Abstract
The development of technologies for the stable genetic transformation of plastid (chloroplast) genomes has been a boon to both basic and applied research. However, the extension of the transplastomic technology to major crops and model plants has proven extremely challenging, and the species range of plastid transformation is still very much limited in that most species currently remain recalcitrant to plastid genome engineering. Here we report an efficient plastid transformation technology for the model plant Arabidopsis thaliana that relies on root-derived microcalli as source tissue for biolistic transformation. The method produces fertile transplastomic plants at high frequency when combined with a CRISPR/Cas9-generated knock-out allele of a nuclear locus that enhances sensitivity to the selection agent used for isolation of transplastomic events. Our work makes the model organism of plant biology amenable to routine engineering of the plastid genome, facilitates the combination of plastid engineering with the power of Arabidopsis nuclear genetics, and informs the future development of plastid transformation protocols for other recalcitrant species.
Stable transformation of chloroplast genomes in the unicellular green alga Chlamydomonas reinhardtii and the seed plant tobacco (Nicotiana tabacum) was achieved more than 25 years ago1,2. Since then, the technology has proven highly valuable in both basic research and biotechnology3–6. It paved the way to the study of all steps in plastid gene expression and their regulation in vivo, facilitated the systematic investigation of the functions of chloroplast genes and open reading frames by reverse genetics7,8 and enabled the experimental reconstruction of key processes in genome evolution9–11. In addition, the transplastomic technology opened up new applications in metabolic engineering12, molecular farming13,14 and resistance engineering15,16.
Given the transformative nature of the technology in so many research areas, enormous efforts have been made in both the academic and the commercial sectors to extend the chloroplast transformation technology to other algae and plants, most importantly model species and agriculturally important crops3,5,17. However, extending the species range of the technology has proven extremely difficult, and over the past 25 years, only very few species could be added to the list of transformable plants. These include a few solanaceous species18,19, lettuce20 and poplar21. What all these plants have in common is that they display favorable properties in in vitro culture and are relatively easy to regenerate. Thus, while biolistic transformation provides a universal, species-independent method for the introduction of foreign DNA into plastids, the efficient selection of transplastomic events and their regeneration into fertile plants represents the major obstacle to the expansion of the species range of the transplastomic technology.
For the above reasons, plastid transformation has proven to be a serious challenge also in the model system of plant biology, Arabidopsis thaliana, a member of the mustard family (Brassicaceae). While the production of plastid-transformed Arabidopsis cells by biolistic bombardment of leaves was accomplished as early as in 1998 (ref. 22), the regenerated plants were male and female sterile and thus, could not be maintained. Recent work has made the generation of transplastomic Arabidopsis cells more efficient23, but has not solved the fertility problem24. This is unsurprising, given that the nuclei of Arabidopsis leaf cells are highly polyploid, with the average ploidy level in mature rosette leaves being 13C (ref. 25). It is for this reason that all methods that have been routinely used for Arabidopsis nuclear transformation rely on non-leafy source tissues (agroinfection of roots, vacuum infiltration of flowers, floral dip).
Here we report the development of an efficient plastid transformation protocol for Arabidopsis. The system uses microcallus material derived from roots as source tissue and, when combined with the knock-out of a nuclear locus that determines sensitivity to the selection agent spectinomycin26,27, produces fertile transplastomic plants at high frequency. Our work makes Arabidopsis amenable to routine engineering of the plastid genome, opens up the possibility to combine the power of Arabidopsis nuclear genetics with chloroplast genome manipulations, and likely will enable new synthetic biology applications in Arabidopsis chloroplasts28.
Results
A root-based tissue culture and selection system for Arabidopsis plastid transformation
We reasoned that the problem with obtaining fertile transplastomic Arabidopsis plants can only be overcome by the use of a source tissue for transformation that readily regenerates and is largely diploid. Regeneration from root tissue initiates from the pericycle, a one-layer cylinder of cells separating the endodermis from the stele. The pericycle cells are meristematic, largely diploid and, in intact plants, play a key role in the initiation of lateral roots29. Protocols for nuclear transformation of Arabidopsis root tissue had been developed30 before vacuum infiltration and floral dip obviated the need for tissue culture in Arabidopsis nuclear transgenesis 25 years ago.
To optimize root regeneration for Arabidopsis chloroplast transformation, we chose Arabidopsis thaliana C24, a standard ecotype that is widely used, for example, in research on abiotic and biotic stresses31, and in studies on the molecular and physiological basis of heterosis32. We revived the protocols for nuclear transformation of Arabidopsis roots30, and modified them for biolistic transformation and spectinomycin selection of transplastomic cells (see Methods; Supplementary Figs. 1-3; Fig. 1). We used roots harvested from a lawn of young seedlings raised on synthetic medium as starting material (Supplementary Fig. 1). Alterations in the hormone composition (i.e., reduction of the concentration of 2-isopentenyladenine to 2 mg/L and inclusion of the growth-promoting peptide hormone phytosulfokine; see Methods) improved the general responsiveness of the root-derived microcallus tissue that was used as source material for transformation experiments to shoot induction and plant regeneration (Supplementary Fig. 2). Nuclear transformation experiments with standard vectors containing the kanamycin resistance gene nptII as selectable marker were conducted to optimize the parameters of the biolistic bombardment and the selection and regeneration conditions (see Methods; Supplementary Fig. 3). The optimized system produced nuclear transgenic lines at high frequency (on average 5 to 10 transgenic lines per bombarded sample; Supplementary Fig. 3).
Fig. 1.
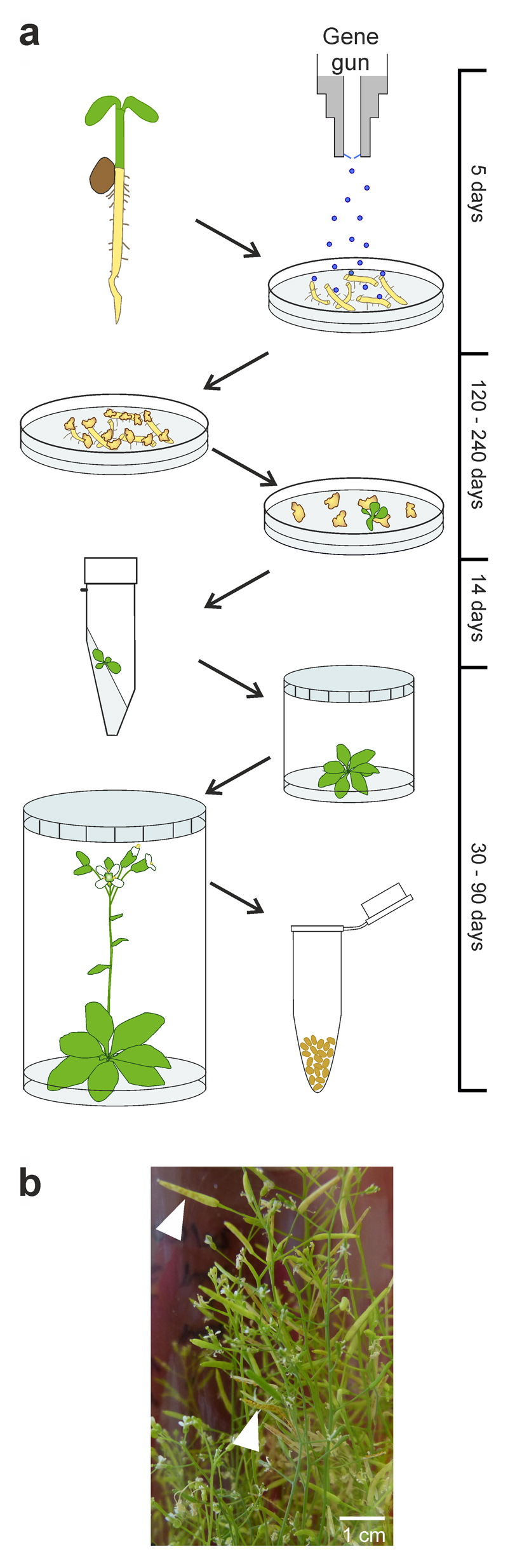
Biolistic nuclear and plastid transformation of Arabidopsis thaliana. (a) Workflow of transformation experiments. Microcalli are induced from root tissue (6 days after germination) harvested from seedlings raised on synthetic medium. Biolistic bombardment is conducted after 5 days of incubation on callus-inducing medium and followed by selection for kanamycin resistance (nuclear transformation) or spectinomycin resistance (chloroplast transformation). Resistant shoots are rooted on agar slants and plants are grown to maturity in sterile containers. A timeline indicating the approximate duration of the individual steps in the protocol is given at the right. See text and Supplementary Figs. 1-4 and 6-8 for details. (b) Fertility of regenerated Arabidopsis plants. The plants are fertile and produce large amounts of seeds. Two ripe siliques in which the seeds can be seen are indicated by white arrowheads. These experiments were repeated independently for 22 transplastomic lines with similar results.
