Abstract
Obesity causes insulin resistance via a chronic low-grade inflammation. This inflammation is characterized by elevated pro-inflammatory markers and macrophage accumulation in the adipose tissue (AT). AT inflammation is a key factor causing insulin resistance and thus type 2 diabetes, both linked to atherosclerotic cardiovascular disease. Osteopontin (OPN), a well-known inflammatory cytokine, is involved in obesity-linked complications including AT inflammation, insulin resistance, atherosclerosis and CVD. During inflammation, OPN is proteolytically cleaved by matrix metalloproteinases or thrombin leading to increased OPN activity. Therefore, OPN provides a new interesting target for immunological prevention and treatment of obesity-associated diseases. The aim of our study was to evaluate peptide-based vaccines against integrin binding sites of OPN and to examine whether these active immunotherapies are functional in reducing metabolic tissue inflammation, insulin resistance, and atherosclerosis in a cardio-metabolic (Ldlr−/− mice) and a diet-induced obesity model (WT mice). However, atherosclerosis, insulin resistance and AT inflammation were not diminished after treatment with OPN-derived peptides in murine models. Lack of efficacy was based on a failure to induce antibodies capable to bind epitopes in the context of functional OPN protein. In conclusion, our data point to unexpected challenges in the immunotherapeutic targeting of adhesive motives, such as RGD containing sequences, on endogenous proteins.
Keywords: Osteopontin, Immunization, Cardio-metabolic disease, Atherosclerosis, Rodent, Cytokine
1. Introduction
Non-communicable diseases, such as cardiovascular disease (CVD), type 2 diabetes and cancer are the major health consequences of obesity, the prevalence of which has more than doubled since 1980 [1]. Obesity develops when caloric intake exceeds energy expenditure [2] and is linked to insulin resistance and a low-grade inflammatory state, primarily originating from visceral adipose tissue (AT) [3,4]. The accumulation of visceral AT is hence a key factor not only of disturbed glucose homeostasis and type 2 diabetes but also of enhanced atherogenesis leading to CVD hence conferring the so-called cardio-metabolic risk [5]. The chronic inflammatory response associated with obesity is characterized by abnormal adipokine production, including tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein (MCP)-1, interleukin (lL)-6 and osteopontin (OPN) [6–9]. The major source of cytokines within the AT are macrophages with markedly increase in abundance in obese rodents and individuals [7,10,11]. In AT and liver tissue-specific metabolic cells, adipocytes and hepatocytes, respectively, and immune cells, particularly macrophages exist in close proximity with immediate access to the blood vessel network [4].
OPN (gene: Spp1) has been recognized to be a multifunctional protein which is mainly expressed in immune cells, smooth muscle as well as endothelial and epithelial cells [12–14]. OPN plays a functional role in several physiological and pathological events, like cell migration and survival, inflammation, and tumor biology [13,15,16]. Moreover, OPN has been identified to be involved in the pathogenesis of obesity-associated diseases including insulin resistance, type 2 diabetes, nonalcoholic fatty liver disease and steatohepatitis, and atherosclerosis [17–20]. OPN contains a 158Gly-Arg-Gly-Asp-Ser162 (GRGDS)-cell binding domain which interacts with integrins mainly of the alpha-v family. Interestingly, this domain is found in many matrix molecules and represents an adhesive motif [21]. Under inflammatory conditions, OPN is cleaved by thrombin and matrix metalloproteinases [22,23] the expression of the latter being largely upregulated in obesity-driven AT inflammation and atherosclerosis [24–27]. Moreover, in obesity and atherosclerosis, an increase of cleaved OPN in human AT and inflamed atherosclerotic plaques, respectively, has been observed [28,29]. Cleavage of OPN results in the exposure of additional integrin-binding sites including 162SVVYGLR168 in humans (SLAYGLR in rodents) and 162SVVYG166 in thrombin- and MMP-cleaved OPN, respectively, which are not accessible in the full-length form and therefore comprise inflammation-specific “neoepitopes” [21–23,28].
We and others reported that OPN is strongly upregulated in AT of obese humans and mice [15,20,30], and showed a relation between increased human plasma OPN concentrations and overweight-obesity [31]. In mice, OPN promotes macrophage infiltration into AT in obesity [30] and plays a key role in liver steatosis [32]. Published data revealed significantly improved AT and liver inflammation, metabolic parameters as well as insulin sensitivity in OPN-deficient mice [30,32]. Importantly, short-term neutralization of OPN successfully diminished the number of hepatic macrophages thereby improving insulin sensitivity in murine models of obesity [15].
Since non-communicable diseases have become the major cause for morbidity and mortality worldwide, new therapeutic strategies have to be developed. Though passive immunotherapies with monoclonal antibodies have dramatically improved the treatment of chronic diseases like rheumatoid arthritis, the drawbacks are considerable. In addition to high costs and frequent administration, tolerability as well as primary and secondary treatment failure of monoclonal antibody therapy have to be mentioned [33,34].
Active immunization approaches have been reported to successfully induce neutralizing antibodies against endogenous proteins [34–36]. Hence, we aimed at evaluating an active immunization approach to specifically target the RGD [21] and additional integrin binding domains, latter are supposed to be generated under pathological conditions such as obesity-associated AT inflammation and atherosclerosis [28,29]. Therefore, immunological targeting of the central OPN region including the integrin binding sites exposed after proteolytic cleavage may inhibit cardio-metabolic complications. We tested this hypothesis in a recently developed rodent model reflecting cardio-metabolic disease [37] and a well-established model of diet-induced obesity (DIO). Ldlr−/− animals on sucrose-enriched high-fat diet (DDC) constitute a suitable model to evaluate cardio-metabolic disease including insulin resistance, adipose tissue inflammation and atherosclerotic plaque formation.
The active immunization with different OPN-derived peptides performed in this study did not improve insulin sensitivity, inflammation and atherosclerosis. Despite significant titers against injection peptides, OPN protein was not substantially bound by post-immune sera. Our data challenges the concept that an active immunization approach may induce high-affinity neutralizing antibodies against highly abundant and conserved adhesive motives, including RGD-containing regions, on endogenous proteins such as OPN.
2. Material and methods
2.1. Ethics statement
The protocol was approved by the Committee on the Ethics of Animal Experiment of the Medical University of Vienna and the Austrian Federal Ministry for Science and Research (Permit Numbers: BMWF-66.009/0096-II/10b/2008; BMWF-66.009/0319-II/3b/2012) and followed the guidelines on accommodation and care of animals formulated by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. All animal experiments were carried out to minimize suffering according to the 3R principals of animal walfare- “replace, reduce and refine”.
