Abstract
Background
Little is known about the epidemiology of β and γ human papillomaviruses (HPVs) in oral cavities of healthy women.
Methods
We performed multiplex polymerase chain reaction analysis for detection of 46 β-HPVs and 51 γ-HPVs in stored oral rinse samples from healthy mid-adult women (age, 30–50 years). A total of 407 women were tested for β-HPVs, and 310 were tested for γ-HPVs. We used log-binomial regression to identify determinants of β-HPV and γ-HPV in separate models. Using paired fingernail data from a subset of 184 women, we also evaluated whether fingernail β-HPV detection was associated with concurrent detection of the same type in the oral cavity.
Results
Oral HPV prevalence was 20.6% for β-HPV and 10.7% for γ-HPV. In multivariate analysis, oral β-HPV detection was associated with increasing age (adjusted prevalence ratio [aPR] per 5-year difference, 1.37; 95% confidence interval [CI], 1.01–1.86) and a greater lifetime number of oral sex partners (aPR for reporting ≥6 vs 0–5 partners, 2.06; 95% CI, 1.01–4.20). In a separate model, concurrent detection of the same β-HPV type in fingernails was strongly associated with oral β-HPV detection (aPR, 31.44; 95% CI, 19.81–49.49). No significant determinants of γ-HPV detection were identified.
Conclusions
Our results suggest a sexual transmission route for β-HPVs and support the hypothesis that fingers may serve as a source of transmission or autoinoculation of β-HPVs to the oral cavity.
Keywords: β-HPV, γ-HPV, oral cavity, mid-adult, women
β– and γ–human papillomaviruses (HPVs) were common in oral samples from healthy mid-adult women. Oral β-HPV detection was associated with increasing age, greater lifetime number of oral sex partners, and concurrent detection of the same β-HPV type in fingernails.
More than 200 types of human papillomavirus (HPV) have been identified, and most are classified into α, β, or γ genera. While α-HPVs are considered mucosal and β- and γ-HPVs cutaneous [1, 2], β- and γ-HPVs have been detected in diverse anatomic sites, including the oral [3, 4] and nasal [5] mucosa, the anus [6–8], and genital [9] and cervical [10] epithelia. While a subset of high-risk α-HPVs are etiologically linked to anogenital and oropharyngeal cancers [11], less is known about β- and γ-HPVs and their potential involvement in carcinogenesis. β-HPVs likely contribute to a portion of skin [12–14] and oropharyngeal [15, 16] cancers through indirect mechanisms that are distinct from those of α-HPVs [17–20]. In addition, in a large nested case-control study, detection of certain β- and γ-HPV types were each positively associated with incident oral and laryngeal squamous cell carcinomas, even after adjustment for HPV-16 (an α type linked to the majority of HPV-positive oropharyngeal cancers), smoking, and alcohol use [21]. Approximately 70% of oropharyngeal cancers (18200 cases; 3400 in women and 14800 in men) in the United States each year are considered to be attributable to HPV infection [22, 23]. However, because most studies have focused exclusively on α-HPVs, the proportion of oropharyngeal cancers that are attributable to HPV may be underestimated [3].
Understanding the epidemiology of β- and γ-HPVs in the oral cavities of healthy individuals is essential to expanding our knowledge of the potential involvement of these types in oral tumorigenesis. To date, there are limited data on the determinants of β- and γ-HPVs in the oral cavity and potential transmission routes [24, 25]. Furthermore, to our knowledge, evaluation of oral β- and/or γ-HPVs has focused primarily on populations of immunosuppressed [3, 26] or older [16, 21] individuals, healthy men [3, 24, 27], and young women aged 20–29 years [25, 27]. A recent population-based study in Hong Kong included a broad age-range of healthy men and women but did not stratify risk factor analyses by age or sex [28].
Our aims were to evaluate prevalence and determinants of β- and γ-HPVs in the oral cavities of healthy mid-adult women. Using stored oral rinse samples, we used multiplex polymerase chain reaction (PCR) assays that covered 46 β-HPV genotypes and 51 γ-HPV genotypes. We used detailed demographic, health, and sexual behavior data to identify predictors of β- and γ-HPV detection in the oral cavity. Finally, we used data on concurrent β-HPV detection in paired fingernail samples to evaluate fingernails as a potential source of transmission of cutaneous HPV types to the oral cavity.
METHODS
Study Population
We used stored samples collected from healthy mid-adult women who were followed between June 2011 and August 2012 in a study of HPV infections. Parent study details have been reported previously [29, 30]. Briefly, we enrolled 409 women aged 30–50 years into a 6-month longitudinal study at the University of Washington. The study protocol included self-collection of oral rinse and fingernail samples for HPV DNA testing and collection of demographic, health, and sexual behavior data via online surveys at enrollment and exit. To collect oral rinse samples, women gargled 10 mL of Scope mouthwash for 30 seconds and expectorated into a collection cup. The sample was centrifuged for 5 minutes and the remaining pellet stored in 1 mL of Qiagen cell lysis solution (Qiagen, Gaithersburg, MD). To collect fingernail samples, women rubbed their fingertips and the underside of the tip of their fingernails on both hands with a cytology brush. The brush was stored in 1 mL of standard transport medium (Qiagen, Gaithersburg, MD). The study protocol was approved by the Institutional Review Board of the University of Washington.
