Abstract
Objective
To investigate T2 mapping as a possible marker for low-grade human articular cartilage lesions during a one-year follow-up, possible changes during the follow-up and compare the reliability and sensitivity of these measurements on high-field (3 T) and ultra-high-field (7 T) MRI scanners.
Design
Twenty-one patients with femoral, tibial and patellar cartilage defect in the knee joint participated in the study. The MRI protocol consisted of morphological, as well as three-dimensional triple-echo steady-state (3D-TESS) T2 mapping sequences with similar parameters at 3T and 7T. Patients were scanned at five time-points up to 12 months. T2 values were evaluated in the lesion and healthy-appearing regions for superficial and deep cartilage zone. The repeated ANOVA was used to determine differences in T2 values at various time points.
Results
A significant decrease in T2 values was observed between baseline and six months in the superficial layer of the lesion in patients at 3 T (decrease from 41.89 ± 9.3 ms to 31.21 ± 7.2 ms, which is a difference of −5.67 ± 2.2 ms (p = 0.031)), and at 12 months in the superficial layer of the lesion in patients at 3 T (decrease from 41.89 ± 9.3 ms to 35.28 ± 4.9 ms, which is a difference of −6.60 ± 4.4 ms (p = 0.044). No significant differences were recorded at 7 T.
Conclusion
The change in T2 values acquired with 3 T 3D-TESS appears to be reflecting subtle changes of cartilage composition in the course of low-grade lesion development. 7 T T2 mapping does not reflect these changes probably due to completely decayed short T2 component.
Keywords: T2 mapping, TESS, Low grade cartilage lesion, 3 T, 7 T
1. Introduction
Cartilage degeneration is a major source of pain and disability in Western societies. Biochemical changes of the extracellular matrix often precede the morphological changes [1]. Thus, early diagnosis of articular cartilage degradation is crucial for successful treatment. However, the detection of low-grade cartilage lesions can be rather challenging. MRI is the modality of choice because of its non-invasive nature, good reliability, and diagnostic power [2–4].
Mature cartilage tissue is characterized by small amount of cells which account only for small volume of hydrated cartilage. Cartilage hydration and the amount various extracellular elements are dependent on age and species. The basic matrix consists of collagen fibers typically oriented in three cartilage layers and glycosaminoglycan molecules responsible for dynamic mechanical properties [5].
There is a strong demand for a noninvasive tool that would enable early diagnosis but also treatment monitoring and benchmark of success for new therapeutic interventions. Such a tool would allow physicians to monitor the patients treated with any treatment options and therapeutic advice to slow down the onset or progression of osteoarthritis (OA) disease. MRI has been previously successfully used for quantification of the cartilage thickness and for volumetry [6], water content [7], as well as proteoglycan and collagen content assessment [8–10]. MRI can also detect focal cartilage disorder that precedes subsequent structural changes of the tissue. The changes in articular cartilage during OA or in acute lesions result in an increased hydrodynamic fluid pressure and increased stress throughout the matrix. This leads to the proteoglycan-collagen matrix degeneration and cartilage tissue loss [11].
Quantitative MRI has shown a great potential to non-invasively detect cartilage tissue alterations, especially with T2 mapping, which is sensitive to collagen matrix anisotropy/organization and water content [9]. There are several methods currently used for T2 mapping in articular cartilage. As a gold standard for T2 mapping, a multi-echo spin echo sequence is usually employed [12,13]. It provides robust, reproducible, and fast T2 mapping; however, its use at ultra-high-fields is compromised by specific-absorption rate (SAR) limitations due to multiple 180° pulses needed for repeated refocusing. The recently introduced technique of 3D Triple-Echo Steady-State (TESS) T2 mapping has been shown a faster and more reproducible alternative to multi-echo spin-echo T2 mapping [14,15]. The reproducibility of T2 mapping with 3D-TESS observed in this study was comparable to the previously published works [16,17]. At ultra-high-field MRI, the advantage of 3D-TESS over multi-echo spin echo is even more prominent since TESS imaging is not prone to specific absorption rate (SAR) issues due to the low optimal excitation flip angles and the derived T2 maps are intrinsically insensitive to transmit field (B1) inhomogeneities. It was shown previously, cartilage tissue has multiple water compartments resulting in multi-component T2 values [18]. Reiter et al. demonstrated in bovine cartilage three components of T2, specifically T2,1 = 2.3 ms, T2,2 = 25.2 ms, and T2,3 = 96.3 ms, with fractions w1 = 6.2%, w2 = 14.5%, and w3 = 79.3%, respectively, using multi-echo spin-echo sequence at 9.4 T [19]. With conventional multi-echo spin-echo the minimal echo time is rarely below 10 ms, however by using TESS T2 mapping with the echo time as short as 5 ms, there are still some residuals from the shortest component which might contribute to the global T2. As this short component is said to be related to bound water molecules, it may bring an interesting information about the cartilage tissue composition. T2* might also provide an interesting information on cartilage composition [20,21], especially because it allows for ultra-short echo times [22,23], however, the previous studies showed this information is not substantially different for T2 [24].
