Abstract
We recently developed anti-OspA human immunoglobulin G1 monoclonal antibodies (HuMAbs) that are effective in preventing Borrelia transmission from ticks in a murine model. Here, we investigated a novel approach of DNA-mediated gene transfer of HuMAbs that provide protection against Lyme disease. Plasmid DNA-encoded anti-OspA HuMAbs inoculated in mice achieved a serum antibody concentration of >6 μg/mL. Among mice injected with DNA-encoded monoclonal antibodies, 75%–77% were protected against an acute challenge by Borrelia-infected ticks. Our results represent the first demonstration of employing DNA transfer as a delivery system for antibodies that block transmission of Borrelia in animal models.
Keywords: Lyme disease, DMAb, OspA, human monoclonal antibody
We investigated a novel approach of DNA-mediated gene transfer of anti-OspA human immunoglobulin G1 monoclonal antibodies that provide protection against Lyme disease. Our results represent the first demonstration of employing DNA transfer to produce antibodies that block transmission by Borrelia-infected ticks.
Lyme disease is a bacterial infection caused by Borrelia burgdorferi, which is transmitted when an infected Ixodes tick feeds on a susceptible human. Tick transmission of Borrelia spirochetes to a human results in significant Lyme disease morbidity in the United States. The Centers for Disease Control and Prevention has estimated that >300000 Americans receive a diagnosis of Lyme disease yearly [1]. Antibodies raised against Borrelia outer surface protein A (OspA) block transmission of spirochetes from ticks to vertebrate hosts in animal models [2–4]. Based on the effectiveness of OspA-specific humoral immunity in these animal models, vaccines containing recombinant OspA of B. burgdorferi were developed to induce a similar antibody response in humans for the prevention of Lyme disease [5]. Despite 76% efficacy, the vaccine was removed from the market due to poor market performance [6]. Currently, no vaccine is available in the United States to prevent human Lyme disease. Thus, there is an urgent need for new Lyme disease prevention strategies.
We have previously reported on the discovery of anti-OspA human monoclonal antibodies (HuMAbs) that completely prevented B. burgdorferi transmission in a mouse model [7]. These HuMAbs can be developed as novel preexposure prophylaxis (PrEP) for at-risk individuals and have many advantages over a conventional vaccine approach, including immediate protection with an antibody of known specificity and concentration, and independence from the age-dependent responses to active immunization. While a passive antibody delivery is an attractive approach for intervention against pathogens, the expense of bioprocessing and cold chain requirements limit this approach for dissemination to global populations. In contrast, DNA-mediated gene transfer utilizes naked DNA plasmids for in vivo delivery of expression of functional antibodies [8]. This approach bypasses conventional antibody production and allows for repeated delivery and in vivo expression that can persist for up to 19 weeks [9]. Our group recently demonstrated that DNA-encoded monoclonal antibodies (DMAbs) delivered by CELLECTRA electroporation (EP) technology can provide protection against dengue virus infection in animal models [10].
Here we describe using the DMAb approach to deliver a potent anti-OspA Lyme HuMAb 319–44. HuMAb 319–44 is an MAb that has strong in vitro borreliacidal activity against B. burgdorferi and Borrelia afzelii and in vivo protection against B. burgdorferi–infected tick challenge [7]. Our results demonstrated that DMAb delivery provided sterilizing protection against Lyme disease by blocking transmission of Borrelia from ticks to animals.
MATERIALS AND METHODS
DMAb Constructs
A wild-type (WT) and modified (Mod1) version of the HuMAbs 319–44 were generated as DMAb constructs. In both variants, the transgenes encode the antibody heavy-chain and light-chain sequences, separated by a furin/picornavirus-2A peptide cleavage site sequence. DMAb Mod1 variant was generated by framework modification of the WT variant to enhance antibody expression. Antibody amino acid sequences were RNA- and codon-optimized for expression in humans and mice. The resulting DNA transgenes were synthesized de novo (Genscript). These synthetic transgenes were cloned into the pGX0001 DNA expression vector (Invitrogen) under the cytomegalovirus immediate-early promoter. Immunoglobulin G (IgG) heavy-chain and light-chain leader sequences were added for cellular processing and secretion.
In Vitro Expression of DMAbs
Human embryonic kidney (HEK) 293T cells were maintained in Dulbecco’s modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum. Cells were plated at 2.5 × 105 cells per well in a 12-well plate and transfected with 0.5μg plasmid DNA using GeneJammer (Agilent Technologies). Forty-eighthours later, supernatants were collected and total antibody expression was quantified by enzyme-linked immunosorbent assay (ELISA).