Laboratory Methods
During the parent study, oral rinse and fingernail samples were genotyped for the presence of α-HPV types using the Roche Linear Array assay [30]. Briefly, genomic DNA from the oral lavage cell pellet was isolated using the Gentra Puregene Buccal Cell kit (catalog no. 158845; Qiagen), and genomic DNA from the fingernail samples was isolated using the QIAamp DNA blood mini kit (catalog no. 51104; Qiagen). Specimens were tested for HPV and β-globin; specimens negative for β-globin were considered insufficient. Overall, 2% of oral rinse samples (16 of 789) and 22% of fingernail samples (140 of 649) were insufficient and not considered for additional testing for cutaneous HPV types [30]. Among 407 women with ≥1 sufficient oral rinse sample at enrollment or exit, 1 sample per woman was selected for cutaneous HPV testing. Because the exit survey captured greater detail on potential oral HPV risk factors, exit samples were prioritized for selection. If the exit sample was unavailable or insufficient in quantity, the enrollment sample was selected instead. A total of 32 enrollment samples and 375 exit samples were selected. In addition, paired fingernail samples from a subset of 204 women were selected.
DNA samples were shipped to the International Agency for Research on Cancer in Lyon, France. Samples were tested for the presence of HPVs, using type-specific PCR bead–based multiplex genotyping assays that combine multiplex PCR and Luminex technology (Luminex, Austin, TX), as described elsewhere [24, 27, 31–35]. Owing to resource limitations that precluded β-HPV and γ-HPV testing of all samples, we prioritized β-HPV testing. β-HPV testing was performed on all oral rinse and fingernail samples, and γ-HPV testing was performed on a random subset of 310 oral rinse samples. The multiplex type-specific PCR method uses specific primers for the detection of 46 β-HPVs, from β-species 1 (5, 8, 12, 14, 19, 20, 21, 24, 25, 36, 47, 93, 98, 99,105, 118, 124, 143, and 152), β-species 2 (9, 15, 17, 22, 23, 37, 38, 80, 100, 104, 107, 110, 111, 113, 120, 122, 145, 151, 159, and 174), β-species 3 (49, 75, 76, and 115), β-species 4 (92), and β-species 5 (96 and 150); and 51 γ-HPVs, from γ-species 1 (4, 65, 95, and 173), γ-species 2 (48 and 200), γ-species 3 (50), γ-species 4 (156), γ-species 5 (60, 88), γ-species 6 (101, 103, and 108), γ-species 7 (109, 123, 134, 149, and 170), γ-species 8 (112, 119, 164, and 168), γ-species 9 (116 and 129), γ-species 10 (121, 130, 133, and 180), γ-species 11 (126, 169, 171, and 202), γ-species 12 (127, 132, 148, 165, and 199), γ-species 13 (128), γ-species 14 (131), γ-species 15 (179), γ-species 18 (156), γ-species 19 (161, 162 and 166), γ-species 20 (163), γ-species 21 (167), γ-species 22 (172), γ-species 23 (175), γ-species 24 (178 and 197), γ-species 25 (184), and γ-species 27 (201). Our assays have been extensively validated in a vast number of epidemiological studies [7, 8, 14, 24, 35–38]. The sensitivity has been evaluated using serial dilutions of DNA from HPV types. This multiplex PCR protocol is highly sensitive, with the ability to detect only 10 copies of the viral genome [32]. All of the Luminex bead probes were designed in a very divergent region of the HPV E7 gene to decrease the risk of cross-reactivity. Cloned HPV genomes have been used to evaluate specificity, and no cross-reactivity was observed. We have also evaluated the reproducibility of the assays by retesting specimens. The results showed high reproducibility of the assay, with a concordance rate >90% [27]. Two primers for the amplification of the β-globin gene were included to provide a positive control for the quality of the DNA in the sample [39]. Following multiplex PCR amplification, 10 µL of each reaction mixture was analyzed by multiplex genotyping using the Luminex technology as previously described [31, 34]. Results were expressed as the median fluorescence intensity (MFI) of at least 100 beads per bead set. For each probe, MFIs with no respective PCR product added to the hybridization mixture were considered background values. The cutoff was computed by adding 5 MFI to 1.1 times the median background value. An oral sample was considered positive for a β- or a γ-HPV type when the corresponding MFI was above the cutoff, calculated specifically for each of the β- and γ-HPV probes.
Statistical Analysis
Fingernail β-HPV results were reported previously [37]; in the present study, fingernail results were used to evaluate concordance between oral and fingernail samples for detecting β-HPV and to evaluate concurrent detection of β-HPV in fingernails as a risk factor for β-HPV detection in the oral cavity.
Within each genera (β-HPV or γ-HPV), oral HPV prevalence was calculated for any HPV, multiple-type HPV, species-specific HPV, and type-specific HPV.
We used proportion positive agreement (PPA) and unweighted κ statistics based on PPA with percentile-bootstrapped 95% confidence intervals (CIs), using women-level clustered sampling [40] to describe the concordance of type-specific β-HPV detection between oral and fingernail samples.
We used log-binomial regression to estimate univariate prevalence ratios (PRs) for the associations between selected correlates (age, race, ethnicity, marital status, smoking status, current alcohol consumption, current immunosuppressive condition, genital warts history, lifetime number of male sex partners, sex with new male partners within the previous year, lifetime number of open-mouth kissing partners, lifetime number of oral sex partners, and detection of the same HPV type in a concurrent fingernail sample) and type-specific oral HPV detection. Separate models were constructed for β-HPV and γ-HPV detection. Each woman contributed multiple “woman-types” to each model, equal to the number of HPV types assessed in her oral sample (eg, each woman contributed 46 woman-types to the β-HPV model). Robust variance estimates were used to account for correlation within women due to multiple HPV types assessed in an oral sample. Variables found to be statistically significant in univariate analysis (P < .1) were entered into final multivariate models.