Chimeric aadA genes that confer resistance to spectinomycin represent the standard selectable marker gene for transformation of the chloroplast genome33,5. Antibiotic sensitivity tests revealed that Arabidopsis cells are much more sensitive to spectinomycin than tobacco cells and bleached out completely when exposed to concentrations as low as 5 µg/mL spectinomycin. However, consistent with previous findings34,26,27, we also noted that the bleached cells displayed a remarkable capacity to continue to proliferate in the presence of spectinomycin (Supplementary Fig. 4). Spectinomycin concentrations of 10-50 µg/mL were found to suppress growth of untransformed callus reasonably well and were, therefore, used for our initial sets of plastid transformation experiments. However, full suppression of background growth turned out to be not possible (Supplementary Fig. 4).
Vector development and Arabidopsis chloroplast transformation
Based on previously developed vectors for plastid transformation of solanaceous species18, a set of vectors for transformation of the chloroplast genome of Arabidopsis was constructed. To this end, the homologous region from the Arabidopsis plastid genome was cloned (Fig. 2a). Initially, both chimeric aadA cassettes and aphA-6 cassettes were tested as selectable markers (Table 1). The aadA gene confers resistance to spectinomycin33, whereas aphA-6 confers resistance to kanamycin35. Since plastid gene expression is generally much less active in non-green tissues than in photosynthetically active leaf tissue36–38, we also constructed marker cassettes with expression signals that had been optimized for high-level expression in root plastids39. In these vectors (Table 1; Fig. 2a), the marker gene is driven by the promoter and 5’ UTR from the plastid clpP gene that were further enhanced by inclusion of the strong Shine-Dalgarno sequence from the gene10 leader sequence of bacteriophage T7 (refs. 39–41). To further optimize marker expression, we also constructed an aadA expression cassette with a synthetic codon-optimized aadA gene. In this cassette, the rrnB terminator sequence (TrrnB) from Escherichia coli was used as 3’ UTR (Fig. 2a). This sequence was reported to confer higher transcript stability than plastid 3’ UTRs (ref. 42). As visual reporters of transgene expression, chimeric yfp and gfp cassettes were additionally incorporated into some vectors (Fig. 2a; Table 1).
Fig. 2.
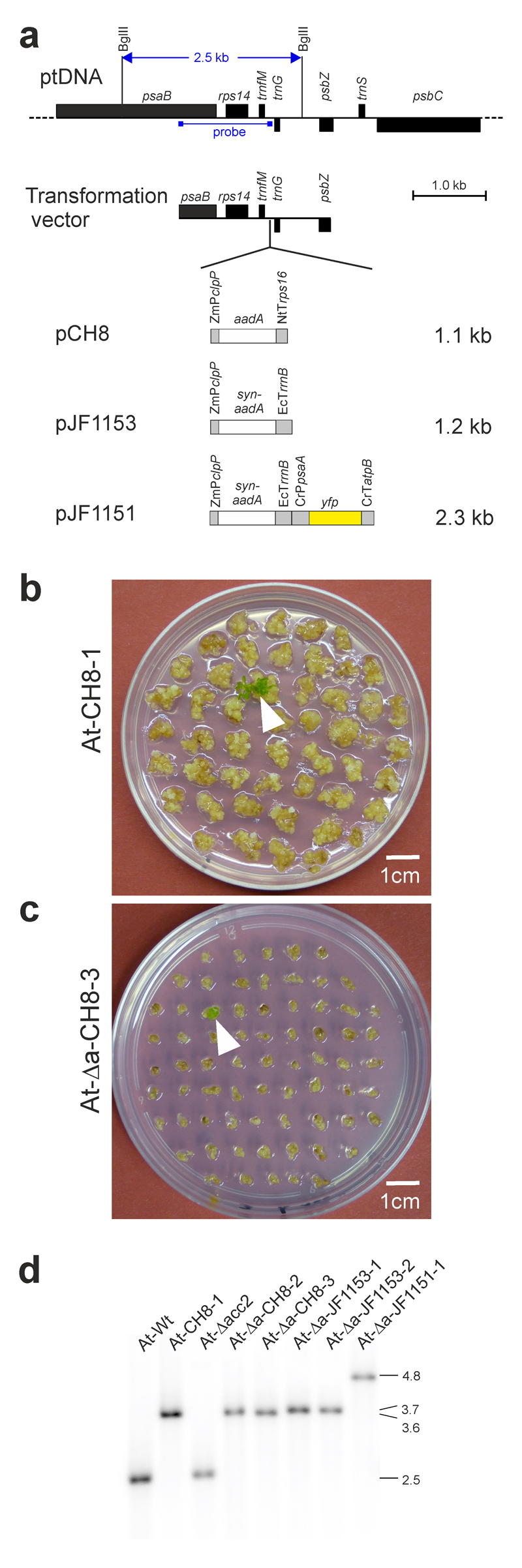
Construction of plastid transformation vectors and selection of transplastomic Arabidopsis plants. (a) Physical map of the targeting region in the plastid genome (ptDNA) of Arabidopsis and plastid transformation vectors. Genes above the line are transcribed from left to right, genes below the line are transcribed from the opposite strand of the ptDNA. The transgenes are inserted into the intergenic spacer between the trnfM and trnG genes within a cloned ptDNA fragment18 (Transformation vector). The location of two BglII restriction sites that were used for Southern blot analysis and the binding site of the hybridization probe is also indicated. The sizes of the transgene cassettes in the three vectors are given in kb. ZmPclpP: promoter of the plastid clpP gene from Zea mays (with the clpP 5’ UTR and the G10-derived Shine-Dalgarno sequence form phage T7; ref. 39); aadA: aadA gene from E. coli; syn-aadA: synthetic codon-optimized aadA gene; NtTrps16: 3’ UTR from the tobacco plastid rps16 gene; EcTrrnB: rRNA operon terminator from E. coli; CrPpsaA: promoter of the plastid psaA gene from Chlamydomonas reinhardtii; CrTatpB: 3’ UTR of the plastid atpB gene from C. reinhardtii. (b) Selection of a transplastomic line (white arrowhead) following bombardment of wild-type tissue with vector pCH8. These transformation experiments were repeated independently 507 times (cf. Table 1), and resulted in similar background growth of the bombarded calli. (c) Selection of a transplastomic line (white arrowhead) after bombardment of acc2 knock-out tissue (At-Δacc2) with vector pCH8. Note the much more efficient suppression of background callus growth from the acc2 knock-out tissue. For additional images, see Supplementary Figs. 4 and 6. The transformation experiments with the At-Δacc2 recipient line and vector pCH8 were repeated independently 98 times with similar results. (d) Southern blot analysis of transplastomic Arabidopsis lines. Total DNA extracted from regenerated plants growing under aseptic conditions was digested with BglII, separated by agarose gel electrophoresis and hybridized to a radiolabelled probe (cf. panel a). The sizes of hybridizing fragments are indicated in kb at the right. These experiments were repeated independently three times with similar results.
Table 1.