Low-grade cartilage lesions are challenging to diagnose since morphological alterations are often subclinical, but are accompanied by changes in water content and disruption of collagen fibers [25]. As a result, T2 mapping could be a very helpful tool for the initial diagnosis of cartilage lesions as well as for monitoring their subsequent development over time. Several longitudinal studies have used quantitative MRI to describe the cartilage repair process. Krusche-Mandl et al. used quantitative MRI parameters to follow-up patients with autologous chondrocyte transplantation where T2 was the only quantitative parameter that correlated with the modified Lysholm score [26]. A strong correlation between qualitative MRI and clinical score was also found by Salzmann et al.; the correlation with quantitative MR (T2 mapping), on the other hand, was only moderate [27]. However, to establish sensitivity of T2 mapping to cartilage tissue alteration, the natural course of cartilage degradation should be established. To date, no study compared the clinical value of quantitative MRI at different field-strengths in patients with untreated, low-grade cartilage lesions.
Therefore, in this study we investigated 3D-TESS T2 mapping as a possible marker of changes in cartilage status over one year after baseline examination in patients with low-grade cartilage lesions and with risk factors for further cartilage degeneration, such as a meniscal or anterior cruciate ligament tear. The sensitivity and reproducibility of T2 mapping were evaluated at two field-strengths, 3 and 7 Tesla.
2. Methods
2.1. Subjects
The ethics committee of the Medical University of Vienna approved the study protocol (No. 1978/2014) and all patients gave written, informed consent. Twenty-one patients (mean age ± standard deviation, 46.3 ± 11.1 years; 12 males/9 females) were enrolled. All of them had femoral (N = 18), patellar (N = 2), and tibial (N = 1) cartilage defect (s) in the knee joint (ICRS Grade I or II [28,29]). The grading of the lesion was performed according to the ICRS classification system by one radiologist with 26 years of experience in musculoskeletal MR (S.T.), using high-resolution morphological MR scans.
Inclusion criteria, in addition to low-grade cartilage lesions Grade I and II, were risk factors for cartilage disease progression, such as the presence of an ACL tear or meniscal tear. Patients with contraindications to MRI, such as pacemakers, implants, or pregnant patients, were excluded from the study.
2.2. MRI examination
All subjects underwent an MR examination on two MR scanners on the same day: a 3 T Trio (Siemens, Erlangen, Germany) with 8-channel knee coil (Quality Electrodynamics, Mayfield Village, Ohio, USA) and a 7 T whole-body investigational MR scanner (Siemens Healthcare, Germany) with 28-channel knee coil (Quality Electrodynamics, Mayfield Village, Ohio, USA). The examination consisted of two parts: morphological and quantitative. Morphological sequences included sagittal intermediate-weighted turbo-spin echo (sag PD TSE), coronal intermediate-weighted turbo-spin echo (cor PD TSE), and transversal intermediate-weighted turbo-spin echo (tra PD TSE) scans at 3 Tesla and an additional three-dimensional double-echo steady-state sequence (3D-DESS) at 7 Tesla. T2 mapping was performed using 3D-TESS with similar parameters at both field-strengths [15]. The maps were reconstructed online on the scanner using an IceLuva script [30]. All sequence parameters are listed in Table 1. The measurements were repeated at four time points: at baseline (B); 8 days (8D); three months (3 M); and six months (6 M) after the baseline examination. Ten patients were also rescanned at 12 months (12 M) after baseline examination.
Table 1. Sequence parameters used for morphological and quantitative 3D-TESS T2 mapping.
| Field strength→ | 3 T | 7 T | ||||
|---|---|---|---|---|---|---|
| ↓Sequence parameters | sag PD TSE | cor PD TSE | 3D-TESS (T2 mapping) | 3D-DESS | 3D-TESS (T2 mapping) | |
| Image plane | Sagittal | Coronal | Sagittal | Sagittal | Sagittal | |
| Slice thickness | 2.0 mm | 3 mm | 3 mm | 0.5 mm | 3 mm | |
| Slice spacing | 2.4 mm | 3.6 mm | 3 mm | 0.5 mm | 3 mm | |
| Repetition time | 2200 ms | 3030 ms | 11.14 ms | 8.86 ms | 9.76 ms | |
| Echo time | 38 ms | 29 ms | 5.53 ms | 2.55 ms | 5.1 ms | |
| Averages | 1 | 1 | 1 | 1 | 1 | |
| Acquisition matrix | 448 × 403 | 448 × 448 | 320 × 288 | 320 × 320 | 384 × 346 | |
| Field-of-view | 120 × 120 | 150 × 150 | 160 × 160 | 160 × 160 | 143 × 143 | |
| Flip angle | 180° | 180° | 15° | 18° | 15° | |
| Total acquisition time | 6:38 min | 3:01 min | 3:45 min | 3:57 min | 3:48 min | |
| Pixel bandwidth | 170 Hz/px | 140 Hz/px | 445 Hz/px | 347 Hz/px | 501 Hz/px | |
2.3. Image analysis
Regions-of-interest (ROI) were defined by the radiologist using morphological images from both 3 and 7 Tesla with JiveX (Visus, Bochum, Germany) based on pathophysiological appearance of cartilage and subchondral bone [31]. The ROIs were then transferred onto T2 maps using the co-registration feature of RadiAnt (Medixant, Poznan, Poland) to define the corresponding slices. The number of evaluated slices depended on the lesion size, and the aim was to cover a majority of the lesion. In each subject, three locations were selected: 1) cartilage lesion; 2) healthy reference cartilage in a weight-bearing area; and 3) healthy reference cartilage in a non-weight bearing area. In each of the locations, the deep and superficial layers were selected by dividing the cartilage thickness into two equal sections. The selection of ROIs is depicted in Fig. 1.