RESULTS
Four of 407 oral samples (1.0%) were determined insufficient for β-HPV testing and 1 of 310 (0.3%) was determined insufficient for γ-HPV testing because of negative results of β-globin testing. In total, 406 women with a sample sufficient for β-HPV and/or γ-HPV testing were included in the analyses. The majority of women were white (79.3%), married or living with a partner (63.4%), and never smokers (74.6%; Table 1). Approximately one quarter of women reported a new male sex partner within the prior year, and almost all women queried (96.0%) reported a history of oral sex.
Table 1.
Demographic, Health, and Sexual Behavior Characteristics of 406 Mid-Adult Women in Seattle, Washington, 2011–2012
| Characteristic | Value |
|---|---|
| Age, y, mean ± SD | 38.8 ± 6.1 |
| Male sex partners,a lifetime no., median (IQR) | 8 (4–16) |
| Race | |
| African American | 10 (2.5) |
| Asian | 45 (11.1) |
| White | 322 (79.3) |
| Otherb | 29 (7.1) |
| Marital statusc | |
| Unmarried or separated | 147 (36.6) |
| Married or living with a partner | 255 (63.4) |
| Ever had genital wartsc | |
| No | 351 (87.3) |
| Yes | 51 (12.7) |
| Has immunosuppressive conditionc,d | |
| No | 396 (98.5) |
| Yes | 6 (1.5) |
| Smoking statusc,e | |
| Never | 299 (74.6) |
| Former | 84 (21.0) |
| Current | 18 (4.5) |
| Currently consumes alcoholic beveragesc | |
| No | 67 (16.7) |
| Yes | 335 (83.3) |
| Sex with new male partner in past 12 moc | |
| No | 297 (75.8) |
| Yes | 95 (24.2) |
| Ever open-mouth or tongue kissedf | |
| Never | 4 (1.4) |
| Male partners only | 196 (66.4) |
| Both male and female partners | 95 (32.2) |
| Ever performed oral sexf | |
| Never | 12 (4.0) |
| Male partners only | 227 (76.4) |
| Female partners only | 6 (2.0) |
| Both male and female partners | 52 (17.5) |
Data are no. (%) of women, unless otherwise indicated.
Abbreviation: IQR, interquartile range.
aRestricted to 394 women (97.0%) who reported ever having had sex with a male partner.
bIncludes individuals indicating the following: American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, other race, or multiple races.
cNumbers do not add up to total because of missing data.
dIncludes human immunodeficiency virus positivity (n = 1) or current receipt of immunosuppressive medications (n = 5).
eSmoking was defined as smoking at least 1 cigarette/day for ≥1 month; former smokers reported ever smoking but not currently smoking, and current smokers reported currently smoking.
fRestricted to 299 women who provided information on these additional potential risk factors for oral HPV. These questions were added after the start of the study and therefore not presented to all participants. Data on kissing were missing for 4 of the 299 women, and data on oral sex were missing for 2 women.
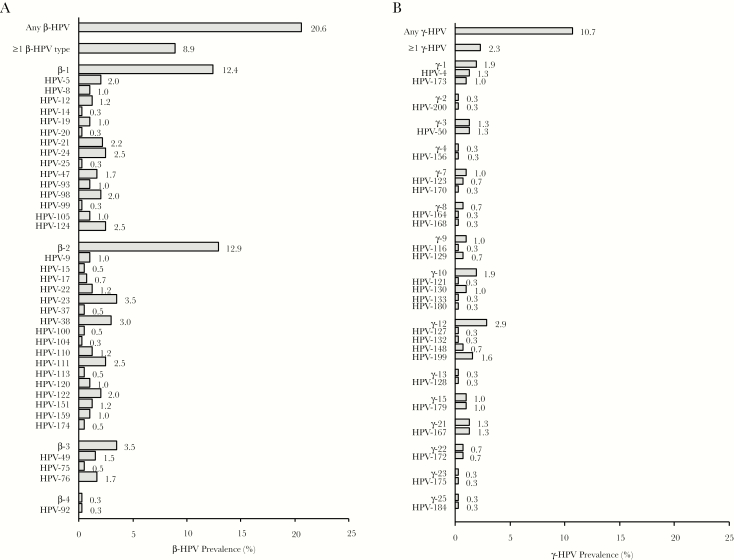
Eighty-three of 403 oral samples (20.6%) were positive for any β-HPV, and 36 (8.9%) were positive for multiple β-HPV types (Figure 1A). Species-specific prevalence was 12.4% for β-1, 12.9% for β-2, 3.5% for β-3, and 0.3% for β-4. β-5 was not detected. In all, 36 different β-HPV types were detected. The most common individual β-HPV types detected were HPV-23 (β-2; 3.5%), HPV-38 (β-2; 3.0%), HPV-24 (β-1; 2.5%), HPV-111 (β-2; 2.5%), and HPV-124 (β-1; 2.5%).
Figure 1.