Summary of transformation experiments conducted in Arabidopsis thaliana. Nt: Nicotiana tabacum; P: promoter; T: 3’ UTR; G10: gene10 leader from bacteriophage T7; Cr: Chlamydomonas reinhardtii; Zm: Zea mays; Ec: Escherichia coli.
| Vector | Transgene cassettes | Selection | Recipient | Bombarded plates | Resistant lines | Resistant lines analyzed | Confirmed transplastomic lines | Spontaneous mutants | Lines grown to maturity | Lines with seeds obtained |
|---|---|---|---|---|---|---|---|---|---|---|
| pSTS5 | NtPrrn:rbcL::aadA::NtTpsbA | spec | WT | 60 | 0 | 0 | 0 | 0 | ||
| pSTS7 | NtPrrn:G10::aadA::NtTpsbA | spec | WT | 155 | 0 | 0 | 0 | 0 | ||
| pSTS10 | NtPrrn:rbcL::aadA::NtTpsbA CrPrrnG10:gfp::CrTatpA | spec | WT | 92 | 0 | 0 | 0 | 0 | ||
| pSTS12 | NtPrrn:G10::aadA::NtTpsbA CrPrrnG10:gfp::CrTatpA | spec | WT | 90 | 1 | 1 | 0 | 1 | ||
| pSTS13 | NtPrrn:G10::aphA-6::NtTpsbA CrPrrnG10:gfp::CrTatpA | kan | WT | 192 | 0 | 0 | 0 | 0 | ||
| pCH7 | ZmPclpP:G10::aphA-6::NtTrps16 | kan | WT | 259 | 0 | 0 | 0 | 0 | ||
| pCH8 | ZmPclpP:G10::aadA::NtTrps16 | spec | WT | 507 | 1 | 1 | 1 | 0 | 1 | 1 |
| pCH8 | ZmPclpP:G10::aadA::NtTrps16 | spec | Δacc2 | 98 | 43 | 30 | 29 | 1 | 8 | 6 |
| pJF1153 | ZmPclpP:G10::synaadA::EcTrrnB | spec | Δacc2 | 141 | 43 | 34 | 30 | 4 | 14 | 12 |
| pJF1151 | ZmPclpP:G10::synaadA::EcTrrnB CrPpsbA::yfp::CrTatpB | spec | Δacc2 | 18 | 6 | 5 | 5 | 0 | 3 | 3 |
Large-scale plastid transformation experiments were conducted using the biolistic method and our optimized root-based regeneration system combined with either spectinomycin or kanamycin selection. Testing seven different vectors and performing more than 1350 biolistic bombardments (Table 1), a single transplastomic event was obtained (Fig. 2b; Supplementary Fig. 4). The line was obtained with vector pCH8 that carries the spectinomycin resistance gene aadA driven by the clpP-derived expression signals optimized for expression in non-green tissues39. This promoter-marker gene combination was, therefore, used in all subsequent transformation experiments.
The transplastomic line At-CH8-1 was characterized by Southern blot analysis (Fig. 2), which confirmed its transplastomic status and revealed homoplasmy (i.e., the absence of residual copies of the wild-type plastid genome). The line could be readily regenerated into fertile plants that produced abundant seeds (Fig. 1b), suggesting that our root-based tissue culture and regeneration protocol may solve the plant sterility problem in Arabidopsis chloroplast transformation.
Generation of acc2 knock-out alleles by CRISPR/Cas9 editing
We reasoned that the very low efficiency at which transplastomic events could be obtained was due to the inefficiency of selection (see above) and the rampant growth of untransformed callus material which likely prevented the successful outgrowth of transplastomic cell lines (Fig. 2b; Supplementary Fig. 4). Recent work has revealed that the sensitivity of Arabidopsis to inhibition of chloroplast translation is largely determined by the nuclear ACC2 locus26,27. ACC2 encodes an alternative acetyl-CoA carboxylase (ACCase) that, upon inhibition of expression of the plastid-encoded accD gene, sustains de novo fatty acid biosynthesis, thus promoting cell proliferation in the presence of chloroplast translational inhibitors. Arabidopsis ecotypes that harbor ACC2 alleles with low activity (or null alleles) display enhanced sensitivity to spectinomycin26,27, but do not show favorable properties in tissue culture. Therefore, although they can produce transplastomic cell lines at higher frequencies, they are not helpful for the generation of fertile transplastomic plants23,24.
To improve the recovery rate of transplastomic events in our plastid transformation system, we set out to generate acc2 null alleles in the Arabidopsis thaliana ecotype C24 with the help of the CRISPR/Cas9 system for genome editing43. Several putative knock-out alleles were obtained two of which were characterized in detail (Supplementary Fig. 5). While one allele turned out to be a deletion allele, the other one showed a fragment inversion at the Cas9 cleavage sites in the ACC2 locus (Supplementary Fig. 5b). Tests for inhibition of seedling growth by spectinomycin revealed complete growth arrest in the two genome-edited mutants (while the C24 wild type continued to proliferate), thus strongly suggesting that both alleles represent functional acc2 knock-out alleles (Supplementary Fig. 5c).
To produce suitable (non-transgenic) recipient lines for plastid transformation, the cas9 gene was segregated out and homozygous acc2 knock-out mutants were produced for both alleles. These transgene-free lines (subsequently referred to as At-Δacc2 recipient line) were used for all subsequent plastid transformation experiments. Consistent with previous reports26,27, our acc2 knock-out mutants showed no visible phenotype and their growth and development was indistinguishable from the wild type under all conditions tested. This is unsurprising, given the redundancy of the ACC2 enzyme and the predominant reliance of de novo fatty acid biosynthesis on the heteromeric ACCase in the plastid.
Greatly enhanced plastid transformation efficiency with acc2 recipient lines
We next used root-derived microcalli from the At-Δacc2 line as source material for large-scale plastid transformation experiments (Table 1). Bombardments were conducted with three vectors, all carrying the aadA marker that is controlled by the expression elements optimized for root tissue39: pCH8 harboring the non-codon-optimized aadA, pJF1153 containing the synthetic, codon-optimized aadA, and pJF1151 carrying a yfp gene in addition to the synthetic aadA (Fig. 2a; Table 1). All three vectors produced large numbers of spectinomycin-resistant lines (At-Δa-CH8, At-Δa-JF1151 and At-Δa-JF1153 lines; Table 1), on average one line per three shots. With the exception of a few lines likely representing spontaneous spectinomycin-resistant mutants44, the transplastomic status of all lines analyzed could be confirmed by PCR assays and/or DNA gel blot analyses (Fig. 2d; Table 1). When compared to transformation experiments with the wild type, growth of untransformed callus tissue was much more strongly suppressed by spectinomycin in the At-Δacc2 tissue (Fig. 2b,c; Supplementary Figs. 4 and 6), indicating that the ACC2 gene knock-out is indeed responsible for the greatly improved transformation efficiency (Table 1).
It is important to note that the transformation efficiency (of approximately one transplastomic event per three shots; Table 1) represents the most conservative estimate and, in reality, is likely to be significantly higher. This is because multiple resistant lines appearing from one bombarded sample were counted as a single transplastomic event. This conservative approach was taken, because due to the callus transfer to fresh plates that is involved in the protocol (Supplementary Fig. 6h,i), it cannot be excluded with certainty that, occasionally, a transplastomic event was split into two calli. Given that, if occurring at all, this should be a rather rare event and that we frequently detected more than one transplastomic callus per plate, we believe that we significantly underestimate the transformation frequency.
YFP reporter expression and maternal transgene inheritance
Transplastomic calli could be readily regenerated into plantlets (Supplementary Figs. 6 and 7). Interestingly, all regenerated plants examined were homoplasmic (Fig. 2d), thus obviating the need to conduct the additional cycles of regeneration and selection that normally need to be performed in plastid transformation to eliminate residual copies of the (highly polyploid) wild-type plastid genome. This observation suggests that, in our tissue culture and selection system, transplastomic lines attain the homoplasmic state very quickly, presumably due to the high selection pressure exerted by the antibiotic.
