Abstract
Naegleria fowleri is the causative agent of primary amoebic meningoencephalitis (PAM), which is fatal in >97% of cases. In this study, we aimed to identify new, rapidly acting drugs to increase survival rates. We conducted phenotypic screens of libraries of Food and Drug Administration–approved compounds and the Medicines for Malaria Venture Pathogen Box and validated 14 hits (defined as a 50% inhibitory concentration of <1 μM). The hits were then prioritized by assessing the rate of action and efficacy in combination with current drugs used to treat PAM. Posaconazole was found to inhibit amoeba growth within the first 12 hours of exposure, which was faster than any currently used drug. In addition, posaconazole cured 33% of N. fowleri–infected mice at a dose of 20 mg/kg and, in combination with azithromycin, increased survival by an additional 20%. Fluconazole, which is currently used for PAM therapy, was ineffective in vitro and vivo. Our results suggest posaconazole could replace fluconazole in the treatment of PAM.
Keywords: Naegleria fowleri, phenotypic screen, drug discovery, posaconazole, azithromycin, miltefosine, primary amoebic meningoencephalitis
Phenotypic screens identified 14 drugs active against Naegleria fowleri, the causative agent of primary amoebic meningoencephalitis. In vitro and in vivo data demonstrate that posaconazole is a potent, rapidly acting drug with potential for enhancing treatment of this usually fatal disease.
Naegleria fowleri is a free-living amoeba that is the causative agent of a rapidly, fulminant disease known as primary amoebic meningoencephalitis (PAM). This amoeba is ubiquitous in the environment, and most patients come in contact with amoebae through recreational water activities, use of a Neti pot, or religious customs, such as ritual ablutions [1–3]. PAM has a worldwide presence, with most cases reported in developed countries that have resources to diagnose the infection [4]. In the United States, from 1969 through 2017, there were only 4 survivors among 143 reported cases of PAM [5, 6]. However, the number of cases is most likely an underrepresentation since PAM is not a notifiable disease in the United States and may easily be misdiagnosed as bacterial meningitis or viral meningitis [7]. Underreporting of PAM is likely in tropical climates. For example, in Karachi, Pakistan, where there was an increased number of diagnosed cases at Aga Khan University Hospital after implementation of policies to perform amoeba diagnostic analysis of cerebrospinal fluid samples from patients who had symptoms of bacterial meningitis [3].
When PAM is diagnosed, patients receive a combination of drugs including amphotericin B, fluconazole, azithromycin, rifampin, and miltefosine [5]. The 2 main concerns with this treatment regimen are toxicity and poor efficacy. Amphotericin B is known to cause severe renal toxicity but has been given to patients with PAM since 1969, following its use in a PAM survivor [5, 8]. Miltefosine, initially developed as a breast cancer drug, has been repurposed for treating leishmaniasis and N. fowleri infection [9, 10]. There is experimental evidence for azithromycin efficacy in vitro and in animal models [11, 12], yet rifampin and fluconazole are used despite the lack of experimental evidence. Steroids (eg, dexamethasone) also are used to manage the increased intracranial pressure caused by the host’s immune response to the amoeba. Recently, patients have been placed in a hypothermic coma because of the neuroprotective effects associated with reducing the intracranial pressure, levels of reactive oxygen species and proinflammatory cytokines, and the frequency of neuronal apoptosis [13, 14]. Although the addition of miltefosine and hypothermic coma appear to have been beneficial in recent cases, the high fatality rates with similar treatments and the toxicity of current drugs still drive the need to identify safer and more-effective treatments [15].
Drug discovery for pathogenic free-living amoeba is not an active area of research, and all drugs currently used for treatment of PAM were selected empirically. The only recent candidate is corifungin, the sodium salt of amphotericin B, which received orphan drug status approval from the Food and Drug Administration (FDA) in 2011 but has yet to be used to treat PAM [16]. This may be due to the perceived risk of replacing amphotericin B or of adding corifungin, owing to concerns about additive toxicity.
In this study, we aimed to discover a drug that can be repurposed to successfully treat PAM. We used our previously developed high-throughput phenotypic screening method [8] to evaluate compound libraries, and we identified 14 potent hits that inhibit amoebae at nanomolar concentrations. In addition, we developed new methods for assessing rates of action and found that posaconazole had a more rapid onset of action than currently used drugs; possessed additive activity in vitro in combination with azithromycin, miltefosine, and amphotericin B, and was effective in vivo.
MATERIALS AND METHODS
Amoeba Culture
The N. fowleri isolate (NF69) used as a reference strain in these studies was isolated from a 9-year-old boy in Adelaide, Australia, who died of PAM in 1969 (ATCC 30215). Additional strains, from the Centers for Disease Control and Prevention (CDC; Supplementary Table 1), were used for comparison of in vitro drug susceptibility. All isolates were grown axenically as trophozoites at 34°C in Nelson’s complete medium. Reagents for the medium were purchased from Sigma-Aldrich (St. Louis, MO).