A, β–Human papillomavirus (HPV) prevalence in oral samples from 403 mid-adult women. The assay included 46 β-HPV types. The following β-HPV types were not detected: HPV-36 (β-1), HPV-118 (β-1), HPV-143 (β-1), HPV-152 (β-1), HPV-80 (β-2), HPV-107 (β-2), HPV-145 (β-2), HPV-115 (β-3), HPV-96 (β-5), and HPV-150 (β-5). B, γ-HPV prevalence in oral samples from 309 mid-adult women. The assay included 51 γ-HPV types. The following γ-HPV types were not detected: HPV-65 (γ-1), HPV-95 (γ-1), HPV-48 (γ-2), HPV-60 (γ-5), HPV-88 (γ-5), HPV-101 (γ-6), HPV-103 (γ-6), HPV-108 (γ-6), HPV-109 (γ-7), HPV-134 (γ-7), HPV-149 (γ-7), HPV-112 (γ-8), HPV-119 (γ-8), HPV-126 (γ-11), HPV-169 (γ-11), HPV-171 (γ-11), HPV-202 (γ-11), HPV-165 (γ-12), HPV-131 (γ-14), HPV-161 (γ-19), HPV-162 (γ-19), HPV-166 (γ-19), HPV-163 (γ-20), HPV-178 (γ-24), HPV-197 (γ-24), and HPV-201 (γ-27).
Thirty-three of 309 oral samples (10.7%) were positive for any γ-HPV, and 7 (2.3%) were positive for multiple γ-HPV types (Figure 1B). Species-specific prevalence was 1.9% for γ-1, 0.3% for γ-2, 1.3% for γ-3, 0.3% for γ-4, 1.0% for γ-7, 0.7% for γ-8, 1.0% for γ-9, 1.9% for γ-10, 2.9% for γ-12, 0.3% for γ-13, 1.0% for γ-15, 1.3% for γ-21, 0.7% for γ-22, 0.3% for γ-23, and 0.3% for γ-25. γ-5, γ-6, γ-11, γ-14, γ-19, γ-20, γ-24, and γ-27 were not detected. In all, 25 different γ-HPV genotypes were detected. The most common γ-HPV-types detected were HPV-199 (γ-12; 1.6%), HPV-4 (γ-1; 1.3%), HPV-50 (γ-3; 1.3%), and HPV-167 (γ-21; 1.3%).
β-HPV testing results were available on 184 paired oral and fingernail samples collected on the same date. PPA between oral and fingernail samples for type-specific β-HPV detection was 19.7% and concordance was poor (κ = 0.19; 95% CI, .13–.25; Table 2).
Table 2.
Concordance Among Oral and Fingernail Samples for Type-Specific β-Human Papillomavirus (HPV) DNA Detection Among Mid-Adult Women in Seattle, Washington, 2011–2012
| Variable | Value |
| HPV positivity, pairs, no.a | |
| Both samples | 70 |
| Oral samples only | 41 |
| Fingernail samples only | 245 |
| Neither sample | 8108 |
| PPA,b % | 19.66 |
| κc (95% CId) | 0.19 (.31–.25) |
Abbreviations: CI, confidence interval; PPA, proportion positive agreement.
aRepresents the number of women (ie, 184) with paired oral and fingernail samples for β-HPV DNA testing) multiplied by the number of β-HPV types (ie, 46) evaluated per woman.
bCalculated as [number positive for HPV DNA in both samples]/[number positive for HPV DNA in either sample] × 100.
cCalculated as [observed PPA – expected PPA]/[1 – expected PPA].
dEstimated using percentile bootstrap methods with 1000 repetitions to account for correlation due to multiple HPV types and dual visits within women.
In univariate analysis, β-HPV detection in the oral cavity was positively associated with older age, white (vs nonwhite) race, current smoking, and greater lifetime number of oral sex partners (Table 3). In the subset of women with paired fingernail β-HPV testing results, having the same β-HPV type detected in the concurrent fingernail sample was also positively associated with oral β-HPV detection. Owing to the small subset of women with paired fingernail results, 2 final multivariate models were constructed, excluding (model 1) and including (model 2) concurrent β-HPV detection in fingernails. β-HPV detection remained significantly associated with older age in both models (adjusted PR, 1.37 and 1.43 in models 1 and 2, respectively), whereas associations with current smoking were attenuated toward the null in both models. Race remained associated with β-HPV in both models (adjusted PR for nonwhite versus white race, 0.52 and 0.09 in models 1 and 2, respectively), although the association was only statistically significant in model 2. Greater lifetime number of oral sex partners also remained positively associated with β-HPV in both models (adjusted PR for reporting at least 6 vs 0–5 oral sex partners, 2.06 and 1.58 in models 1 and 2, respectively), but the association was attenuated and not statistically significant in model 2. Concurrent detection of the same β-HPV type in the fingernails remained a strong risk factor for detection of β-HPV in the oral cavity after adjustment for age, race, smoking, and number of oral sex partners (adjusted PR, 31.44; 95% CI, 19.81–49.89).
Table 3.
