Abstract
Background
Indoor residual spraying of insecticide (IRS) has been associated with reductions in the incidence of malaria, but its impact on malaria parasitemia is unclear.
Methods
We followed 469 participants from August 2011 to May 2016 in Tororo, Uganda, a historically high malaria transmission setting. Three rounds of IRS with bendiocarb were implemented from December 2014 to December 2015. Symptomatic malaria episodes were identified by passive surveillance. Parasitemia was identified by active surveillance every 1–3 months using microscopy and Plasmodium falciparum–specific loop-mediated isothermal amplification.
Results
IRS was associated with a significant decline in the incidence of symptomatic malaria irrespective of age (episodes per person per year declined from 3.98 to 0.13 in children aged <5 years, 2.30 to 0.15 in children aged 5–10 years, and 0.41 to 0 in adults; P < .001 for all). IRS significantly reduced the prevalence of parasitemia, but the prevalence remained high (pre-IRS to post–third round: 58.5% to 11.3% in children aged <5 years, 73.3% to 23.7% in children aged 5–10 years, and 52.2% to 15.4% in adults; P < .001 for all).
Conclusions
Although IRS was associated with significant reductions in the incidence of malaria and prevalence of parasitemia, a proportion of the population remained parasitemic, providing a potential reservoir for malaria transmission.
Keywords: malaria, infectious reservoir, indoor residual spraying, parasitemia
Three rounds of indoor residual spraying were associated with significant reduction in clinical malaria and parasite prevalence; however, a proportion of the proportion remained parasitemic. These parasites could serve as a reservoir for onward transmission.
Important advances have been made in malaria control in Africa, with declines in malaria burden and progress toward elimination in some countries [1–3]. This progress has been attributed to the scale-up of proven malaria control interventions including indoor residual spraying of insecticide (IRS), insecticide-treated nets (ITNs), and effective case management. IRS involves the application of long-acting insecticides on the walls of houses to kill mosquitoes and reduce the risk of infectious bites from the Anopheles vector. IRS has been used to control malaria since the 1950s [4] and has been associated in many countries with a significant reduction in malaria burden, including prevalence of parasitemia, morbidity, mortality, and entomological indices of transmission [5–7]. However, although IRS is effective, it is difficult to maintain due to high implementation costs, and downscales have been reported in several countries in Africa [8].
After a gap of 40 years, IRS was reestablished in Uganda in 2006 in 10 districts in the north; an additional 14 districts were added in 2014. Use of IRS in these districts has been associated with a significant reduction in key malaria indicators, including the slide positivity rate (defined as the number of laboratory-confirmed malaria cases per 100 suspected cases examined), incidence of malaria, human biting rate, preterm birth risk, and prevalence of anemia [2, 6, 9–13]. Although significant benefits have been documented following the reinitiation of IRS in Uganda, the observed declines in malaria burden have been temporary, with resurgence observed following the cessation of the intervention in northern Uganda [14]. This phenomenon has been reported elsewhere; studies from many endemic settings show that when IRS is scaled back, there is a rapid resurgence of malaria [15]. The reason for the rapidity of resurgence following cessation of IRS is not clear; however, one explanation could be that a significant reservoir of infections remained following the implementation of vector control, contributing to a rapid rebound in transmission once insecticide effects diminished.
In highly endemic countries like Uganda, the malaria parasite reservoir is dynamic, and includes symptomatic and asymptomatic infections. Symptomatic infections are detected and treated when patients seek healthcare. Most evaluations of IRS have focused on measures of malaria morbidity or vector abundance [5, 7, 9, 16, 17]. Less studied are the effects of IRS on the prevalence of asymptomatic infections, especially low-density infections below the level of detection by microscopy, referred to as submicroscopic parasitemia. These asymptomatic infections can only be detected by active surveillance, ideally using highly sensitive molecular techniques such as polymerase chain reaction (PCR) or loop-mediated isothermal amplification (LAMP) assays. Despite the lack of attention paid to asymptomatic infections, these infections are known to comprise a large proportion of the parasitic reservoir, and recent studies have demonstrated that even low-density infections can transmit parasites to mosquitoes [18, 19]. It is therefore important to understand the impact of IRS on all parasite reservoirs (symptomatic and asymptomatic) to more effectively evaluate and direct vector control measures and determine the need for additional measures (such as mass drug administration) to address the parasite reservoir that persists after IRS.