In Vitro Drug Susceptibility Assay
Drug susceptibility assays with the CellTiter-Glo 2.0 system (CTG; Promega, Madison, WI) were performed as previously described [8]. A collection of 1134 FDA-approved drugs assembled at the St Jude Children’s Research Hospital were prepared in 384-well plates at starting concentrations ranging from 1 to 17 mM. We first screened drugs in a single-point assay at both 1:100 and 1:1000 dilutions with 3000 amoebae/well. We also screened the Medicines for Malaria Venture (MMV) Pathogen Box, which is a collection of 400 drug and probe-like molecules that are active against various pathogens [17]. Compounds were prepared in 96-well plates at a 1-mM concentration in dimethyl sulfoxide (DMSO) and were screened in a single-point assay in 96-well plates at 10 and 1 μM. To verify active hits from both collections, putative hits were cherry picked and serially diluted in duplicate from 10 μM to 10 nM in a 96-well plate with 4000 amoebae/well, to generate dose-response curves. Curve fitting using nonlinear regression was analyzed with DataAspects Plate Manager analysis software [8].
Drug Combination Assay
Miltefosine was purchased from Cayman Chemical (Ann Arbor, MI), and posaconazole, amphotericin B, and azithromycin were purchased from Sigma Aldrich (St. Louis, MO). Isobolar combination studies with drugs were conducted by combining compounds in ratios of their 50% inhibitory concentration (IC50) and serially diluting them. CTG assay methods were followed as previously described [8].
Rate of Action Assay
The RealTime-Glo MT Cell Viability Assay (RTG; Promega, Madison, WI) was used to assess the rate of action of active compounds against N. fowleri. Compounds were plated at 1 and 5 times their IC50, with 4 replicates/plate. The 96-well plates were prepared with 10 μL of drug, 40 μL with 4000 cells, and 50 μL of RTG reagent. The positive-growth control comprised cells in medium with RTG reagent; the negative-growth control comprised medium and RTG reagent. Plates were incubated at 34°C for 72 hours in a SpectraMax i3x plate reader (Molecular Devices; Sunnyvale, CA). Relative luminescence units (RLUs) were recorded every hour for 72 hours and analyzed using Prism 7 (GraphPad Software; La Jolla, CA).
Animal Studies
In vivo efficacy studies were performed in accordance with the protocols approved by the institutional animal care and use committees of the University of South Florida and the University of Georgia. Female ICR mice were intranasally infected with 10 μL of 10000 N. fowleri trophozoites. Drug treatments began 3 days after infection and were administered once daily for 3 consecutive days. Posaconazole (Noxafil; Merck) was obtained from the St Jude Children’s Research Hospital and administered intravenously through tail vein injections once daily at 20, 10, or 5 mg/kg. Phosphate-buffered saline and 20 mg/kg miltefosine were administered intraperitoneally for the monotherapy study. Animals displaying symptoms (ie, papilledema, hunched, ataxic, stiff neck, 20% decrease in body weight, and shallow breathing) were euthanized to relieve suffering per the approved protocols. Sham controls were infected mice dosed with drug vehicle. Mice were monitored twice daily, but deaths in the monotherapy study were pooled and recorded daily. Samples of brain tissue were collected at autopsy to confirm the presence of amoebae.
A similar protocol was used for the combination studies. Mice in monotherapy groups were dosed with 20 mg/kg of posaconazole intravenously, 30 mg/kg of fluconazole intraperitoneally, 25 mg/kg ketoconazole intraperitoneally, or 25 mg/kg azithromycin intraperitoneally. Mice in combination groups were dose with 25 mg/kg of azithromycin intraperitoneally and either 20 mg/kg of posaconazole intravenously, 30 mg/kg of fluconazole intraperitoneally, or 25 mg/kg of ketoconazole intraperitoneally. Intravenously administered PBS and intraperitoneally administered 0.5% HEC/0.1% Tween 80 with 10% DMSO were used as vehicle controls. Mice were monitored twice daily, and survival data (deaths or compassionate euthanasia) were recorded twice per day.
RESULTS
Phenotypic Screen of Bioactive Compound Libraries
Following the development of new quantitative methods for high-throughput screening with N. fowleri [8], we screened 2 libraries of known bioactive compounds. The first was a library of 1134 FDA-approved drugs that was assembled at the St Jude Children’s Research Hospital. The second library was the MMV Pathogen Box; this collection contains 400 compounds with known activities against parasites that cause malaria, leishmaniasis, trypanosomiasis, and 10 other infectious diseases [17].
