjuvant also significantly increased anti-MDA-LDL IgM titers in CD4–/–apoE–/–when compared to untreated mice but was lower when compared to treated apoE–/–[33]. Thus, the protective effect of CFA/IFA seems to be dependent on CD4+ T-cells, which may also be needed for efficiently promoting protective anti-MDA-LDL IgM Abs; for example via IL-5. Dissecting the mechanistic details of this protective effect may be of particular interest, as the use of adjuvants such as Alum that shows an atheroprotective capacity in mice are approved for clinical use in humans.
Summary
A number of different strategies have demonstrated the effectiveness of immunotherapeutic interventions to decrease the extent of atherosclerosis or alter the plaque phenotype to a more stable one. While the general interference with certain immune functions may not be useful for long-term therapeutic purposes, as required by the inherently long period until clinically relevant lesions develop, such approaches may be especially useful for acute conditions with disease acceleration. The potential adverse effects of interfering with immune signalling and cell function in general (e.g. an increased susceptibility to infections) may otherwise outweigh the potential benefits on atherogenesis in case of long-term interventions.
By targeting antigen-specific responses, therapeutic interventions provide much higher disease specificity. Indeed, both active immunization as well as tolerization approaches have been successful. However, despite extensive experimental evidence mechanistic insight from these studies is surprisingly limited. This is largely influenced by the fact that in most cases experiments were designed to prove the principle of a given intervention, rather than to identify how these may work. Nevertheless, more detailed insights will be necessary in order to further optimize such intervention strategies but also to identify appropriate biomarkers that could allow the monitoring of successful immunotherapeutic interventions and act as surrogates for the protective effect in the vessel wall.
While immunotherapeutic interventions have been largely successful in animal models of atherosclerosis, the utility of such approaches in humans is still unclear. Although the approaches discussed above to not aim at the interference with lipoprotein metabolism, it is important to point out that atherosclerosis in rodents exhibits differences to the human disease, which are partially based on differences in lipoprotein metabolism. Moreover, the homologous genetic background in animal models certainly does not reflect the different disease-modifying genetic variances found in humans. However; despite these caveats possibilities to translate the experimental immunotherapeutic approaches for atherosclerosis to humans are worthwhile to be explored. A number of issues will be critical to allow this translation to humans. First, the optimally protective antigens/epitopes need to be identified, which should also provide better insights into the protective mechanisms. Secondly, reliable biomarkers for successful interventions need to be defined. These are an absolute requirement for successful monitoring of such interventions, given the chronic character of atherosclerosis before clinical events occur. Likely, an in depth understanding about the mechanism by which a given intervention mediates atheroprotection will identify measurable immune responses that reflect the protective activity.Asthe regulation of the immune system in humans differs from the murine system at various levels, the exact definition and corroboration of immune mediated atheroprotective mechanisms need to be scrutinized vigorously in humans. For example, certain OxLDL-specific antibody responses in humans seem to be dominated by IgG antibodies, and strategies would need to be developed to selectively induce potentially protective IgM antibodies. Lastly, future studies should also focus on interventions that either induce lesion regression of established atherosclerosis or alter plaque phenotype, as such strategies will more likely meet the clinical needs and possibilities. Thus, much work remains to be done before a new generation of atheroprotective therapeutics will be available. Nevertheless, given the great success of “biologicals” in other chronic inflammatory diseases such as rheumatoid arthritis, there is a realistic chance that immunological interventions may oneday become an additional tool in fighting heart disease. Be it as therapeutic vaccines for long lasting immune modulation or as monoclonal antibodies for short term interventions during special clinical settings.