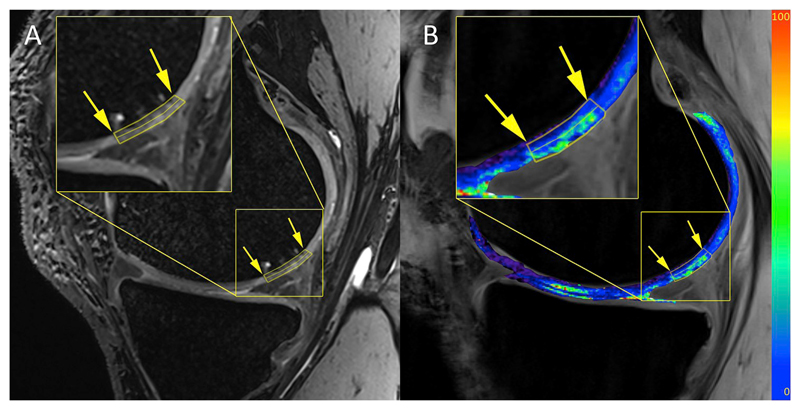
Fig. 1.
An example of T2 maps and a cartilage bilayer segmentation of a patient with a low-grade cartilage lesion acquired at 7 T, A) 3D-DESS image with the lesion delineated by the yellow arrows, B) T2 map overlaid on F0 contrast of a 3D-TESS image. The values of the color bars are T2 in milliseconds. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.4. Reproducibility and inter-observer variability analysis
To assess the reproducibility of T2 mapping with 3D-TESS, all subjects were scanned eight days after the baseline measurement. The difference between T2 values at these two time-points was expressed as a coefficient of variation (CV) in percent for each location and layer separately. Ten subjects were evaluated by three readers to assess the inter-observer variability, calculated as an intra-class coefficient (ICC), at the baseline examination.
2.5. Statistical analysis
A repeated ANOVA test was used to determine the differences in T2 at various time points for 3 and 7 Tesla. The T2 values were compared at baseline with T2 values at 3 M, 6 M, and 12 M, respectively. A P-value < 0.05 was considered statistically significant. All statistical analyses were done using IBM SPSS Statistical Package version 21.0 (IBM, Armonk, North Castle, New York, United States).
3. Results
3.1. Morphological appearance
Morphologically, no change in lesion grade in the time course was found for any patients. The size of the lesions ranged from 23.4 mm3 to 368.1 mm3 (on average, 162.5 ± 107 mm3) and did not change significantly during the observation period.
3.2. Reproducibility and inter-observer variability
The mean coefficient of variation of T2 mapping between the baseline and the eight-day follow-up measurement ranged, in various cartilage regions, from 4.5% to 10.1% at 3 T (on average, 7.8 ± 2.0%) and from 8.9% to 12.8% at 7 T (on average, 11.0 ± 1.5%). The intra-class correlation coefficient between readers ranged from 0.81 to 0.92 at 3 T (on average, 0.85 ± 0.1) and from 0.79 to 0.91 at 7 T (on average, 0.82 ± 0.15).
3.3. T2 mapping
An example of T2 maps is depicted in Fig. 2. In patients with low-grade lesions, the mean T2 values found at 3 T were (mean ± standard deviation) 31.83 ± 10.6, 41.89 ± 9.3, 29.20 ± 7.1, 34.90 ± 7.8, 25.03 ± 5.3 and 36.93 ± 5.5 ms in deep zone, superficial zone, weight-bearing deep reference, weight-bearing superficial reference, non-weight-bearing deep reference, and non-weight-bearing superficial reference, respectively. At 7 T, the values were 25.09 ± 6.9, 31.40 ± 8.6, 21.47 ± 7.0, 23.46 ± 6.0, 23.76 ± 4.7 and 32.32 ± 9.0 ms. All T2 values for the patients with low-grade cartilage lesions are listed in Table 2.
