Abstract
Although permanent His bundle pacing (HBP) was first reported almost two decades ago, it is only recently gaining wider adoption, following facilitation of the implant procedure by dedicated tools. An additional challenge is programming the system, as HBP may have specific configurations and require special considerations which current implantable pulse generators are not designed for. The aim of this article is to provide practical recommendations for programming HBP in order to deliver optimal therapy and ensure patient safety.
Keywords: pacing, pacemaker optimization, implanted cardioverter defibrillator, His bundle pacing, device programming
Journal Subject Terms: Catheter Ablation and Implantable Cardioverter-Defibrillator, Pacemaker
Introduction
Permanent His bundle pacing (HBP) as an alternative to pacing from the right ventricular (RV) apex was first performed in 2000 by Desmukh et al,1 and was introduced as an alternative to cardiac resynchronization therapy (CRT) by Barba-Pichardo et al. in 2006.2 Although pacing of the native conduction system has been a milestone in the quest for physiological pacing, this technique has not been readily adopted because of its technical complexity and elevated capture thresholds. Recently, a sheath with a fixed curve specifically designed to locate the His bundle (C315His catheter, Medtronic Inc, Minneapolis, MN) has greatly facilitated the procedure, with improved capture thresholds, and >90% implant success in experienced hands.3, 4 The improved implantation success rate, combined with the advent of MRI-conditionality of the SelectSecure 3830 lead (Medtronic) in 2017 and FDA labelling for HBP in July 2018, have led to a marked increase in adoption of HBP as a means of pacing. A number of excellent reviews have recently been written on general aspects HBP.4, 5
Although implantation tools for HBP have been the focus of attention and are continuing to be developed, specific requirements for implantable pulse generator (IPG) programming and follow-up have as yet not been properly addressed. A first step in this direction has been the recent publication of recommendations to standardize definitions, implant measurements and follow-up for HBP.6 Although timing cycles, algorithms and programming options are likely to be adapted in the future to better suit the needs of HBP, this is unlikely to happen soon. The aim of this article is to provide practical recommendations for programming HBP.
General Considerations
There are a number of general considerations, which need to be taken into account with HBP.
His pacing labelling
As presently there are no IPGs available with a dedicated His pacing port, it should be made clear in the patient’s pacemaker card and on the device free text that the pacing system has a His pacing lead and which port it is plugged into. This is particularly relevant when the His lead is connected to the atrial port of the IPG, which may result in confusion during follow-up, as it may be mistaken that the atrial lead has dislodged into the ventricle.
Choice of generator and His pacing lead port position
Different IPGs and lead configurations are used according to 1) the aim of HBP 2) the presence of a backup ventricular lead and 3) baseline rhythm – see table 1. Further configurations using Y-adapters are possible but are not covered here.7 The aim of HBP may be to provide therapy in lieu of RV pacing, in lieu of biventricular (BiV) pacing (e.g. in case of failed coronary sinus lead implantation or to optimize prolonged AV intervals in heart failure8) or as His-optimized CRT. For this last novel entity, HBP is used in addition to RV, LV, or BiV pacing to optimize CRT.9, 10 HBP may only partially correct bundle branch block (BBB), and ventricular pacing may serve to further improve synchrony by activating regions which remain delayed. In case of selective His capture without correction, His-optimized CRT enables fusion pacing with consistent timing between activation wavefronts via the intrinsic conduction system and ventricular pacing,9 even in case of complete AV block (e.g. after AV node ablation).
Table 1. Different device types and configurations for HBP.
| Aim | Indications | Presence of additional V lead(s)* | Sinus rhythm | AF |
|---|---|---|---|---|
| In lieu of RV pacing | AV block Slow AF/flutter |
+ | CRT: His in LV port + RV + A leads in corresponding ports |
DDD: His in A port + RV lead in ventricular port |
| - | DDD: His in RV port† + A lead in A port | VVI: His in only port† | ||
| In lieu of BiV | Failed LV lead implantation Low likelihood of response to BiV Optimization of long AV intervals in heart failure (e.g. in patients with narrow QRS) |
+ | CRT: His in LV port + RV + A leads in corresponding ports CRT: His in RV port† + LV + A leads in corresponding ports |
CRT: His in A port + RV + LV leads in corresponding ports DDD: His in A port and RV or LV# lead in ventricular port |
| - | DDD: His in RV port† + A lead in A port |
VVI: His in only port†) |
||
| His-optimized CRT | Improvement of response to CRT RBBB and selective His capture without correction |
+ | CRT: His in RV port† + LV + A leads in corresponding ports or CRT: His in LV port‖ + LV lead in RV port‡,# + A lead in A port |
DDD: His in A port + LV‡ lead in RV port or CRT: His in A port + RV + LV leads in corresponding ports |
A: atrial; AF: atrial fibrillation; AV: atrioventricular; BiV: biventricular pacing; CRT: cardiac resynchronization therapy; LV: left ventricle; RBBB: right bundle branch block; RV: right ventricle; V: ventricular.
The additional ventricular lead(s) may be used for backup pacing, as an alternative therapy in case HBP is inactivated, or to optimize therapy.
In all instances, if the device is a defibrillator, and a His or LV lead is connected to the RV port, a DF-1 lead should be used, with capping of the IS-1 pin of the lead.
With a His lead in the RV port, sensing must be acceptable
Alternatively, in case of RBBB, an RV lead may be implanted instead of an LV lead (likely to have more shortening effect on ventricular activation time).
Alternatively, the His lead may be connected to the RV port if sensing is good and there is concern for LV dislodgment
Requires a bipolar (not quadripolar) LV lead. Electrical repositioning of the LV lead (e.g. using the ring electrode as cathode) will not be possible.
Furthermore, the NBG code11 for pacing was implemented before the era of HBP, and does not therefore accurately reflect His pacing, although the closest approximation is ventricular pacing. A revision of this code to accommodate for HBP would be timely.