The initial screens of both compound libraries were conducted as single-point assays of each compound at approximately 10 μM and approximately 1 μM. The quality control of the single point assay screens was characterized by a Z′ of 0.95 for 384-well plates and a Z′ of 0.93 for 96-well plates. From the FDA library of bioactive compounds, 24 hits were identified that produced ≥70% inhibition of growth of N. fowleri in the 72-hour assay (Supplementary Figure 1). Several of the hits were identified more than once, since different salts of some drugs (eg, azithromycin and azithromycin dihydrate) were included. These hits were next evaluated in dose response assays to determine the IC50. From these phenotypic screen data, 20 hits from multiple drug classes were confirmed (Table 1). Among the most potent were antibiotics that target protein synthesis. Azithromycin was the most potent compound, with an IC50 of 20 nM, and several related antibiotics also were found to produce an IC50 of ≤440 nM; these included clarithromycin, erythromycin, and dirithromycin. Tilmicosin, a macrolide antibiotic for veterinary use, was among the most potent compounds (IC50, 60 nM). For the first time, 2 pleuromutilin antibiotics, valnemulin and retapamulin, were found to be active against N. fowleri. Antifungals were the next major class that produced multiple hits. These included the known inhibitors itraconazole and ketoconazole, but posaconazole was also found to be potent (IC50, 240 nM). In addition, a few nucleosides were identified as hits. The pyrimidine analog gemcitabine was a potent inhibitor (IC50, 340 nM) and the purine nucleoside analogs fludarabine and clofarabine produced IC50 values of 460 nM and 1.76 μM, respectively.
Table 1.
Active Food and Drug Administration–Approved Compounds Identified from the St. Jude Children’s Research Hospital Library
| Class, Compound | IC50, μM |
|---|---|
| Antibiotics | |
| Azithromycina | 0.02 |
| Azithromycin dihydratea | 0.02 |
| Clarithromycin | 0.03 |
| Valnemulin hydrochloride | 0.04 |
| Tilmicosin | 0.06 |
| Erythromycin ethylsuccinatea | 0.10 |
| (-)-Erythromycina | 0.12 |
| Roxithromycin | 0.20 |
| Dirithromycin | 0.39 |
| Retapamulin | 0.44 |
| Antifungals | |
| Itraconazole | 0.08 |
| Posaconazole | 0.24 |
| Butenafine hydrochloride | 0.42 |
| Ketoconazole | 1.37 |
| Terbinafine hydrochloridea | 3.00 |
| Liranaftate | 5.42 |
| Terbinafinea | 6.05 |
| Nucleosides | |
| Gemcitabinea | 0.34 |
| Gemcitabine hydrochloridea | 0.36 |
| Fludarabine phosphate | 0.58 |
| Clofarabine | 1.76 |
| Proteasome inhibitor | |
| Bortezomib | 0.60 |
| HDAC inhibitor | |
| Vorinostat | 1.26 |
| HMG-CoA reductase | |
| Pitavastatin | 3.52 |
Abbreviations: CoA, coenzyme A; HDAC, histone deacetylase; IC50, 50% inhibitory concentration.
aDifferent salts were included in the compound library.
The screen of the MMV Pathogen Box produced fewer hits (n = 13), yet posaconazole was confirmed again in this screen (Table 2). Posaconazole was the most potent compound identified in the Pathogen Box and was included in the library as a reference compound (used for the treatment of Chagas disease). Interestingly, the majority of hits with IC50 values of 1–2 μM were compounds with demonstrated activity against kinetoplastid parasites.
Table 2.
Active Compounds From the Medicines for Malaria Venture (MMV) Pathogen Box
| Compound | Other Name | IC50, μM | Disease Seta | Source(s) |
|---|---|---|---|---|
| MMV688774 | Posaconazole | 0.86 | Reference | NA |
| MMV652003 | AN3520 | 1.10 | Kinetoplastids | [23, 25] |
| MMV688180 | DDD85646 | 1.44 | Kinetoplastids | [26–28] |
| MMV024406 | NA | 1.61 | Kinetoplastids | [26–28] |
| MMV676602 | Milciclib | 1.78 | Kinetoplastids | [29, 30] |
| MMV676604 | NA | 2.17 | Kinetoplastids | [30] |
| MMV688943 | Difenoconazole | >10 | Kinetoplastids | [30] |
| MMV688942 | Bitertanol | >10 | Kinetoplastids | [30] |
| MMV689244 | NA | >10 | Kinetoplastids | [30] |
| MMV689243 | NA | >10 | Kinetoplastids | [30] |
| MMV006741 | NA | >10 | Malaria | [30] |
| MMV1019989 | NA | >10 | Malaria | [30] |
| MMV1037162 | NA | >10 | Malaria | [30] |
Abbreviations: IC50, 50% inhibitory concentration; NA, not applicable.
aRefers to the group of organisms that have known activity with the compounds in the collection, as detailed by the MMV.
We also screened a small set of miscellaneous compounds with known activity against Plasmodium falciparum and other parasites (Supplementary Table 2). The most-potent compounds in this library were auranofin (IC50, 8.37 μM), trans-mirincamycin (IC50, 9.29 μM), and tafenoquine succinate (IC50, 9.56 μM). Most compounds in this library were inactive at the concentrations screened (Supplementary Table 2).