In Tororo District, Uganda, 3 rounds of IRS were conducted with the carbamate bendiocarb between December 2014 and December 2015. Our cohort, in which participants were actively assessed for parasitemia every 3 months prior to the initiation of IRS and then monthly starting in December 2014, provided a unique opportunity to evaluate the effect of IRS on the reservoir of parasitemia in a high-transmission setting.
METHODS
Study Setting
The study was conducted in Nagongera subcounty, Tororo district, in southeastern Uganda. Tororo had high transmission of primarily Plasmodium falciparum infections, with an estimated entomological inoculation rate of 310 infective bites per person per year prior to initiation of IRS [20]. Prior to this study, malaria control in Tororo was limited to the distribution of ITNs through antenatal care services, promotion of intermittent preventive treatment during pregnancy, and malaria case management with artemisinin-based combination therapy. One round of mass distribution of free ITNs was conducted as part of a national campaign in November 2013. IRS with bendiocarb was initiated for the first time in Tororo District in December 2014 (December 2014–January 2015); 2 additional rounds of IRS with bendiocarb were implemented in June–July 2015 and November–December 2015.
Study Design, Enrollment, and Follow-up
Details of the cohort study have been described previously [20]. In brief, 100 households were randomly selected using a list generated from enumeration and mapping of all households in Nagongera subcounty in 2011. All children aged 0.5–10 years who fulfilled the selection criteria and had written informed consent from a parent or guardian and 1 adult primary caregiver from each household were enrolled into the cohort in August 2011. The cohort was dynamic, such that all newly eligible children in a household were enrolled, and participants who reached 11 years of age were excluded. All participants were given a long-lasting ITN at enrollment. The enrollment visit and all subsequent study visits took place at a designated study clinic at Nagongera Health Center. The study clinic was open every day from 8 am to 5 pm for scheduled routine visits and nonroutine visits due to illness.
Routine visits were conducted at least every 90 days, and a standardized evaluation was conducted. Blood was collected by finger prick to perform thick and thin blood smears for malaria parasites and estimate hemoglobin levels; an additional sample was stored on filter paper for future molecular testing using LAMP. Beginning in December 2014, blood samples were collected every 30 days. Participants were encouraged to seek all medical care at the study clinic and to avoid the use of any antimalarial medication outside of the clinic. Participants with fever (>38.0°C tympanic) or history of fever in the previous 24 hours at the time of routine or nonroutine visits had a thick blood smear read urgently. If the smear was positive, the patient was diagnosed with malaria and managed according to Uganda national guidelines (for uncomplicated malaria treatment with artemether-lumefantrine) [21].
Laboratory Evaluations
Blood smears were stained with 2% Giemsa for 30 minutes. Parasite densities were calculated by counting the number of asexual parasites per 200 leukocytes (or per 500, if the count was <10 parasites per 200 leukocytes), assuming a leukocyte count of 8000/µL. A thick blood smear was considered negative when the examination of 100 high-power fields revealed no parasites. When malaria was diagnosed, thin smears were read for species identification based on standard morphology criteria. For quality control, all slides were read by a second microscopist, and a third reader was used to settle any discrepant readings.
LAMP assays were performed on DNA from dried blood spots of all participants who had a negative blood smear during a routine visit to detect submicroscopic infections. DNA was extracted using the Chelex extraction method as previously described [22]. LAMP was performed using Eiken Loopamp MALARIA Pan Detection Kit reaction tubes and 15 µL of extracted DNA, per the manufacturer’s guidelines. For each assay, 46 samples plus 1 positive and 1 negative control were run. The LAMP results were read based on visual detection of fluorescence under an ultraviolet lamp.
Data Analysis
Statistical data analysis was conducted using Stata version 14. Baseline characteristics were summarized using proportions. The primary exposure of interest was calendar time in relationship to the implementation of IRS. The pre-IRS period was August 2011 to January 2015, and the post-IRS periods were February 2015–June 2015 (after first round), July 2015 to November 2015 (after second round), and December 2015–May 2016 (after third round). The date that IRS was considered potentially protective against malaria infection was 30 days after initiation of spraying.