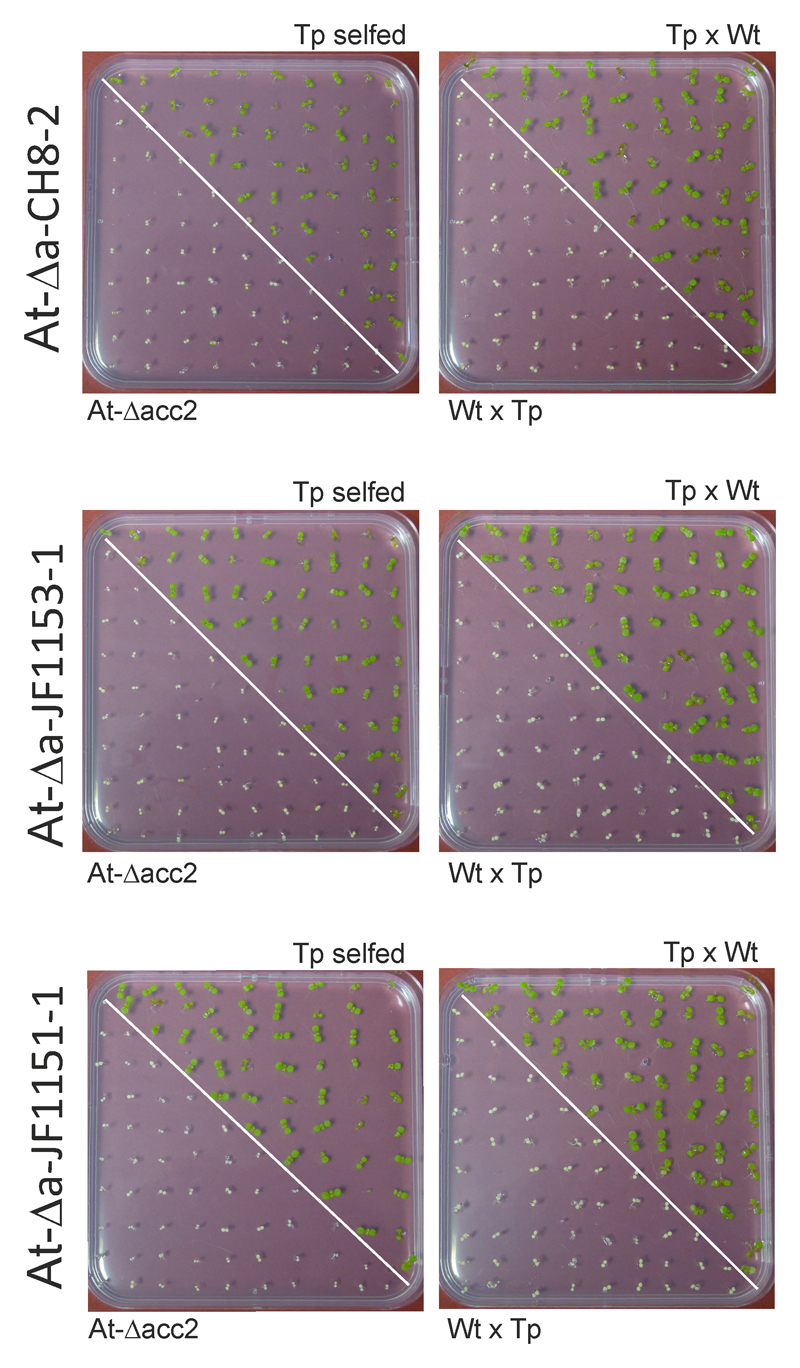
The transplastomic lines flowered readily and produced abundant amounts of seeds (Fig. 1b; Supplementary Figs. 7 and 8). To ultimately confirm homoplasmy, transgene inheritance assays were performed. To this end, the transplastomic lines were selfed and reciprocally crossed to wild-type plants. Germination of seeds on medium with spectinomycin revealed a uniform population of resistant seedlings in the progeny of selfed plants and crosses with the transplastomic plant as maternal parent. By contrast, crosses with the transplastomic plant as paternal parent (and the wild type as maternal parent) yielded a uniform population of antibiotic-sensitive seedlings, in line with the maternal inheritance of the plastid genome44–46.
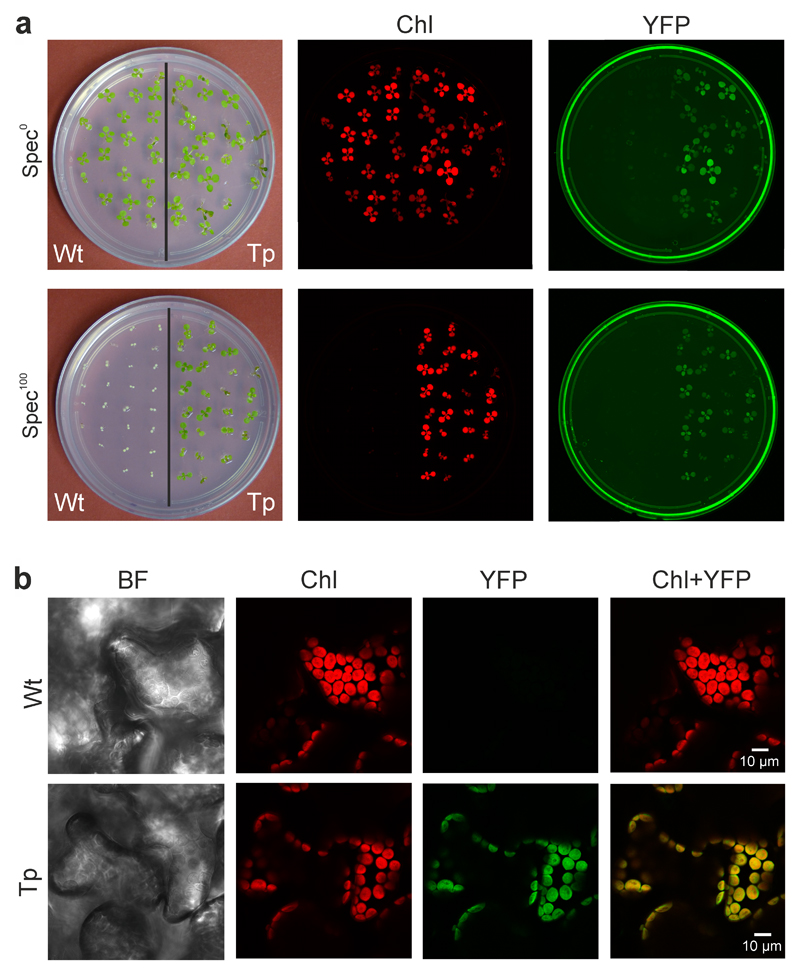
The successful production of transplastomic At-Δa-JF1151 plants, that additionally carry a yfp cassette, enabled us to (i) also test for uniparental inheritance of the fluorescent reporter, and (ii) analyze the subcellular localization of the YFP fluorescence (Fig. 4). The presence of bright YFP fluorescence in chloroplasts (Fig. 4b) and the strong fluorescence of the entire At-Δa-JF1151 seedlings (Fig. 4a) confirmed high-level expression of the reporter protein and its confinement to the chloroplast compartment.
Fig. 4.
Expression of the YFP reporter in transplastomic At-Δa-JF1151 plants. (a) Chlorophyll and YFP fluorescence of wild-type (Wt) and transplastomic (Tp) seedlings grown in the absence (Spec0) or presence (Spec100) of spectinomycin. The plates were scanned with a Typhoon imager that produces a red image of the chlorophyll fluorescence and a green image for the (yellow) YFP fluorescence. The images were taken 20 days after sowing. (b) Confirmation of chloroplast YFP expression in leaf mesophyll cells by confocal laser-scanning microscopy. BF: bright-field image; Chl: chlorophyll fluorescence; YFP: YFP fluorescence (colored in green, to match the color of the Typhoon image); Chl+YFP: overlay of the chlorophyll and YFP fluorescences. These experiments were repeated independently three times with similar results.