Rate of Action Studies
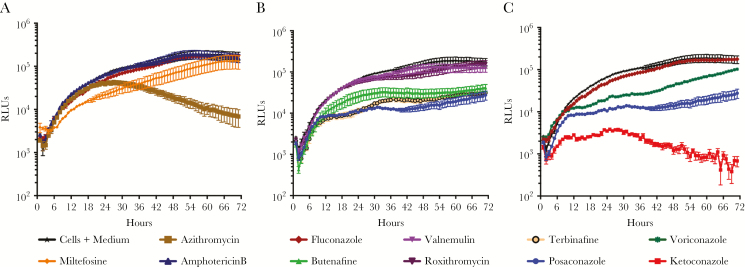
PAM is a rapidly fulminant disease, with a short onset of symptoms followed by coma and death. Therefore, an important pharmacodynamic property of drugs to treat PAM is a rapid onset of action. Few studies have addressed this issue, and, more importantly, the current treatment drugs have been shown to require long exposure times (≥72 hours) to produce dose response data in vitro [8]. In this study, we developed a new kinetic read assay, which assesses the rate of action against N. fowleri. This assay is based on metabolically active cells, and the RLUs produced were found to be proportional to growing amoebae (Supplementary Figure 2). To assess the rate of action, we first determined the IC50 for each compound, and then we exposed amoeba to 1 times the IC50 (Figure 1) or 5 times the IC50 (Supplementary Figure 3), and luciferase expression was monitored every hour for 72 hours. The results demonstrate that drugs currently used to treat PAM (ie, miltefosine, amphotericin B, azithromycin, and fluconazole) have a slow onset of action in this assay (Figure 1). Interestingly, azithromycin had a lag phase of reduced activity for 30 hours before rapid onset of significant inhibition of amoeba growth (Figure 1A); increasing the dose of azithromycin to 5 times the IC50 did not abrogate the lag phase (Supplementary Figure 3A). At 1 times the IC50 of miltefosine, we did not see any shift differing from the control, but at the higher concentration tested the miltefosine curve dropped below the background level; this may be due to solubility of drug in the growth medium (Supplementary Figure 3A). Importantly fluconazole, a drug that is included in the current treatment regimens, was not effective in the rate of action assay at either concentration (Figure 1A) and was not potent in standard in vitro drug susceptibility assays (IC50, 1.9 μM).
Figure 1.
Rate of inhibition at 1 times the 50% inhibitory concentration (IC50) for drugs currently used to treat primary amoebic meningoencephalitis (A), Food and Drug Administration–approved compounds identified through the St. Jude Children’s Research Hospital library screen (B), and known antifungals (C). Data are from a single experiment with 4 replicates but are representative of independent biological repeats (n = 3). RLU, relative luminescence unit.
Next, we examined the rate of action of hits identified in the library of FDA-approved drugs. Posaconazole and terbinafine exhibited the most rapid onset of action at the 1 times the IC50 concentration (Figure 1B). At 5 times the IC50, valnemulin and roxithromycin showed a lag phase similar to that observed with azithromycin (Supplementary Figure 3B). A comparison of different conazoles identified both ketoconazole and posaconazole to be the most rapidly acting compounds in this study, at both the high and low drug concentrations used (Figure 1C and Supplementary Figure 3C).
Drug Combinations
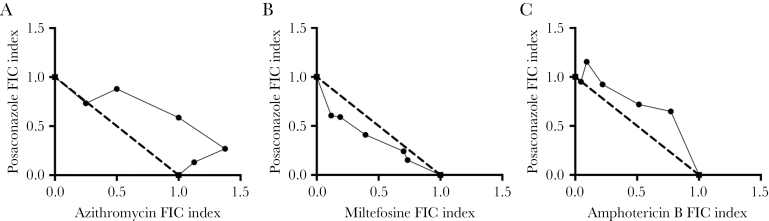
Isobolograms from the fixed-ratio combinations between posaconazole and miltefosine, amphotericin B, or azithromycin showed additivity. Importantly no antagonistic activity, (defined as a summation fractional inhibitory concentration [ΣFIC] index of >2) or synergistic activity (defined as a ΣFIC of <0.5) was seen with posaconazole in combination with any drug tested in this study (Figure 2).
Figure 2.
Isobolograms of posaconazole with azithromycin (A), miltefosine (B), or amphotericin B (C). The dotted line represents the line of additivity. Data are from a single experiment with 4 replicates but are representative of independent biological repeats (n = 3). FIC, fractional inhibitory concentration.