The outcomes of interest were malaria incidence and prevalence of parasitemia. Incidence of malaria was defined as number of symptomatic malaria episodes per time at risk. The prevalence of microscopic parasitemia was defined as the number of participant visits with a positive blood smear divided by the number of routine blood smears performed. The prevalence of any parasitemia was defined as the number of participant visits with a positive blood smear or LAMP test divided by the total number of routine blood smears performed. All data were stratified by 3 age groups a priori: 0.5 to < 5 years, 5–10 years, and ≥18 years. Negative binomial regression models were used to calculate incidence rate ratios (IRRs) comparing each post-IRS time period to the pre-IRS period. Comparisons of proportions with repeated measures were made with generalized estimating equations, with the use of log-binomial regression and robust standard errors to generate prevalence ratios (PRs). A P value <.05 was considered statistically significant for all analyses.
RESULTS
Characteristics of the Study Population
Between August 2011 and May 2016, a total of 469 participants were enrolled in the cohort (Table 1). The majority of the participants were children aged 5–10 years, and most (94.9%) were enrolled between August 2011 and January 2015, prior to initiation of IRS. Of the enrolled participants, 152 (32.6%) were withdrawn or lost to follow-up. The commonest reason for leaving the study was reaching 11 years of age (74 [48.4%]), which was an exclusion criterion. Other reasons for leaving the study included participants missing a visit for >120 days (18.4%), withdrawal of consent (12.5%), relocation out of the study area (11.2%), failure to comply with study procedures (7.9%), and death (1.3%). The total follow-up time was 1604 person-years (PY), with the majority of the follow-up time before the initiation of IRS (1195 PY before IRS vs 408 PY after IRS). There were 17956 clinic visits within the study period, of which 8894 (49.5%) were routine visits. At each clinic visit, bed net use reported for the prior evening was high (99.9%).
Table 1.
Characteristics of the Study Population
| Characteristic | Pre-IRSa |
After First Rounda of IRS | After Second Rounda of IRS | After Third Rounda of IRS |
|---|---|---|---|---|
| Data at the participant level | ||||
| No. of participants, by age group | ||||
| 0.5 to <5 y | 192 | 80 | 79 | 82 |
| 5–10 y | 148 | 179 | 168 | 161 |
| ≥18 y | 106 | 80 | 77 | 74 |
| Total follow-up time, person-years, by age group | ||||
| 0.5 to <5 y | 345.7 | 29.4 | 29.5 | 37.5 |
| 5–10 y | 539.2 | 69.2 | 66.7 | 75.4 |
| ≥18 y | 310.3 | 32.6 | 31.6 | 36.6 |
| Data at the visit level, No. (%) | ||||
| Type of visit | ||||
| Enrollment | 445 (3.5) | 5 (0.3) | 14 (0.9) | 5 (0.3) |
| Unscheduled | 7790 (60.1) | 460 (26.5) | 496 (30.1) | 316 (19.6) |
| Routine | 4725 (36.5) | 1270 (73.2) | 1139 (69.1) | 1291 (80.1) |
| Febrile during study visit | 3094 (40.7) | 155 (11.2) | 67 (5.6) | 30 (2.3) |
Abbreviation: IRS, indoor residual spraying.
aPre-IRS: August 2011–January 2015; first round of IRS: February–June 2015; second round of IRS: July–November 2015; third round of IRS: December 2015–May 2016.