Enzyme-Linked Immunosorbent Assay
For quantification of total human IgG1κ, cell supernatants or mouse sera were added to plates coated with 10μg/mL goat antihuman IgG Fc fragment (Bethyl Laboratories). Plates were stained with horseradish peroxidase–conjugated goat antihuman κ light-chain (1:20000; Bethyl Laboratories) and developed using SigmaFast o-Phenylenediamine dihydrochloride (Sigma-Aldrich). For binding activity against OspA proteins, purified antibody or mouse sera were added to plates coated with 2 μg/mL of recombinant his-OspA B. burgdorferi B31. Plates were stained with alkaline phosphatase (AP)–conjugated goat antihuman IgG Fcγ (1:1000; Jackson ImmunoResearch Laboratories) and developed using p-nitrophenyl phosphate (PNPP; ThermoFisher Scientific). To detect total anti-Borrelia mouse antibodies, mouse sera were added to plates coated with 1 μg/mL of B. burgdorferi N40 lysate prepared as previously described [7]. Plates were stained with AP-conjugated goat antimouse IgG Fcγ (1:1000; Jackson ImmunoResearch Laboratories) and developed using PNPP. Absorbance for all ELISAs was measured at 405nm using an Emax precision plate reader (Molecular Devices).
Borreliacidal Assay by Bac-Titer Glo Detection
The borreliacidal assay was conducted using B. burgdorferi B31 (ATCC35210) as previously described [7]. In brief, serial dilutions of antibodies were made in 100 µL of Barbour–Stonner–Kelly (BSK-H) medium containing 10% of guinea pig complement (Sigma) in a 96-well plate (ThermoFisher Scientific). One hundred microliters of B. burgdorferi (5 × 106 spirochetes/mL) in BSK-H medium was added to each well. After 3 days, spirochete viability was quantified by luciferase detection using Bac-Titer Glo reagent (Promega). Fluorescence was detected using a Victor3 multilabel counter (Applied Biosystems). Relative viability was calculated by comparing 319–44 antibodies cultures to an irrelevant control antibody culture. The assay was conducted in triplicate, and half maximal effective concentrations were reported as the mean and standard deviation.
Animals
C3H and severe combined immunodeficient (SCID) mice (Jackson Laboratory) housed and treated in a temperature-controlled, light-cycled facility were used to analyze in vivo DMAb expression and for tick challenge studies. Animal protocols were approved by the Institutional Animal Care and Use Committee at Wistar and Tufts University according to guidelines.
Intramuscular DNA CELLECTRA Electroporation
Six- to 8-week-old female C3H mice were administered 100 μg, 200 μg, or 300 μg doses of DMAb DNA by intramuscular injections (100 μg/leg) into the tibialis anterior or quadriceps muscles. The leg was also injected intramuscularly with hyaluronidase (200 U/mL, Sigma-Aldrich) in the same site as the DMAb injection. The injections were followed by EP using a CELLECTRA 3P adaptive constant current device (Inovio Pharmaceuticals, Plymouth Meeting, Pennsylvania) [11]. Serum was collected to monitor in vivo antibody expression 1 week postadministration.
Mouse Challenge With Infected Ticks
DMAb-injected mice were challenged with B. burgdorferi–infected nymphs using an established murine tick challenge model as previously described [7]. In brief, infected nymphs were prepared by placing Ixodes larva on B. burgdorferi N40–infected SCID mice for a blood meal. Larvae were harvested and allowed to molt to the nymphal stage before use for challenge. The infection rate of infected nymphs was checked by polymerase chain reaction (PCR) to ensure >80% infection. C3H mice were given a 300-μg dose of 319–44 WT DMAb, 319–44 Mod1 DMAb, or an irrelevant IgG DMAb by IM injection followed by CELLECTRA EP. An additional group of C3H mice was intraperitoneally injected with 319–44 WT HuMAb at a dose of 1.5 mg/kg. This dose was chosen based on preliminary studies showing that it would provide a similar antibody concentration in the serum as the DMAbs (data not shown). Serum was collected using submandibular bleeds 5 days postinjection. Following the blood collection, mice were challenged by placing 6 Borrelia-infected nymphs behind the ear of each mouse. Nymphs were collected after taking a blood meal, approximately 3–4 days later. Three weeks later, serum samples were collected to analyze DMAb concentration and mouse anti-Borrelia antibody presence. Tissue samples from ear, bladder, heart, and joint were harvested and cultured in BSK-H medium for twice-weekly observations for 4 weeks by dark field microscopy to detect the presence of spirochetes. PCR was conducted on the liquid cultures weekly using OspA-specific PCR primers, for 4 weeks [7]. Animals were considered infected if any of the 3 assays was positive for the presence of Borrelia.