In Vivo Efficacy
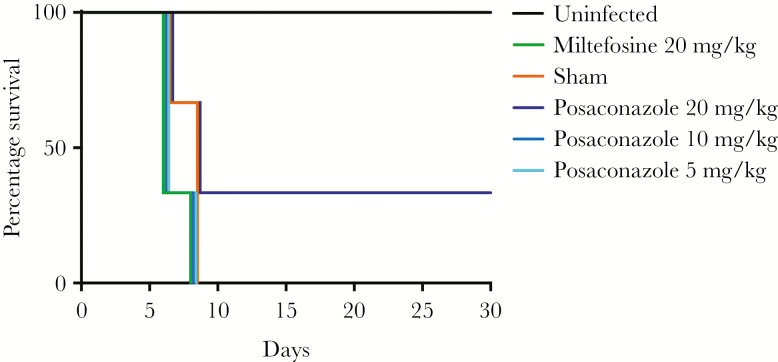
Posaconazole was among the most-potent drugs identified in the in vitro study, possessed a rapid onset of action, and was additive in in vitro combination assays with azithromycin, amphotericin B, and miltefosine. Therefore, we next assessed the efficacy of posaconazole in the mouse model of PAM (Figure 3 and Supplementary Figure 4). We infected mice with NF69 strain on day 0 and dosed them once daily on days 3–5. Posaconazole at 20 mg/kg (intravenously administered) showed significant results by curing 2 of 6 mice (P < .05), whereas lower doses of posaconazole and miltefosine were not effective. Importantly, the model demonstrated the high degree of pathogenicity of N. fowleri, with all sham controls dying of disease by day 8. A repeat study with a less virulent challenge produced similar cure rates with 20 mg/kg posaconazole (Supplementary Figure 4). These data suggest that posaconazole is moderately effective when used alone, a result that suggests it is as potent as any drug ever used as monotherapy in the mouse model.
Figure 3.
Kaplan-Meier survival curves for 6 female ICR mice dosed intravenously with posaconazole at 20 mg/kg, 10 mg/kg, and 5 mg/kg; phosphate-buffered saline; or 20 mg/kg miltefosine. A survival rate of 33% was observed with mice treated with intravenous posaconazole (20 mg/kg; P < .05 by the log-rank test for trend).
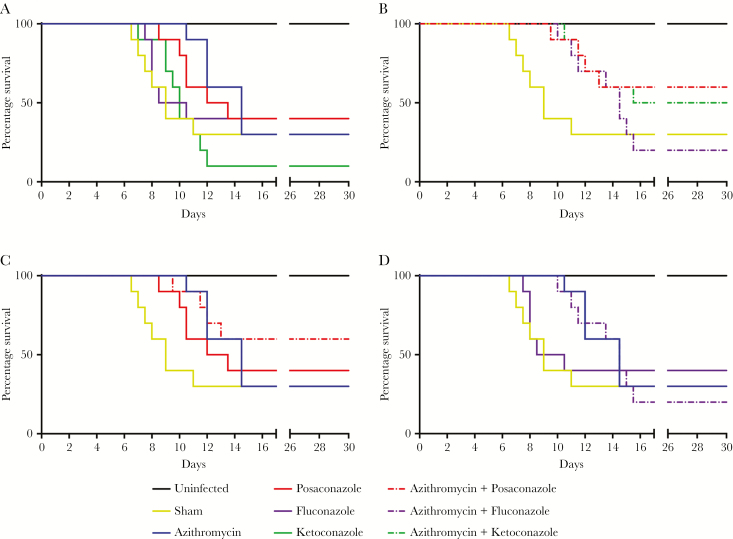
To assess whether posaconazole is more effective than fluconazole, we tested posaconazole (20 mg/kg), ketoconazole (25 mg/kg), and fluconazole (30 mg/kg) alone and in combination with azithromycin (25 mg/kg) in the PAM murine model. While the survival rate of azithromycin alone and vehicle were the same (30%), azithromycin alone prolonged survival for up to 5 days (Figure 4A). There was no significant difference in survival rate with posaconazole alone, but we observed prolonged survival of approximately 2.5 days. However, there was a significant increase in survival in the group of animals treated with a combination of posaconazole and azithromycin as compared to sham-treated mice (P < .05; Figure 4B). In contrast, prolonged survival in mice that received other combination treatments (azithromycin-fluconazole and azithromycin-ketoconazole) was no different than observed in mice treated with azithromycin alone (Figure 4B–D).
Figure 4.
Survival curves for 10 female ICR mice infected with Naegleria fowleri trophozoites and dosed with azithromycin at 25 mg/kg (A), posaconazole at 20 mg/kg alone and in combination with azithromycin (B), fluconazole 30 mg/kg alone and in combination with azithromycin (C), and ketoconazole alone at 25 mg/kg and in combination with azithromycin (D). Solid lines represent drug alone, and dashed lines represent combination with azithromycin. Deaths are reported in half days.