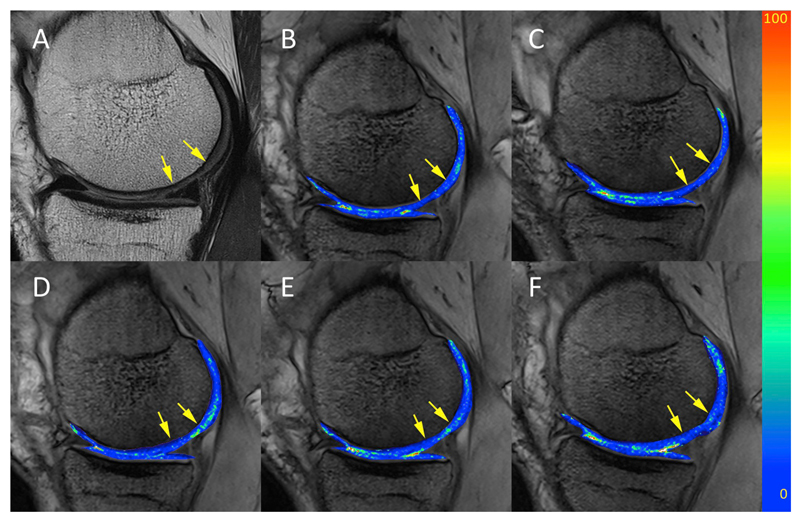
Fig. 2.
A representative T2 maps in a patient with low-grade cartilage lesion scanned at five time-points: A) morphological image; T2 map acquired at B) baseline; C) eight days; D) three months; E) six months; and F) twelve months. T2 maps of the segmented cartilage are overlaid on F0 contrast of the 3D-TESS sequence. The values of the color bars are T2 in milliseconds.
Table 2. T2 values at different time-points and different field-strengths in patients low grade cartilage lesions.
| Time-point | Location | T2 [ms] ± (st.dev.) | Change from baseline | p-values | T2 [ms] ± (st.dev.) | Change from baseline | p-values |
|---|---|---|---|---|---|---|---|
| 3 T | 7 T | ||||||
| Baseline | Lesion deep | 31.83 ± 10.6 | 25.09 ± 6.9 | ||||
| Lesion superficial | 41.89 ± 9.3 | 31.40 ± 8.6 | |||||
| Healthy weight-bearing deep | 29.20 ± 7.1 | 21.47 ± 7.0 | |||||
| Healthy weight-bearing superficial | 34.90 ± 7.8 | 23.46 ± 6.0 | |||||
| Healthy non-weight-bearing deep | 25.03 ± 5.3 | 23.76 ± 4.7 | |||||
| Healthy non-weight-bearing superficial | 36.93 ± 5.5 | 32.32 ± 9.0 | |||||
| Month 3 | Lesion deep | 32.66 ± 9.8 | 0.83 ± −0.8 | 0.487 | 26.84 ± 7.9 | 1.75 ± 1.0 | 0.309 |
| Lesion superficial | 41.81 ± 10.4 | −0.08 ± 1.1 | 0.406 | 34.36 ± 10.0 | 2.96 ± 1.4 | 0.237 | |
| Healthy weight-bearing deep | 28.74 ± 7.0 | −0.47 ± −0.1 | 0.368 | 19.57 ± 3.5 | −1.90 ± −3.5 | 0.343 | |
| Healthy weight-bearing superficial | 34.82 ± 7.2 | −0.08 ± −0.5 | 0.377 | 24.02 ± 2.8 | 0.55 ± −3.2 | 0.211 | |
| Healthy non-weight-bearing deep | 28.44 ± 6.2 | 3.41 ± 0.9 | 0.469 | 21.75 ± 3.7 | −2.01 ± −1.0 | 0.186 | |
| Healthy non-weight-bearing superficial | 37.72 ± 7.3 | 0.79 ± 1.7 | 0.104 | 30.07 ± 6.1 | −2.25 ± −2.9 | 0.066 | |
| Month 6 | Lesion deep | 29.83 ± 7.1 | −2.00 ± −3.5 | 0.200 | 27.21 ± 8.1 | 2.12 ± 1.4 | 0.082 |
| Lesion superficial | 36.21 ± 7.2 | −5.67 ± −2.2* | 0.016 | 30.13 ± 10.3 | −1.27 ± 2.1 | 0.183 | |
| Healthy weight-bearing deep | 28.42 ± 5.2 | −0.78 ± −1.9 | 0.318 | 21.12 ± 5.2 | −0.35 ± −1.8 | 0.340 | |
| Healthy weight-bearing superficial | 34.10 ± 6.2 | −0.80 ± −1.5 | 0.325 | 24.22 ± 3.6 | 0.76 ± −2.4 | 0.454 | |
| Healthy non-weight-bearing deep | 27.09 ± 5.6 | 2.06 ± 0.3 | 0.493 | 24.34 ± 4.2 | 0.58 ± −0.5 | 0.203 | |
| Healthy non-weight-bearing superficial | 36.80 ± 5.9 | −0.14 ± 0.4 | 0.221 | 30.87 ± 3.1 | −1.45 ± −5.9 | 0.408 | |
| Month 12 | Lesion deep | 27.31 ± 3.7 | −4.52 ± −6.9 | 0.052 | 21.12 ± 3.4 | −3.97 ± −1.7 | 0.270 |
| Lesion superficial | 35.28 ± 4.9 | −6.60 ± −4.4* | 0.020 | 30.08 ± 4.4 | −1.32 ± −2.1 | 0.416 | |
| Healthy weight-bearing deep | 29.81 ± 3.5 | 0.61 ± −3.6 | 0.088 | 20.42 ± 2.5 | −1.05 ± −4.5 | 0.428 | |
| Healthy weight-bearing superficial | 31.99 ± 2.3 | −2.91 ± −5.5 | 0.365 | 26.01 ± 5.5 | 2.55 ± −0.5 | 0.189 | |
| Healthy non-weight-bearing deep | 27.92 ± 1.4 | 2.90 ± −3.9 | 0.104 | 22.84 ± 3.1 | −0.92 ± −1.6 | 0.048 | |
| Healthy non-weight-bearing superficial | 39.79 ± 2.8 | 2.85 ± −2.7 | 0.201 | 26.42 ± 5.6 | −5.90 ± −3.4 | 0.158 | |
The T2 values are presented as mean values (standard deviation) in milliseconds.