2.2. Animals and diets
Male C57BL/6JRj wild-type (WT) mice were purchased from Janvier Labs (Saint Berthevin, France). At 9 weeks of age, 150 mice were separated into ten different groups and set either on a high-fat (HF, 60 kcal%, D12492; Research Diets, New Brunswisk, NJ) to serve as a diet-induced obesity (DIO) model or a low-fat diet (LF, 10 kcal%, D12450B; Research Diets, New Brunswisk, NJ) for 14 weeks. For the recently established cardio-metabolic mouse model [37], 120 B6.129S7-Ldlrtm1Her/J (Ldlr−/−) mice were obtained from Charles River (Sulzfeld, Germany), separated into eight groups and kept either on a sucrose-enriched high-fat diet (DDC, 58 kcal% fat and 218 g sucrose, D09071704, Research Diets, New Brunswisk, NJ) or normal chow (NC) for 17 weeks. Mice fed LF and NC served as control diets for DIO and the cardio-metabolic model, respectively (Table 1).
Table 1.
Treatment groups including mouse strain, animal number per group, dietary treatment, abbreviations of OPN-derived peptide-based vaccines, amino acid sequences and description of vaccines.
| Model | Genotype | Number of animals | Dietary treatment | ID No | Amino acid sequence | Description |
|---|---|---|---|---|---|---|
| DIO | WT | 15 | HF | Control pep 1 | C-LYRGLAS-COO | scrambled |
| cardio-metabolic | Ldlr–/– | 15 | DDC | Control pep 1 | C-LYRGLAS-COO | scrambled |
| DIO | WT | 15 | HF | Control pep 2 | C-GYSAGLD-COO | scrambled |
| DIO | WT | 15 | HF | Peptide 1 | C-LAYGLR-COO | free thrombin cleavage site |
| cardio-metabolic | Ldlr–/– | 15 | DDC | Peptide 1 | C-LAYGLR-COO | free thrombin cleavage site |
| DIO | WT/ | 15 | HF | Peptide 2 | C-PTVDVPNGRGDS-NH2 | GRGDS in OPN context |
| cardio-metabolic | Ldlr–/– | 15 | DDC | Peptide 2 | C-PTVDVPNGRGDS-NH2 | GRGDS in OPN context |
| DIO | WT | 15 | HF | Peptide 3 | C-GDSLAYG-COO | free MMP cleavage site |
| cardio-metabolic | Ldlr–/– | 15 | DDC | Peptide 3 | C-GDSLAYG-COO | free MMP cleavage site |
| DIO | WT | 15 | HF | Peptide 4 | C-RGDSLAYG-COO | free MMP cleavage site |
| DIO | WT | 15 | LF | PBS | ||
| DIO | WT | 15 | HF | PBS | ||
| cardio-metabolic | Ldlr–/– | 15 | NC | PBS | ||
| cardio-metabolic | Ldlr–/– | 15 | DDC | PBS | ||
| DIO | WT | 15 | LF | Adj-KLH | ||
| DIO | WT/ | 15 | HF | Adj-KLH | ||
| cardio-metabolic | Ldlr–/– | 15 | NC | Adj-KLH | ||
| cardio-metabolic | Ldlr–/– | 15 | DDC | Adj-KLH |
DIO = diet-induced obesity; WT = wild-type mice; Ldlr–/– = low-density lipoprotein receptor knock-out; HF = high-fat diet; DDC = sucrose-enriched high-fat diet with cholesterol; LF = low-fat diet; NC = normal chow; Adj = adjuvant; OPN = osteopontin; MMP = matrix metalloproteinase.
All mice were housed in a specific pathogen-free facility, which maintained a 12 h light/dark cycle and had free access to water and food. Weight was determined biweekly. Gonadal white AT (GWAT) and liver were collected and stored for further experiments. Additionally, aortae were excised from Ldlr−/− mice, fixed in 4% paraformaldehyde for 24 h and stored in PBS at 4 °C.
2.3. Vaccination experiments using OPN-derived peptides
Peptides were conjugated to the carrier protein keyhole limpet hemocyanin (KLH), formulated with 0.2% Alhydrogel (Adj; Brenntag Biosector, Denmark) and stored at 4 °C. Mice were subcutaneously (s.c) immunized with 20 µg peptide antigen per mouse, including OPN-derived peptides and scrambled OPN (see Table 1) controls as well as adjuvant with carrier protein (Adj-KLH) and PBS, respectively. The primary immunization was performed one week before as well as one, three and seven weeks after the start of the dietary treatment. Plasma was collected before the primary immunization (P1) and after the 2nd (P2) and 3rd (P3) boost by using heparin-coated minicaps (Hirschmann, Eberstadt, Germany). Serum samples collected after study duration (end-serum) were prepared before mice were sacrificed and stored at −20 °C for further analysis.
2.4. Titer determination
To evaluate antibody titers and check potential cross-reactivity between peptides and OPN protein, isolated P1, P2 and P3 plasma as well as end-serum was used to perform enzyme-linked immunosorbent assays (ELISA), as described elsewhere [35]. In short, plates were coated with injected peptides coupled to bovine serum albumin, and full-length (FL) OPN (R&D Systems, Minneapolis, MN), blocked with 1% bovine serum albumin in PBS and incubated with obtained plasma and end-serum samples. Streptavidin-horse radish peroxidase was used to measure bound antibodies at 405 nm.
2.5. Metabolic measurements
Serum cholesterol and triglyceride concentrations were assessed by an automated analyzer (Cobas c 111 analyzer). Commercially available ELISA kits were used to measure serum insulin (Mercodia, Uppsala, Sweden) and OPN (R&D Systems). Fast protein liquid chromatography (FPLC) analysis of serum lipoproteins was performed with pooled (5 mice per pool) end-serum as described previously [37]. Insulin tolerance tests (ITT) were performed after 13 and 15 weeks on indicated diets measuring glucose levels before, 30, 60, 90 and 120 min after an intraperitoneal injection (i.p.) of recombinant human insulin (0.75 IU/kg bodyweight NovoRapid for HF and DDC and 0.25 IU/kg bodyweight for LF and NC. respectively; Novo Nordisk, Bagsværd, Denmark).
2.6. Reverse transcription and gene expression
Samples of GWAT and liver were stored in RNAlater (Qiagen, Hilden, Germany) overnight at 4 °C. Next day, RNAlater was removed and tissues were snap frozen and stored at −80 °C. To isolate RNA, tissues were homogenized in TRIzol Reagent (Invitrogen, Carlsbad, CA,USA) using an automated homogenizer (Precellys 24, Erlangen, Germany). One microgram RNA was transcribed to cDNA with M-MLV Reverse Transcriptase (Promega, Madison, USA). Gene expression was normalized to ubiquitin C (Ubc) and analyzed by quantitative real-time RT-PCR on an ABI Prism 7000 cycler, using commercially available assays-on demand (Applied Biosystems, Foster City, CA)(Supplementary Table S1).