Drug Susceptibility Across Various N. fowleri Strains
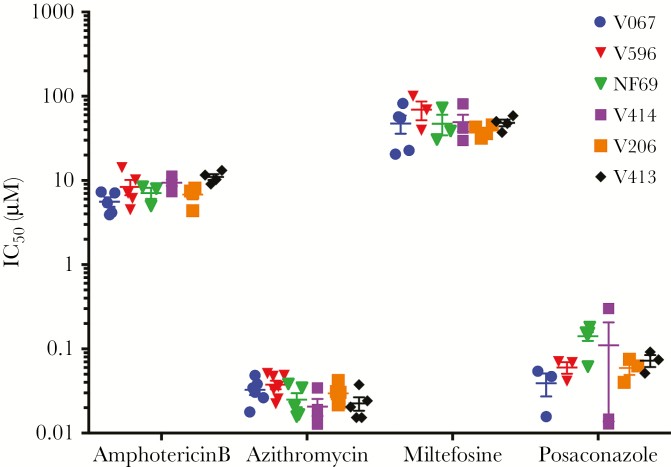
N. fowleri is ubiquitous in the environment, especially in warm climates, yet little is known about genetic heterogeneity and response to chemotherapeutic drugs. Therefore, we assessed the in vitro potency of amphotericin B, miltefosine, azithromycin, and posaconazole against 5 patient isolates of N. fowleri that were obtained from the CDC and compared the data to NF69 (Figure 5). Using the Dunnett multiple comparisons test, we found no significant differences in IC50 between NF69 and the CDC isolates for the 4 most potent drugs (P > .01). These data demonstrate that azithromycin and posaconazole are much more potent than the currently used drugs (ie, amphotericin B and miltefosine) against multiple N. fowleri isolates, further demonstrating the potential of posaconazole and azithromycin as effective combination drugs to be used in the treatment regimen of PAM.
Figure 5.
Fifty percent inhibitory concentration (IC50) of multiple Centers for Disease Control and Prevention isolates and NF69 (reference strain). Each point represents 1 biological repeat with 2 replicates (n ≥ 3). Error bars represent standard errors of the mean.
DISCUSSION
Phenotypic whole-cell screens have provided a major boost for discovery of new hits and leads for drugs targeting multiple parasitic protozoan diseases [18, 19]. In particular, the repurposing of approved drugs for new indications is an attractive option for diseases such as PAM. In this study, we used a high-throughput assay and identified numerous new hits that can accelerate drug discovery, as well as compounds that can be repurposed and used immediately for treatment of this neglected, nearly uniformly fatal disease.
Multiple antibiotics were identified that provided nanomolar potency against N. fowleri. The most active compound was azithromycin, a drug already used to treat PAM. We also found that clarithromycin, erythromycin, and roxithromycin have nanomolar activity as previously reported from in vitro studies [20]. We also identified 3 veterinary antibiotics—valnemulin, retapamulin, and tilmicosin [21, 22]—that potentially could be used to treat animal infections. Further research is warranted to assess whether these are viable candidates for treatment of amoeba infections.
From the MMV Pathogen Box we identified 6 compounds with known antiparasitic activity that had low micromolar potency. MMV652003 is a benzoxaborole analog that targets leucyl–transfer RNA synthetase and is active against Trypanosoma brucei brucei, P. falciparum, and Caenorhabditis elegans [23–25]. MMV688180 is a pyrazole sulfonamide inhibitor of N-myristoyltransferase; it is known to have trypanocidal activity and cured all mice in HAT models [26] and was effective yet less potent against Trypanosoma cruzi [27]. Interestingly, MMV688180 (DDD85646) has been optimized to produce analogs with a lower molecular weight and polar surface area to increase blood brain barrier permeability [28]. MMV676602, a mammalian cyclin-dependent protein kinase inhibitor, has shown nanomolar activity in Neospora caninum but was ineffective in vivo [29]. MMV676602 and MMV676604 are known to be potent against Toxoplasma gondii tachyzoites [30]. All of these hits provide starting points for new lead optimization studies for drugs active against amoebae.
As expected we found multiple active antifungals, with itraconazole and posaconazole being 2 of the most active. Posaconazole was initially developed for invasive Candida and Aspergillus infections in immunocompromised individuals [31] and was a hit in both library screens. Conazoles are of interest because they target CYP51, a key enzyme in sterol biosynthesis and a verified target in N. fowleri [32]. The amine antifungals butenafine and terbinafine were identified as hits. Butenafine is a benzyl amine derivative that is used to treat tinea pedis and Candida albicans [33]. Terbinafine is mainly used to treat fungal infections but has also been used in combination to treat osteocutaneous acanthamoebiasis [34].
The most important result of our phenotypic screens was the identification of posaconazole as a repurposing candidate. Posaconazole possessed the key pharmacodynamic properties required for a new drug for PAM. Since PAM is such a rapidly fulminant disease that often is diagnosed just before (or after) a patient dies, rapidity of drug action is the most important property of a new drug. We validated a new rate of action method and demonstrated that posaconazole and ketoconazole are the fastest acting compounds ever identified for inhibiting amoeba growth. In this assay, posaconazole inhibited amoeba growth significantly by 12 hours; in contrast, azithromycin did not begin to inhibit growth until 30 hours, and other currently used drugs had an even slower onset of action. Another important pharmacodynamic property is the ability to use it in the cocktail of existing drugs currently used to treat PAM. We assessed synergistic and antagonistic potential for posaconazole and found that posaconazole had additive activity with amphotericin B, miltefosine, and azithromycin. Although no synergy was observed, additive drug interactions are beneficial, especially since there is no indication posaconazole would reduce the efficacy of the other drugs currently used.
