Abstract
Background:
Serum folate concentrations in the United States have risen since dietary folic acid fortification was first mandated in 1998. Although maternal folic acid offers protection against neural tube defects in conceptuses, its impact on other organ systems and life stages have not been fully examined. Here, we used a mouse model to investigate the impact of a Folic acid (FA) enriched diet on prostate homeostasis and response to androgen deprivation.
Methods:
Male mice were fed a control diet (4 mg FA/kg feed) or a folic acid supplemented diet (24 mg FA/kg feed) beginning at conception and continuing through early adulthood, when mice were castrated.
Results:
We made the surprising observation that dietary FA supplementation confers partial resistance to castration-mediated prostate involution. At 3, 10 and 14 days post-castration, FA enriched diet fed mice had larger prostates as assessed by wet weight, taller prostatic luminal epithelial cells and more abundant RNAs encoding prostate secretory proteins than castrated control diet fed mice. Diet did not significantly affect prostate weights of intact mice or serum testosterone concentrations of castrated mice. RNA-Seq analysis revealed that the FA enriched diet was associated with a unique prostate gene expression signature, affecting several signaling and metabolic pathways.
Conclusions:
Continuous exposure to a FA enriched diet slows prostate involution in response to androgen deprivation. Prostates from FA diet mice have increased secretory gene expression and increased luminal cell heights. The influence of dietary FA supplementation on the prostate response to androgen deprivation raises a future need to consider how dietary folic acid supplementation affects efficacy of androgen-reducing therapies for treating prostate disease.
Keywords: Folate, In utero, Castration, Spermine binding protein, testosterone
1. Introduction
The prostate is susceptible to aging related hyperplastic growth, which contributes to urethral obstruction and urinary dysfunction 1,2. Diet is a potential risk modifier for prostate-related urinary disorders. For example, excessive caloric intake is associated with obesity and diabetes, which in turn increases risk of benign prostatic hyperplasia (BPH) and associated Lower Urinary Tract symptoms (LUTS) 3–6. On the other hand, dietary modifications and supplements are increasingly used alone or in combination with pharmacological and surgical interventions in attempt to control BPH symptoms7. Environmental factors, including diet, have also been linked to prostate cancer incidence. Dietary modifications are gaining popularity as inexpensive and minimally invasive strategies for reducing prostate cancer risk, slowing its progression and preventing advanced disease 8,9.
Folates are water soluble vitamins involved in numerous biochemical processes including one-carbon transfer, DNA synthesis, cell growth, hematopoiesis, metabolism and DNA methylation. Folates are the naturally occurring form of Vitamin B9 and are particularly concentrated in green leafy vegetables 10. Folic acid is the synthetic form in fortified food stuffs and most multi-vitamin supplements, the use of which is prevalent across all age groups, and particularly in older men 11–13. Folic acid consumption by pregnant women is so effective in reducing the incidence of neural tube defects in offspring 14,15 that the United States mandated folic acid fortification of cereal grains beginning in 1998 16. This action has affected many individuals, not just pregnant women. In fact, 34% of American men over 60 years of age have serum biomarkers consistent with folic acid excess 17.
Despite the overwhelming positive effects of folic acid in preventing neural tube defects, the consequences of folate supplementation at various stages of life and during disease processes are not fully understood. The complex roles of folates in the body could yield protective or deleterious effects depending on the context. Unmetabolized folic acid reduces natural killer cell function in post-menopausal women 18. Maternal and post-weaning folic acid supplementation increases mammary cancer risk in rats 19 and induces anxiety-like behavior in mice 20. Folic acid supplementation changes DNA methylation at several gene loci in mice and humans with unknown consequences 21–25. Folates are also involved in the synthesis of polyamines 26,27, depletion of which has been proposed as a prostate cancer therapy 28. We reported previously that a FA enriched diet given from conception through adulthood diminishes urinary symptom severity in male mice with hormone induced urinary obstruction 29. However, the impact of folic acid supplementation on adult prostate homeostasis has not been specifically investigated.