Statistical Analysis
Analysis of variance with Tukey test post-hoc analysis was performed for multiple comparisons, and Fisher exact test was used to analyze protection. All statistical tests were conducted using GraphPad Prism software. P values <.05 were considered to be statistically significant.
RESULTS
319–44 DMAb Optimization and Characterization
To develop an anti-OspA–specific HuMAb in the DMAb format, we designed and engineered synthetic plasmid DNA encoding the heavy and light chains of the most potent HuMAb, 319–44, in a single reading frame, as described previously [10, 12]. A second DMAb construct (319–44 Mod1) was constructed by further modifications through specific mutations to the framework region to enhance expression while retaining functionality as previously described [10, 12].
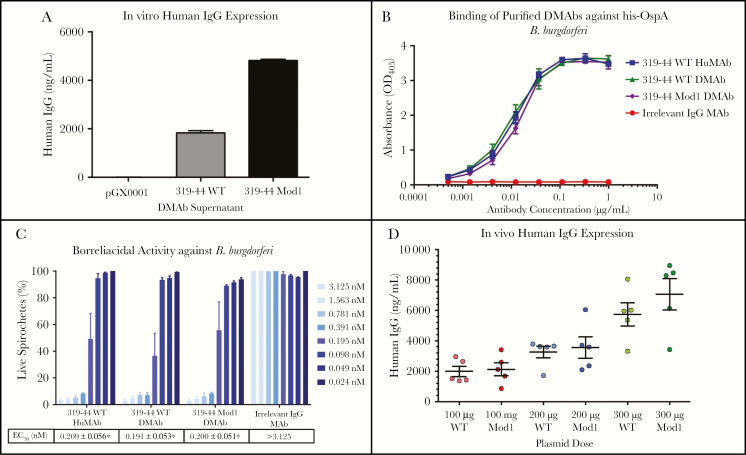
Both the 319–44 WT DMAb and Mod1 DMAb plasmids were transfected into HEK cells, and secreted antibody levels were quantified after 48 hours by ELISA. Modifications to the DMAb plasmids increased in vitro human IgG expression in HEK cells by 2.5-fold (Figure 1A) without losing antigen binding (Figure 1B) or borreliacidal activity (Figure 1C). DMAbs inoculated into C3H mice at 100-μg, 200-μg, or 300-μg doses resulted in a dose-dependent increase in in vivo antibody concentrations 1 week postadministration (Figure 1D). The modifications led to a modest increase in vivo antibody expression (6.7 µg/mL) compared to the unmodified WT variant (5.7 µg/mL).
Figure 1.
DNA-encoded monoclonal antibody (DMAb) expression and functional characterization. A, Enzyme-linked immunosorbent assay (ELISA) quantification of human immunoglobulin G (IgG) in supernatants of 319–44 wild-type (WT) or 319–44 modified (Mod1)–transfected human embryonic kidney (HEK) 293T cells. B, OspA Borrelia burgdorferi binding by purified 319–44 WT or 319–44 Mod1 DMAbs from transfected HEK 293T cells. C, Borreliacidal activity of 319–44 WT or 319–44 Mod1 DMAbs against B. burgdorferi. The half maximal effective concentration was calculated for each of the antibodies. D, Total serum-detectable levels of human IgG were measured by ELISA after various doses of 319–44 WT DMAb or 319–44 Mod1 DMAb were injected intramuscularly in C3H mice. The data displayed are the mean ± standard error of the mean and are representative of 3 independent experiments. *Statistically different compared to irrelevant IgG DMAb control (P < .05). Abbreviations: DMAb, DNA-encoded monoclonal antibody; EC50, half maximal effective concentration; HuMAb, human monoclonal antibody; IgG, immunoglobulin G; MAb, monoclonal antibody; Mod1, modified; OD405, optical density at 405 nm; WT, wild type.