2.7. Histology and oil-red O staining
Murine liver tissue was embedded in OCT (Sakura, Netherlands) and cut into 5 µm sections. Liver sections were fixed in 60% 2-Propanol for 5 min and lipids stained with 0.5% oil-red O (Sigma-Aldrich) in 60% 2-Propanol for 20 min. Counterstaining was performed by using hematoxylin-eosin [19] and sections were analyzed with standard light microscopy. Relative lipid accumulation was quantified by detecting oil-red O stained areas with ImageJ software, thereby assessing five independent fields per sample by a blinded person.
2.8. Atherosclerotic lesion quantification
To quantify atherosclerotic plaque formation en face staining of lipids by Sudan IV on the luminal side of opened aortae was performed as described elsewhere [37]. In short, aortae were excised from Ldlr−/− mice and cleaned by removing connective tissue and surrounded fat. Next, aortae were opened longitudinally, cleaned and pinned on black silicon plates. Then aortae were fixed overnight at 4 °C with 2% paraformaldehyde, 5% sucrose, 20 µM EDTA (pH 7.4). Aortic lesions were stained with 0.5% Sudan IV and destaining was performed using 75% ethanol. Pictures were taken with a Sony Z-1000 camera and samples were quantified by using ImageJ software observer blinded.
2.9. Statistical analysis
Data are expressed as mean ± SEM or mean ± SD. For both experiments, effects of immunization within the HFand DDC diet groups, respectively, were analyzed with one-way ANOVA using Tukey’s multiple comparisons test. Effects of dietary treatment of PBS and Adj-KLH control groups (LF vs. HF; NC vs. DDC, respectively) and Adj-KLH (LF and HF; NC and DDC) was assessed by unpaired Student’s t-test. A P value of ≤0.05 was considered statistically significant
3. Results
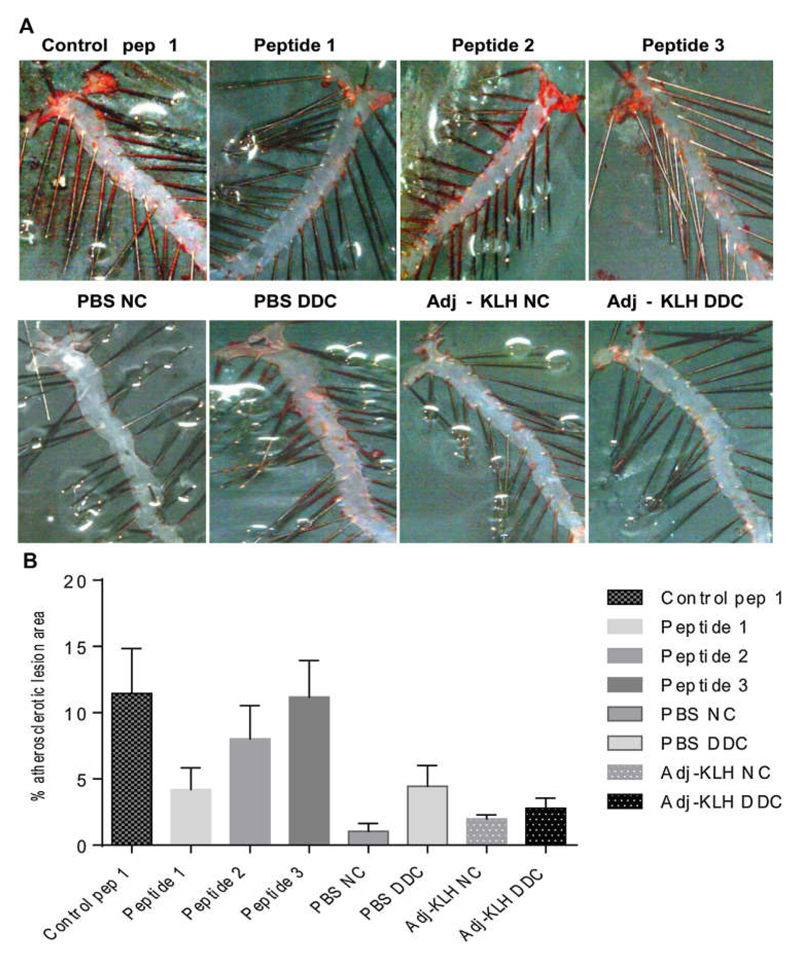
To evaluate an active immunization approach against the central, integrin binding sites-comprising region of OPN, we employed our recently developed cardio-metabolic model for atherosclerosis and insulin resistance [37] as well as the well-established DIO model. Male Ldlr−/− and WT mice were set either on a DDC for 17 weeks and a HF diet for 14 weeks to induce atherosclerosis and obesity, or on a NC and LF diet to serve as lean controls, respectively. For each experiment, mice from each group were immunized four times using different peptides derived from OPN (Peptide 1–4; Pep 1–4), scrambled controls based on OPN-peptides (Ctrl 1; Control pep 1 and Ctrl 2; Control pep 2), Adj-KLH and PBS. As expected, dietary treatment with DDC and HF generated DIO by significantly increasing bodyweight and GWAT weight, compared to the respective lean controls, which after 17 and 14 weeks on DDC and HF diet did not differ between the treatment groups in both models (Supplementary Table S2). The co-primary efficacy parameters of this study were insulin resistance, adipose tissue inflammation, hepaticsteatosis, and atherosclerotic plaque formation. En face staining of aortae excised from the cardio-metabolic model (Ldlr−/− mice) after 17 weeks on DDC and NC diet suggested that immunization with Peptide 1 reduced plaque formation compared to the Control pep 1, which, however, appeared to increase the atherosclerotic lesion area as compared to PBS or Adj-KLH. Of note, atherosclerotic lesion formation was generally lower in PBS LF and Adj-KLH LF groups than expected [37]. Due to the high variation and the low number of animals that could be analyzed none of these results were statistically significant (Fig. 1).
Fig. 1.
Immunization with OPN-derived peptides does not significantly affect atherosclerotic lesion formation. The cardio-metabolic model (Ldlr−/− mice) (n = 14–15 per group) was fed a DDC and NC and immunized with scrambled OPN controls, OPN-derived peptides, PBS or Adj-KLH, as indicated. Atherosclerotic lesion formation was quantified with en face staining after dietary treatment for 17 weeks (n = 3 per group). Representative image of Sudan IV staining is given (A) and quantification of plaque formation was performed by ImageJ software (B). Data is expressed as mean ± SEM. Effects of immunization within the HF and DDC diet groups were analyzed with one-way ANOVA using Tukey’s multiple comparisons test. Effects of dietary treatment of PBS and Adj-KLH control groups (LF vs. HF; NC vs. DDC, respectively) and Adj-KLH (LF and HF; NC and DDC) was assessed by unpaired Student’s t-test.