Analysis of the Electrocardiogram
Unless it is known that a His lead has been implanted, diagnosis of loss of ventricular capture may be made in some cases due to the relatively long pacing stimulus to QRS onset. Another potential source of confusion when interpreting the 12-lead ECG is the pseudo delta wave seen during non-selective His capture (see below), which may be interpreted as the presence of an accessory pathway.
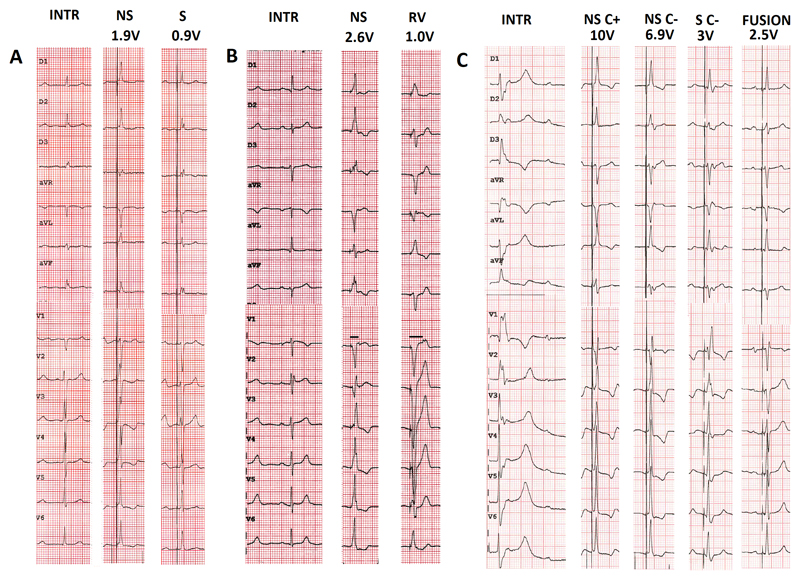
We cannot stress enough the importance of recording a standard 12-lead electrocardiogram (ECG) for determining whether HBP is achieved, for evaluating capture thresholds and also the type of capture. The different types of capture have been previously defined by a consensus paper,6 and are illustrated in figure 1.
Figure 1.
Examples of types of His capture at different programmed pacing outputs in three patients. A. Patient in atrial fibrillation awaiting atrioventricular nodal ablation. Transition from non-selective to selective His capture. Note the presence of a pseudo-delta wave without an isoelectric interval after the pacing spike with non-selective capture, visible in aVL and the precordial leads B. Patient with exercise-induced Mobitz 2 atrioventricular block and narrow intrinsic QRS. Transition from non-selective His capture to right ventricular capture. Note the increase in stimulus to retrograde P duration with right ventricular capture compared to during His bundle capture (horizontal lines in lead V1). C. Patient in sinus rhythm with complete atrioventricular block and ventricular escape rhythm. Selective capture without correction shows a typical right bundle branch block pattern. Note how it is difficult to distinguish between non-selective capture with and without correction (a difference in QRS morphology is visible in lead II). The patient had a biventricular pacemaker with an atrial, right ventricular, and His lead (connected to the left ventricular port). Due to high capture thresholds for non-selective His capture, fusion pacing with sequential selective His capture (threshold was 1.5V/0.5ms) and right ventricular pacing was programmed.
INTR: intrinsic rhythm; NS: non-selective His capture; S: selective His capture; C+: with correction; C-: without correction.
Nonselective His bundle capture
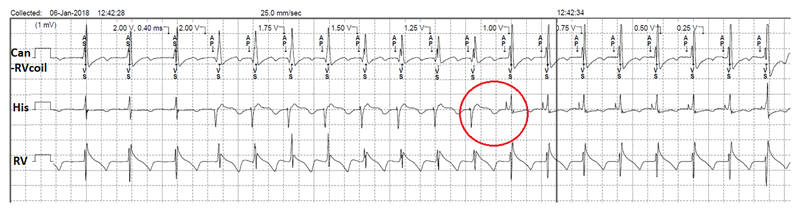
This is characterized by a pseudo-delta wave, reflecting local anteroseptal myocardial depolarization prior to the rapid activation of the ventricle via the His-Purkinje system (similar to a para-Hissian accessory pathway). It is important to analyze all 12 leads, as the pseudo-delta wave may be isoelectric in some leads, thereby masquerading as selective capture (see figure 1A). If the local ventricular electrogram on the His lead directly follows the pacing spike, this indicates non-selective capture (see figure 2). It is at present unclear if hemodynamics with non-selective capture differ compared to selective capture. Non-selective capture provides backup ventricular pacing in case of loss of His capture in patients with AV block, and may also be desirable in case of His capture without correction of right bundle branch block (RBBB) as it serves to narrow the QRS complex (see below).
Figure 2.
Threshold test with 0.4ms pulse width with the His lead connected to the atrial port of a dual-chamber pacemaker. Non-selective His capture down to 1V, with selective capture thereafter (down to the minimum amplitude of 0.25V). Note the change in electrogram morphology during the transition of non-selective to selective capture (circle). This patient had underlying right bundle branch block, which explains why sensed events by the His lead are labelled as “AS” and not “Ab”.
Selective His bundle capture
This involves activation of the ventricles exclusively over the His-Purkinje system, with an isoelectric interval in all 12 leads. Paced QRS morphology may be identical to that in intrinsic rhythm or show complete or partial correction of underlying BBB. The HV interval is equal to the stimulus-V interval, although this criterion is more useful during implantation (where these intervals can be accurately measured using an electrophysiology recording system) than for follow-up. Analysis of the device intracardiac electrogram can be useful to identify selective His bundle capture which is suggested by an interval between the pacing artefact and the local ventricular electrogram (see figure 2).
Correction of bundle branch block
In case of baseline BBB, HBP may correct the conduction delay (sometimes to varying degrees depending upon the pacing output) with either selective or non-selective His capture (see figure 1C). In case of baseline RBBB, it may be difficult to distinguish between non-selective His capture with or without correction, as capture of the right anteroseptal myocardium results in a fusion beat which narrows the QRS complex (see figure 1C).