319–44 DMAb Protects Against B. burgdorferi–Infected Tick Challenge in Mice
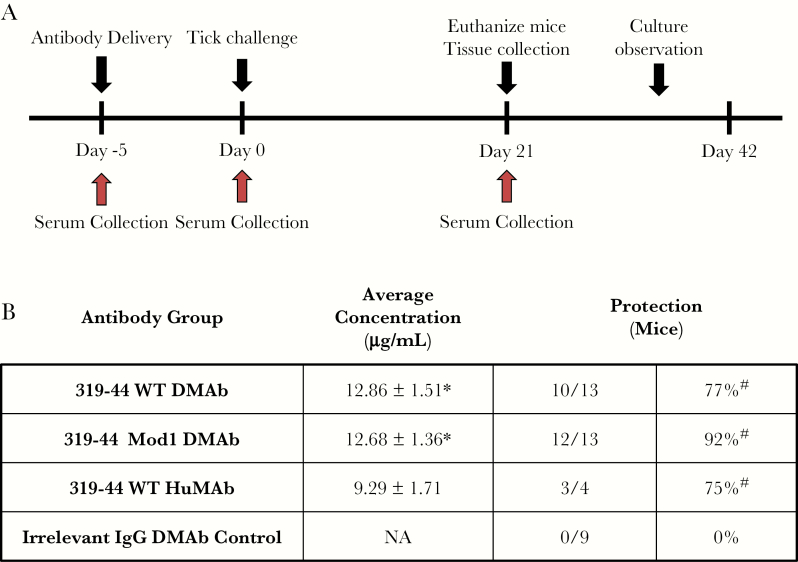
The protective efficacy of 319–44 WT and Mod1 DMAbs was tested in C3H mice acutely challenged with B. burgdorferi–infected nymphs. C3H mice were administered 300 μg of 319–44 WT, Mod1, or an irrelevant IgG DMAb delivered by IM CELLECTRA EP (Figure 2A). An additional group of mice was also given a 1.5 mg/kg IP injection of purified 319–44 WT HuMAb protein. Prior to tick challenge on day 5, the serum concentrations for 319–44 WT DMAb (12.86 ± 1.51 µg/mL) and 319–44 Mod1 DMAb (12.68 ± 1.36 µg/mL) were significantly greater (P < .05) than the purified 319–44 WT HuMAb (9.29 ± 1.71 µg/mL) (Figure 2B). However, protection between these groups was not statistically different. Protection was defined as failure of Borrelia transmission evidenced by observations of tissue in liquid culture, OspA-specific PCR, and mouse anti-Borrelia antibodies (Supplementary Figure 1). As 6 infected ticks are placed on each mouse, the B. burgdorferi infective dose can vary by mouse and may explain the lack of correlation between DMAb sera levels and protection. The purified 319–44 WT HuMAb protected 3 out of 4 mice. 319–44 WT DMAb and 319–44 Mod1 DMAb protected 10 of 13 and 12 of 13 of the mice, respectively. (Figure 2B). All 319–44 groups showed a significantly greater level of protection (P < .05) than the irrelevant IgG DMAb, which did not protect against Borrelia transmission.
Figure 2.
DNA-encoded monoclonal antibodies (DMAbs) protect tick transmission of Borrelia burgdorferi infection in C3H mice. A, Murine challenge schedule. C3H mice received 300 µg of DMAb or 1.5 mg/kg soluble 319–44 wild-type (WT) antibody 5 days prior to challenge. At day 0, each mouse was challenged by the placement of 6 ticks. Serum was collected at days 0 and 21 to measure antibody concentration. Tissue samples were collected on day 21 and observed for 3 weeks for the presence of Borrelia. B, 319–44 WT DMAb, 319–44 Mod1 DMAb, and 319–44 WT monoclonal antibody resulted in comparable in vivo antibody expression (9–12 µg/mL). This led to similar levels of protection when the mice were challenged by B. burgdorferi–infected nymphs (85%–100%). Protection was determined by tissue observations, polymerase chain reaction, and enzyme-linked immunosorbent assay. Animals were considered infected if any of the 3 assays were positive for the presence of Borrelia. Data displayed are the mean ± standard error of the mean. *Statistically different compared to 319–44 WT human monoclonal antibody (P < .05). #Statistically different compared to irrelevant immunoglobulin G DMAb control (P < .05). Abbreviations: DMAb, DNA-encoded monoclonal antibody; HuMAb, human monoclonal antibody; IgG, immunoglobulin G; Mod1, modified; NA, not applicable; WT, wild type.
DISCUSSION
Passive immunization with HuMAbs offers a prophylactic strategy for the prevention of Lyme disease. Here, we explored a strategy to deliver anti-OspA HuMAbs for protection against infected tick challenge. Our results demonstrate that DNA-mediated gene transfer serves as a potential PrEP against Lyme disease and are consistent with our previous DMAb studies against dengue and chikungunya viruses [10, 12] and other infectious diseases [9, 13].