Here we examine how exposure to a FA enriched diet during the in utero period and continuing into adulthood affects the mouse prostate and its response to androgen deprivation by castration. Though intact mouse prostate weight weights are unaffected by diet, mice exposed to the FA enriched diet have significantly higher prostate wet weights compared to mice on a control diet after castration. The FA enriched diet also leads to taller prostate luminal epithelial cells and more abundant secretory protein-encoding mRNAs compared to control diet mice after castration. Together, our results indicate that dietary folic acid attenuates the prostate response to androgen deprivation. This has important implications for androgen deprivation therapies that are commonly used to treat prostate cancer and benign prostatic hyperplasia 30–32. Elevated folate levels could diminish the efficacy of these therapies.
2. Materials and Methods
2.1. Mice
C57BL/6J (000664, The Jackson laboratory) nulliparous females were housed as previously described 29. At sexual maturity, females were placed on a base diet (control diet, Envigo 2019, Harlan Teklad, Madison, WI) containing 4 mg folic acid / kg feed or the same base diet containing 24 mg folic acid / kg feed (FA enriched diet, Envigo 120256, Harlan Teklad, Madison, WI) for 2 weeks prior to mating. The manufacturer of the diet estimates 4 mg folic acid/kg feed in the Control diet. This estimate includes folates derived from cereal grains and yeast extract. The folic acid enriched diet is prepared by adding 20 mg folic acid/kg feed to the Control diet. While absolute values of folic acid may differ seasonally, the magnitude of difference is consistently the same: 20 mg folic acid / kg feed. The control diet (4 mg folic acid/kg feed) provides twice the daily folic acid requirement for mice (2 mg folic acid/kg feed)33. The FA enriched diet provides approximately 12 times the daily folic acid requirement for mice 34 which is comparable to the 10X higher dose consumed by pregnant women at risk for neural tube defects (0.4 mg/day vs 4 mg/day) 35. The 2-week loading period has been shown to raise maternal serum folic acid concentrations 36.
Dams were placed on the diets throughout pregnancy and lactation and resulting offspring were also maintained on the same diet throughout the experiment. At 7 weeks of age, male mice were euthanized by CO2 asphyxiation for tissue collection (Intact) or subject to bilateral orchiectomy (castration) under isoflurane anesthesia and given 5 mg/kg ketoprofen for analgesia. Castrated mice were euthanized 3, 10 or 14 days post-castration. Body weights were recorded at time of necropsy and prostate, seminal vesicles and serum were collected. Prostate and seminal vesicle absolute wet weights were measured using an analytical balance and divided by mouse whole body weights to obtain relative weights. All procedures involving mice were approved by the University of Wisconsin Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Fluorescent Immunohistochemistry
Fluorescent immunohistochemistry was performed as described previously 37. Dissected tissues were fixed overnight in 4% paraformaldehyde solution and embedded in paraffin for sectioning. 5 μm paraffin sections were deparaffinized in xylene and hydrated through a series of ethanol washes. Heat mediated antigen retrieval was performed by boiling slides in 10 mM sodium citrate (pH 6.0) for 20 mins in a conventional microwave oven. Tissues were washed with a solution containing 25 mM Tris-HCl, pH 7.5, 140 mM NaCl, 2.7 mM KCl, and 0.1% Tween-20 (TBSTw) and non-specific binding sites were blocked for 1 hr in TBSTw containing 1% Blocking Reagent (Roche Diagnostics, Indianapolis, IN), 5% normal goat sera, and 1% bovine serum albumin fraction 5 (RGBTw). Tissues were incubated overnight at 40C with primary antibodies diluted in RGBTw. Primary antibody sources and dilutions are: Ki67 (ab15580, 1:400 dilution) from Abcam, Cambridge, MA, USA. CDH1 (610181, 1:500 dilution) from BD Biosciences, San Jose, CA, USA. Cleaved Caspase 3 Asp175 (9664, 1:200 dilution) from Cell Signaling Technology, Beverly, MA, USA. Androgen Receptor (sc-816, 1:200 dilution) from Santa Cruz Biotechnology, Dallas, TX, USA. Tissues were washed several times in TBSTw and incubated with secondary antibodies diluted in RGBTw for 1 hour at room temperature. The secondary antibodies and dilutions are: goat anti-Mouse AlexaFluor488 (115–547-003, 1:500 dilution), goat anti-Rabbit AlexaFluor594 (111–586-045, 1:500 dilution), goat anti-Mouse AlexaFluor594 (115–585-062, 1:500 dilution), goat anti-Rabbit AlexaFluor488 (111–487-003, 1:500 dilution) from Jackson ImmunoResearch, West Grove, PA, USA. Following several washes with TBSTw, tissues sections were incubated with 4’,6-diamidino-2-phenylindole, dilactate (DAPI) to visualize cell nuclei and mounted in phosphate buffered saline containing 80% glycerol and 0.2% n-propyl gallate. Images were captured using a Leica SP8 Confocal Microscope fitted with a 20X oil immersion objective (HC PL Apo CS2 NA = 0.75) (Leica, Wetzlar, Germany) or a Nikon Eclipse E600 compound microscope fitted with 10X (Plan Fluor NA = 0.30) and 20X (Plan Fluor NA = 0.50) objectives (Nikon Instruments Inc., Tokyo, Japan). Tissue sections from both diet groups were imaged using the same exposure settings.