Impact of Multiple Rounds of IRS on Incidence of Malaria
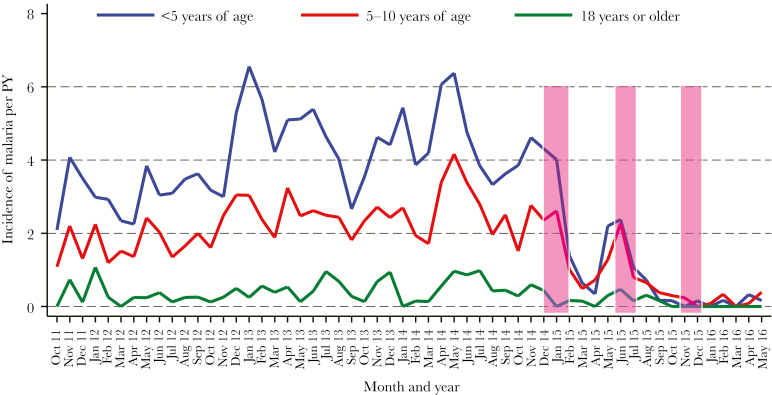
There were 2933 episodes of symptomatic malaria over the course of the study, giving an overall incidence of 1.82 episodes per person per year. The majority of malaria episodes (93.5%) occurred prior to initiation of IRS (incidence 2.29 episodes per person per year vs 0.47 episodes per person per year after initiation, P < .001). As expected, the incidence of malaria was highest in children <5 years of age, followed by children aged 5–10 years, and lowest in adults (Table 2 and Figure 1). A decline in the overall risk of symptomatic malaria was observed with each round of IRS, with the largest decline in absolute numbers of cases per PY observed following the first round. After 3 rounds of IRS, only 5 episodes of symptomatic malaria (0.13 episodes per PY) were reported in children aged <5 years, 6 episodes in children aged 5–10 years (0.15 episodes per PY), and no episodes in adults.
Table 2.
Incidence of Malaria and Prevalence of Parasitemia Before and After Implementation of Indoor Residual Spraying, Stratified by Age of the Participants
| Time Perioda | Age Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 to <5 y | 5–10 y | ≥18 y | |||||||
| Incidence per PY | IRR (95% CI) | P Value | Incidence per PY |
IRR (95% CI) | P Value | Incidence per PY |
IRR (95% CI) | P Value | |
| Clinical malaria | |||||||||
| Pre-IRS | 3.98 | 1 | 2.30 | 1 | 0.41 | 1 | |||
| After first round of IRS | 1.37 | 0.29 (.20–.41) | <.001 | 1.15 | 0.31 (.24–.40) | <.001 | 0.22 | 0.63 (.28–1.46) | .295 |
| After second round of IRS | 0.41 | 0.10 (.05–.18) | <.001 | 0.48 | 0.13 (.09–.19) | <.001 | 0.13 | 0.36 (.12–1.06) | .064 |
| After third round of IRS | 0.13 | 0.04 (.02–.10) | <.001 | 0.15 | 0.04 (.02–.08) | <.001 | 0 | 0 (NA) | NA |
| Parasite Prevalence, % (No.) | Prevalence Ratio (95% CI) | P Value | Parasite Prevalence, % (No.) | Prevalence Ratio (95% CI) | P Value | Parasite Prevalence, % (No.) | Prevalence Ratio (95% CI) | P Value | |
| Microscopic parasitemiab | |||||||||
| Pre-IRS | 24.0 (366) | 1 | 37.3 (878) | 1 | 6.1 (78) | 1 | |||
| After first round of IRS | 13.3 (45) | 0.7 (.5–.8) | .001 | 30.9 (240) | 0.8 (.8–.9) | .004 | 9.6 (15) | 1.6 (.9–2.8) | .073 |
| After second round of IRS | 10.5 (31) | 0.5 (.4–.7) | <.001 | 20.9 (148) | 0.6 (.5–.7) | <.001 | 7.2 (10) | 1.2 (.6–2.2) | .624 |
| After third round of IRS | 5.1 (17) | 0.3 (.2–.4) | <.001 | 10.2 (82) | 0.3 (.2–.3) | <.001 | 2.1 (3) | 0.3 (.1–1.0) | .058 |
| Any parasitemiab | |||||||||
| Pre-IRS | 58.5 (891) | 1 | 73.3 (1178) | 1 | 52.2 (666) | 1 | |||
| After first round of IRS | 26.8 (91) | 0.5 (.4–.6) | <.001 | 51.5 (400) | 0.8 (.7–.8) | <.001 | 37.8 (59) | 0.7 (.6–.9) | .001 |
| After second round of IRS | 19.6 (58) | 0.4 (.3–.5) | <.001 | 39.2 (278) | 0.6 (.5–.6) | <.001 | 24.5 (34) | 0.5 (.4–.6) | <.001 |
| After third round of IRS | 11.3 (38) | 0.3 (.2–.3) | <.001 | 23.7 (187) | 0.4 (.3–.4) | <.001 | 15.4 (22) | 0.3 (.2–.4) | <.001 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; IRS, indoor residual spraying; NA, not applicable; PY, person-year.