N. fowleri–infected mice are a good model for PAM; however, very few drugs have shown efficacy in this model, and none have produced high cure rates as monotherapy [35]. We elected to use a stringent model to assess drug efficacy. We waited 3 days after infection before dosing and then dosed only once daily for 3 days to assess survival and cure rates. Presumably, dosing more frequently or starting treatment earlier could have increased the efficacy, yet we aimed to test drugs in a model that is similar to the current situation, in which diagnosis is often very late during an infection. Posaconazole (20 mg/kg administered intravenously) cured 33% of infected mice; this rate was as high as that for any other reported drug when used as monotherapy. Furthermore, the combination of posaconazole (25 mg/kg) plus azithromycin (20 mg/kg) was the most effective combination we tested, as evidenced by prolonged survival and increased cure rate. These data suggest that posaconazole is a potential combination partner for the treatment of PAM.
Fluconazole has been used empirically in the cocktail of drugs for PAM [8]. Presumably it was included because of its low molecular weight, low degree of binding to plasma protein, and ability to cross the BBB more effectively than other conazoles [36]. Importantly, there are no published data that fluconazole is effective against N. fowleri in vitro or in vivo, and our data demonstrated a very poor efficacy of fluconazole, both in vitro and in vivo, thus bringing into question its usefulness in the current therapeutic regimen. Our studies demonstrate that posaconazole is much more potent than fluconazole, has a rapid onset of action, is equally active against diverse strains, and produces greater efficacy in the murine model of PAM, both in monotherapy and in combination with azithromycin.
In summary, our data suggest posaconazole is among the most potent, rapidly acting drugs with the potential for enhancing treatment of this usually fatal disease. Our data suggest that the inclusion of fluconazole may not be warranted in the current PAM treatment regimen and that posaconazole could replace fluconazole in the treatment regimen for future PAM cases.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments We thank Dr Ibne Ali (Centers for Disease Control and Prevention) for the clinical isolates of N. fowleri used in this study and Emma Troth (University of Georgia) for technical assistance.
B. L. C., C. A. R., R. K. G., and D. E. K. designed the experiments; B.L.C and C.A.R. performed the experiments; B.L.C, C.A.R., R.K.G., and D.E.K analyzed the data; and B.L.C, C.A.R., R.K.G., and D.E.K wrote the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (R21AI103664 and R21AI119787), the University of South Florida, College of Public Health, and the Georgia Research Alliance.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Yoder JS, Eddy BA, Visvesvara GS, Capewell L, Beach MJ. The epidemiology of primary amoebic meningoencephalitis in the USA, 1962–2008. Epidemiol Infect 2010; 138:968–75. [DOI] [PubMed] [Google Scholar]
- 2. Yoder JS, Straif-Bourgeois S, Roy SL, et al. Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin Infect Dis 2012; 55:e79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shakoor S, Beg MA, Mahmood SF, et al. Primary amebic meningoencephalitis caused by Naegleria fowleri, Karachi, Pakistan. Emerg Infect Dis 2011; 17:258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Visvesvara GS, Stehr-Green JK. Epidemiology of free-living ameba infections. J Protozool 1990; 37:25S–33S. [DOI] [PubMed] [Google Scholar]
- 5. Capewell LG, Harris AM, Yoder JS, et al. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937–2013. J Pediatric Infect Dis Soc 2015; 4:68–75. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Naegleria fowleri—primary amebic meningoencephalitis (PAM)—amebic encephalitis. 2014. http://www.cdc.gov/parasites/naegleria/diagnosis.html. Accessed 11 March 2018. [Google Scholar]
- 7. Matanock A, Mehal JM, Liu L, Blau DM, Cope JR. Estimation of undiagnosed Naegleria fowleri primary amebic meningoencephalitis, United States1. Emerg Infect Dis 2018; 24:162–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rice CA, Colon BL, Alp M, Göker H, Boykin DW, Kyle DE. Bis-benzimidazole hits against Naegleria fowleri discovered with new high-throughput screens. Antimicrob Agents Chemother 2015; 59:2037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention (CDC). Investigational drug available directly from CDC for the treatment of infections with free-living amebae. MMWR Morb Mortal Wkly Rep 2013; 62:666. [PMC free article] [PubMed] [Google Scholar]
- 10. Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol 2006; 53:121–6. [DOI] [PubMed] [Google Scholar]
- 11. Goswick SM, Brenner GM. Activities of therapeutic agents against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. J Parasitol 2003; 89:837–42. [DOI] [PubMed] [Google Scholar]
- 12. Soltow SM, Brenner GM. Synergistic activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. Antimicrob Agents Chemother 2007; 51:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Irazuzta JE, Pretzlaff R, Rowin M, Milam K, Zemlan FP, Zingarelli B. Hypothermia as an adjunctive treatment for severe bacterial meningitis. Brain Res 2000; 881:88–97. [DOI] [PubMed] [Google Scholar]
- 14. Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 2009; 37:1101–20. [DOI] [PubMed] [Google Scholar]
- 15. Cope JR, Ali IK. Primary amebic meningoencephalitis: what have we learned in the last 5 years?Curr Infect Dis Rep 2016; 18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Debnath A, Tunac JB, Galindo-Gómez S, Silva-Olivares A, Shibayama M, McKerrow JH. Corifungin, a new drug lead against Naegleria, identified from a high-throughput screen. Antimicrob Agents Chemother 2012; 56:5450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Medicines for Malaria Venture. The Pathogen Box. Catalysing neglected disease drug discovery. 2016. https://www.pathogenbox.org/. Accessed 3 May 2018.