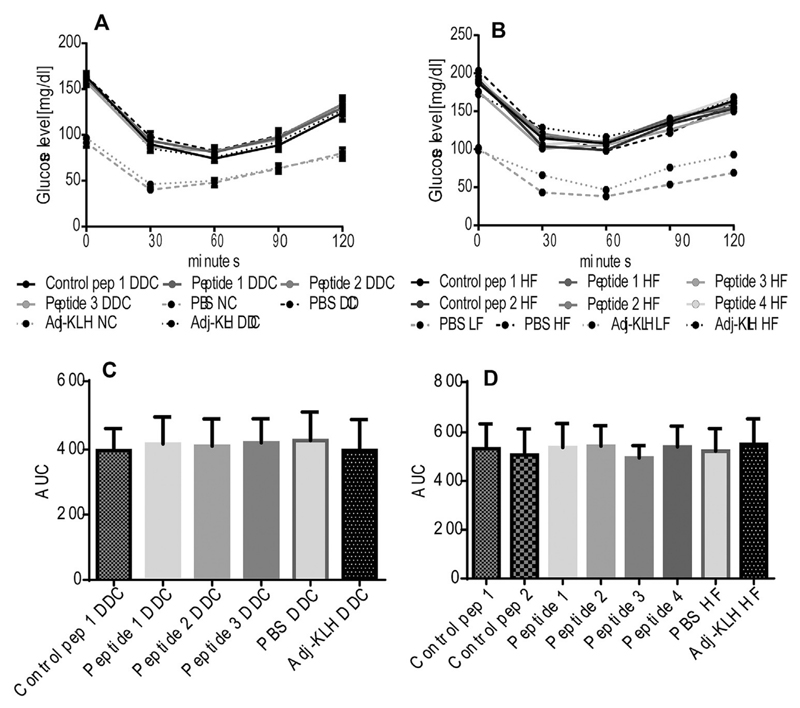
To evaluate, whether insulin resistance in obese mice is ameliorated by active immunization with OPN-derived peptides, an ITT was performed. Obesity-associated diet-induced insulin resistance was not improved after treatment with anti-OPN vaccines in both animal models (Fig. 2).
Fig. 2.
Insulin sensitivity is not improved after immunization with OPN-derived peptides. Both models (Ldlr−/− and WT mice) (n = 11–15 per group) were immunized with scrambled OPN controls, OPN-derived peptides, PBS or Adj-KLH, as indicated. ITT was performed after 15 and 13 weeks of dietary treatment. Glucose concentrations are given for Ldlr−/− (A) treated with DDC or NC, and WT mice treated with HF or LF diet as indicated (B). Area under the curve (AUC) was calculated for all DDC-fed groups of Ldlr−/− mice (C) and for all HF diet-fed groups of WT mice (D). Data is expressed as mean ± SD, one-way ANOVA.
In order to elucidate, whether immunization with peptides derived from OPN diminish hepatic lipid droplet formation, cryo-sectioned liver probes of the DIO model were stained with oil-Red O. As expected, HF-induced obesity resulted in accumulation of lipid droplets indicating the development of hepatic steatosis in the DIO model (WT animals). However, treatment with OPN-derived peptides did not affect lipid droplet formation in the liver of the DIO model (data not shown).
3.1. Immunization of obese mice does not ameliorate tissue inflammation and systemic parameters
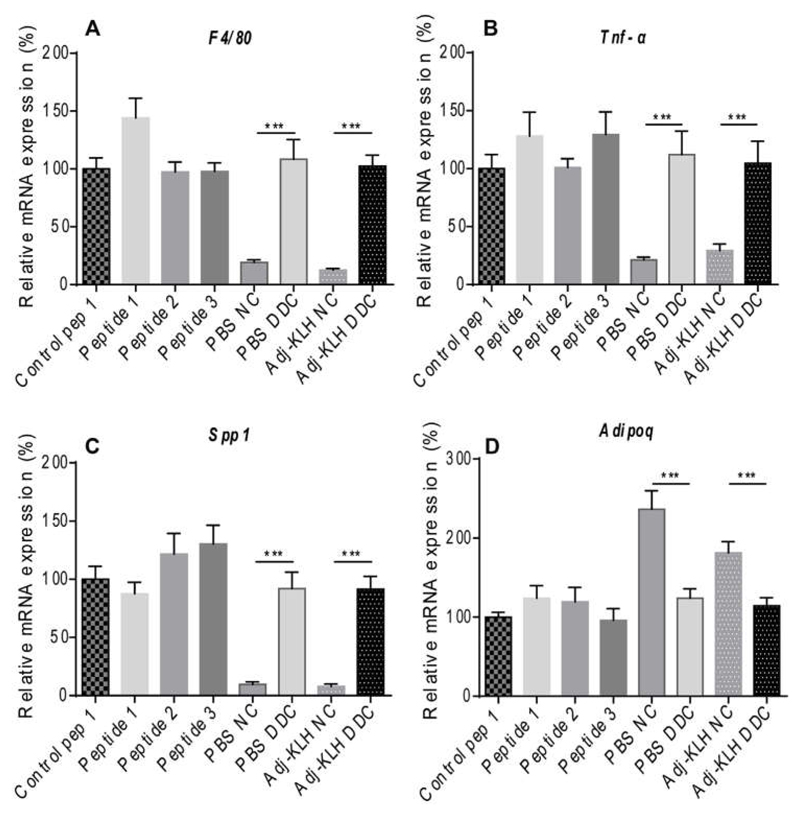
To clarify at which stage the treatment was insufficient, we investigated if and which upstream events of atherosclerosis and insulin resistance were affected by the immunization. Therefore, we investigated mRNA expression of inflammatory parameters and adiponectin in AT and liver in the immunized DDC-treated cardio-metabolic model (Ldlr−/− mice) (Fig. 3) and the HF-treated DIO model (WT mice) (data not shown). As expected, dietary treatment significantly enhanced Emr1 (F4/80), Tnf-α and Spp1 (Fig. 3A-C) mRNA expression, whereas adipogenic marker Adipoq (Fig. 3D) gene expression levels were reduced in the cardio-metabolic model. However, immunization with OPN-derived peptides did not change these markers in AT of both models (data not shown). Similarly, immunization with OPN-derived peptides did not show any differences in hepatic mRNA expression of Emr1, Ccl2, Tnf-α and Spp1 in both animal models (data not shown).