In bold, significantly different from the baseline, p < 0.05.
The significant continuous decrease in T2 values patients with lesions was observed between baseline and six months in the superficial layer of the lesions at 3 T, where the T2 value decreased from 41.89 ± 9.3 ms to 36.21 ± 7.2 ms, which was a difference of 5.67 ± 2.2 ms (p = 0.031). A significant decrease in T2 values was also observed between baseline and twelve months in the superficial layer of the lesions at 3 T, where the T2 value decreased from 41.89 ± 9.3 ms to 35.28 ± 2.3 ms, which was a difference of 6.6 ± 4.4 ms (p = 0.044).
At 7 T, no significant differences were observed for any of the time-points, although the continuous decrease in T2 values of patients with lesions was also observed between baseline and six months in the superficial layer. The T2 value decreased from 31.4 ± 8.6 ms to 30.13 ± 10.3 ms, which was a difference of 1.27 ± −2.1 ms (p = 0.083). A non-significant decrease in T2 values was also observed between baseline and twelve months in the superficial layer, where the T2 value decreased from 31.4 ± 8.6 ms to 30.08 ± 4.4 ms, which was a difference of 1.32 ± −2.1 ms (p = 0.521) (Fig. 3).
Fig. 3.
An example of a patient with a traumatic cartilage lesion on a femoral condyle, where the hyper-intense region became hypo-intense over the course of four time-points, suggesting the disappearance of edema (depicted by the arrowheads). A to D) morphological images acquired by sagittal intermediate-weighted turbo-spin echo at baseline, three months, six and twelve months follow-up, respectively; E to H) the T2 maps acquired with a 3D-TESS sequence at 3 T at the corresponding time-points; and I to L) the T2 maps acquired with a 3D-TESS sequence at 7 T at the corresponding time-points. The values of the color bars are T2 in milliseconds.
4. Discussion
The results of this study showed that the cartilage structure can be detected in patients with low-grade lesions and followed-up over time. The results also suggest that the T2 values at 3 T might be more beneficial for detecting collagen organization than those obtained at 7 T. It is generally accepted that T2 values slightly decrease as the field-strength increases [32]. Indeed, T2 values in this study appeared lower when measured at 7 T compared to 3 T.
Conventional MR sequences typically used for the evaluation of cartilage can identify mostly morphological changes, such as partial- and full-thickness defects, cracks, fissures, and fibrillations; however, they are not capable of detecting any early stage alterations [1]. Recently, there has been an increasing effort to employ quantitative MR approaches to assess the composition of cartilage. These methods are either proteoglycan-specific (sodium imaging [33], dGEMRIC [34]), collagen-specific (T2 and T2* mapping [9]), or a mixture of methods (diffusion-weighted imaging [35], T1ρ mapping [36]). To date, the most often used quantitative MR technique is T2 mapping for its relative uncomplicated use and no requirements for special hardware or for high magnetic field. The T2 value itself provides information about the collagen fiber content and orientation, as well as water distribution. One of the earliest pathologic changes in cartilage degeneration is the elevated matrix permeability, leading to increased water content and faster motion of water molecules resulting in an increased stress on cartilage because the hydrodynamic pressure is not sustained by the matrix [1]. As the T2 value is strongly dependent on collagen orientation, this value decreases from the superficial cartilage toward the deep zone and also varies between condyles and regions within the condyles [37,38]. Based on these assumptions, the reference cartilage in this study was selected in both weight-bearing and non-weight-bearing regions. However, due to potentially confounding magic angle effect, i.e., the T2 dependence on the angle between the fibers and the static magnetic field resulting in a substantial T2 increase at 55°, none of the regions-of-interest were selected in this region.