2.3. Prostate luminal cell height measurements
Fluorescent immunohistochemistry for the epithelial marker E-cadherin (CDH1) was performed as described above on 5 μm paraffin sections of ventral prostate tissue. Images were obtained using a 20X objective on a Nikon Eclipse E600 compound microscope. Prostate luminal cells were identified by their characteristic tall, columnar morphology. Cell heights were measured using the Straight-line tool and Analyze>Measure function in ImageJ. Measurements were taken from 7 individual cells separated by > 5 cells in each field. Ventral prostate tissue sections from at least three mice per diet group were used for analyses.
2.4. Proliferative and apoptotic index measurements
Fluorescent immunohistochemistry for Ki67 and cleaved Caspase 3 was performed as described above on 5 μm paraffin sections of ventral prostate tissue. Slides were counterstained with DAPI to visualize nuclei. Images were obtained using a 20X objective on a Nikon Eclipse E600 compound microscope. DAPI stained nuclei in the field were counted using the Analyze Particles function in ImageJ. Ki67 and cleaved Caspase 3 positive cells were manually counted using the Cell counter plugin for ImageJ. Proliferative index is defined as Ki67 positive cells as a percent of total cells and the apoptotic index is defined as cleaved Caspase 3 positive cells as a percent of total cells. Ventral prostate tissue sections from at least three mice per diet group were used for analyses.
2.5. Real time quantitative PCR
Quantitative PCR was carried out as previously described 38 on ventral prostate tissue using gene-specific primers. Primer sequences are provided in Supplemental Table 1. Relative mRNA abundance was determined using the ΔCt method 39 and normalized to the abundance of beta-2-microglobulin (B2m). Ventral prostate tissue from at least three mice per diet group were used for analyses.
2.6. Serum testosterone measurements
Mice were euthanized by CO2 asphyxiation and blood was collected by cardiac puncture, clotted for 45 mins and centrifuged at 6000g for 10 mins to clear the serum. Serum testosterone measurements were carried out using a testosterone ELISA kit (#55-TESMS-E01, ALPCO, Salem, NH, USA) following the manufacturer’s instructions. Sera from at least three mice per diet group were used for the analysis.
2.7. RNA-Seq
RNA was isolated from the ventral prostates of mice belonging to six experimental groups: Control diet-Intact (N=4), FA diet-Intact (N=4), Control diet-3d post-castration (N=3), FA diet-3d post-castration (N=4), Control diet-10d post-castration (N=4) and FA diet-10d post-castration (N=4) using the Illustra RNAspin Mini kit (#25–0500-72, GE Healthcare, Chicago, Illinois, USA). Approximately 100 ng of total RNA from each diet group was used to construct the RNA-Seq library according to the LM-seq protocol 40. The reads generated from the Illumina HiSeq 3000 (78 cycles of insert read and 10 cycles of index read) were processed with CASAVA-1.8.2 basecalling software (Illumina). The demultiplexing step allotted approximately 253 million total reads across all the samples, ranging from ~6.5 million to ~15.8 million reads assigned per sample. Reads were mapped to Mus musculus reference mm10 assembly with an average of ~82.4% mapping rate using Bowtie 41, and gene expression estimates were obtained using RSEM 42. Differentially expressed genes were identified using the EBSeq package 43. Gene Ontology enrichment analysis for biological processes was conducted using WebGestalt 44. RNA-seq data are available in Gene Expression Omnibus under GSE116299.