Performing his Threshold Tests
When performing threshold tests, it is mandatory to record a 12-lead ECG to compare QRS morphologies at different pacing outputs during the threshold test. It is useful to start the test at maximal output and to allow at least 3 pulses at all decrementing amplitudes.
It is important to note transitions in QRS morphology. Transition between non-selective and selective His bundle capture can be recognized by disappearance of the pseudo-delta wave (see figure 1A), sometimes with subtle changes in QRS morphology. Another possible transition with initial non-selective capture is loss of His capture, resulting only in myocardial capture. In case of 1:1 retrograde conduction, prolongation of the stimulus to P interval may be observed in this instance (see figure 1B).
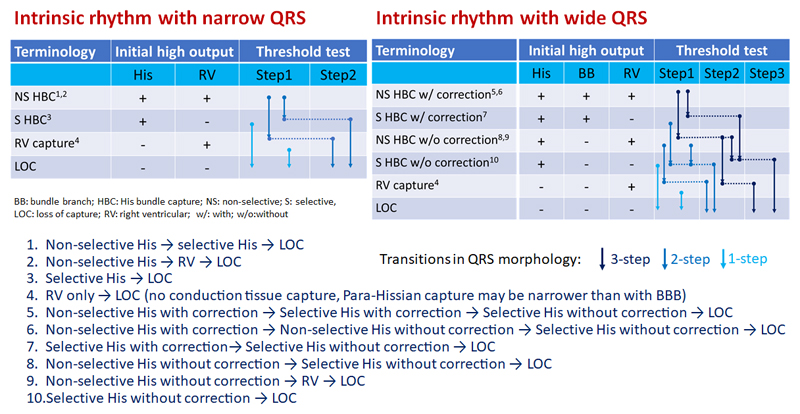
There are a total of 10 different possible main transitions which are shown in figure 3. It is important to stress that His capture may not be achieved at all, resulting in para-Hisian pacing with myocardial capture only (i.e. no capture of conduction tissue). The QRS complex may nevertheless be narrower than during conventional RV pacing or even compared to intrinsic rhythm with BBB, but there is no transition in QRS morphology before loss of capture with para-Hisian pacing.
Figure 3.
Summary of the main possible transitions during threshold testing of direct His bundle pacing. Note that only initial selective His bundle capture or initial right ventricular myocardial capture (without His capture) result in a single step. Transitions may however be missed if the decrement in pacing output is greater than the difference in thresholds (e.g. between myocardial and His thresholds). Additional minor transitions are possible in case of varying degrees of correction of BBB in a given patient, or with anodal capture if the His lead is programmed to a bipolar or extended bipolar vector, and are not shown here.
The only instances during a threshold test where no transitions in QRS morphology are observed (i.e. transition directly from a paced QRS complex to loss of capture) are:
-
1)
Initial myocardial capture only (i.e. no HBP achieved)
-
2)
Initial selective His capture
-
3)
Near-identical thresholds for the conduction tissue and myocardium (which are not distinguished by the decremental steps during threshold tests, which are usually 0.25V).
Impossible transitions with HBP during threshold testing are:
-
1)
Selective → non-selective His capture (the pacing output for His+RV myocardial capture needs to be higher than for His capture alone).
-
2)
Selective His capture → RV capture (if His capture is selective, there is no initial RV myocardial capture).
-
3)
Without BBB correction → with BBB correction (output for correction of BBB needs to be higher than without correction).
The His lead may also result in direct atrial capture. When performing threshold tests in a single-chamber mode in patients in sinus rhythm, it is possible to observe His bundle capture with higher outputs and only atrial capture with lower amplitudes, or vice-versa.
It is recommended to specify the different thresholds for correction of BBB, non-selective, selective, or RV myocardial capture.6
Programming Requirements with Different Configurations
Programming recommendations are summarized in table 2. The pacing mode depends upon the IPG type and which port the His lead is connected to. If the His lead is connected to a ventricular port, one should bear in mind the latency in ventricular activation resulting from HV conduction, and shorten the programmed atrioventricular interval (AVI) accordingly. The HV interval can be measured by the stimulus to QRS onset in case of selective capture. With non-selective capture, it is at present unclear if the AVI should be adjusted. It is sometimes useful to program a unipolar or extended bipolar (His tip to RV defibrillator coil) pacing vector in order that the pacing spike is clearly visible on the surface ECG (to avoid confusion with intrinsic rhythm, and because thresholds may be lower than with bipolar pacing12). The tradeoff is that impedance is lower than with a bipolar vector, thereby increasing current drain. As automatic capture management algorithms are often ineffective with HBP, sufficient safety margin should be provided (e.g. twice the amplitude or three times the duration – by analogy with myocardial pacing). In the interest of battery longevity, the safety margin is sometimes programmed lower (especially if there is backup ventricular pacing or if the patient is not pacemaker dependent), or if selective His capture (as opposed to non-selective capture) is desired. The pacing amplitude should if possible be programmed below that of the battery voltage, in order to avoid requirement of voltage multipliers which result in premature battery drain. In case of high thresholds, the pulse width should be increased to e.g. 0.8-1.5ms (in clinical practice, pulse widths are often programmed to 1ms). Sensing can be an issue and should be handled differently according to which port the lead is connected to.