In this study, a statistically significant decrease in T2 values acquired at 3 T was observed in the superficial zone of the lesion after one year; a decrease in T2 values was observed in the deep zone of the lesion, too, although not a statistically significant decrease. In the degenerative patients, no statistically significant change in T2 over time was recorded. Desrochers et al. studied structural and biochemical changes in degenerated articular cartilage and concluded that the early changes include proteoglycan loss and collagen network disorganization at or near the articular surface [39]. This is in agreement with our results, as we observed substantial T2 alterations predominantly in the superficial zone. Interestingly, T2 values at 7 T did not demonstrate the same trend as seen at 3 T, which might be due to a multi-component T2 relaxation. Previously, several studies have shown a multi-component T2 decay in cartilage. Reiter et al. observed three-component T2 decay in cartilage T(2,1) = 2.3 ms, T(2,2) = 25.2 ms, and T(2,3) = 96.3 ms, with fractions 6.2%, 14.5%, and 79.3%, respectively [19]. In another study, Liu et al. determined only two-components of T2 in the knee cartilage: a short component ranging from 13.6 ms to 22.3 ms and a long component ranging from 63.8 ms to 75.4 ms, using the multicomponent-driven equilibrium, single-shot observation of T1 and T2 (mcDESPOT) [40]. Qian et al. found two components of T2* in articular cartilage, a short component ranging from 1.5 to 3.6 ms in diseased cartilage and from 4.4 to 4.9 ms in healthy cartilage, while the long component had a mixed distribution between the healthy and diseased cartilage, with values ranging from 7.3 to 23.4 ms [21]. In this study, the echo time used in 3D-TESS T2 mapping was 5.53 ms (at 3 T) and 5.1 ms (at 7 T). Thus, assuming the short component to be around 3 ms at 3 T, but much shorter at 7 T, the contribution of the short component to the ‘averaged’ T2 value at 3 T might be much more substantial than that at 7 T at the given echo time. Different outcome at 3 and 7 T might be also caused by different in-plane resolution of TESS (0.5 × 0.5 mm at 3 T vs 0.37 × 0.37 mm at 7 T) leading to increased measurement error at 7 T due to lower signal gain. Also, the contribution of diffusion is higher at 7 T resulting in lower sensitivity of T2 change. Comparing our 3 T results with the previously conducted longitudinal studies, such as [16,17,41], we were not able to observe any changes in the whole cartilage since the follow-up delay was too short to capture the slowly progressing cartilage degeneration.
In our study, 3D-TESS was used rather than conventional multi-echo spin echo (CPMG). The 3D-TESS relaxometry method was introduced to enable fast and accurate T2 and T1 mapping. This sequence has several advantages over the conventional T2 mapping: it is very fast, the data are acquired in a single scan, and the quantification of T2 is markedly insensitive to B1. In addition, due to the low excitation flip angles (in this study 15°), TESS imaging is not affected by SAR constraints making it suited to be applied at ultra-high-field strength. It has also been shown previously that the T2 values acquired with 3D-TESS strongly correlate with those acquired with CPMG sequences, with the same regional and zonal distribution [14]. However, since multi-echo spin-echo techniques like CPMG tend to overestimate T2 due to stimulated echo contributions, 3D-TESS T2 values were found to be generally lower in comparison to CPMG-based T2 quantification [14,15]. For T2 mapping, 3D-TESS uses a thresholding based on the second (F0) contrast images. This allows for a robust cartilage segmentation due to elimination of the partial volume effect from the bone and synovial fluid. A good reproducibility of 3D-TESS T2 mapping was demonstrated in this study: the coefficient of variation between baseline and the eight-day re-test was on average as low as 7.8 ± 2.0% and 11.0 ± 1.5% at 3 T and 7 T, respectively.
This study has some limitations. Not all patients were scanned at all time-points due to either defective coil, drop-outs, or data reconstruction failure. From the total of 21 patients, the total number of full scans (morphological + T2 mapping) at baseline, 8D, 3 M, 6 M, and 12 M was 17, 16, 16, 17, and 8, respectively (3 T) and 20, 20, 18, 18, and 10 (7 T). However, as we pooled all values through the time-points, we consider our results to be valid and conclusive. Second, although 3D-TESS T2 mapping is B1-insensitive by its nature, in some cases of extreme B1 (and/or B0) imperfections, the signal drops so much that the reconstruction could not iterate to reasonable T2 values. These artefacts occurred mostly in outer slices that were not used for the evaluation. In cases where the slices of interest were affected, the pixels with obvious alterations were omitted. These cases, however, were rather rare and did not affect the overall evaluation.
5. Conclusion
3D-TESS T2 mapping is highly sensitive for the detection of the status of low-grade lesions in human articular cartilage and for observing the development of the lesions over time. 3 T T2 mapping appears to be more sensitive to subtle alteration in cartilage tissue due to residuals of short T2 component (usually attributed to bound water molecules) which is mostly decayed at 7 T. In the future, T2 mapping might be a valuable marker for monitoring cartilage development after regenerative therapy. Using 3D-TESS for obtaining T2 values in human articular cartilage demonstrates many benefits in comparison with conventional techniques, i.e., a decrease of the scan time, lower SAR demands and an increase of the reliability and reproducibility of T2 mapping on both high-field and ultra-high-field MR scanners.