2.8. Statistics
Statistical analyses were conducted with R version 3.2.4. Homogeneity of variance was determined using Bartlett’s test or Levene’s test packages for R. Student’s t-test was performed on parametric data with two groups. P values less than 0.05 were considered statistically significant. Results are presented as mean ± standard error of the mean (SEM). NS: Not significant, * p<0.05, ** p<0.01, *** p<0.001.
3. Results
3.1. FA enriched diet blunts castration-mediated mouse prostate gland involution
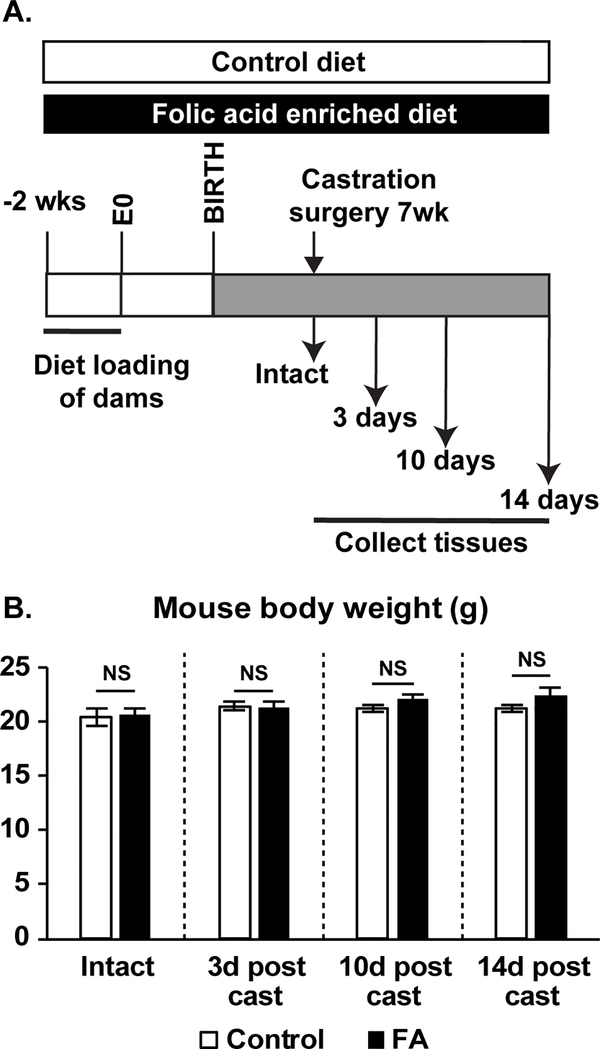
Reducing androgen synthesis is a therapeutic strategy for prostate-related growth diseases including BPH and prostate cancer. Our primary goal was to determine if continuous dietary FA supplementation modifies the prostate involution response to castration. Folate levels have risen across all age groups in the US, including women of child bearing age, children and older adults, since cereal grain fortification was mandated in 1998 45–47. Our study design parallels this historical change by continuously exposing male mice to high levels of folic acid across all life stages. C57Bl6/J female mice were loaded on a control or FA enriched diet 2 weeks before mating and through pregnancy and lactation. Male offspring were continued on a matched diet through study completion (Figure 1A).
Figure 1.
(A) Study design. (B) Mouse body weights do not change across diet or treatment groups. Number of mice per group: Control-Intact N=8, FA-Intact N=11, Control-3d post cast N=8, FA-3d post cast N=7, Control-10d post cast N=14, FA-10d post cast N=15, Control-14d post cast N=5, FA-14d post cast N=5. Results are presented as mean ± SEM. Homogeneity of variance was tested using Bartlett’s test. p-values represent results from unpaired Student’s t-test between diet groups for each treatment. NS: Not significant p>0.05. Abbreviations FA: Folic Acid, d post cast: days post castration.
The first objective was to test whether the FA enriched diet affects prostate homeostatic regulation by changing prostate wet weight. We did not find significant diet group differences in body weight (Figure 1B), relative prostate weight (all prostate lobes combined) (Figure 2A) or relative seminal vesicle weight in intact mice (Figure 2E).