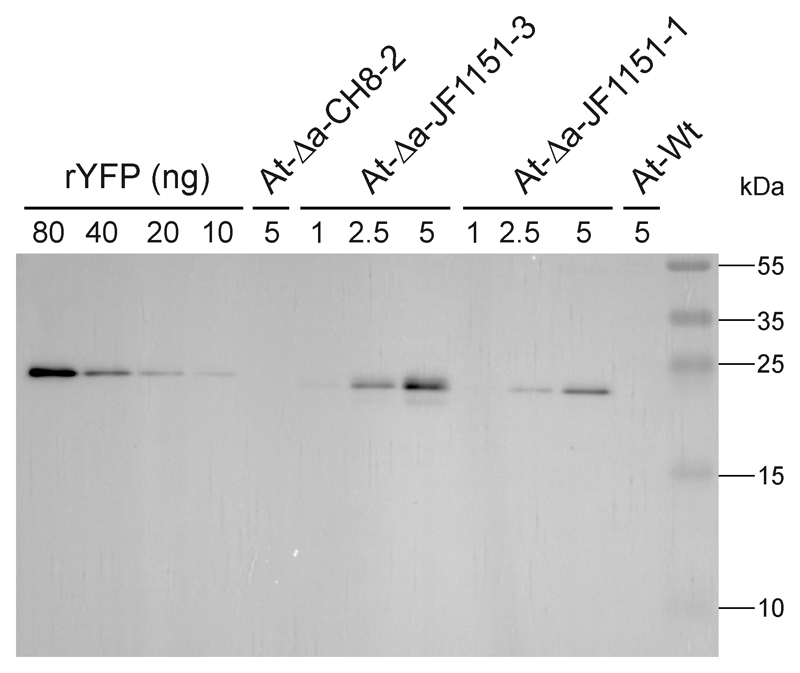
Finally, we performed immunoblot analyses to determine the YFP expression level in the transplastomic lines. Using a dilution series of recombinant YFP as standard, we estimated YFP to accumulate to approximately 1 to 2% of the total soluble protein (Fig. 5).
Fig. 5.
Immunoblot analysis of YFP accumulation in transplastomic Arabidopsis plants. Two independently generated transplastomic At-Δa-JF1151 lines were analyzed. The wild type (At-Wt) and a transplastomic At-Δa-CH8 line were included as negative controls. Samples of total soluble protein extracted from leaves (with the amounts given in µg) were separated by polyacrylamide gel electrophoresis and blotted. A dilution series of YFP purified from Escherichia coli (with the amounts given in ng) was loaded for semiquantitative assessment of protein accumulation in transplastomic plants. Note that the YFP recombinantly expressed in bacteria migrates slightly slower than the YFP in the transplastomic samples. This is due to the presence of a purification tag (His-tag) in the YFP isolated from E. coli. See Methods for further details. These experiments were repeated independently two times with similar results.
Discussion
Our work reported here has overcome the main obstacle that had prevented the extension of the transplastomic technology to the model plant Arabidopsis thaliana. Given that the introduction of foreign DNA into chloroplasts by biolistic transformation solely relies on physical principles and, therefore, occurs in a largely species-independent manner, the development of efficient protocols for the selection and regeneration of fertile transplastomic lines is key to the establishment of plastid transformation in any new species. The inefficiency of regeneration from leaves and the high incidence of (male and female) infertility of the regenerated plants has hampered the development of a transplastomic technology for Arabidopsis, even though transplastomic cells could be readily produced22,23. As noted previously24, the major “challenge is therefore to identify procedures and ecotypes that facilitate conversion of transplastomic callus of Arabidopsis into stable and heritable plant material”.
The key to our success with the establishment of a workable transplastomic protocol for Arabidopsis were (i) the use of a root-based selection and regeneration system that does not suffer from somatic endopolyploidization, and therefore facilitates the efficient regeneration of fertile plants, and (ii) the knock-out of the nuclear ACC2 locus that had been shown previously to determine the tolerance of Arabidopsis cells to spectinomycin26,27, thus facilitating the selection of spectinomycin-resistant cells23. Although we were also able to obtain fertile transplastomic Arabidopsis plants in the wild-type background (Figure 2c), the acc2 knock-out produced by CRISPR/Cas editing greatly enhanced the selection efficiency of transplastomic events (Table 1). This is largely due to suppression of undesired background growth of untransformed callus material that hinders proliferation of transplastomic cells (Supplementary Fig. 4).
Combination of the At-Δacc2 recipient line with our root-based tissue culture and selection system produced transplastomic events at high frequency and solved the infertility problem inherent in leaf-based regeneration systems. The transformation frequency determined (of approximately 1 transplastomic event per 3 shots; Table 1) is by far high enough for routine use of the technology for plastid genome engineering in Arabidopsis. Although the root tissue-based method developed here is more laborious and time consuming than the leaf-based plastid transformation protocol that is useable in the well-established tobacco system, the early attainment of homoplasmy and the nonnecessity to conduct additional regeneration rounds partly offset these extra investments in time and work.
Importantly, the acc2 knock-out alleles produced as recipient lines for chloroplast transformation do not cause any visible phenotype, consistent with the presence of natural null alleles in some Arabidopsis ecotypes26,27. Nonetheless, in certain cases, it may be desirable to obtain transplastomic lines in the ACC2 wild-type background. Taking advantage of the maternal inheritance of plastids44–46, this can be readily achieved by reintroducing the ACC2 allele into transplastomic lines through a simple cross. Similarly, transfer of transgenic plastids to other ecotypes of Arabidopsis can easily be done by crossing.
The development of a workable method for chloroplast genome engineering closes a large gap in the spectrum of technologies available for the model plant Arabidopsis. The technology will be particularly useful to study plastid-nuclear interactions and to set up genetic screens for factors that regulate plastid gene expression at all levels (transcription, RNA processing and turnover, translation, protein stability and assembly). This has not been possible in tobacco, the species currently used for chloroplast transformation experiments, because tobacco is an allotetraploid and lacks sufficiently developed resources for nuclear genetics.
Given the growing interest in using the plastid as a chassis for synthetic biology and synthetic genomics28,47,48 the availability of a transplastomic technology will also enable synthetic biology applications in Arabidopsis. Moreover, the development of an efficient method for biolistic transformation of Arabidopsis will facilitate new transgenic applications in the nucleus, especially multigene engineering approaches such as combinatorial transformation (a large-scale co-transformation approach depending on the introduction of multiple plasmids) that cannot be done by Agrobacterium-mediated transformation methods49,50.
Finally, the protocols developed in the course of this work will inform approaches to develop plastid transformation technology for other species, especially the many plant species that are recalcitrant to efficient selection and regeneration of transgenic and transplastomic cells from leaf explants.
Methods
Plant material, growth conditions and genetic crosses
Arabidopsis thaliana ecotype C24 was used for all experiments. Root material for transformation experiments was harvested from seedlings raised under aseptic conditions from surface-sterilized seeds on synthetic medium (see below) at an average light intensity of 75 µE m-2 s-1. The day length was 12 h (at 20°C) followed by an 12 h dark period at 18°C.
To confirm the homoplasmic state of transplastomic lines and maternal transgene inheritance, transplastomic plants were selfed and reciprocally crossed to wild-type plants. Seeds were harvested and assayed by germination on spectinomycin-containing (100 mg/L) AtGM medium (see below).
Plants in soil were grown under standard greenhouse conditions (16 h daylength, temperature regime: 21°C during the day, 18°C at night) at an average light intensity of 300 µE m-2 s-1.
Construction of plastid transformation vectors
Plasmids pCH8, pJF1151 and pJF1153 (GenBank accession numbers MH590891, MH590893 and MH590894) are all based on pBlueScript II SK (+). The homologous sequences for transformation of the A. thaliana chloroplast genome were inserted between the KpnI and EcoICRI sites. The codon-optimized aadA sequence present in pJF1151 and pJF1153 was chemically synthesized (MWG-Biotech). The three constructs were created by consecutive rounds of classical cloning using type II restriction endonucleases and DNA ligase. The intergenic region between trnfM and trnG was chosen as transgene integration site18 (Fig. 2a) and the corresponding flanking sequences for homologous recombination were amplified from the A. thaliana chloroplast genome (NC_000932.1, nucleotide positions 35,431-36,604 and 36,606-37,895) by PCR. The selectable marker gene cassette (conferring spectinomycin resistance) was generated by combining the maize clpP promoter and 5’ UTR (ref.39), the aadA coding sequence from E. coli (pCH8) or the synthetic version codon optimized for A. thaliana chloroplasts (pJF1151, pJF1153), and the rps16 terminator from N. tabacum51 (pCH8) or the rrnB terminator from E. coli42 (pJF1151, pJF1153; Fig. 2a). The psaA promoter and atpB terminator from the Chlamydomonas reinhardtii chloroplast genome were used for expression of the yfp gene52. Plastid transformation vectors pSTS5, pSTS7, pSTS10, pSTS12, pSTS13 and pCH7 are based on the same vector backbone, and their transgenes and expression elements are listed in Table 1. Vector pSTS5 harbors an aadA cassette developed for tobacco plastids33 and pSTS7 a cassette designed for very high-level aadA expression40. pSTS10 and pSTS12 are derived from pSTS5 and pSTS7, respectively, but additionally contain a gfp cassette. pSTS13 and pCH7 contain an aphA-6 cassette53 conferring kanamycin resistance as selectable marker gene.