Acknowledgements
The authors would like to thank Harry Haber for his help with statistics. This work was supported by the NOVARTIS PHARMA AG PJMR0062112, Austrian Science Fund (FWF) KLI541-B30 and Slovak Grant Agency APVV-15-0029.
References
- [1].Choi JA, Gold GE. MR imaging of articular cartilage physiology. Magn Reson Imaging Clin N Am. 2011;19(2):249–82. doi: 10.1016/j.mric.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mohr A. The value of water-excitation 3D FLASH and fat-saturated PDw TSE MR imaging for detecting and grading articular cartilage lesions of the knee. Skeletal Radiol. 2003;32(7):396–402. doi: 10.1007/s00256-003-0635-z. [DOI] [PubMed] [Google Scholar]
- [3].Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- [4].Springer E, Bohndorf K, Juras V, Szomolanyi P, Zbyn S, Schreiner MM, et al. Comparison of routine knee magnetic resonance imaging at 3 T and 7 T. Invest Radiol. 2017;52(1):42–54. doi: 10.1097/RLI.0000000000000303. [DOI] [PubMed] [Google Scholar]
- [5].McDevitt CA. Biochemistry of articular cartilage. Nature of proteoglycans and collagen of articular cartilage and their role in ageing and in osteoarthrosis. Ann Rheum Dis. 1973;32(4):364–78. doi: 10.1136/ard.32.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cohen ZA, McCarthy DM, Kwak SD, Legrand P, Fogarasi F, Ciaccio EJ, et al. Knee cartilage topography, thickness, and contact areas from MRI: in-vitro calibration and in-vivo measurements. Osteoarthritis Cartilage. 1999;7(1):95–109. doi: 10.1053/joca.1998.0165. [DOI] [PubMed] [Google Scholar]
- [7].Liess C, Lusse S, Karger N, Heller M, Gluer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10(12):907–13. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- [8].Trattnig S, Mlynarik V, Breitenseher M, Huber M, Zembsch A, Rand T, et al. MRI visualization of proteoglycan depletion in articular cartilage via intravenous administration of Gd-DTPA. Magn Reson Imaging. 1999;17(4):577–83. doi: 10.1016/s0730-725x(98)00215-x. [DOI] [PubMed] [Google Scholar]
- [9].Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8(4):355–68. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- [10].Zbyn S, Mlynarik V, Juras V, Szomolanyi P, Trattnig S. Evaluation of cartilage repair and osteoarthritis with sodium MRI. NMR Biomed. 2016;29(2):206–15. doi: 10.1002/nbm.3280. [DOI] [PubMed] [Google Scholar]
- [11].Nissi MJ, Toyras J, Laasanen MS, Rieppo J, Saarakkala S, Lappalainen R, et al. Proteoglycan and collagen sensitive MRI evaluation of normal and degenerated articular cartilage. J Orthop Res. 2004;22(3):557–64. doi: 10.1016/j.orthres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- [12].Mamisch TC, Trattnig S, Quirbach S, Marlovits S, White LM, Welsch GH. Quantitative T2 mapping of knee cartilage: differentiation of healthy control cartilage and cartilage repair tissue in the knee with unloading–initial results. Radiology. 2010;254(3):818–26. doi: 10.1148/radiol.09090335. [DOI] [PubMed] [Google Scholar]
- [13].Roemer FW, Crema MD, Trattnig S, Guermazi A. Advances in imaging of osteoarthritis and cartilage. Radiology. 2011;260(2):332–54. doi: 10.1148/radiol.11101359. [DOI] [PubMed] [Google Scholar]
- [14].Juras V, Bohndorf K, Heule R, Kronnerwetter C, Szomolanyi P, Hager B, et al. A comparison of multi-echo spin-echo and triple-echo steady-state T2 mapping for in vivo evaluation of articular cartilage. Eur Radiol. 2016;26(6):1905–12. doi: 10.1007/s00330-015-3979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Heule R, Ganter C, Bieri O. Triple echo steady-state (TESS) relaxometry. Magn Reson Med. 2014;71(1):230–7. doi: 10.1002/mrm.24659. [DOI] [PubMed] [Google Scholar]
- [16].Lin W, Alizai H, Joseph GB, Srikhum W, Nevitt MC, Lynch JA, et al. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2013;21(10):1558–66. doi: 10.1016/j.joca.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pan JD, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the Normal control cohort from the osteoarthritis initiative. Radiology. 2011;261(2):507–15. doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Henkelman RM, Stanisz GJ, Kim JK, Bronskill MJ. Anisotropy of NMR properties of tissues. Magn Reson Med. 1994;32(5):592–601. doi: 10.1002/mrm.1910320508. [DOI] [PubMed] [Google Scholar]
- [19].Reiter DA, Lin PC, Fishbein KW, Spencer RG. Multicomponent T2 relaxation analysis in cartilage. Magn Reson Med. 2009;61(4):803–9. doi: 10.1002/mrm.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mamisch TC, Hughes T, Mosher TJ, Mueller C, Trattnig S, Boesch C, et al. T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skeletal Radiol. 2012;41(3):287–92. doi: 10.1007/s00256-011-1171-x. [DOI] [PubMed] [Google Scholar]