Prevalence Ratios (PRs) for the Associations Between Select Risk Factors and Type-Specific Oral β–Human Papillomavirus (HPV) Detection Among 403 Mid-Adult Women in Seattle, Washington, 2011–2012
| Characteristic | Woman-Types, No. | PR (95% CI) | Adjusted PR (95% CI) | ||
|---|---|---|---|---|---|
| Analyzed | Determined to Be Oral β-HPV Positive | ||||
| Model 1a | Model 2b | ||||
| Age5 | 18538 | 178 | 1.36c (1.07–1.74) | 1.37c (1.01–1.86) | 1.43c (1.05–1.96) |
| Marital status | |||||
| Unmarried or separated | 6716 | 68 | 1.00 (reference) | … | … |
| Married or living with a partner | 11638 | 110 | 0.93 (.55–1.58) | … | … |
| Race | |||||
| White | 14766 | 162 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Nonwhite | 3772 | 16 | 0.39 (.19–.79) | 0.52 (.20–1.37) | 0.09 (.01–.68) |
| Ever had genital warts | |||||
| No | 16008 | 162 | 1.00 (reference) | … | … |
| Yes | 2346 | 16 | 0.67 (.33–1.39) | … | … |
| Smoking statusd | |||||
| Never | 13662 | 128 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Former | 3818 | 32 | 0.89 (.42–1.89) | 0.93 (.39–2.20) | 0.97 (.35–2.66) |
| Current | 828 | 18 | 2.32 (1.00–5.41) | 1.06 (.24–4.71) | 1.03 (.40–2.69) |
| Consumes alcoholic beverages | |||||
| No | 3082 | 22 | 1.00 (reference) | … | … |
| Yes | 15272 | 156 | 1.43 (.70–2.91) | … | … |
| Has immunosuppressive conditione | |||||
| No | 18078 | 172 | 1.00 (reference) | … | … |
| Yes | 276 | 6 | 2.28 (.71–7.33) | … | … |
| Male sex partners, lifetime no. (tertiles) | |||||
| 0–4 | 5980 | 48 | 1.00 (reference) | … | … |
| 5–11 | 5796 | 51 | 1.10 (.54–2.21) | … | … |
| ≥12 | 6164 | 77 | 1.56 (.77–3.14) | … | … |
| Male or female open-mouth kissing partners, lifetime no. (median split)f | |||||
| 0–15 | 6578 | 52 | 1.00 (reference) | … | … |
| ≥16 | 6578 | 73 | 1.40 (.70–2.83) | … | … |
| New male sex partner in past 12 mo | |||||
| No | 13524 | 124 | 1.00 (reference) | … | … |
| Yes | 4370 | 45 | 1.12 (.57–2.20) | … | … |
| Male or female oral sex partners, lifetime no. (median split)f | |||||
| 0–5 | 6992 | 44 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ≥6 | 6440 | 84 | 2.07 (1.02–4.21) | 2.06 (1.01–4.20) | 1.58 (.74–3.40) |
| Detection of same β-HPV type in concurrent fingernail sampleg | |||||
| No | 8149 | 41 | 1.00 (reference) | … | 1.00 (reference) |
| Yes | 315 | 70 | 44.17 (29.36–66.44) | … | 31.44 (19.81–49.89) |
A total of 403 women contributed 18538 women-types to the oral β-HPV analysis.
Abbreviations: Age5, age in years divided by 5; CI, confidence interval.
aIncludes age, race, smoking status, and lifetime number of oral sex partners. Data are for 13386 women-types.
bIncludes age, race, smoking status, lifetime number of oral sex partners, and detection of same β-HPV type in concurrent fingernail sample. Data are for 5750 women-types.
cReflects the relative change in the likelihood of β-HPV detection associated with a 5-year difference in age.
dSmoking was defined as smoking at least 1 cigarette/day for ≥1 month; former smokers reported ever smoking but not currently smoking, and current smokers reported currently smoking.
eIncludes human immunodeficiency virus positivity or current receipt of immunosuppressive medications.
fRestricted to 296 women who provided information on these additional potential risk factors for oral HPV. These questions were added after the start of the study and therefore not presented to all participants.
gRestricted to 184 women with β-HPV test results for a concurrent fingernail sample.
No statistically significant associations were observed between any of the selected risk factors and detection of γ-HPV in the oral cavity (Table 4). Therefore, a multivariate model was not constructed.
Table 4.
Prevalence Ratios (PRs) for the Associations Between Selected Risk Factors and Type-Specific Oral γ–Human Papillomavirus (HPV) Detection Among 309 Mid-Adult Women in Seattle, Washington, 2011–2012
| Characteristic | Woman-Types | PR (95% CI) | |
|---|---|---|---|
| Analyzed | Determined to Be Oral γ-HPV Positive | ||
| Age5 | 15675 | 48 | 1.01a (.66–1.52) |
| Marital status | |||
| Unmarried or separated | 5786 | 26 | 1.00 (reference) |
| Married or living with a partner | 9736 | 22 | 0.50 (.22–1.13) |
| Race | |||
| White | 12745 | 41 | 1.00 (reference) |
| Nonwhite | 2930 | 7 | 0.74 (.30–1.86) |
| Ever had genital warts | |||
| No | 13380 | 41 | 1.00 (reference) |
| Yes | 2142 | 7 | 1.07 (.37–3.09) |
| Smoking statusb | |||
| Never | 11521 | 31 | 1.00 (reference) |
| Former | 3287 | 14 | 1.58 (.62–4.03) |
| Current | 663 | 3 | 1.68 (.24–11.77) |
| Has immunosuppressive conditionc | |||
| No | 15318 | 47 | 1.00 (reference) |
| Yes | 204 | 1 | 1.60 (.28–9.19) |
| Consumes alcoholic beverages | |||
| No | 2346 | 8 | 1.00 (reference) |
| Yes | 13176 | 40 | 0.89 (.33–2.38) |
| Male sex partners, lifetime no. (tertiles) | |||
| 0–4 | 4868 | 11 | 1.00 (reference) |
| 5–11 | 4845 | 17 | 1.55 (.47–5.13) |
| ≥12 | 5531 | 20 | 1.60 (.52–4.91) |
| New male sex partner in past 12 mo | |||
| No | 11391 | 32 | 1.00 (reference) |
| Yes | 3672 | 12 | 1.16 (.40–3.42) |
| Male or female open-mouth kissing partners, lifetime no. (median split)d | |||
| 0–15 | 5888 | 15 | 1.00 (reference) |
| ≥16 | 6064 | 22 | 1.42 (.54–3.77) |
| Male or female oral sex partners, lifetime no. (median split)d | |||
| 0–5 | 6217 | 18 | 1.00 (reference) |
| ≥6 | 5990 | 19 | 1.10 (.42–2.86) |
A total of 309 women contributed 15675 women-types to the oral γ-HPV analysis.