aPre-IRS: August 2011–January 2015; first round of IRS: February–June 2015; second round of IRS: July–November 2015; third round of IRS: December 2015–May 2016.
bOnly includes enrollment and routine visits.
Figure 1.
Temporal changes in the incidence of malaria, stratified by age. Monthly trends of the incidence of clinical malaria following multiple rounds of indoor residual spraying with the carbamate bendiocarb (bars). Abbreviations: PY, person-years.
Impact of Multiple Rounds of IRS on the Prevalence of Parasitemia
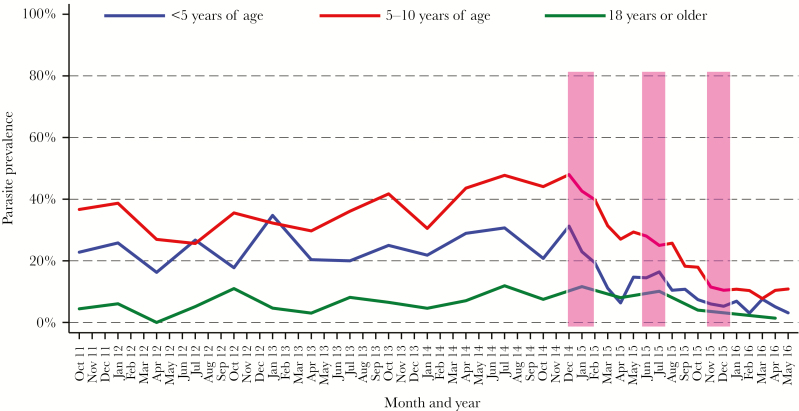
There were 4451 (50.4%) events of malaria parasitemia recorded at routine visits in the study period. The majority of these parasitemia events were asymptomatic (4197 [94.3%]), with more asymptomatic events detected by LAMP (2540 [60.5%]) than microscopy (1657 [39.5%]). Prior to initiation of IRS, overall prevalence of microscopic parasitemia was 25.6%, decreasing to 16% (P < .001) following 3 rounds of IRS. The baseline prevalence of microscopic parasitemia was highest among children 5–10 years of age (37.3%) and lowest in the adults (6.1%). As with incidence, a decline in the prevalence of microscopic parasitemia in children was observed with each round of IRS. Three rounds of IRS reduced the prevalence of microscopic parasitemia from 24% to 5.1% in children <5 years, and from 37.3% to 10.2% in children 5–10 years of age (P < .001 for both). However, in adults, prevalence after the second round of IRS remained similar to that before initiation of IRS, with an observable decrease noted only after the third round (6.1% pre-IRS vs 2.1% post–third round of IRS; P = .058; Table 2 and Figure 2).
Figure 2.
Temporal changes in the prevalence of microscopic parasitemia, stratified by age. Monthly trends of microscopic parasitemia following multiple rounds of indoor residual spraying with the carbamate bendiocarb (bars).