Fig. 3.
Obesity-associated AT inflammation is not diminished after immunization with OPN-derived peptides, The cardio-metabolic model (Ldlr−/− mice) was fed either a DDC or NC for 17 weeks (n = 11–15 per group) and immunized with three different OPN-based peptides, scrambled control. Adj-KLH and PBS, as indicated. Expression of macrophage markers Emr1 (F4/80) (A) and Itgax (B) by qRT-PCR as well as cytokines like Spp1 (C) and Adipoq (D) was assessed in GWAT. Ubiquitin was used as a housekeeping gene. Data is expressed as mean ± SEM and shown after normalization to the mean of control peptide 1. ***p ≤ 0.001, one-way ANOVA and unpaired Student’s t-test, as indicated.
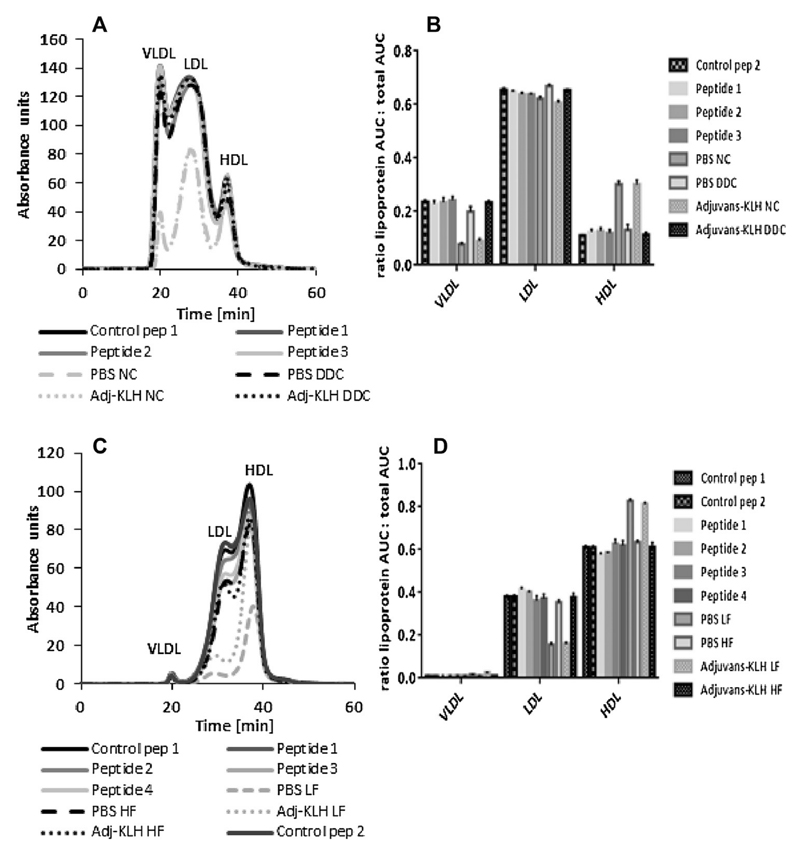
To test, whether immunization affected systemic inflammation associated with obesity, serum characteristics were determined. Diet administration deteriorated serum triglycerides (TAGs), total cholesterol, insulin and OPN, whereas treatment with OPN-derived peptides did not reduce the systemic inflammatory state in both models (Supplementary Table S2). As expected, lipoprotein fractionation by FPLC analysis revealed comparable cholesterol distribution between all respective animals on DDC and HF diet (Fig. 4) without changes in immunization groups.
Fig. 4.
Analysis of serum lipoproteins after dietary treatment. Serum cholesterol distribution in both models (n = 14–15 per group) was assessed by FPLC analysis after treatment with indicated diets and immunization with OPN-derived peptides, scrambled controls and PBS for 17 weeks (A,B) and 14 weeks (C,D), as indicated. Specific lipoprotein area under the curve (AUC) was normalized to total AUC of the cardio-metabolic (Ldlr−/− mice) (B) and DIO model (WT mice) (D). Data is expressed as mean ± SEM.
3.2. Active immunization with OPN-derived peptides generates stable moderate antibody titers
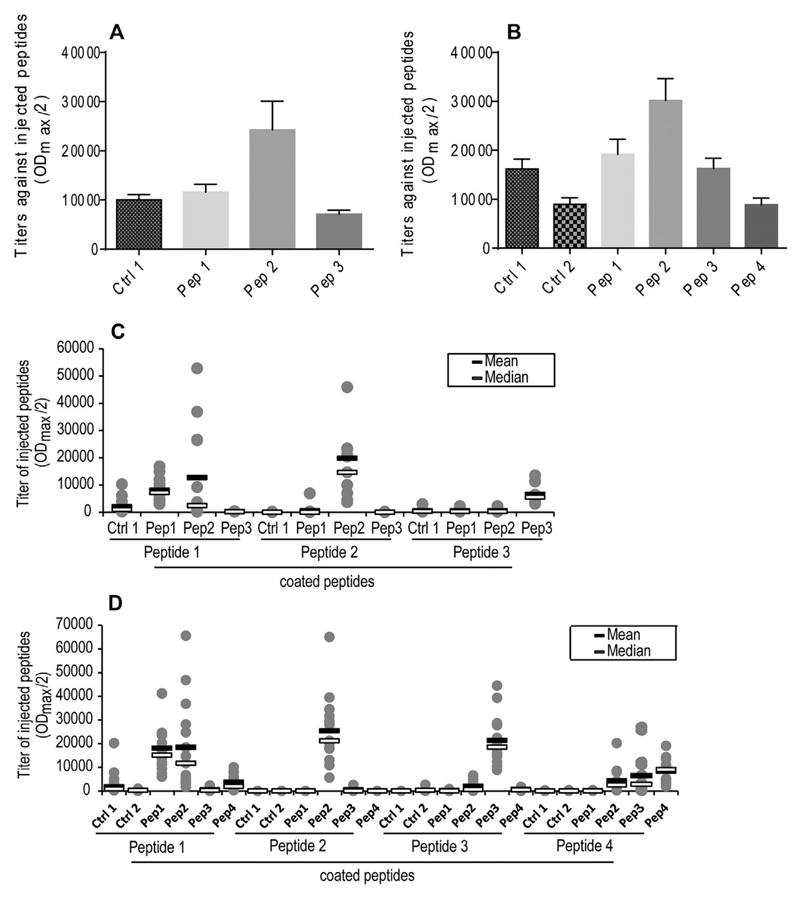
In order to examine the efficacy and specificity of immunization-induced antibodies against the integrin binding sites of OPN, obtained plasma and end-sera were tested against injected peptides. All vaccines tested in both models were found to elicit a profound antibody response (Fig. 5A and B). Both models under treatment with Peptide 2 (Pep 2) showed significantly higher antibody titers compared to the other peptides. Also antibody titer progression over 17 and 14 weeks, respectively, on indicated diets showed highest response for Peptide 2 but still a moderate and stable response in the other groups (Supplementary Fig. S1A and B).