During tick feeding, Borrelia spirochetes rapidly undergo changes in the expression of outer surface proteins. OspA is involved in the attachment of Borrelia spirochetes to the tick midgut. OspA is gradually down-regulated whereas OspC, which is associated with migration of spirochetes from the midgut to the salivary gland, is rapidly up-regulated [14]. Our previous study suggested that HuMAbs, when consumed by the tick, block the OspA to OspC gene switch in the tick midgut, though the mechanism remains unclear [7]. This may inhibit spirochete dissemination to the salivary gland and prevent transmission. Similar to the protection seen when soluble anti-OspA antibody is administered, in vivo delivery of the DMAbs showed comparable results by several measurements, indicating that the antibodies produced by DNA-mediated gene transfer may block spirochete transmission via the same mechanism.
The potential of delivering anti-OspA antibodies by injection of DNA, followed by CELLECTRA EP and subsequent antibody production in the host, could be a unique alternative to traditional passive administration of antibodies produced by Chinese hamster ovary cells without the limitation of expensive downstream production and associated cold-chain handling, which increases cost and can limit widespread use. As a transmission-blocking PrEP, 319–44 DMAb could be highly beneficial in combating Lyme disease in at-risk populations. Through our studies, we established the feasibility of using DNA-encoded antibodies to block the transmission of Lyme disease, as well as a proof of concept for other tick-borne illnesses.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Defense Advanced Research Projects Agency (grant number W911NF-13-1-0346 to M. K.); the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1-TR001453 to Y. W.); and a National Institutes of Health Emerging Infectious Disease Training Grant (grant number T32-AI055400 to R. N. E.).
Potential conflicts of interest. K. B. and M. W. are employees of Inovio Pharmaceuticals and as such receive salary and benefits, including ownership of stock and stock options, from the company. D. B. W. has received grant funding, participates in industry collaborations, has received speaking honoraria, and has received fees for consulting, including serving on scientific review committees and board services. Remuneration received by D. B. W. includes direct payments or stock or stock options, and in the interest of disclosure he notes potential conflicts associated with this work with Inovio and possibly others. In addition, he has a patent DNA vaccine delivery pending to Inovio. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Shapiro ED. Lyme disease. N Engl J Med 2014; 371:684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Golde WT, Piesman J, Dolan MC, et al. Reactivity with a specific epitope of outer surface protein A predicts protection from infection with the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun 1997; 65:882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schaible UE, Kramer MD, Eichmann K, Modolell M, Museteanu C, Simon MM. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A 1990; 87:3768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson BJ, Sviat SL, Happ CM, et al. Incomplete protection of hamsters vaccinated with unlipidated OspA from Borrelia burgdorferi infection is associated with low levels of antibody to an epitope defined by mAb LA-2. Vaccine 1995; 13:1086–94. [DOI] [PubMed] [Google Scholar]
- 5. Sigal LH, Zahradnik JM, Lavin P, et al. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant outer-surface protein a Lyme disease vaccine study consortium. N Engl J Med 1998; 339:216–22. [DOI] [PubMed] [Google Scholar]
- 6. Lathrop SL, Ball R, Haber P, et al. Adverse event reports following vaccination for Lyme disease: December 1998–July 2000. Vaccine 2002; 20:1603–8. [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Kern A, Boatright NK, et al. Pre-exposure prophylaxis with OspA-specific human monoclonal antibodies protects mice against tick transmission of Lyme disease spirochetes. J Infect Dis 2016; 214:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muthumani K, Flingai S, Wise M, Tingey C, Ugen KE, Weiner DB. Optimized and enhanced DNA plasmid vector based in vivo construction of a neutralizing anti-HIV-1 envelope glycoprotein Fab. Hum Vaccin Immunother 2013; 9:2253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patel A, DiGiandomenico A, Keller AE, et al. An engineered bispecific DNA-encoded IgG antibody protects against Pseudomonas aeruginosa in a pneumonia challenge model. Nat Commun 2017; 8:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flingai S, Plummer EM, Patel A, et al. Protection against dengue disease by synthetic nucleic acid antibody prophylaxis/immunotherapy. Sci Rep 2015; 5:12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amante DH, Smith TR, Mendoza JM, et al. Skin transfection patterns and expression kinetics of electroporation-enhanced plasmid delivery using the CELLECTRA-3P, a portable next-generation dermal electroporation device. Hum Gene Ther Methods 2015; 26:134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muthumani K, Block P, Flingai S, et al. Rapid and long-term immunity elicited by DNA-encoded antibody prophylaxis and DNA vaccination against chikungunya virus. J Infect Dis 2016; 214:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elliott STC, Kallewaard NL, Benjamin E, et al. DMAb inoculation of synthetic cross reactive antibodies protects against lethal influenza A and B infections. NPJ Vaccines 2017; 2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol 2000; 38:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.