References
- [1].Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- [2].Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- [3].Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- [4].Dansky HM, Charlton SA, Harper MM, Smith JD. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci. 1997;94:4642–4646. doi: 10.1073/pnas.94.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Daugherty A, Puré E, Delfel-Butteiger D, Chen S, Leferovich J, Roselaar SE, Rader DJ. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E–/– mice. J Clin Invest. 1997;100:1575–1580. doi: 10.1172/JCI119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–259. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, Bluestone J, Getz GS. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler Thromb Vase Biol. 2001;21:1011–1016. doi: 10.1161/01.atv.21.6.1011. [DOI] [PubMed] [Google Scholar]
- [8].Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Steffens S, Burger F, Pelli G, Dean Y, Elson G, Kosco-Vilbois M, Chatenoud L, Mach F. Short-term treatment with anti-CD3 antibody reduces the development and progression of atherosclerosis in mice. Circulation. 2006;114:1977–1984. doi: 10.1161/CIRCULATIONAHA.106.627430. [DOI] [PubMed] [Google Scholar]
- [10].Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- [11].Schönbeck U, Sukhova GK, Shimizu K, Mach F, Libby P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci. 2000;97:7458–7463. doi: 10.1073/pnas.97.13.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lutgens E, Cleutjens KB, Heeneman S, Koteliansky VE, Burkly LC, Daemen MJ. Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype. Proc Natl Acad Sci U S A. 2000;97:7464–7469. doi: 10.1073/pnas.97.13.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baum PR, Gayle RB, Ramsdell F, Srinivasan S, Sorensen RA, Watson ML, Seldin MF, Baker E, Sutherland GR, Clifford KN. Molecular characterization of murine and human OX40/0X40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paigen B, Mitchell D, Reue K, Morrow A, Lusis AJ, LeBoeuf RC. Ath-1, a gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Proc Natl Acad Sci. 1987;84:3763–3767. doi: 10.1073/pnas.84.11.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang X, Ria M, Kelmenson PM, Eriksson P, Higgins DC, Samneård A, Petros C, Rollins J, Bennet AM, Wiman B, Faire Ud, et al. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat Genet. 2005;37:365–372. doi: 10.1038/ng1524. [DOI] [PubMed] [Google Scholar]
- [16].Wanrooij EJv, Puijvelde GHv, Vos Pd, Yagita H, Berkel TJv, Kuiper J. Interruption of the Tnfrsf4/Tnfsf4 (OX40/OX40L) pathway attenuates atherogenesis In low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:204–210. doi: 10.1161/01.ATV.0000251007.07648.81. [DOI] [PubMed] [Google Scholar]
- [17].Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- [18].Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- [19].Hörkkö S, Binder CJ, Shaw PX, Chang MK, Silverman G, Palinski W, Witztum JL. Immunological responses to oxidized LDL. Free Radic Biol Med. 2000;28:1771–1779. doi: 10.1016/s0891-5849(00)00333-6. [DOI] [PubMed] [Google Scholar]
- [20].Chou MY, Hartvigsen K, Hansen LF, Fogelstrand L, Shaw PX, Boullier A, Binder CJ, Witztum JL. Oxidation-specific epitopes are Important targets of Innate Immunity. J Intern Med. 2008;263:479–488. doi: 10.1111/j.1365-2796.2008.01968.x. [DOI] [PubMed] [Google Scholar]
- [21].Ylä-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb vasc Biol. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- [22].Shaw PX, Hörkkö s, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hulthe J. Antibodies to oxidized LDL in atherosclerosis development—clinical and animal studies. Clin Chim Acta. 2004;348:1–8. doi: 10.1016/j.cccn.2004.05.021. [DOI] [PubMed] [Google Scholar]
- [24].Karvonen J, Päivänsalo M, Kesäniemi YA, Hörkkö S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- [25].Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- [26].Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ameli S, Hultgårdh-Nilsson A, Regnström J, Calara F, Yano J, Cercek B, Shah PK, Nilsson J. Effect of Immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemlc rabbits. Arterioscler Thromb vasc Biol. 1996;16:1074–1079. doi: 10.1161/01.atv.16.8.1074. [DOI] [PubMed] [Google Scholar]
- [29].Nilsson J, Calara F, Regnstrom J, Hultgardh-Nilsson A, Ameli S, Cercek B, Shah PK. Immunization with homologous oxidized low density lipoprotein reduces neointimal formation after balloon injury in hypercholesterolemic rabbits. Atherosclerosis. 1998;30:1886–1891. doi: 10.1016/s0735-1097(97)00366-5. [DOI] [PubMed] [Google Scholar]