Fig. 5.
Antibody titers and cross-reactivity of DDC and HF diet groups. The cardio-metabolic (Ldlr−/− mice) and the DIO model (WT mice) were fed either a DDC and HF diet for 17 and 14 weeks, or a NC and LF diet to serve as lean controls (n = 14–15 per group). Immunization was performed four times with scrambled control OPN-peptides (Control pep 1 and 2; Ctrl 1 and 2) and four different OPN-derived peptides (Peptides 1–4; Pep 1–4). Mean progression of OPN-specific antibody production in Ldlr−/− (A) and WT mice (B) is given. Possible cross-reactivity of obtained end-sera against peptides was determined in Ldlr−/− (C) and WT mice (D) using ELISA. Data is expressed as mean ± SEM as well as mean (black band) and median (white band).
In order to check the selectivity of vaccination-induced antibodies, end-sera were tested against all used peptides. Antibodies raised by Pep 2 and Pep 3 specifically reacted with corresponding peptides (Fig. 5C and D). However, in the DIO model (WT mice) Peptide (Pep) 1 and Peptide 4 induced antibodies cross-reacted with Peptide 2 and Peptide 3, respectively (Fig. 5D).
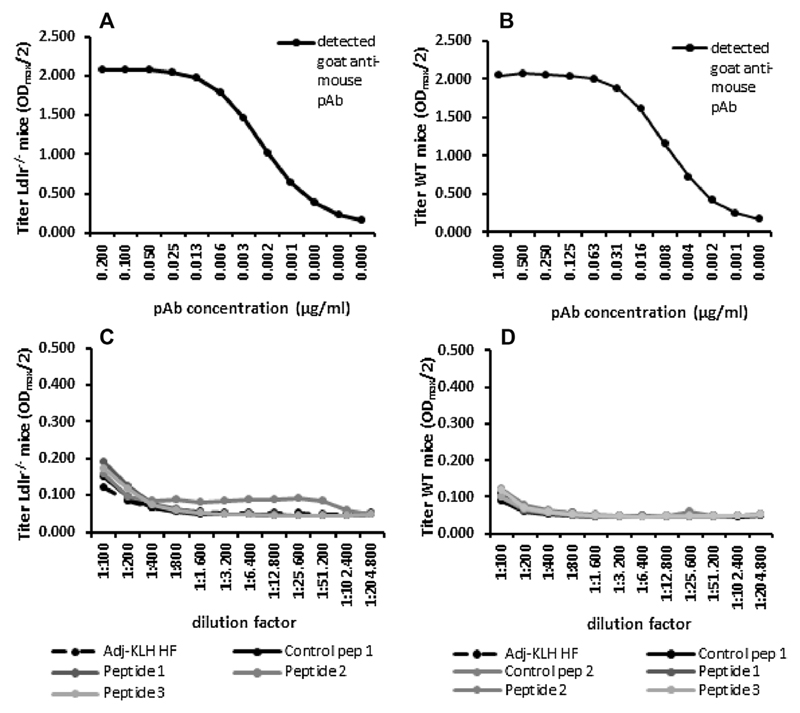
These results raised the question, whether the absence of functional effects despite proper titers against vaccination peptides indicates a failure to induce OPN-neutralizing antibodies. Therefore we tested the induced antisera for their binding of recombinant murine OPN. Strikingly, essentially no binding of the Peptide 2-vaccinated group could be detected, indicating insufficiency of induced antibodies to recognize full-length OPN protein (Fig. 6). As expected, this also accounts for groups vaccinated with Peptide 1, 3 and 4 which are supposed to induce functional antibodies merely against cleaved OPN forms.
Fig. 6.
Cross-reactivity of polyclonal antibody and antisera against recombinant mouse OPN. Both animal models were fed either a DDC or HF and NC or LF diet for 17 and 14 weeks, respectively (n = 14–15 per group). Immunization was performed using scrambled control OPN-peptides (Control pep 1 and 2; Ctrl 1 and 2), different OPN-derived peptides (Peptide 1–4; Pep 1–4) and Adj-KLH, as indicated. Mean titer of polyclonal antibody (pAB) titration of the cardio-metabolic (Ldlr−/− mice) (A) and the DIO model (WT mice) (B) as well as possible cross-reactivity of obtained end-sera from Ldlr−/− (C) and WT mice (D) against recombinant mouse OPN was determined with ELISA. Data is expressed as mean.
4. Discussion
The increasing prevalence of obesity-associated non-communicable diseases like type 2 diabetes, cancer and CVD requires new therapeutic interventions to combat 38 million deaths per year [1]. Obesity-induced inflammation and cardio-metabolic diseases based on insulin resistance and atherosclerosis are constantly investigated to find new targets for modern therapies. We and others could demonstrate that OPN is drastically increased in obese human and rodent models. Moreover, data published from our lab showed improved insulin resistance as well as AT and liver inflammation after short-term neutralization with anti-OPN antibodies in obese mice [15,29,30,38]. Moreover, Shojaei et al. reported an inhibition of metastatic lesion growth after treatment with an anti-OPN monoclonal antibody, thereby targeting the 162SVVYGLRSKS171 motif next to the RGD region, and the main thrombin cleavage site, in mice [39]. However, mapping of human OPN epitopes to generate novel monoclonal anti-OPN antibodies revealed the 2K1 antibody, established against the region VDTYDRGDSVVYGLRS and able to react with full-length and thrombin-cleaved OPN, thereby indicating a pivotal role in monocyte binding and migration. Additionally, treatment with the modified 2K1 anti-OPN antibody after fusion with human IgG1, improved joint swelling in a rheumatoid arthritis model [40,41].