Abbreviations: Age5, age in years divided by 5; CI, confidence interval.
aReflects the relative change in the likelihood of γ-HPV detection associated with a 5-year difference in age.
bSmoking was defined as smoking at least 1 cigarette/day for ≥1 month; former smokers reported ever smoking but not currently smoking, and current smokers reported currently smoking.
cIncludes human immunodeficiency virus positivity or current receipt of immunosuppressive medications.
dRestricted to 245 women who provided information on these additional potential risk factors for oral HPV. These questions were added after the start of the study and therefore not presented to all participants.
DISCUSSION
To our knowledge, this is the first study to evaluate both the prevalence and determinants of cutaneous HPV types in the oral cavities of healthy mid-adult women. Using multiplex assays for 46 β-HPVs and 51 γ-HPVs, we detected β-HPVs in 20.6% and γ-HPVs in 10.7% of oral rinse samples. In the same cohort of women, we previously detected α-HPVs in only 2.4% of oral cavity samples, using the Roche Linear Array assay [30]. Other studies evaluating HPVs in oral cavity samples from healthy men and women [3, 21, 25, 28] and HIV-positive individuals [3] have similarly reported a higher prevalence of cutaneous mucosal HPV types, compared with mucosal HPV types. Furthermore, while absolute comparisons across studies are complicated by differences in sampling methods and numbers of HPV types evaluated, oral cutaneous HPVs appear to be more abundant in men than in women. Among young women (aged 22–29 years) enrolled in the control arm of the Costa Rica HPV vaccine trial, β-HPVs were detected in 18.6% of oral rinse samples [25], similar to our results. In a multinational study of healthy men from the United States, Brazil, and Mexico, the oral β-HPV prevalence was higher, at 29.3% [24]. In a small study of cutaneous HPVs in young heterosexual couples aged 20–29 years, β-HPV prevalence was only 6% in women, compared with 15% in men [27]. In a recent population-based study of healthy adults in Hong Kong, oral β-HPV prevalence was also lower in women (9.5%) than in men (14.4%) [28]. Of note, the prevalence among a subset of 297 women aged 35–54 years was 8.4%, considerably lower than the oral β-HPV prevalence in our study of women aged 30–50 years. The highest β-HPV prevalence estimates (56.7% [16] and 58.8% [21]) have been reported among control subjects (predominantly older males) from case-control studies of head and neck cancer. These studies also highlight age-related trends in prevalence, as the oral β-HPV prevalence tends to be higher in studies in older as compared to younger participants. This is consistent with our finding in mid-adult women that oral β-HPV detection increased with age, a finding also reported in the multinational study of men [24] and the study of men and women in Hong Kong [28]. Interpreting γ-HPV prevalence is more challenging owing to greater variations in the number of types evaluated across studies. Similar to our results, the few studies evaluating both β-and γ-HPVs in the same population have reported a lower prevalence of γ-HPVs [3, 21, 25, 27]. Using a next-generation sequencing assay to detect a broad number of γ-HPV types, Agalliu et al [21] detected γ-HPVs in oral rinse samples from 35.4% of their control subjects. On the other hand, Wong et al detected γ-HPVs in only 2.9% of adults, using a similar next-generation sequencing assay [28]. Other studies evaluating few specific types detected γ-HPVs in 1%–9% of oral samples [3, 25, 27].
Cutaneous HPVs may contribute to some skin [12–14] and oropharyngeal [15, 16] cancers through indirect mechanisms. β-and γ-HPVs may promote carcinogenesis by initiating tumorigenesis, by modifying other exposures (such as UV exposure or smoking), or through host immune or genetic factors [17–20, 41]. Case-control studies have identified β and γ species and individual genotypes associated with head and neck squamous cell carcinomas, including HPV-5 (β-1), HPV-38 (β-2), and γ-7, γ-11, and γ-12 species [16, 21]. In our sample of healthy mid-adult women, we detected HPV-5 and HPV-38 in 2.0% and 3.0% of oral rinse samples, respectively. γ-7 and γ-12 species types were detected in 1.0% and 2.9% of samples, respectively; we did not detect any samples positive for γ-11 types.
We observed that β-HPV detection was more likely in women reporting a history of ≥6 oral sex partners than in women reporting <6 partners, supporting a potential sexual transmission route for these types. To our knowledge, this is the first study to report an association between sexual behavior and detection of cutaneous HPVs in the oral cavity. No sexual behavior risk factors for oral cutaneous HPVs were identified in either the young female Costa Rica vaccine trial participants [25], the men enrolled in the multinational study [24], or the Hong Kong study participants (risk factor analyses were not stratified by age or sex) [28]. We also observed that the prevalence was lower in white as compared to nonwhite participants. We did not identify any notable demographic or behavioral characteristics associated with γ-HPV detection. Associations between our selected variables and γ-HPV detection were generally in the same direction as observed for β-HPV but were attenuated and not statistically significant. The lack of significant associations may be due to differences in the epidemiology or natural history of β-HPVs as compared to γ-HPVs in the oral cavity, or reduced power to detect associations, owing to the smaller sample size and lower prevalence of γ-HPVs as compared to β-HPVs.