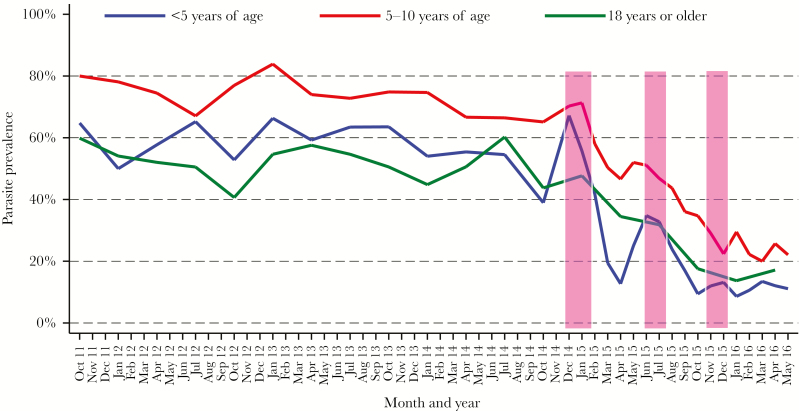
Compared with microscopy alone, the prevalence of any parasitemia was approximately twice as high in children and 8-fold higher in adults prior to IRS when LAMP results were included. The majority of infections were submicroscopic in children <5 years (59%) and in adults (88.3%), and nearly half of infections (48.1%) were submicroscopic in children 5–10 years of age. As observed with microscopic parasitemia, there was a significant reduction in parasite prevalence with each successive round of IRS in children (Table 2; Figure 3). However, in adults, in contrast to the results for microscopic parasitemia, one round of IRS was sufficient to significantly reduce the prevalence of parasitemia detected by microscopy and LAMP (PR, 0.72; 95% confidence interval [CI], .58–.88; P = .001). Critically, though 3 rounds of IRS significantly reduced parasitemia across all age groups, residual parasite prevalence after 3 rounds remained quite high, especially in children aged 5–10 years (23.7%) and adults (14.8%). After 3 rounds of IRS, submicroscopic parasitemia accounted for the majority of parasitemia in all age groups (56.8% in children <5 years of age, 56.7% in children 5–10 years, and 85.7% in adults). There was a rebound in symptomatic malaria and, to a lesser degree, in parasitemia prevalence (both microscopic and all parasitemia) after the first and second rounds of IRS, but not after the third round (Figures 1–3). The overall prevalence of gametocytes was very low (273 [2.9%]), with the majority of gametocytes seen prior to initiation of IRS (182/273 [66.7%]). There was a significant reduction in the overall prevalence of gametocytes following 3 rounds of IRS (PR, 0.76; 95% CI, .59–.97; P = .02).
Figure 3.
Temporal changes in the prevalence of any parasitemia (microscopic and submicroscopic), stratified by age. Monthly trends of microscopic and submicroscopic parasitemia following multiple rounds of indoor residual spraying with the carbamate bendiocarb (bars).
DISCUSSION
We investigated the impact of multiple rounds of IRS on the incidence of malaria and prevalence of parasitemia and how the impact varied with age, to better characterize the parasite reservoir available to contribute to transmission. IRS significantly reduced both the incidence of symptomatic malaria and the prevalence of parasitemia; however, a large proportion of the population remained parasitemic even after 3 consecutive rounds of IRS. Notably, the majority of this residual parasite reservoir was submicroscopic in all age groups.
Although significant progress has been realized in the last decade, the 2017 World Malaria Report suggested that the decline in malaria burden has stalled [1]. Indeed, current control strategies are unlikely to be sufficient to eliminate malaria in many countries, prompting calls for innovation in developing new control tools and in using available methods in novel ways. IRS has long been documented to reduce the malaria burden and improve entomological outcomes; however, its benefits are typically short-lived, as it is too expensive to continue indefinitely [14]. To our knowledge, the effect of IRS on the different reservoirs of malaria parasites has not previously been reported. Because parasitemic individuals may serve as a reservoir for transmission, particularly if IRS is delayed, interrupted, or discontinued, it is vitally important to better characterize this reservoir. Notably, similar to results from other studies [9, 16, 23–25], in our cohort 3 rounds of IRS provided almost total protection against symptomatic malaria, irrespective of age group.
Unlike previous studies, our study explored the longitudinal impact of multiple rounds of IRS on all parasitemia. Intriguingly, at baseline (pre-IRS), adults had a prevalence of all parasitemia that was nearly as high as that of children <5 years of age (52.2% vs 58.5%, respectively), primarily due to submicroscopic infections. Thus, though compared to children adults have lower-density infections and are less likely to become symptomatic, they represent a large portion of the reservoir of malaria parasitemia. Therefore, surveillance for submicroscopic infections in both children and adults is needed to fully understand the efficacy of malaria control interventions on transmission.