Construction of a vector for CRISPR/Cas editing of ACC2
Plasmid pHEE2E-TRI (ref. 43) was ordered from Addgene (plasmid no. 71288; https://www.addgene.org/71288/) and used for cloning of CRISPR/Cas9 vectors. The plasmid harbors a cas9 gene driven by an egg cell-specific promoter, thus minimizing off-target effects while editing the target locus with high efficiency43. To facilitate easy exchange of the gene-specific sgRNA protospacer sequences, an intermediate vector (pJF1031) was constructed in which the sequence between the U6-26 promoter and the second sgRNA scaffold was replaced by two BsaI sites. To this end, the U6-26 promoter from pHEE2E-TRI (flanked by HindIII and AscI sites) was amplified with primers oJF212 and oJF213, and the sgRNA scaffold and the U6-26 terminator from pHEE2E-TRI (flanked by AscI and SpeI sites) were amplified with primers oJF214 and oJF215 (Supplementary Table 1). The PCR products were then digested with HindIII/AscI/SpeI and inserted back into the pHEE2E-TRI backbone cut with HindIII and SpeI. The final plasmid pJF1046 was created by amplifying the sgRNA scaffold, the U6-26 terminator and the U6-29 promoter from pHEE2E-TRI with primers oJF217 and oJF218 (flanked by BsaI sites and adding an sgRNA target sequence at each side). Both protospacers target the Arabidopsis thaliana ACC2 (At1g36180) locus, the sequence of the first one being 5’-CCATGGAGATATATTCGTG-3’, and that of the second one 5’-GTAGTACCCGGTAGAAATG-3’ (Supplementary Fig. 5). The resulting PCR product was cloned into pJF1031 in a simultaneous digestion (BsaI) and ligation reaction. The final transformation vector pJF1046 is identical to pHEE2E-TRI except for the two protospacer sequences. The two anti-ACC2 (At1g36180) sgRNA sequences used to construct pJF1046 were selected to have no potential off-target site in the (sequence-related) upstream ACC1 gene (At1g36160).
Agrobacterium-mediated nuclear transformation of Arabidopsis and genotyping of transgenic plants
Arabidopsis thaliana ecotype C24 plants were transformed by floral dip transformation using Agrobacterium tumefaciens strain GV3101 pmp90 harboring vector pJF1046. Transgenic T1 seedlings were selected on synthetic medium (1/2 MS + 1 % sucrose54) containing 25 mg/L hygromycin.
For genotyping of transgenic lines, the genomic ACC2 sequence surrounding the sgRNA binding sites was amplified with primers oJF324 and oJF325, yielding a PCR product of 752 bp in A. thaliana C24 wild-type plants and a 243 bp product for the deletion allele. To characterize the inversion allele (Supplementary Fig. 5b), primer oJF325 was combined with primer oJF219 amplifying a fragment of 195 bp.
Biolistic transformation and regeneration of transgenic and transplastomic plants
Tissue culture media and regeneration procedures were adapted with modifications from previously published protocols for nuclear transformation of Arabidopsis roots with Agrobacterium30. All chemicals were purchased from Duchefa Biochemie B.V.
Medium AtGM was used for generation of root material for transformation, and contains half-concentrated MS salts54, 1 % sucrose and 0.5 g/L 2-(n-morpholino)ethanesulfonic acid (MES) monohydrate. The pH was adjusted to 5.7, and the medium was solidified with 0.68 % Micro agar. Seeds were germinated on netting (polyester netting PES1 cut to circles of ~80 mm diameter; mesh size 210 µm, A. Hartenstein GmbH, Würzburg, Germany). After harvest (Supplementary Fig. 1), the root tissue was exposed to medium AtCIM1 for microcallus induction in the dark (under a diurnal temperature cycle of 25°C for 16 h and 20°C for 8 h). AtCIM1 consists of Gamborg B5 salts supplemented with McCown woody plant vitamin mixture, 2.2 % glucose monohydrate, 0.5 g/L MES monohydrate, 0.05 mg/L kinetin, 0.5 mg/L 2,4-dichlorophenoxyacetic acid (2,4 D), 30 nM α-phytosulfokine55 (α-PSK). The pH was adjusted to 5.7 and the medium solidified with 0.54 % Daishin agar. Selection of transgenic and transplastomic lines from bombarded tissue was conducted on medium AtSIM3 (in a 16 h light at 25 °C / 8 h dark at 20 °C regime). AtSIM3 consists of Gamborg B5 salts supplemented with McCown Woody plant vitamin mixture, 2 % sucrose, 0.5 g/L MES monohydrate, 0.15 mg/L indole-3-acetic acid (IAA), 2.0 mg/L N6-[2-isopentenyl]adenine (2-iP), and 50 mg/L kanamycin for selection of nuclear transgenic lines or 10-50 mg/L spectinomycin for selection of transplastomic lines (for details, see text and Supplementary Figs. 2-4 and 6). The pH was adjusted to 5.7 and the medium solidified with 0.54 % Daishin agar. For growth of regenerated plantlets and root induction, shoots were transferred to medium AtRIM1 for 14 days (Supplementary Fig. 7). It consists of MS salts supplemented with MS vitamins, 1 % sucrose, 0.5 g/L MES monohydrate, 1 mg/L 3-indolebutyric acid (IBA). The pH was adjusted to 5.7 and the medium solidified with 0.65 % Daishin agar. Plantlets were incubated in a 12 h light/12 h dark regime at 20°C/18°C. For seed production, plants were transferred to medium AtPM1 and grown to maturity (Supplementary Fig. 8). AtPM1 consists of MS salts supplemented with MS vitamins, 1 % sucrose and 0.5 g/L MES monohydrate. The pH was adjusted to 5.7 and the medium solidified with 0.65 % Daishin agar.
Biolistic transformation experiments were performed with a helium-driven particle gun (PDS-1000He; Bio-Rad) using DNA-coated gold particles of 0.6 µm diameter and 1800 psi rupture disks. Samples were bombarded one to three times. For nuclear transformation experiments, vector pGreenII0029 with a gfp transgene driven by CaMV 35S expression elements was used56.
Isolation of nucleic acids and gel blot analyses
Total plant DNA was extracted using a cetyltrimethylammoniumbromide (CTAB)-based method57. For Southern blot analyses, DNA samples were digested with the restriction enzyme BglII, followed by separation of the fragments by electrophoresis in 1% agarose gels and blotting onto Hybond XL membranes (GE Healthcare). A hybridization probe was prepared by PCR amplification with primers oSR2 and oSR1 (Supplementary Table 1) and purification by agarose gel electrophoresis using the NucleoSpin Extract II kit (Macherey-Nagel). Probes were labelled with α[32P]dCTP by random priming (GE Healthcare), and hybridizations were performed at 65°C in Rapid-Hyb buffer (GE Healthcare) according to the manufacturer’s protocol.