In the same cohort of mid-adult women, β-HPV prevalence was 3 times higher in the fingernail cavity, compared with the oral cavity (61.1% in fingernail samples [37], compared with 20.6% in oral samples in the present study). This is consistent with previous studies demonstrating a higher prevalence of cutaneous HPVs in skin as compared to oral samples. In the study of young heterosexual couples, β- and γ-HPV genera were each detected more frequently in hand samples, compared with oral samples [27], and in the multinational study of men, β-HPVs were detected more frequently in forearm skin samples than in oral samples [42]. While concordance between oral and fingernail samples for detecting the same β-HPV type was low in our study, concurrent detection in the fingernails was strongly associated with oral β-HPV detection, supporting the hypothesis that fingers may serve as a source of transmission or autoinoculation of β-HPVs to the oral cavity.
Study limitations should be noted. Because of limited resources, we were only able to test 76% of our oral samples for γ-HPV, limiting power to detect significant associations. In addition, β-HPV results were available on only a subset of paired fingernail samples, and we did not perform any γ-HPV testing on fingernail samples. In addition, data were cross-sectional, thus precluding any evaluation of acquisition or persistence of cutaneous HPVs in the oral cavity.
In conclusion, cutaneous HPV types were commonly detected in the oral cavities of healthy mid-adult women. Our results suggest a sexual transmission route for β-HPVs and support the hypothesis that fingers may serve as a source of transmission or autoinoculation of β-HPVs to the oral cavity. These epidemiologic data further our understanding of the tropism and transmission of these cutaneous HPV genera. As our knowledge of the role of cutaneous HPV types in oral tumorigenesis advances, additional longitudinal studies should be conducted to further elucidate the epidemiology and natural history of these genera.
Notes
Financial support. This work was supported by the National Institutes of Health (grant R03AI103322 to R. L. W.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chouhy D, Bolatti EM, Pérez GR, Giri AA. Analysis of the genetic diversity and phylogenetic relationships of putative human papillomavirus types. J Gen Virol 2013; 94:2480–8. [DOI] [PubMed] [Google Scholar]
- 2. de Villiers EM. Cross-roads in the classification of papillomaviruses. Virology 2013; 445:2–10. [DOI] [PubMed] [Google Scholar]
- 3. Bottalico D, Chen Z, Dunne A, et al. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J Infect Dis 2011; 204:787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paolini F, Rizzo C, Sperduti I, et al. Both mucosal and cutaneous papillomaviruses are in the oral cavity but only alpha genus seems to be associated with cancer. J Clin Virol 2013; 56:72–6. [DOI] [PubMed] [Google Scholar]
- 5. Forslund O, Johansson H, Madsen KG, Kofoed K. The nasal mucosa contains a large spectrum of human papillomavirus types from the Betapapillomavirus and Gammapapillomavirus genera. J Infect Dis 2013; 208:1335–41. [DOI] [PubMed] [Google Scholar]
- 6. Smelov V, Hanisch R, McKay-Chopin S, et al. Prevalence of cutaneous beta and gamma human papillomaviruses in the anal canal of men who have sex with women. Papillomavirus Res 2017; 3:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Torres M, Gheit T, McKay-Chopin S, et al. Prevalence of beta and gamma human papillomaviruses in the anal canal of men who have sex with men is influenced by HIV status. J Clin Virol 2015; 67:47–51. [DOI] [PubMed] [Google Scholar]
- 8. Donà MG, Gheit T, Latini A, et al. Alpha, beta and gamma Human Papillomaviruses in the anal canal of HIV-infected and uninfected men who have sex with men. J Infect 2015; 71:74–84. [DOI] [PubMed] [Google Scholar]
- 9. Pierce Campbell CM, Messina JL, Stoler MH, et al. Cutaneous human papillomavirus types detected on the surface of male external genital lesions: a case series within the HPV Infection in Men Study. J Clin Virol 2013; 58:652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sichero L, El-Zein M, Nunes EM, Ferreira S, Franco EL, Villa LL; Ludwig-McGill Cohort Study Cervical infection with cutaneous beta and mucosal alpha Papillomaviruses. Cancer Epidemiol Biomarkers Prev 2017; 26:1312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bosch FX, Broker TR, Forman D, et al. ; authors of ICO Monograph Comprehensive Control of HPV Infections and Related Diseases Vaccine Volume 30, Supplement 5, 2012 Comprehensive control of human papillomavirus infections and related diseases. Vaccine 2013; 31(Suppl 7):H1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neale RE, Weissenborn S, Abeni D, et al. Human papillomavirus load in eyebrow hair follicles and risk of cutaneous squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2013; 22:719–27. [DOI] [PubMed] [Google Scholar]
- 13. Proby CM, Harwood CA, Neale RE, et al. ; EPI-HPV-UV-CA group A case-control study of betapapillomavirus infection and cutaneous squamous cell carcinoma in organ transplant recipients. Am J Transplant 2011; 11:1498–508. [DOI] [PubMed] [Google Scholar]
- 14. Iannacone MR, Gheit T, Pfister H, et al. Case-control study of genus-beta human papillomaviruses in plucked eyebrow hairs and cutaneous squamous cell carcinoma. Int J Cancer 2014; 134:2231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koppikar P, deVilliers EM, Mulherkar R. Identification of human papillomaviruses in tumors of the oral cavity in an Indian community. Int J Cancer 2005; 113:946–50. [DOI] [PubMed] [Google Scholar]