Table 2. Summary of programming recommendations.
| Parameter | Setting | Comment |
|---|---|---|
| Pacing mode | VVI(R) | Single-chamber system |
| AAI(R) | In case of single-chamber system with low detection amplitude of the His lead - allows higher sensitivity setting than with VVI(R). | |
| DDD(R) | Do not programme long AVI (e.g. 200ms) if the His lead is connected to the A port (due to risk of His pacing on the T-wave in case of intermittent loss of His lead capture). | |
| AAI(R)/DDD(R)* ADI(R)/DDD(R) |
Avoid programming if His lead connected to the A port (cf comment above), or if pacing from the LV port is desired (as only RV pacing is delivered in these modes. | |
| DVI(R) | May be programmed if the His lead is connected to the A port, but results in pacing above the upper sensor-driven rate due to ventricular-based timing. | |
| DDI(R) | With the His lead in the A port, avoid programming long AVI (c.f. comment on DDD). With the His lead in the A port, results in pacing above the upper sensor-driven rate due to V-based timing. | |
| Pacing rates | According to clinical requirement | |
| AVI | ||
| - His lead in atrial port | Delay depends on requirement | If V backup pacing is desired, programme paced AVI> Stim His-VS interval (but ≤180ms cf. comment on DDD). If His+RV/LV pacing is desired, programme a short AVI (shortest is 30ms*). |
| - His lead in RV/LV port | Usual AVI – HV interval (or stimulus-QRS onset) | AV hysteresis may be programmed if V pacing needs to be avoided. |
| Sensing vector | Bipolar | Unipolar may be tried if low sensing amplitude or if issues with P-wave oversensing. |
| Sensitivity | ||
| - His lead in A port | Lowest possible (e.g. 4mV) | Program lowest sensitivity to avoid issues with oversensing of A or His potentials (V is sensed by RV lead). |
| - His lead in RV port | As usual practice | Ensure no oversensing of A or His potentials. Consider automatic sensing threshold in case of low R-waves. |
| - His lead in LV port | Not available or inactivate | V sensing by RV lead. |
| Pacing vector | Consider unipolar or extended bipolar (if available). | Capture thresholds are usually lower than with bipolar vector, and pacing spike is visible (avoids confusion with intrinsic rhythm on ECG). Trade-off is lower impedance compared to bipolar (i.e. higher current drain). |
| Capture management algorithms | Off or monitor | Rarely yields accurate measurements. Must be inactivated in Medtronic CRT devices if configuration is His in LV port and RV output is subthreshold (as backup pacing during atrial, RV and LV automatic threshold tests is RV only at the programmed output*). Default setting is “adaptive”.* The same also applies to Boston Scientific CRT devices for the LV automatic capture threshold tests. |
| Ventricular safety pacing | On or off (non-programmable in some devices | Consider turning off in case of His lead in A port, to avoid unnecessary V pacing (and inform of loss of His lead capture -corresponding to the percentage of AP-VP). However, presence of AV crosstalk should be tested first with “worst case” scenario. |
| AdaptivCRT* | “Off” | Will yield suboptimal intervals. Nominal setting is “Adaptive BiV and LV”. |
| VV delay | His lead first. Delay dependant on requirement. | If His-only pacing is desired (and backup pacing by the ventricular lead), programme to maximum delay (with His first). In case of His-optimized CRT, programme the VV delay to optimize fusion with V lead. |
| EffectivCRT diagnostic* | Non-programmable | Feature designed to indicate LV capture. If His lead in LV port, selective His capture may be indicated by “ineffective” pacing. |
| EffectivCRT during AF* | “Off” if His lead in LV port | Feature designed to increase pacing rate during AF if “ineffective” LV pacing is diagnosed (even in case of RV pacing), which may be the case with selective His capture. Default is “on”. |
| Conducted AF response | “On” | Algorithm increases pacing rate in case of V sensing during AF, to promote CRT. Will not be activated if the His lead is in the A port. Default is “on”. |
| Ventricular sense response | “Off” | VVT pacing designed to resynchronize conducted beats (e.g. in AF). Is ineffective for HBP and will result in unnecessary battery drain. Default is “on”. |
| Non-competitive atrial pacing | “On” | Algorithm designed to avoid pacing shortly after an A refractory sensed event. Does not affect HBP. Nominal is “on”. |
| PVC response | “On” | Post PVC PVARP extension designed to avoid endless loop tachycardia. Does not affect HBP. Nominal is “on”. |
| Ventricular refractory period | >200ms (usually by default) | If His is connected to the LV port with pacing only from the LV channel, RV double-counting may occur if the Stim His to RVS interval > ventricular blanking. |
| ICD rhythm discrimination algorithms | Inactivate dual-chamber algorithms (e.g. PR logic) if His lead in atrial port! | Use single chamber discriminators instead (sudden onset, stability, morphology discrimination). Inactivate rhythm discriminators if the patient is in complete AV block. |
A: atrial; AF: atrial fibrillation; AV: atrioventricular; AVI: atrioventricular interval; CRT: cardiac resynchronization therapy; DHBP: direct His bundle pacing; ICD: implantable cardioverter defibrillator; LV: left ventricular; RV:right ventricular; RVS: right ventricular sense; Stim: stimulation; V: ventricle; VS: ventricular sense;
applicable to current Medtronic devices
His lead in the RV port
This may involve either:
-
1)
a single-chamber system in the case of atrial fibrillation (AF) or flutter
-
2)
a dual chamber device and either sinus rhythm (atrial + His leads) or AF (His + LV leads)
-
3)
a CRT device (RA + His + LV leads).
Pacing mode
With dual-chamber systems (atrial lead + His lead in a ventricular port), the DDD(R) mode or managed ventricular pacing (MVP) algorithms such as AAI(R)/DDD(R) mode and atrioventricular (AV) hysteresis, or DDI(R) mode may be activated if the intent is to promote intrinsic conduction (although long PR intervals may result). In case of a BiV system (atrial lead + His lead in the RV port and LV lead in the LV port), the ADI(R)/DDD(R) and AAI(R)/DDD(R) modes should not be programmed in Biotronik and Medtronic devices respectively if the intent is to pace with the LV lead (as a backup or for His-optimized CRT), as only pacing from the RV channel is currently possible in these modes. With Boston Scientific (Marlborough, MA, USA) devices, the Rythmiq mode is currently not programmable. However, Microport (Shanghai, China) CRT devices can be programmed in the SafeR mode, with pacing delivered from one or both ventricular channels.
Sensing
A major issue with a His lead connected to the RV port is sensing, as amplitudes may be low (due to a “far-field” ventricular signal with low frequency content which is filtered by the sense amplifier), and oversensing of atrial or His potentials, which may be potentially disastrous in a patient with complete AV block. A single-chamber pacemaker may be programmed to AAI(R), as fixed sensitivity may be set to a higher level/lower value (e.g. 0.25mV) compared to the VVI(R) mode (usually limited to 1mV). Otherwise, automatic sensitivity should be considered. In devices with automatic sensitivity, the maximum sensitivity varies from 0.3mV (Abbott, St Paul, MN) to 0.5mV (Biotronik).