Importantly, in our study the prevalence of parasitemia remained high even after 3 rounds of IRS, especially in older children and adults. A possible explanation for why we observed a less pronounced drop in prevalence vs incidence is because IRS likely decreases the rate of acquisition of new infections, which are most likely to cause symptoms, without impact on existing blood stage infections, which may last for a year or longer [23]. In addition, reduction of malaria incidence after IRS will lead to fewer courses of treatment so that the average duration of infection may increase. Thus, the residual reservoir following IRS may represent infections that are on average older and less dynamic than those prior to IRS. If this is true, targeting this reservoir directly, for example via administration of antimalarials, may hasten reductions in incidence and prevalence and make these gains more robust despite delays, temporary interruptions, or otherwise decreased efficacy of vector control.
Previous studies have shown that asymptomatic infections may be responsible for the majority of onward mosquito infections, especially following control efforts [24]. Studies have also shown that though older children and adults transmit fewer parasites to mosquitoes than young children, they receive more mosquito bites and thus are likely to contribute equally or more to transmission [25]. In our high-transmission setting, 3 rounds of IRS were highly effective in reducing morbidity but did not eliminate the infectious reservoir, and a large proportion of this reservoir was composed of older children and adults. These study findings may help explain the rapidity of resurgence of malaria following the withdrawal of IRS observed in northern Uganda [16, 17] and elsewhere. The findings highlight that the combination of IRS with other effective interventions—for example, integrated vector control [26] and/or chemoprevention [27]—is likely to bring us closer to the goal of eliminating the infectious reservoir.
Limitations to our study included the observational study design and lack of a control group. However, it is likely that the observed changes in malaria incidence and parasitemia were related to the initiation of IRS given the longitudinal nature of the data and the timing and magnitude of changes [6]. Another limitation was the use of LAMP to assess for submicroscopic parasitemia; a recent study showed that LAMP failed to identify more than half of all infections diagnosed by ultrasensitive quantitative PCR [28]. Thus, we likely missed a proportion of very low-density infections, and so the true prevalence of submicroscopic parasitemia after multiple rounds of IRS is likely to be even higher than observed in our study. Finally, we were unable to tell whether the infections remaining after IRS were new or old infections; this would require genotyping these infections, which was beyond the scope of this work.
Our study highlights the need to include molecular diagnostics in epidemiological studies of malaria, as a substantial proportion of malaria infections are undetected by conventional microscopy and rapid diagnostic tests. Our current understanding of the importance of submicroscopic infections for malaria transmission is incomplete, and the longitudinal dynamics of these infections need to be further characterized, especially in the context of ongoing malaria control interventions, so that the use of these interventions can be optimized.
Notes
Acknowledgments. We thank all study participants for agreeing to participate in this study; all members of the Program for Resistance Immunology Surveillance and Modelling Study Team; and the Tororo molecular laboratory staff for the great work and a job well done.
Disclaimer. The funders of the study had no role in the study design, data collection, data interpretation, or writing of the report. The authors had the final responsibility for the decision to submit for publication.
Financial support. This work was supported by the /National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) (grant number U19A1089674); the Fogarty International Center of the NIH (Emerging Global Leader Award grant number K43TW010365); and the NURTURE Career Development Award of the NIH (grant number D43TW010132).
Potential conflicts of interest. All authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World malaria report 2017 http://www.who.int/malaria/publications/world-malaria- report-2017/report/en/. Accessed 15 February 2018.
- 2. Ministry of Health Uganda. The Uganda malaria indicator survey 2014–2015 http://www.ubos.org/2015/11/06/the-uganda-malaria-indicator-survey-2014–2015/. Accessed 15 February 2018.