Fluorescence imaging and microscopic analyses
To visualize YFP fluorescence of transplastomic seedlings growing in Petri dishes (Fig. 4a), the entire Petri dish was scanned with an Amersham Typhoon RGB Biomolecular Imager (GE Healthcare) using 488 nm laser light for excitation and the Cy2 Filter (525BP20) for detection of YFP fluorescence, and 635 nm laser light for excitation and the Cy5 Filter (670BP30) for detection of chlorophyll fluorescence. The subcellular localization of YFP fluorescence was determined by confocal laser-scanning microscopy (TCS SP5; Leica, Wetzlar, Germany) using an argon laser for excitation (at 514 nm), a 524-566 nm filter for detection of YFP fluorescence and a 650-700 nm filter for detection of chlorophyll fluorescence.
Protein extraction and immunoblot analysis
Total soluble protein from leaf tissue samples was extracted in 250 μL lysis buffer [50 mm HEPES/KOH pH 7.5, 10 mm KAc, 5 mm MgAc, 1 mm EDTA, 1 mm DTT, 1× protease inhibitor cocktail cOmplete (Roche)]. Gel electrophoretic separation and immunoblot analysis were done according to standard protocols using a dilution series of recombinantly expressed YFP (purified from Escherichia coli) for semiquantitative assessment of protein accumulation52. A commercial anti-GFP antibody (Living Colors® A.v. Monoclonal Antibody JL-8, TaKaRa Bio, Clontech Laboratories) was used for detection.
Supplementary Material
Fig. 3.
Homoplasmy of transplastomic lines obtained with vectors pCH8, pJF1153 and pJF1151, and demonstration of maternal transgene inheritance. The left plates show the comparison between the progenies of the selfed transplastomic line (Tp) and the selfed At-Δacc2 recipient line used for transformation. The right plates show the progenies of reciprocal crosses between the wild type (Wt) and the transplastomic line. Seeds were germinated in the presence of 100 mg/L spectinomycin. These assays were repeated independently two times with similar results.
Acknowledgements
We thank Marta M. Bednarska, Carolin Runge and Margit Rößner for excellent technical assistance, Katrin Kiemel and Regina Narawitz for media preparation and help with tissue culture, Dennis Kleinschmidt for microscopy, and Drs. Jiang Zhang and Daniel Karcher for help with vector design. This research was financed by the Max Planck Society and a grant from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (ERC-ADG-2014; grant agreement 669982) to R.B.
Footnotes
Data availability
The data supporting the findings of this study are available within the paper and its supplementary information files. Annotated sequences of plastid transformation vectors pCH8, pJF1151 and pJF1153 were deposited in GenBank (accession numbers MH590891, MH590893 and MH590894).
Author contributions
S.R., J.F. and R.B. designed the research. C.H., X.K., S.S., A.S., L.S. and J.F. performed the experiments. All authors participated in data evaluation. R.B. wrote the manuscript, with input from S.R. and J.F.
Competing interests
The authors declare no competing interests.
References
- 1.Boynton JE, Gillham NW, Harris EH, Hosler JP, Johnson AM, Jones AR, Randolph-Anderson BL, Robertson D, Klein TM, Shark KB, Sanford JC. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988;240:1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- 2.Svab Z, Hajdukiewicz P, Maliga P. Stable transformation of plastids in higher plants. Proc Natl Acad Sci USA. 1990;87:8526–8530. doi: 10.1073/pnas.87.21.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maliga P. Plastid transformation in higher plants. Annu Rev Plant Biol. 2004;55:289–313. doi: 10.1146/annurev.arplant.55.031903.141633. [DOI] [PubMed] [Google Scholar]
- 4.Maliga P, Bock R. Plastid biotechnology: food, fuel, and medicine for the 21st century. Plant Physiol. 2011;155:1501–1510. doi: 10.1104/pp.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock R. Engineering plastid genomes: Methods, tools, and applications in basic research and biotechnology. Annu Rev Plant Biol. 2015;66:211–241. doi: 10.1146/annurev-arplant-050213-040212. [DOI] [PubMed] [Google Scholar]
- 6.Fuentes P, Armarego-Marriott T, Bock R. Plastid transformation and its application in metabolic engineering. Curr Op Biotechnol. 2018;49:10–15. doi: 10.1016/j.copbio.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi Y, Goldschmidt-Clermont M, Soen S-Y, Franzen LG, Rochaix J-D. Direct chloroplast transformation in Chlamydomonas reinhardtii: insertional inactivation of the psaC gene encoding the iron-sulfur protein destabilizes photosystem I. EMBO J. 1991;10:2033–2040. doi: 10.1002/j.1460-2075.1991.tb07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hager M, Biehler K, Illerhaus J, Ruf S, Bock R. Targeted inactivation of the smallest plastid genome-encoded open reading frame reveals a novel and essential subunit of the cytochrome b6f complex. EMBO J. 1999;18:5834–5842. doi: 10.1093/emboj/18.21.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang CY, Ayliffe MA, Timmis JN. Direct measurement of the transfer rate of chloroplast DNA into the nucleus. Nature. 2003;422:72–76. doi: 10.1038/nature01435. [DOI] [PubMed] [Google Scholar]
- 10.Stegemann S, Bock R. Exchange of genetic material between cells in plant tissue grafts. Science. 2009;324:649–651. doi: 10.1126/science.1170397. [DOI] [PubMed] [Google Scholar]
- 11.Bock R. Witnessing genome evolution: experimental reconstruction of endosymbiotic and horizontal gene transfer. Annu Rev Genet. 2017;51:1–22. doi: 10.1146/annurev-genet-120215-035329. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Rijzaani H, Karcher D, Ruf S, Bock R. Efficient metabolic pathway engineering in transgenic tobacco and tomato plastids with synthetic multigene operons. Proc Natl Acad Sci USA. 2013;110:E623–E632. doi: 10.1073/pnas.1216898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tregoning JS, Nixon P, Kuroda H, Svab Z, Clare S, Bowe F, Fairweather N, Ytterberg J, van Wijk KJ, Dougan G, Maliga P. Expression of tetanus toxin fragment C in tobacco chloroplasts. Nucleic Acids Res. 2003;31:1174–1179. doi: 10.1093/nar/gkg221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bock R, Warzecha H. Solar-powered factories for new vaccines and antibiotics. Trends Biotechnol. 2010;28:246–252. doi: 10.1016/j.tibtech.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 15.McBride KE, Svab Z, Schaaf DJ, Hogan PS, Stalker DM, Maliga P. Amplification of a chimeric Bacillus gene in chloroplasts leads to an extraordinary level of an insecticidal protein in tobacco. Bio/Technology. 1995;13:362–365. doi: 10.1038/nbt0495-362. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Khan SA, Hasse C, Ruf S, Heckel DG, Bock R. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science. 2015;347:991–994. doi: 10.1126/science.1261680. [DOI] [PubMed] [Google Scholar]
- 17.Bock R. Genetic engineering of the chloroplast: novel tools and new applications. Curr Opin Biotechnol. 2014;26:7–13. doi: 10.1016/j.copbio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Ruf S, Hermann M, Berger IJ, Carrer H, Bock R. Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nature Biotechnol. 2001;19:870–875. doi: 10.1038/nbt0901-870. [DOI] [PubMed] [Google Scholar]
- 19.Sidorov VA, Kasten D, Pang S-Z, Hajdukiewicz PTJ, Staub JM, Nehra NS. Stable chloroplast transformation in potato: use of green fluorescent protein as a plastid marker. Plant J. 1999;19:209–216. doi: 10.1046/j.1365-313x.1999.00508.x. [DOI] [PubMed] [Google Scholar]
- 20.Kanamoto H, Yamashita A, Asao H, Okumura S, Takase H, Hattori M, Yokota A, Tomizawa K-I. Efficient and stable transformation of Lactuca sativa L. cv. Cisco (lettuce) plastids. Transgenic Res. 2006;15:205–217. doi: 10.1007/s11248-005-3997-2. [DOI] [PubMed] [Google Scholar]
- 21.Okumura S, Sawada M, Park YW, Hayashi T, Shimamura M, Takase H, Tomizawa K-I. Transformation of poplar (Populus alba) plastids and expression of foreign proteins in tree chloroplasts. Transgenic Res. 2006;15:637–646. doi: 10.1007/s11248-006-9009-3. [DOI] [PubMed] [Google Scholar]