Capture Management Algorithm
RV capture management algorithms are based upon detection of the evoked potential, which is absent in case of selective His bundle capture. Even in case of non-selective capture, the algorithm seldom yields accurate results, and the feature should be either inactivated or programmed to “monitor”.
His lead in the LV port
In patients implanted with a CRT generator, the His lead is usually connected to the LV port and the RV port is connected to an RV lead (for ventricular sensing and backup pacing) or to an LV lead (due to fewer sensing issues compared to a His lead – see above). The LV port is not used for ventricular detection in Medtronic and Microport devices, and rate sensing can be programmed to the RV channel only in those manufacturers that do sense from both channels. This configuration also allows programming an extended bipolar pacing vector with the His lead, which has been shown to lower capture thresholds.12 However, the tradeoffs in case of CRT with an LV lead in the RV port is that only bipolar LV leads can be used (whereas an IS-4 LV lead may be used if it is connected to the LV port), and that electrical repositioning (with pacing from the LV ring only) is not possible.
Pacing mode
The device should be programmed to DDD(R) or DDI(R) modes. As mentioned above, the AAI/DDD mode should not be programmed with Medtronic devices as only pacing from the RV channel is currently possible in this mode.
Programming of sequential pacing
When His pacing is used in lieu of RV or BiV pacing in patients with a His lead connected to the LV port (+ atrial + RV leads in their respective ports), the RV lead essentially serves for ventricular sensing or backup pacing in case of loss of His capture. RV pacing is therefore usually unnecessary or even undesirable in these instances, and His-only pacing may be the best option. However, backup RV pacing is useful in case of AV block. In this setting, sequential BiV pacing with initial pacing from the LV channel (His lead) and a VV interval set to the maximum value (which varies between manufacturers from 64ms for Abbott to 120ms for Medico,Rubano, Italy), can be programmed to minimize undesirable RV fusion. RV pacing will usually be delivered independently of His capture, because the programmed VV delay is shorter and the ventricular blanking period longer (usually >200ms) than the delay between His pace to RV sense interval (reported to be 94 ± 14 ms13).In some situations, fusion between HBP and RV pacing may be desirable and is a form of His-optimized CRT (see table 1). For example, His capture may be selective without correction of RBBB, or with correction/non-selective capture at an unacceptably high output. Sequential RV pacing (with His pacing first, at approximately the HV interval) will result in narrowing of the QRS complex, similar to non-selective capture in this instance (see figure 1C).
Pacing only from the LV (His) channel
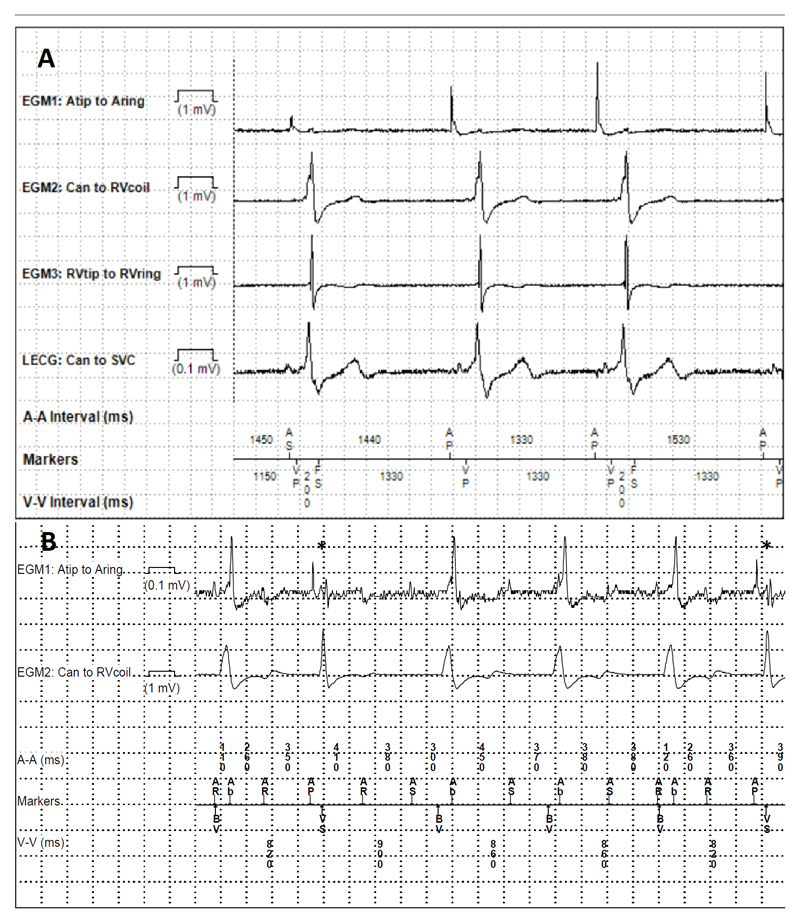
This is an option to avoid unnecessary/undesirable RV pacing (if backup ventricular pacing is not required), with the RV lead serving only for ventricular sensing or delivering ICD therapy. A potential issue is R-wave double counting if the delay between His pacing and RV sensing is longer than the ventricular blanking period (see figure 4A). This may result from prolonged HV intervals or non-corrected RBBB. The same phenomenon can be encountered in CRT with programming of LV-only pacing (outside the context of fusion pacing) or in case of loss of RV capture. Ventricular blanking after ventricular pacing should therefore be set to >200ms (which is the default setting in most current devices). However, one should avoid prolonging ventricular blanking after ventricular sensing, to avoid undersensing of ventricular fibrillation.
Figure 4.