In this study we investigated peptide-based active immunization against the central domain of OPN and could not observe any influence on obesity-related consequences including atherosclerosis, insulin resistance, hepatic steatosis and metabolic tissue-specific inflammation in both murine models. A possible reason for this lack of effect may be insufficiency of the peptide-based vaccination approach used here to induce functional neutralizing antibodies against integrin binding sites on endogenous protein despite binding to linear peptides. Of note, similar situations have been described in humans [42]. OPN in particular is highly concentrated in murine compared to the human serum [10,29,37]. Our approach to include peptides that correspond to the free thrombin and MMP cleavage site, thus comprising neoepitopes, was designed to circumvent this problem. Although an anti-SLAYGYR monoclonal antibody was successfully tested in a model of rheumatoid arthritis [43], it is not known whether cleaved OPN is increased under inflammatory conditions in mice, which would be necessary for its targeting. While OPN cleavage products were detected in human AT and atherosclerotic plaque samples [28,29], it is insufficiently known whether and to which extent obesity and atherosclerosis induces protease cleavage of OPN in mice. On the other hand, the presence of considerable amounts of cleaved OPN under normal conditions could prevent induction of OPN-specific auto-antibodies. As a limitation of our study, we could not test the antisera for binding of cleaved murine OPN for technical reasons, so that the complete cryptic region might not have been exposed in the full-length protein. In addition to limited functionality of our peptide-induced antibodies, we detected specific binding only after immunization with Peptide 2 and 3, but not with Peptide 1 and 4. Though a thorough peptide design and in silico testing was performed, cross-reactivity of sera obtained after immunization with peptides without any amino acid sequence similarities occurred.
In general, the RGD region constitutes a highly abundant and relevant domain in several proteins. Concerning these facts, induction of sufficient antibodies against the RGD domain may pose severe drawbacks and uncertainties, e.g. by attacking different vital proteins. Interestingly, another study exhibited OPN-specific antibodies after active immunization with human OPN-derived peptides in the DIO model (WT mice) [44]. Concerning these results, the question is raised whether naïve lymphocytes specific for the original (murine) central OPN region-exist or whether T- and/or B-cells will be eliminated by negative depletion. Here we suggest that insufficient induction of protein-specific antibody titers in mice after vaccination with murine OPN-derived peptides supports the hypothesis that OPN specific lymphocytes are eliminated during their development. Another hypothesis is based on the existence of B-cells in a state of anergy. Methods to break tolerance include the use of more potent adjuvants such as toll-like receptor agonists [45] to counter poor immunogenicity or other novel immunization approaches utilizing viral vectors or antigen-loaded mature dendritic cells [46–48]. However, a more effective adjuvant may bear the risk of inducing a general inflammatory state, which in this case, could ignite the inflammatory disorder underlying insulin resistance and atherosclerosis rather that quench it by the specific antibodies to be elicited.
It is noteworthy that there is increasing evidence that neutralization of OPN improves obesity-associated complications including AT inflammation, insulin resistance and CVD [15]. Therefore, novel strategies to test OPN-related treatment strategies have to be evolved and the need for the generation of a humanized OPN mouse model is given.
Concluding this study, although knockout data and passive immunization experiments clearly revealed functional improvements by deficiency or neutralization of OPN in obesity, active immunization with peptide-based OPN vaccines directed against the central integrin-binding region did not elicit antibodies that improved obesity-driven cardio-metabolic sequelae. Due to its crucial role in a variety of inflammatory and cardio-metabolic diseases further investigations on active immunization against OPN are warranted with particular emphasis on epitope design and novel vaccination approaches.
Supplementary Material
Acknowledgements
We gratefully thank Melina Amor and Matteo Tardelli for technical support. This work was supported by the Federal Ministry of Economy, Family and Youth and the National Foundation for Research, Technology and Development (to T.M.S.).
Footnotes
Conflict of interest
The authors have read the journal’s authorship statement and policy on conflicts of interest and declare no commercial or financial conflict of interest.
The article has been reviewed and approved by all authors.
References
- [1].WHO. WHO; [zitiert 20. August 2015]. Obesity and overweight. [Internet]. Verfügbar unter: http://www.who.int/mediacentre/factsheets/fs311/en/. [Google Scholar]
- [2].Jung UJ, Choi M-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014 Apr;11(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bastard J-P, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006 Mar;1(1):4–12. [PubMed] [Google Scholar]
- [4].Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006 Dec;14(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- [5].Rega-Kaun G, Kaun C, Wojta J. More than a simple storage organ: adipose tissue as a source of adipokines involved in cardiovascular disease. Thromb Haemost. 2013 Oct;110(4):641–650. doi: 10.1160/TH13-03-0212. [DOI] [PubMed] [Google Scholar]
- [6].Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003 Dec;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003 Dec;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004 Nov;18(14):1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- [9].Kiefer FW, Zeyda M, Todoric J, Huber J, Geyeregger R, Weichhart T, et al. Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology. 2008 Mar;149(3):1350–1357. doi: 10.1210/en.2007-1312. [DOI] [PubMed] [Google Scholar]
- [10].Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Györi G, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes. 2007 Jun;26(9):1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- [11].Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61–67. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- [12].O’Brien ER, Garvin MR, Stewart DK, Hinohara T, Simpson JB, Schwartz SM, et al. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler Thromb J Vasc Biol Am Heart Assoc. 1994 Oct;14(10):1648–1656. doi: 10.1161/01.atv.14.10.1648. [DOI] [PubMed] [Google Scholar]
- [13].Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J. Osteopontin-a molecule for all seasons. QJM Mon J Assoc Phys. 2002 Jan;95(1):3–13. doi: 10.1093/qjmed/95.1.3. [DOI] [PubMed] [Google Scholar]
- [14].Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 2004 Sep;26(3):179–184. [PubMed] [Google Scholar]
- [15].Kiefer FW, Zeyda M, Gollinger K, Pfau B, Neuhofer A, Weichhart T, et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes. 2010 Apr;59(4):935–946. doi: 10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mormile R. Multiple sclerosis and susceptibility to celiac disease: an osteopontin gene haplotypes affair? Immunol Lett. 2015 Jan;163(1):132–133. doi: 10.1016/j.imlet.2014.11.015. [DOI] [PubMed] [Google Scholar]