- [30].George J, Afek A, Gilburd B, Levkovitz H, Shaish A, Goldberg I, Kopolovic Y, Wick G, Shoenfeld Y. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138:147–152. doi: 10.1016/s0021-9150(98)00015-x. [DOI] [PubMed] [Google Scholar]
- [31].Freigang S, Hörkkö S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb vasc Biol. 1998;18:1972–1982. doi: 10.1161/01.atv.18.12.1972. [DOI] [PubMed] [Google Scholar]
- [32].Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb vasc Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- [33].Zhou X, Robertson AK, Rudling M, Parini P, Hansson GK. Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ Res. 2005;96:427–434. doi: 10.1161/01.RES.0000156889.22364.f1. [DOI] [PubMed] [Google Scholar]
- [34].Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Bäckhed F, Miller YI, Hörkkö S, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009 doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Binder CJ, Chou MY, Fogelstrand L, Hartvigsen K, Shaw PX, Boullier A, Witztum JL. Natural antibodies in murine atherosclerosis. Curr Drug Targets. 2008;9:190–195. doi: 10.2174/138945008783755520. [DOI] [PubMed] [Google Scholar]
- [37].Virella G, Lopes-Virella MF. Atherogenesis and the humoral immune response to modified lipoproteins. Atherosclerosis. 2008;200:239–246. doi: 10.1016/j.atherosclerosis.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid–protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- [42].Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston AT, Delignat S, Mandet C, Kohler HV, Kaveri SV, Nicoletti A. Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol. 2007;50:540–546. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- [43].Fredrikson GN, Hedblad B, Berglund G, Alm R, Ares M, Cercek B, Chyu KY, Shah PK, Nilsson J. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:872–878. doi: 10.1161/01.ATV.0000067935.02679.B0. [DOI] [PubMed] [Google Scholar]
- [44].Fredrikson GN, Söderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23:879–884. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- [45].Fredrikson GN, Andersson L, Söderberg I, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Atheroprotective immunization with MDA-modified apo B-100 peptide sequences is associated with activation of Th2 specific antibody expression. Autoimmunity. 2005;38:171–179. doi: 10.1080/08916930500050525. [DOI] [PubMed] [Google Scholar]
- [46].Fredrikson GN, Björkbacka H, Söderberg I, Ljungcrantz I, Nilsson J. Treatment with apo B peptide vaccines inhibits atherosclerosis in human apo B-100 transgenic mice without inducing an increase in peptide-specific antibodies. J Intern Med. 2008;264:563–570. doi: 10.1111/j.1365-2796.2008.01995.x. [DOI] [PubMed] [Google Scholar]
- [47].Faria-Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, Cercek B, Shah PK. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006;189:83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- [48].Schiopu A, Bengtsson J, Söderberg I, Janciauskiene S, Lindgren S, Ares MP, Shah PK, Carlsson R, Nilsson J, Fredrikson GN. Recombinant human antibodies against aldehyde-modified apolipoprotein B-100 peptide sequences inhibit atherosclerosis. Circulation. 2004;110:2047–2052. doi: 10.1161/01.CIR.0000143162.56057.B5. [DOI] [PubMed] [Google Scholar]
- [49].Schiopu A, Frendéus B, Jansson B, Söderberg I, Ljungcrantz I, Araya Z, Shah PK, Carlsson R, Nilsson J, Fredrikson GN. Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1 (−/−)/low-density lipoprotein receptor(−/−) mice. J Am Coll Cardiol. 2007;50:2313–2318. doi: 10.1016/j.jacc.2007.07.081. [DOI] [PubMed] [Google Scholar]
- [50].Goncalves I, Nitulescu M, Ares MP, Fredrikson GN, Jansson B, Li ZC, Nilsson J. Identification of the target for therapeutic recombinant anti-apoB-100 peptide antibodies in human atherosclerotic lesions. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.11.020. [DOI] [PubMed] [Google Scholar]
- [51].Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- [52].Melamed D, Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993;23:935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- [53].Friedman A. Induction of anergy in Th1 lymphocytes by oral tolerance. Importance of antigen dosage and frequency of feeding. Ann NY Acad Sci. 1996;778:103–110. doi: 10.1111/j.1749-6632.1996.tb21119.x. [DOI] [PubMed] [Google Scholar]