Oversensing issues with HBP. A. Ventricular double-counting. Biventricular defibrillator with a His lead connected to the left ventricular port. Pacing from the His lead only, with sensing from the right ventricular channel at 200ms as shown by the “FS” (fibrillation sense) events (first and third cycles). Ventricular blanking was extended from 200ms to 230ms, which solved the issue. B. Atrial oversensing. Patient in atypical flutter who had undergone atrioventricular nodal ablation, implanted with a biventricular defibrillator and a His lead connected to the atrial port with nominal sensitivity settings. Note sensing of the atrial flutter in the His lead, with mode switch and pacing in the DDIR mode at the baseline rate of 70bpm, mostly with biventricular pacing, and inhibition of His bundle pacing except in two instances denoted by the asterisks. Atrial sensitivity was decreased from 0.3mV to 4mV, with resumption of His pacing.
Capture management algorithms
Left ventricular capture management algorithms are based on AV and interventricular (VV) conduction delays, and do not usually yield accurate values when a His lead is connected to the LV port. It is important to inactivate all capture management algorithms in Medtronic CRT devices if the RV lead is programmed with a subthreshold output (to reduce current drain or to avoid RV capture), as backup pacing during the threshold test is only delivered with RV pacing at the programmed amplitude (also for LV threshold tests with Boston Scienfic devices), and may result in transient asystole in case of complete AV block.14 Other manufacturers deliver BiV backup pulses (Biotronik) or high-output RV backup pulses (e.g. 5V/≥0.5ms for Abbott).
CRT optimization algorithms
With CRT devices, algorithms to optimize AV and VV should be inactivated when the His lead is connected to one of the ventricular ports, as these algorithms are not adapted to HBP and are likely to yield suboptimal values. For instance, the Medtronic AdaptivCRT algorithm automatically adjusts the AVI based on far-field P-wave duration, or on intrinsic AV conduction delay. The “optimized” AVI may be too long by about 40-60ms, as the algorithm does not take into account the HV interval following the pacing stimulus prior to ventricular activation in HBP. Furthermore, the algorithm may propose LV-only pacing for fusion with intrinsic AV conduction, which may not be desirable in certain situations (e.g. fusion with RV pacing for selective HBP with RBBB, c.f. above). Also, asystole may result in case of loss of capture in a patient with transient complete AV block (this is not an issue in case of persistent AV block as the algorithm does not activate LV-only pacing in this instance). Likewise, triggered ventricular pacing algorithms (e.g. Medtronic Ventricular Sense Response) should be inactivated as they result in pseudofusion (in most CRT devices, this feature is nominally activated).
Diagnostic features
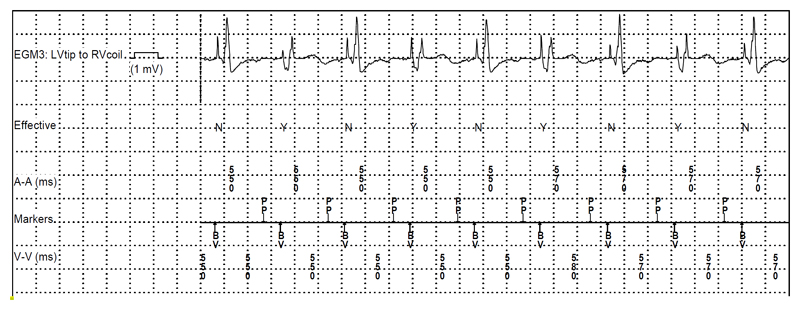
The Medtronic EffectivCRT diagnostic algorithm evaluates whether there is LV capture based upon an initial negative deflection of the intracardiac electrogram immediately following the pacing spike. In case of selective His bundle capture, the algorithm will indicate ineffective capture (due to the initial isoelectric interval in the electrogram), and effective capture in case of non-selective His capture (see figure 5).
Figure 5.
Episode of an “ineffective” ventricular capture event retrieved from a patient with a His lead connected to the left ventricular port of a biventricular defibrillator (with His pacing in lieu of biventricular pacing due to anomaly of the coronary sinus). The Medtronic EffectivCRT algorithm annotates “ineffective” capture cycles with an “N”, which correspond in this case to selective His capture and “effective” cycles with a “Y”, which correspond here to non-selective capture (markers are displayed in the second line). Note the differences in electrogram morphology with “QR” waveforms of the “effective” cycles corresponding to local myocardial capture of non-selective His capture and an isoelectric interval with a far-field ventricular electrogram corresponding to the “ineffective” cycle (selective His capture). It could be deduced from the counters that the patient had selective His capture in 98% of the time (the threshold for selective capture was 1.0V/0.4ms and for non-selective capture, 2.75V/0.4ms, with a programmed output of 2.5V/0.4ms). This patient also had episodes of ectopic atrial rhythm originating close to the coronary sinus with short PR intervals, resulting in loss of His pacing; the “preferential pacing” algorithm was activated to override this rhythm (indicated by the “PP” annotation of the atrial marker channel).
His lead in the atrial port
This configuration may cause most confusion, as sensed and paced events correspond to ventricular rather than atrial events. It is exclusively indicated in patients with chronic atrial flutter/AF, where there is usually no need for an atrial lead and may involve dual-chamber or CRT devices (see table 1).
Pacing mode
The device should be programmed to a DDD(R) mode. The DDI(R) mode or DVI(R) modes (if available) are also options. A consideration resulting from short AP-VS timing with the His lead in the atrial port is the risk of pacing well above the programmed upper rate limit in case of ventricular-based timing, which may be applied in the DDIR and DVIR modes. For example, in a device with a programmed upper sensor-driven rate of 120bpm (500ms) and a paced AV interval of 180ms (the nominal value of current Medtronic dual-chamber devices), the VA interval will be 320ms. If the AP-VS interval is 80ms, the actual pacing rate will be 150bpm (400ms). As most devices function with atrial-based timing in the DDD(R) mode, short AP-VS intervals will not affect pacing rate in this mode.