- [17].Takemoto M, Yokote K, Nishimura M, Shigematsu T, Hasegawa T, Kon S, et al. Enhanced expression of osteopontin in human diabetic artery and analysis of its functional role in accelerated atherogenesis. Arterioscler Thromb Vasc Biol. 2000 Mar;20(3):624–628. doi: 10.1161/01.atv.20.3.624. [DOI] [PubMed] [Google Scholar]
- [18].Isoda K, Kamezawa Y, Ayaori M, Kusuhara M, Tada N, Ohsuzu F. Osteopontin transgenic mice fed a high-cholesterol diet develop early fatty-streak lesions. Circulation. 2003 Feb;11(5):679–681. doi: 10.1161/01.cir.0000055739.13639.d7. [DOI] [PubMed] [Google Scholar]
- [19].Sahai A, Malladi P, Melin-Aldana H, Green RM, Whitington PF. Upregulation of osteopontin expression is involved in the development of nonalcoholic steatohepatitis in a dietary murine model. Am J Physiol Gastrointest Liver Physiol. 2004 Jul;287(1):G264–G273. doi: 10.1152/ajpgi.00002.2004. [DOI] [PubMed] [Google Scholar]
- [20].Bertola A, Deveaux V, Bonnafous S, Rousseau D, Anty R, Wakkach A, et al. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes. 2009 Jan;58(1):125–133. doi: 10.2337/db08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001 Jul;27(30):28261–28267. doi: 10.1074/jbc.M103608200. [DOI] [PubMed] [Google Scholar]
- [22].Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem. 2007 Nov;1(4):912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- [23].Yokosaki Y, Tanaka K, Higashikawa F, Yamashita K, Eboshida A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol J Int Soc Matrix Biol. 2005;24(6):418–427. doi: 10.1016/j.matbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [24].Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002 Apr;51(4):1093–1101. doi: 10.2337/diabetes.51.4.1093. [DOI] [PubMed] [Google Scholar]
- [25].Chavey C, Mari B, Monthouel M-N, Bonnafous S, Anglard P, Van Obberghen E, et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003 Apr;4(14):11888–11896. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- [26].Vacek TP, Rehman S, Neamtu D, Yu S, Givimani S, Tyagi SC. Matrix metalloproteinases in atherosclerosis: role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc Health Risk Manag. 2015;11:173–183. doi: 10.2147/VHRM.S68415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huber J, Löffler M, Bilban M, Reimers M, Kadl A, Todoric J, et al. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. lnt J Obes. 2005 Jun;31(6):1004–1013. doi: 10.1038/sj.ijo.0803511. [DOI] [PubMed] [Google Scholar]
- [28].Leitner L, Schuch K, Jürets A, Itariu BK, Keck M, Grablowitz V, et al. Immunological blockade of adipocyte inflammation caused by increased matrix metalloproteinase-cleaved osteopontin in obesity. Obes Silver Spring Md. 2015;23(4):779–785. doi: 10.1002/oby.21024. [DOI] [PubMed] [Google Scholar]
- [29].Wolak T, Sion-Vardi N, Novack V, Greenberg G, Szendro G, Tarnovscki T, et al. N-terminal rather than full-length osteopontin or its C-terminal fragment is associated with carotid-plaque inflammation in hypertensive patients. Am J Hypertens. 2013 Mar;26(3):326–333. doi: 10.1093/ajh/hps043. [DOI] [PubMed] [Google Scholar]
- [30].Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007 Oct;1(10):2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gómez-Ambrosi J, Catalán V, Ramírez B, Rodríguez A, Colina I, Silva C, et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J Clin Endocrinol Metab. 2007;92(9):3719–3727. doi: 10.1210/jc.2007-0349. [DOI] [PubMed] [Google Scholar]
- [32].Kiefer FW, Neschen S, Pfau B, Legerer B, Neuhofer A, Kahle M, et al. Osteopontin deficiency protects against obesity-induced hepatic steatosis and attenuates glucose production in mice. Diabetologia. 2011;54(8):2132–2142. doi: 10.1007/s00125-011-2170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Röhn TA, Bachmann MF. Vaccines against non-communicable diseases. Curr Opin Immunol. 2010 Jun;22(3):391–396. doi: 10.1016/j.coi.2010.02.009. [DOI] [PubMed] [Google Scholar]
- [34].Bachmann MF, Whitehead P. Active immunotherapy for chronic diseases. Vaccine. 2013 Apr;3(14):1777–1784. doi: 10.1016/j.vaccine.2013.02.001. [DOI] [PubMed] [Google Scholar]
- [35].Galabova G, Brunner S, Winsauer G, Juno C, Wanko B, Mairhofer A, et al. Peptide-based anti-PCSK9 vaccines – an approach for long-term LDLc management. PLoS One. 2014;9(12):e114469. doi: 10.1371/journal.pone.0114469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Landlinger C, Oberleitner L, Gruber P, Noiges B, Yatsyk K, Santic R, et al. Active immunization against complement factor C5a: a new therapeutic approach for Alzheimer’s disease. J Neuroinflammation. 2015;12:150. doi: 10.1186/s12974-015-0369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Neuhofer A, Wernly B, Leitner L, Sarabi A, Sommer NG, Staffler G, et al. An accelerated mouse model for atherosclerosis and adipose tissue inflammation. Cardiovasc Diabetol. 2014;13:23. doi: 10.1186/1475-2840-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, et al. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112(9):1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shojaei F, Scott N, Kang X, Lappin PB, Fitzgerald AA, Karlicek S, et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J Exp Clin Cancer Res CR. 2012 Mar;23(1):26. doi: 10.1186/1756-9966-31-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kon S, Yokosaki Y, Maeda M, Segawa T, Horikoshi Y, Tsukagoshi H, et al. Mapping of functional epitopes of osteopontin by monoclonal antibodies raised against defined internal sequences. J Cell Biochem. 2002 Jan;84(2):420–432. doi: 10.1002/jcb.10039. [DOI] [PubMed] [Google Scholar]
- [41].Yamamoto N, Nakashima T, Torikai M, Naruse T, Morimoto J, Kon S, et al. Successful treatment of collagen-induced arthritis in non-human primates by chimeric anti-osteopontin antibody. Int Immunopharmacol. 2007;7(11):1460–1470. doi: 10.1016/j.intimp.2007.06.009. [DOI] [PubMed] [Google Scholar]
- [42].Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. 2007 May;6(5):404–414. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- [43].Yamamoto N, Sakai F, Kon S, Morimoto J, Kimura C, Yamazaki H, et al. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest. 2003 Jul;15(2):181–188. doi: 10.1172/JCI17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jürets A, Le Bras M, Staffler G, Stein G, Leitner L, Neuhofer A, et al. Inhibition of cellular adhesion by immunological targeting of osteopontin neoepitopes generated through matrix metalloproteinase and thrombin cleavage. PLoS One. 2016;11(2):e0148333. doi: 10.1371/journal.pone.0148333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pasquale AD, Preiss S, Silva FTD, Garçon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines. 2015;3(2):320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Moser M, Leo O. Key concepts in immunology. Vaccine. 2010;31(Suppl 3):C2–C13. doi: 10.1016/j.vaccine.2010.07.022. [DOI] [PubMed] [Google Scholar]
- [47].Ludewig B, Bonilla WV, Dumrese T, Odermatt B, Zinkemagel RM, Hengartner H. Perforin-independent regulation of dendritic cell homeostasis by CD8(+) T cells in vivo: implications for adaptive immunotherapy. Eur J Immunol. 2001 Jun;31(6):1772–1779. doi: 10.1002/1521-4141(200106)31:6<1772::aid-immu1772>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- [48].Booga CJP. Principles of vaccination and possible development strategies for rational design. Immunol Lett. 2009;122:104–107. doi: 10.1016/j.imlet.2008.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.