- [54].Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- [55].Faria AM, Weiner HL. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13:143–157. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Harats D, Yacov N, Gilburd B, Shoenfeld Y, George J. Oral tolerance with heat shock protein 65 attenuates Mycobacterium tuberculosis-induced and high-fat-diet-driven atherosclerotic lesions. J Am Coll Cardiol. 2002;40:1333–1338. doi: 10.1016/s0735-1097(02)02135-6. [DOI] [PubMed] [Google Scholar]
- [57].Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708–1715. doi: 10.1161/01.cir.0000029750.99462.30. [DOI] [PubMed] [Google Scholar]
- [58].Puijvelde GHv, Es Tv, Wanrooij EJv, Habets KL, Vos Pd, Zee Rvd, Eden Wv, Berkel TJv, Kuiper J. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb vasc Biol. 2007;27(12):2677–2683. doi: 10.1161/ATVBAHA.107.151274. [DOI] [PubMed] [Google Scholar]
- [59].George J, Yacov N, Breitbart E, Bangio L, Shaish A, Gilburd B, Shoenfeld Y, Harats D. Suppression of early atherosclerosis in LDL-receptor deficient mice by oral tolerance with beta 2-glycoprotein I. Cardiovasc Res. 2004;62:603–609. doi: 10.1016/j.cardiores.2004.01.028. [DOI] [PubMed] [Google Scholar]
- [60].Puijvelde GHv, Hauer AD, Vos Pd, Heuvel Rvd, Herwijnen MJv, Zee Rvd, Eden Wv, Berkel TJv, Kuiper J. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–1976. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- [61].Hansen PR, Chew M, Zhou J, Daugherty A, Heegaard N, Jensen P, Mouritsen S, Falk E. Freunds adjuvant alone is antiatherogenic in apoE-deficient mice and specific immunization against TNFalpha confers no additional benefit. Atherosclerosis. 2001;158:87–94. doi: 10.1016/s0021-9150(01)00418-x. [DOI] [PubMed] [Google Scholar]
- [62].Khallou-Laschet J, Tupin E, Caligiuri G, Poirier B, Thieblemont N, Gaston AT, Vandaele M, Bleton J, Tchapla A, Kaveri SV, Rudling M, et al. Atheroprotective effect of adjuvants in apolipoprotein E knockout mice. Atherosclerosis. 2006;184:330–341. doi: 10.1016/j.atherosclerosis.2005.04.021. [DOI] [PubMed] [Google Scholar]
- [63].Xiong Q, Li J, Jin L, Liu J. Nasal immunization with heat shock protein 65 attenuates atherosclerosis and reduces serum lipids in cholesterol-fed wild-type rabbits probably through different mechanisms. Immunol Lett. 2009 doi: 10.1016/j.imlet.2009.05.007. [DOI] [PubMed] [Google Scholar]
- [64].Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- [65].Hauer AD, Uyttenhove C, Vos Pd, Stroobant V, Renauld JC, Berkel TJv, Snick Jv, Kuiper J. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation. 2005;112:1054–1062. doi: 10.1161/CIRCULATIONAHA.104.533463. [DOI] [PubMed] [Google Scholar]
- [66].Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- [67].Schober A, Bernhagen J, Thiele M, Zeiffer U, Knarren S, Roller M, Bucala R, Weber C. Stabilization of atherosclerotic plaques by blockade of macrophage migration inhibitory factor after vascular injury in apolipoprotein E-deficient mice. Circulation. 2004;109:380–385. doi: 10.1161/01.CIR.0000109201.72441.09. [DOI] [PubMed] [Google Scholar]
- [68].Burger-Kentischer A, Göbel H, Kleemann R, Zernecke A, Bucala R, Leng L, Finkelmeier D, Geiger G, Schaefer HE, Schober A, Weber C, et al. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF) Atherosclerosis. 2006;184:28–38. doi: 10.1016/j.atherosclerosis.2005.03.028. [DOI] [PubMed] [Google Scholar]
- [69].Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- [70].Wanrooij EJv, Happé H, Hauer AD, Vos Pd, Imanishi T, Fujiwara H, Berkel TJv, Kuiper J. HIV entry inhibitor TAK-779 attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb vasc Biol. 2005;25:2642–2647. doi: 10.1161/01.ATV.0000192018.90021.c0. [DOI] [PubMed] [Google Scholar]
- [71].Wanrooij EJv, Jager SCd, Es Tv, Vos Pd, Birch HL, Owen DA, watson RJ, Biessen EA, Chapman GA, Berkel TJv, Kuiper J. CXCR3 antagonist NBI-74330 attenuates atherosclerotic plaque formation in LDL receptor-deficient mice. Arterioscler Thromb vasc Biol. 2008;28:251–257. doi: 10.1161/ATVBAHA.107.147827. [DOI] [PubMed] [Google Scholar]
- [72].Braunersreuther V, Steffens S, Arnaud C, Pelli G, Burger F, Proudfoot A, Mach F. A novel RANTES antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscler Thromb vasc Biol. 2008;28:1090–1096. doi: 10.1161/ATVBAHA.108.165423. [DOI] [PubMed] [Google Scholar]
- [73].Ström A, Fredrikson GN, Schiopu A, Ljungcrantz I, Söderberg I, Jansson B, Carlsson R, Hultgårdh-Nilsson A, Nilsson J. Inhibition of injury-induced arterial remodelling and carotid atherosclerosis by recombinant human antibodies against aldehyde-modified apoB-100. Atherosclerosis. 2007;190:298–305. doi: 10.1016/j.atherosclerosis.2006.03.032. [DOI] [PubMed] [Google Scholar]