Programming the AV interval
When HBP is used in lieu of RV or BiV pacing, a fixed paced AV interval should be programmed to a value greater than the His pace – RV sense interval, which is usually 80-100ms.13 Long AVI (e.g. >180ms), as well as the AAI/DDD mode or AV hysteresis should however be avoided, as these may result in His pacing on the preceding T-wave in case of intermittent loss of His capture. For example, in a device pacing at the sensor rate of 120bpm (500ms) with a paced AVI of 200ms, the resulting VA interval will be 300ms. In case of intermittent loss of capture of the His lead, an AP-VP-AP sequence will result, with risk of pacing by the His lead on the preceding T-wave. In case of consistent capture of the His lead, the sequence will be a short AP-VS interval (usually 80-100ms13) with a longer VA interval (around 400ms in the example above).
When HBP is used for His-optimized CRT, a short fixed paced AVI corresponding roughly to the HV interval (30-60ms) should be activated, with BiV or LV pacing (the minimum programmable interval is 30ms for most manufacturers). The optimal interval also depends on latency of LV capture, and needs to be verified by other means such as QRS width and morphology on the ECG.
Ventricular safety pacing
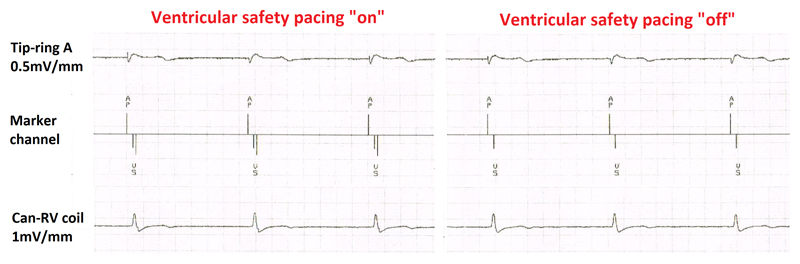
The short AP-VS interval almost always falls within the ventricular safety pacing (VSP) window which varies between 64ms (Abbott) and 110ms (Medtronic), resulting in automatic delivery of ventricular pacing at the end of this window (see figure 6), with pseudofusion. Although this is not harmful, it results in unnecessary battery drain, and inactivation of this feature should be considered. However, risk of AV crosstalk needs to be tested first by programming a maximum unipolar output in the atrial (His) channel with maximum sensitivity in the RV channel. Analyzing timing of the “VS” event with respect to the ventricular electrogram is crucial to distinguish between crosstalk (detection of the afterpotential of the pacing spike) and true ventricular sensing of the captured beat. The feature cannot be inactivated in Biotronik devices (with a VSP window of 100ms) nor in Microport devices (VSP window of 95ms), and does not exist in Boston Scientific devices, which rely on retriggerable noise windows to avoid crosstalk. Also, inactivation of VSP will indirectly inform on the percentage of loss of His lead capture, corresponding to the percentage of AP-VP events.
Figure 6.
Medtronic biventricular defibrillator in a patient in atrial fibrillation who had undergone atrioventricular nodal ablation. Left: Pacing by the His lead connected to the atrial port with detection of the local ventricular electrogram by the right ventricular lead after approximately 80ms, which triggers ventricular safety pacing (depicted in Medtronic devices by the double downward lines in the marker channel) at the end of the 110ms window. Right: After inactivation of ventricular safety pacing (after having ruled out crosstalk), unnecessary ventricular pacing with pseudofusion is avoided.
Sensing
Nominal sensitivity levels may lead to atrial oversensing of AF by the His lead resulting either in mode switch and inhibition or HBP (see figure 4B) or in irregular ventricular paced rhythm in case of intermittent atrial oversensing. It is important to stress that ventricular sensing by the His lead connected to the atrial port is not required as this is ensured by the ventricular lead. Sensitivity in the atrial channel may be programmed to the lowest level (highest value) or the DVI(R) mode may be programmed if available.
In case of intrinsic conduction and detection of the ventricular electrograms despite low sensitivity settings, sensing in the His lead usually follows shortly that of the RV lead, falling in the post-ventricular atrial blanking period. Current Biotronik and Medtronic devices show “Ab” markers for these events, which are however not displayed on rate histograms. Exceptions to this are presence of RBBB or ventricular premature beats, where events may be detected in the His lead before the ventricular lead, with “AS” events (see figure 2).
Diagnostic features
Medtronic devices perform automatic checks for atrial lead position (based on short AP-VS intervals), and warnings will appear as observations during device follow-up, but these do not affect device function for HBP.
ICD considerations
It should be emphasized that in ICDs with a His lead implanted in the atrial channel, dual-chamber rhythm discrimination algorithms should be inactivated, otherwise true ventricular tachycardia may be misdiagnosed as junctional tachycardia if the ventricular electrogram is sensed by the His lead. If the His lead does not sense the ventricular electrogram (e.g. because sensitivity has been set to a high value, as is generally recommended), all supra-ventricular tachycardia will be classified as ventricular tachycardia due to fulfillment of the V>A criterion. Single-chamber algorithms such as sudden onset, stability and morphology matching, should therefore be used instead.
Unmet Needs and Future Directions
As illustrated by this article, programming of HBP may be complex, and it would be very useful to have automated optimization of device parameters based upon His lead connection. Adaptation of filter settings to increase sensing amplitude of far-field ventricular signals of His leads is desirable (but T-wave and atrial oversensing needs to be avoided). Specific refractory periods to prevent oversensing of atrial or His potentials, and alternatives to VSP to avoid crosstalk, would be useful. A useful modification would be to increase the programmable VV delay and modify ventricular refractory periods to allow crosschamber sensing for a defined interval after pacing to avoid unnecessary stimulation from the RV lead when the His lead is connected to the LV port. With the IS-4 standard and advent of sequential multipoint pacing, a Y-adaptor (ideally MRI-conditional) allowing the connection of two IS-1 leads (His and LV) would offer new possibilities, especially as the DF-4 standard is likely to phase out DF-1 connectors due to driving market forces. High capture thresholds are still an issue with HBP, and in addition to changes in lead design, battery longevity, capture management algorithms which accurately measure thresholds and adapt output accordingly would be an important step forward.