- [21].Qian YX, Williams AA, Chu CR, Boada FE. Multicomponent T-2(star) mapping of knee cartilage: technical feasibility ex vivo. Magn Reson Med. 2010;64(5):1427–32. doi: 10.1002/mrm.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Williams A, Qian Y, Bear D, Chu CR. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthritis Cartilage. 2010;18(4):539–46. doi: 10.1016/j.joca.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chu CR, Williams AA, West RV, Qian Y, Fu FH, Do BH, et al. Quantitative magnetic resonance imaging UTE-T2* mapping of cartilage and meniscus healing after anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(8):1847–56. doi: 10.1177/0363546514532227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Welsch GH, Mamisch TC, Hughes T, Zilkens C, Quirbach S, Scheffler K, et al. In vivo biochemical 7.0 Tesla magnetic resonance: preliminary results of dGEMRIC, zonal T2, and T2* mapping of articular cartilage. Invest Radiol. 2008;43(9):619–26. doi: 10.1097/RLI.0b013e31817e9122. [DOI] [PubMed] [Google Scholar]
- [25].Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- [26].Krusche-Mandl I, Schmitt B, Zak L, Apprich S, Aldrian S, Juras V, et al. Long-term results 8 years after autologous osteochondral transplantation: 7 T gagCEST and sodium magnetic resonance imaging with morphological and clinical correlation. Osteoarthritis Cartilage. 2012;20(5):357–63. doi: 10.1016/j.joca.2012.01.020. [DOI] [PubMed] [Google Scholar]
- [27].Salzmann GM, Erdle B, Porichis S, Uhl M, Ghanem N, Schmal H, et al. Long-term T2 and qualitative MRI morphology after first-generation knee autologous chondrocyte implantation cartilage ultrastructure is not correlated to clinical or qualitative MRI outcome. Am J Sports Med. 2014;42(8):1832–40. doi: 10.1177/0363546514536682. [DOI] [PubMed] [Google Scholar]
- [28].Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–34. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- [29].Brittberg M, Peterson L. Introduction of an articular cartilage classification. ICRS Newsl. 1998;1:5–8. [Google Scholar]
- [30].Santini F, Patil S, Scheffler K. IceLuva: a scripting framework for MR image reconstruction based on free software. Concept Magn Reson B. 2011;39B(1):1–10. [Google Scholar]
- [31].Rodrigues MB, Camanho GL. Mri evaluation of knee cartilage. Rev Bras Ortop. 2010;45(4):340–6. doi: 10.1016/S2255-4971(15)30379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Regatte RR, Schweitzer ME. Ultra-high-field MRI of the musculoskeletal system at 7.0 T. J Magn Reson Imaging. 2007;25(2):262–9. doi: 10.1002/jmri.20814. [DOI] [PubMed] [Google Scholar]
- [33].Reddy R, Insko EK, Noyszewski EA, Dandora R, Kneeland JB, Leigh JS. Sodium MRI of human articular cartilage in vivo. Magn Reson Med. 1998;39(5):697–701. doi: 10.1002/mrm.1910390505. [DOI] [PubMed] [Google Scholar]
- [34].Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [35].Mlynarik V, Sulzbacher I, Bittsansky M, Fuiko R, Trattnig S. Investigation of apparent diffusion constant as an indicator of early degenerative disease in articular cartilage. J Magn Reson Imaging. 2003;17(4):440–4. doi: 10.1002/jmri.10276. [DOI] [PubMed] [Google Scholar]
- [36].Keenan KE, Besier TF, Pauly JM, Han E, Rosenberg J, Smith RL, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage. 2011;19(2):171–9. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14(1):50–5. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- [38].Mosher TJ, Smith HE, Smith MB. In vivo spatial variation in cartilage MRI-T2 values of the knee. Arthritis Rheum. 2000;43(9):S220–S. [Google Scholar]
- [39].Desrochers J, Amrein MW, Matyas JR. Viscoelasticity of the articular cartilage surface in early osteoarthritis. Osteoarthritis Cartilage. 2012;20(5):413–21. doi: 10.1016/j.joca.2012.01.011. [DOI] [PubMed] [Google Scholar]
- [40].Liu F, Chaudhary R, Hurley SA, del Rio AM, Alexander AL, Samsonov A, et al. Rapid multicomponent T2 analysis of the articular cartilage of the human knee joint at 3.0T. J Magn Reson Imaging. 2014;39(5):1191–7. doi: 10.1002/jmri.24290. [DOI] [PubMed] [Google Scholar]
- [41].Wei ZM, Du XK, Huo TL, Li XB, Quan GN, Li TR, et al. Quantitative T2 mapping evaluation for articular cartilage lesions in a rabbit model of anterior cruciate ligament transection osteoarthritis. Chin Med J (Engl) 2012;125(5):843–50. [PubMed] [Google Scholar]