- 16. Sabol I, Smahelova J, Klozar J, et al. Beta-HPV types in patients with head and neck pathology and in healthy subjects. J Clin Virol 2016; 82:159–65. [DOI] [PubMed] [Google Scholar]
- 17. Wallace NA, Robinson K, Howie HL, Galloway DA. HPV 5 and 8 E6 abrogate ATR activity resulting in increased persistence of UVB induced DNA damage. PLoS Pathog 2012; 8:e1002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viarisio D, Gissmann L, Tommasino M. Human papillomaviruses and carcinogenesis: well-established and novel models. Curr Opin Virol 2017; 26:56–62. [DOI] [PubMed] [Google Scholar]
- 19. Viarisio D, Mueller-Decker K, Kloz U, et al. E6 and E7 from beta HPV38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice. PLoS Pathog 2011; 7:e1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viarisio D, Müller-Decker K, Accardi R, et al. Beta HPV38 oncoproteins act with a hit-and-run mechanism in ultraviolet radiation-induced skin carcinogenesis in mice. PLoS Pathog 2018; 14:e1006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agalliu I, Gapstur S, Chen Z, et al. Associations of oral alpha-, beta-, and gamma-human papillomavirus types with risk of incident head and neck cancer. JAMA Oncology 2016; 2:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers - United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2016; 65:661–6. [DOI] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention. Cancers associated with human papillomavirus, United States—2011–2015 USCS data brief, no. 4. Atlanta, GA: Centers for Disease Control and Prevention. 2018. [Google Scholar]
- 24. Nunes EM, Sudenga SL, Gheit T, et al. ; HIM Study group Diversity of beta-papillomavirus at anogenital and oral anatomic sites of men: The HIM Study. Virology 2016; 495:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang Kuhs KA, Gonzalez P, Struijk L, et al. ; Costa Rica Vaccine Trial Group Prevalence of and risk factors for oral human papillomavirus among young women in Costa Rica. J Infect Dis 2013; 208:1643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fatahzadeh M, Schlecht NF, Chen Z, et al. Oral human papillomavirus detection in older adults who have human immunodeficiency virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 115:505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moscicki AB, Ma Y, Gheit T, et al. Prevalence and transmission of beta and gamma human papillomavirus in heterosexual couples. Open Forum Infect Dis 2017; 4:ofw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong MCS, Vlantis AC, Liang M, et al. Prevalence and epidemiologic profile of oral infection with alpha, beta, and gamma papillomaviruses in an Asian Chinese population. J Infect Dis 2018; 218:388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fu TC, Fu Xi L, Hulbert A, et al. Short-term natural history of high-risk human papillomavirus infection in mid-adult women sampled monthly. Int J Cancer 2015; 137:2432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fu TC, Hughes JP, Feng Q, et al. Epidemiology of human papillomavirus detected in the oral cavity and fingernails of mid-adult women. Sex Transm Dis 2015; 42:677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmitt M, Bravo IG, Snijders PJ, Gissmann L, Pawlita M, Waterboer T. Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol 2006; 44:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gheit T, Billoud G, de Koning MN, et al. Development of a sensitive and specific multiplex PCR method combined with DNA microarray primer extension to detect Betapapillomavirus types. J Clin Microbiol 2007; 45:2537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruer JB, Pépin L, Gheit T, et al. Detection of alpha- and beta-human papillomavirus (HPV) in cutaneous melanoma: a matched and controlled study using specific multiplex PCR combined with DNA microarray primer extension. Exp Dermatol 2009; 18:857–62. [DOI] [PubMed] [Google Scholar]
- 34. Schmitt M, Dondog B, Waterboer T, Pawlita M, Tommasino M, Gheit T. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J Clin Microbiol 2010; 48:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hampras SS, Giuliano AR, Lin HY, et al. Natural history of cutaneous human papillomavirus (HPV) infection in men: the HIM study. PLoS One 2014; 9:e104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hampras SS, Reed RA, Bezalel S, et al. cutaneous human papillomavirus infection and development of subsequent squamous cell carcinoma of the skin. J Skin Cancer 2016; 2016:1368103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winer RL, Gheit T, Cherne S, et al. Prevalence and correlates of beta human papillomavirus detection in fingernail samples from mid-adult women. Papillomavirus Res 2018; 5:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smelov V, Muwonge R, Sokolova O, et al. Beta and gamma human papillomaviruses in anal and genital sites among men: prevalence and determinants. Sci Rep 2018; 8:8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saiki RK, Gelfand DH, Stoffel S, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988; 239:487–91. [DOI] [PubMed] [Google Scholar]
- 40. Wolfrum SG, Koutsky LA, Hughes JP, et al. Evaluation of dry and wet transport of at-home self-collected vaginal swabs for human papillomavirus testing. J Med Microbiol 2012; 61:1538–45. [DOI] [PubMed] [Google Scholar]
- 41. Rollison DE, Gillison ML. The alpha, beta, gammas of oral human papillomavirus infection and head and neck cancer risk. JAMA Oncol 2016; 2:606–7. [DOI] [PubMed] [Google Scholar]
- 42. Hampras SS, Rollison DE, Giuliano AR, et al. Prevalence and concordance of cutaneous beta human papillomavirus infection at mucosal and cutaneous sites. J Infect Dis 2017; 216:92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]