- 22.Sikdar SR, Serino G, Chaudhuri S, Maliga P. Plastid transformation in Arabidopsis thaliana. Plant Cell Rep. 1998;18:20–24. [Google Scholar]
- 23.Yu Q, Lutz KA, Maliga P. Efficient plastid transformation in Arabidopsis. Plant Physiol. 2017;175:186–193. doi: 10.1104/pp.17.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibberd JM. A major advance in plastid transformation. Plant Physiol. 2017;175:5. doi: 10.1104/pp.17.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preuten T, Cincu E, Fuchs J, Zoschke R, Liere K, Börner T. Fewer genes than organelles: extremely low and variable gene copy numbers in mitochondria of somatic plant cells. Plant J. 2010;64:948–959. doi: 10.1111/j.1365-313X.2010.04389.x. [DOI] [PubMed] [Google Scholar]
- 26.Parker N, Wang Y, Meinke D. Natural variation in sensitivity to a loss of chloroplast translation in Arabidopsis. Plant Physiol. 2014;166:2013–2027. doi: 10.1104/pp.114.249052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker N, Wang Y, Meinke D. Analysis of Arabidopsis accessions hypersensitive to a loss of chloroplast translation. Plant Physiol. 2016;172:1862–1875. doi: 10.1104/pp.16.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharff LB, Bock R. Synthetic biology in plastids. Plant J. 2014;78:783–798. doi: 10.1111/tpj.12356. [DOI] [PubMed] [Google Scholar]
- 29.Ikeuchi M, Ogawa Y, Iwase A, Sugimoto K. Plant regeneration: cellular origins and molecular mechanisms. Development. 2016;143:1442–1451. doi: 10.1242/dev.134668. [DOI] [PubMed] [Google Scholar]
- 30.Weigel D, Glazebrook J. Root transformation of Arabidopsis. CSH Protoc. 2006;7 doi: 10.1101/pdb.prot4671. [DOI] [PubMed] [Google Scholar]
- 31.Bechtold U, Ferguson JN, Mullineaux PM. To defend or to grow: lessons from Arabidopsis C24. J Exp Bot. 2018;69:2809–2821. doi: 10.1093/jxb/ery106. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto R, Taylor JM, Shirasawa S, Peacock WJ, Dennis ES. Heterosis of Arabidopsis hybrids between C24 and Col is associated with increased photosynthesis capacity. Proc Natl Acad Sci USA. 2012;109:7109–7114. doi: 10.1073/pnas.1204464109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svab Z, Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA. 1993;90:913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zubko MK, Day A. Stable albinism induced without mutagenesis: a model for ribosome-free plastid inheritance. Plant J. 1998;15:265–271. doi: 10.1046/j.1365-313x.1998.00195.x. [DOI] [PubMed] [Google Scholar]
- 35.Huang F-C, Klaus SMJ, Herz S, Zou Z, Koop H-U, Golds TJ. Efficient plastid transformation in tobacco using the aphA-6 gene and kanamycin selection. Mol Genet Genomics. 2002;268:19–27. doi: 10.1007/s00438-002-0738-6. [DOI] [PubMed] [Google Scholar]
- 36.Kahlau S, Bock R. Plastid transcriptomics and translatomics of tomato fruit development and chloroplast-to-chromoplast differentiation: Chromoplast gene expression largely serves the production of a single protein. Plant Cell. 2008;20:856–874. doi: 10.1105/tpc.107.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valkov VT, Scotti N, Kahlau S, MacLean D, Grillo S, Gray JC, Bock R, Cardi T. Genome-wide analysis of plastid gene expression in potato leaf chloroplasts and tuber amyloplasts: transcriptional and posttranscriptional control. Plant Physiol. 2009;150:2030–2044. doi: 10.1104/pp.109.140483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caroca R, Howell KA, Hasse C, Ruf S, Bock R. Design of chimeric expression elements that confer high-level gene activity in chromoplasts. Plant J. 2013;73:368–379. doi: 10.1111/tpj.12031. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Ruf S, Hasse C, Childs L, Scharff LB, Bock R. Identification of cis-elements conferring high levels of gene expression in non-green plastids. Plant J. 2012;72:115–128. doi: 10.1111/j.1365-313X.2012.05065.x. [DOI] [PubMed] [Google Scholar]
- 40.Kuroda H, Maliga P. Complementarity of the 16S rRNA penultimate stem with sequences downstream of the AUG destabilizes the plastid mRNAs. Nucleic Acids Res. 2001;29:970–975. doi: 10.1093/nar/29.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye G-N, Hajdukiewicz PTJ, Broyles D, Rodriguez D, Xu CW, Nehra N, Staub JM. Plastid-expressed 5-enolpyruvylshikimate-3-phosphate synthase genes provide high level glyphosate tolerance in tobacco. Plant J. 2001;25:261–270. doi: 10.1046/j.1365-313x.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 42.Tangphatsornruang S, Birch-Machin I, Newell CA, Gray JC. The effect of different 3' untranslated regions on the accumulation and stability of transcripts of a gfp transgene in chloroplasts of transplastomic tobacco. Plant Mol Biol. 2011;76:385–396. doi: 10.1007/s11103-010-9689-1. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z-P, Xing H-L, Dong L, Zhang H-Y, Han C-Y, Wang X-C, Chen Q-J. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 2015;16:144. doi: 10.1186/s13059-015-0715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azhagiri AK, Maliga P. Exceptional paternal inheritance of plastids in Arabidopsis suggests that low-frequency leakage of plastids via pollen may be universal in plants. Plant J. 2007;52:817–823. doi: 10.1111/j.1365-313X.2007.03278.x. [DOI] [PubMed] [Google Scholar]
- 45.Ruf S, Karcher D, Bock R. Determining the transgene containment level provided by chloroplast transformation. Proc Natl Acad Sci USA. 2007;104:6998–7002. doi: 10.1073/pnas.0700008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greiner S, Sobanski J, Bock R. Why are most organelle genomes transmitted maternally? Bioessays. 2014;37:80–94. doi: 10.1002/bies.201400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, Long SP, Moore TA, et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci USA. 2015;112:8529–8536. doi: 10.1073/pnas.1424031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharwood RE. Engineering chloroplasts to improve Rubisco catalysis: prospects for translating improvements into food and fiber crops. New Phytol. 2017;213:494–510. doi: 10.1111/nph.14351. [DOI] [PubMed] [Google Scholar]
- 49.Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, Conesa DP, Ros G, Sandmann G, Capell T, Christou P. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci USA. 2009;106:7762–7767. doi: 10.1073/pnas.0901412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuentes P, Zhou F, Erban A, Karcher D, Kopka J, Bock R. A new synthetic biology approach allows transfer of an entire metabolic pathway from a medicinal plant to a biomass crop. eLife. 2016;5:e13664. doi: 10.7554/eLife.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staub JM, Maliga P. Translation of the psbA mRNA is regulated by light via the 5'-untranslated region in tobacco plastids. Plant J. 1994;6:547–553. doi: 10.1046/j.1365-313x.1994.6040547.x. [DOI] [PubMed] [Google Scholar]
- 52.Barahimipour R, Strenkert D, Neupert J, Schroda M, Merchant SS, Bock R. Dissecting the contributions of GC content and codon usage to gene expression in the model alga Chlamydomonas reinhardtii. Plant J. 2015;84:704–717. doi: 10.1111/tpj.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehrnthaler M, Scharff LB, Fleischmann TT, Hasse C, Ruf S, Bock R. Synthetic lethality in the tobacco plastid ribosome and its rescue at elevated growth temperatures. Plant Cell. 2014;26:765–776. doi: 10.1105/tpc.114.123240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 55.Matsubayashi Y, Goto T, Sakagami Y. Chemical nursing: phytosulfokine improves genetic transformation efficiency by promoting the proliferation of surviving cells on selective media. Plant Cell Rep. 2004;23:155–158. doi: 10.1007/s00299-004-0816-9. [DOI] [PubMed] [Google Scholar]
- 56.Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol. 2000;42:819–832. doi: 10.1023/a:1006496308160. [DOI] [PubMed] [Google Scholar]
- 57.Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.