- 3. Xu JW, Li JJ, Guo HP, et al. Malaria from hyperendemicity to elimination in Hekou County on China-Vietnam border: an ecological study. Malar J 2017; 16:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bruce-Chwatt LJ. Malaria eradication. In: Essential malariology. London: Hodder-Arnold, 1985. [Google Scholar]
- 5. Zhou G, Githeko A, Minakawa N, Yan G. Community-wide benefits of targeted indoor residual spray for malaria control in the western Kenya highland. Malar J 2010; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katureebe A, Zinszer K, Arinaitwe E, et al. Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: a prospective observational study. PLoS Med 2016; 13:e1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coleman S, Dadzie SK, Seyoum A, et al. A reduction in malaria transmission intensity in northern Ghana after 7 years of indoor residual spraying. Malar J 2017; 16:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oxborough RM. Trends in US President’s malaria initiative-funded indoor residual spray coverage and insecticide choice in sub-Saharan Africa (2008–2015): urgent need for affordable, long-lasting insecticides. Malar J 2016; 15:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bukirwa H, Yau V, Kigozi R, et al. Assessing the impact of indoor residual spraying on malaria morbidity using a sentinel site surveillance system in western Uganda. Am J Trop Med Hyg 2009; 81:611–4. [DOI] [PubMed] [Google Scholar]
- 10. Kigozi R, Baxi SM, Gasasira A, et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS One 2012; 7:e42857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alegana VA, Kigozi SP, Nankabirwa J, et al. Spatio-temporal analysis of malaria vector density from baseline through intervention in a high transmission setting. Parasit Vectors 2016; 9:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roh ME, Shiboski S, Natureeba P, et al. Protective effect of indoor residual spraying of insecticide on preterm birth among pregnant women with HIV infection in Uganda: a secondary data analysis. J Infect Dis 2017; 216:1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oguttu DW, Matovu JKB, Okumu DC, et al. Rapid reduction of malaria following introduction of vector control interventions in Tororo District, Uganda: a descriptive study. Malar J 2017; 16:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raouf S, Mpimbaza A, Kigozi R, et al. Resurgence of malaria following discontinuation of indoor residual spraying of insecticide in an area of Uganda with previously high-transmission intensity. Clin Infect Dis 2017; 65:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen JM, Smith DL, Cotter C, et al. Malaria resurgence: a systematic review and assessment of its causes. Malar J 2012; 11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okullo AE, Matovu JKB, Ario AR, et al. Malaria incidence among children less than 5 years during and after cessation of indoor residual spraying in northern Uganda. Malar J 2017; 16:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tukei BB, Beke A, Lamadrid-Figueroa H. Assessing the effect of indoor residual spraying (IRS) on malaria morbidity in northern Uganda: a before and after study. Malar J 2017; 16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 2014; 12:833–40. [DOI] [PubMed] [Google Scholar]
- 19. Hassanpour G, Mohebali M, Zeraati H, Raeisi A, Keshavarz H. Asymptomatic malaria and its challenges in the malaria elimination program in Iran: a systematic review. J Arthropod Borne Dis 2017; 11:172–81. [PMC free article] [PubMed] [Google Scholar]
- 20. Kamya MR, Arinaitwe E, Wanzira H, et al. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg 2015; 92:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uganda Ministry of Health. Uganda clinical guidelines 2016 http://www.health.go.ug/content/uganda-clinical- guidelines-2016. Accessed 20 February 2018.
- 22. Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg 1995; 52:565–8. [DOI] [PubMed] [Google Scholar]
- 23. Bretscher MT, Maire N, Felger I, Owusu-Agyei S, Smith T. Asymptomatic Plasmodium falciparum infections may not be shortened by acquired immunity. Malar J 2015; 14:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tadesse FG, Slater HC, Chali W, et al. the relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis 2018; 66:1883–91. [DOI] [PubMed] [Google Scholar]
- 25. Gonçalves BP, Kapulu MC, Sawa P, et al. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun 2017; 8:1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiware SS, Chitnis N, Tatarsky A, et al. Attacking the mosquito on multiple fronts: Insights from the Vector Control Optimization Model (VCOM) for malaria elimination. PLoS One 2017; 12:e0187680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walker PG, Griffin JT, Ferguson NM, Ghani AC. Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: a modelling study. Lancet Glob Health 2016; 4:e474–84. [DOI] [PubMed] [Google Scholar]
- 28. Katrak S, Murphy M, Nayebare P, et al. Performance of loop-mediated isothermal amplification for the identification of submicroscopic Plasmodium falciparum infection in Uganda. Am J Trop Med Hyg 2017; 97:1777–81. [DOI] [PMC free article] [PubMed] [Google Scholar]