- 18. Lotharius J, Gamo-Benito FJ, Angulo-Barturen I, et al. Repositioning: the fast track to new anti-malarial medicines?Malar J 2014; 13:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voorhis WC, Van Adams JH, Adelfio R, et al. Open source drug discovery with the malaria box compound collection for neglected diseases and beyond. PLoS Pathog 2016; 12:e1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JH, Lee YJ, Sohn HJ, et al. Therapeutic effect of rokitamycin in vitro and on experimental meningoencephalitis due to Naegleria fowleri. Int J Antimicrob Agents 2008; 32:411–7. [DOI] [PubMed] [Google Scholar]
- 21. Šperling D, Čížek A, Smola J. Effect of zinc chelate and valnemulin for the treatment of swine dysentery in an experimental challenge study. Res Vet Sci 2014; 96:30–2. [DOI] [PubMed] [Google Scholar]
- 22. Gorham PE, Carroll LH, McAskill JW, et al. Tilmicosin as a single injection treatment for respiratory disease of feedlot cattle. Can Vet J 1990; 31:826–9. [PMC free article] [PubMed] [Google Scholar]
- 23. Nare B, Wring S, Bacchi C, et al. Discovery of novel orally bioavailable oxaborole 6-carboxamides that demonstrate cure in a murine model of late-stage central nervous system African trypanosomiasis. Antimicrob Agents Chemother 2010; 54:4379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duffy S, Sykes ML, Jones AJ, et al. Screening the medicines for malaria venture pathogen box across multiple pathogens reclassifies starting points for open-source drug discovery. Antimicrob Agents Chemother 2017; 61:e00379–01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Partridge FA, Brown AE, Buckingham SD, et al. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int J Parasitol Drugs Drug Resist 2018; 8:8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frearson JA, Brand S, McElroy SP, et al. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature 2010; 464:728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts AJ, Torrie LS, Wyllie S, Fairlamb AH. Biochemical and genetic characterization of Trypanosoma cruzi N-myristoyltransferase. Biochem J 2014; 459:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bayliss T, Robinson DA, Smith VC, et al. Design and synthesis of brain penetrant trypanocidal N-myristoyltransferase inhibitors. J Med Chem 2017; 60:9790–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Müller J, Aguado A, Laleu B, Balmer V, Ritler D, Hemphill A. In vitro screening of the open source Pathogen Box identifies novel compounds with profound activities against Neospora caninum. Int J Parasitol 2017; 47:801–9. [DOI] [PubMed] [Google Scholar]
- 30. Spalenka J, Escotte-Binet S, Bakiri A, et al. Discovery of new inhibitors of toxoplasma gondii via the pathogen box. Antimicrob Agents Chemother 2018; 62:e01640–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Greer ND. Posaconazole (Noxafil): a new triazole antifungal agent. Proc (Bayl Univ Med Cent). 2007; 20:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Debnath A, Calvet CM, Jennings G, et al. CYP51 is an essential drug target for the treatment of primary amoebic meningoencephalitis (PAM). PLoS Negl Trop Dis 2017; 11:e0006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iwatani W, Arika T, Yamaguchi H. Two mechanisms of butenafine action in Candida albicans. Antimicrob Agents Chemother. 1993; 37:785–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma M, Sudhan SS, Sharma S, Megha K, Nada R, Khurana S. Osteo-cutaneous acanthamoebiasis in a non-immunocompromised patient with a favorable outcome. Parasitol Int 2017; 66:727–30. [DOI] [PubMed] [Google Scholar]
- 35. Visvesvara GS. Amebic meningoencephalitides and keratitis: challenges in diagnosis and treatment. Curr Opin Infect Dis 2010; 23:590–4. [DOI] [PubMed] [Google Scholar]
- 36. Kethireddy S, Andes D. CNS pharmacokinetics of antifungal agents. Expert Opin Drug Metab Toxicol 2007; 3:573–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.