Conclusions
As a direct consequence of increasing adoption of HBP, there is a need for more widespread awareness of ECG analysis and specific programming requirements relating to this therapy. IPGs are currently not designed for HBP, and the different permutations and combinations of lead connections and system configurations leading to unusual programming settings can be confusing. The industry is no doubt going to adapt its technology to meet these challenges and facilitate device programming. However, this is likely to take time to develop and gain regulatory approval before becoming available in routine clinical practice. In the meantime, device specialists need to familiarize themselves with tailoring HBP programming to ensure safe and effective therapy for their patients.
Acknowledgments
The authors would like to thank Mr Thierry Offner (Abbott), Dr Thomas Brueggemann (Biotronik), Mr Wyatt Stahl (Boston Scientific), Mr Gary Nygaard (Medtronic) and Mr Piero Modena (Microport) for having replied to our technical queries.
Sources of Funding: Dr Keene is supported by a British Heart Foundation grant (FS/15/53/31615)
Footnotes
Disclosures: Haran Burri has served as a speaker and received institutional research support from Abbott, Biotronik, Boston Scientific, Medtronic and Microport. Daniel Keene has served as a speaker for Medtronic. Pugazhendi Vijayaraman has served as a speaker for and received research support from Medtronic; has served as a consultant for Medtronic, Boston Scientific, Merritt Medical, and Abbott; and has a patent pending for a His delivery tool. Zachary Whinett has received speakers fees from Medtronic, consultant fees from Medtronic, Abbot and Boston and research funding from Medtronic and the British Heart foundation. Francesco Zanon has received speaker fees from Abbot, Biotronik, Boston Scientific, Medtronic and LivaNova-Microport.
References
- 1.Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation. 2000;101:869–77. doi: 10.1161/01.cir.101.8.869. [DOI] [PubMed] [Google Scholar]
- 2.Barba-Pichardo R, Moriña-Vázquez P, Venegas-Gamero J, Maroto-Monserrat F, Cid-Cumplido M, Herrera-Carranza M. Permanent His-Bundle Pacing in Patients With Infra-Hisian Atrioventricular Block. Revista Española de Cardiología (English Edition) 2006;59:553–558. [PubMed] [Google Scholar]
- 3.Sharma PS, Dandamudi G, Naperkowski A, Oren JW, Storm RH, Ellenbogen KA, Vijayaraman P. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm. 2015;12:305–12. doi: 10.1016/j.hrthm.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Zanon F, Ellenbogen KA, Dandamudi G, Sharma PS, Huang W, Lustgarten DL, Tung R, Tada H, Koneru JN, Bergemann T, Fagan DH, et al. Permanent His-bundle pacing: a systematic literature review and meta-analysis. EP Europace. 2018 doi: 10.1093/europace/euy058. in press. [DOI] [PubMed] [Google Scholar]
- 5.Vijayaraman P, Chung MK, Dandamudi G, Upadhyay GA, Krishnan K, Crossley G, Bova Campbell K, Lee BK, Refaat MM, Saksena S, Fisher JD, et al. His Bundle Pacing. Journal of the American College of Cardiology. 2018;72:927–947. doi: 10.1016/j.jacc.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Vijayaraman P, Dandamudi G, Zanon F, Sharma PS, Tung R, Huang W, Koneru J, Tada H, Ellenbogen KA, Lustgarten DL. Permanent His bundle pacing: Recommendations from a Multicenter His Bundle Pacing Collaborative Working Group for standardization of definitions, implant measurements, and follow-up. Heart Rhythm. 2018;15:460–468. doi: 10.1016/j.hrthm.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Lustgarten DL, Crespo EM, Arkhipova-Jenkins I, Lobel R, Winget J, Koehler J, Liberman E, Sheldon T. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart Rhythm. 2015;12:1548–57. doi: 10.1016/j.hrthm.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 8.Keene D, Arnold A, Shun-Shin MJ, Howard JP, Sohaib SA, Moore P, Tanner M, Quereshi N, Muthumala A, Chandresekeran B, Foley P, et al. Rationale and design of the randomized multicentre His Optimized Pacing Evaluated for Heart Failure (HOPE-HF) trial. ESC Heart Fail. 2018 doi: 10.1002/ehf2.12315. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padeletti L, Pieragnoli P, Ricciardi G, Innocenti L, Checchi L, Padeletti M, Michelucci A, Picariello F, Valsecchi S. Simultaneous His Bundle and Left Ventricular Pacing for Optimal Cardiac Resynchronization Therapy Delivery: Acute Hemodynamic Assessment by Pressure-Volume Loops. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003793. [DOI] [PubMed] [Google Scholar]
- 10.Vijayaraman P, Ellenbogen KA, Gajek J. His-OpTimized Cardiac Resnychronization Therapy (HOT-CRT): A novel approach to enhance CRT response. Heart Rhythm Annual Scientific Sessions. 2018 Abstract AB16-03. [Google Scholar]
- 11.Bernstein AD, Daubert JC, Fletcher RD, Hayes DL, Luderitz B, Reynolds DW, Schoenfeld MH, Sutton R. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group. Pacing and clinical electrophysiology : PACE. 2002;25:260–4. doi: 10.1046/j.1460-9592.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 12.Su L, Xu L, Wu SJ, Huang WJ. Pacing and sensing optimization of permanent His-bundle pacing in cardiac resynchronization therapy/implantable cardioverter defibrillators patients: value of integrated bipolar configuration. Europace. 2016;18:1399–405. doi: 10.1093/europace/euv306. [DOI] [PubMed] [Google Scholar]
- 13.Zanon F, Marcantoni L, Pastore G, Baracca E, Picariello C, Lanza D, Giatti S, Aggio S, Conte L, K'Elia K, Roncon L, et al. Hisian pacing with apical back-up on demand is safe and effective (abstract). Paper presented at: Annual congress of the European Heart Rhythm Association; Barcelona. 2018. [Google Scholar]
- 14.Padala SK, Ellenbogen KA, Koneru JN. Intermittent loss of capture in a His bundle pacemaker: What is the cause? HeartRhythm Case Rep. 2017;3:555–558. doi: 10.1016/j.hrcr.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]