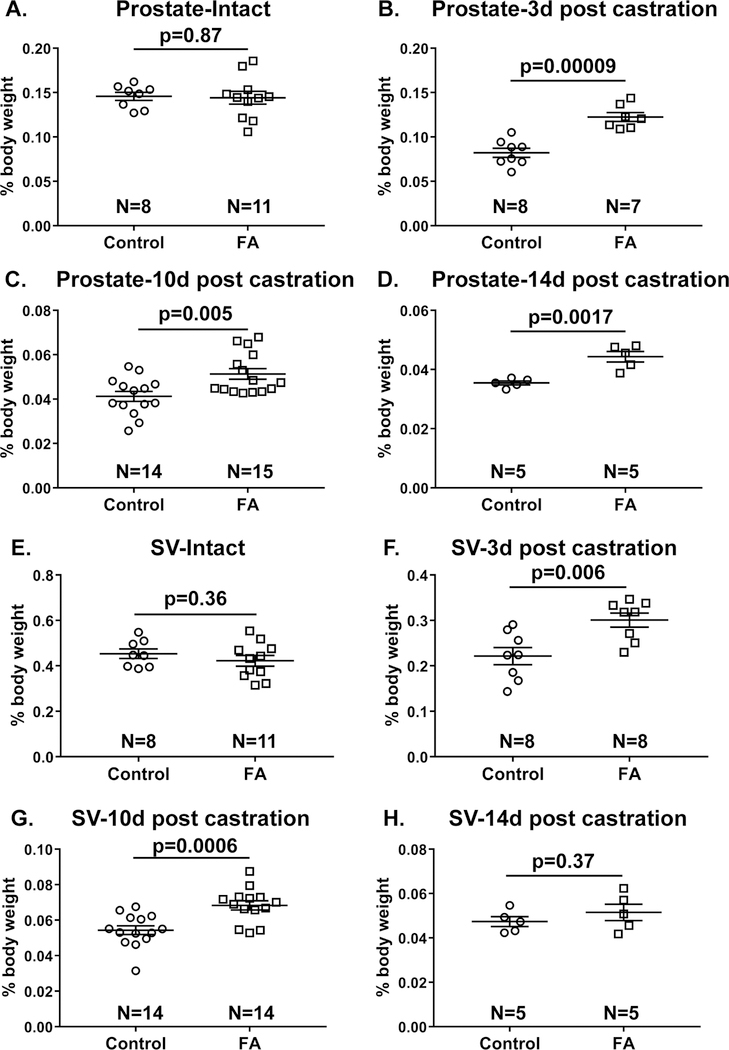
Figure 2. Mice fed a FA enriched diet and then castrated have greater prostate and seminal vesicle weights than mice fed a control diet and castrated.
Relative prostate and seminal vesicle (SV) wet weights were determined as percentage of mouse body weight for: (A, E) Intact mice or mice (B, F) 3 days (C, G) 10 days (D, H) or 14 days post castration. N value indicates number of mice/group. Results are presented as mean ± SEM. Homogeneity of variance was tested using Bartlett’s test. p-values represent results from unpaired Student’s t-test between diet groups for each treatment.
We next tested whether a FA enriched diet interferes with castration-induced prostate involution. 7-week old sexually mature mice were castrated and tissues weighed at 3, 10 and 14 days post-castration. The relevance of these time points is that prostate apoptosis peaks approximately 3 days post-castration and prostate involution is complete by 14 days post-castration 48,49. Body weights did not differ between diet groups for each treatment (Figure 1B). Relative prostate and seminal vesicle weights were reduced by castration in both diet groups as expected. However, castration-mediated prostate gland involution was unexpectedly incomplete in FA enriched diet fed mice, as evidenced by significantly greater relative prostate weights than castrated control diet fed mice at 3, 10 and 14 days post-castration (Figure 2B-D). Similarly, FA enriched diet fed mice had significantly greater relative seminal vesicle weights than castrated control diet fed mice at 3 and 10 days post-castration (2F-H).
3.2. FA enriched diet prostates retain greater luminal epithelial secretory gene expression and luminal cell heights after castration
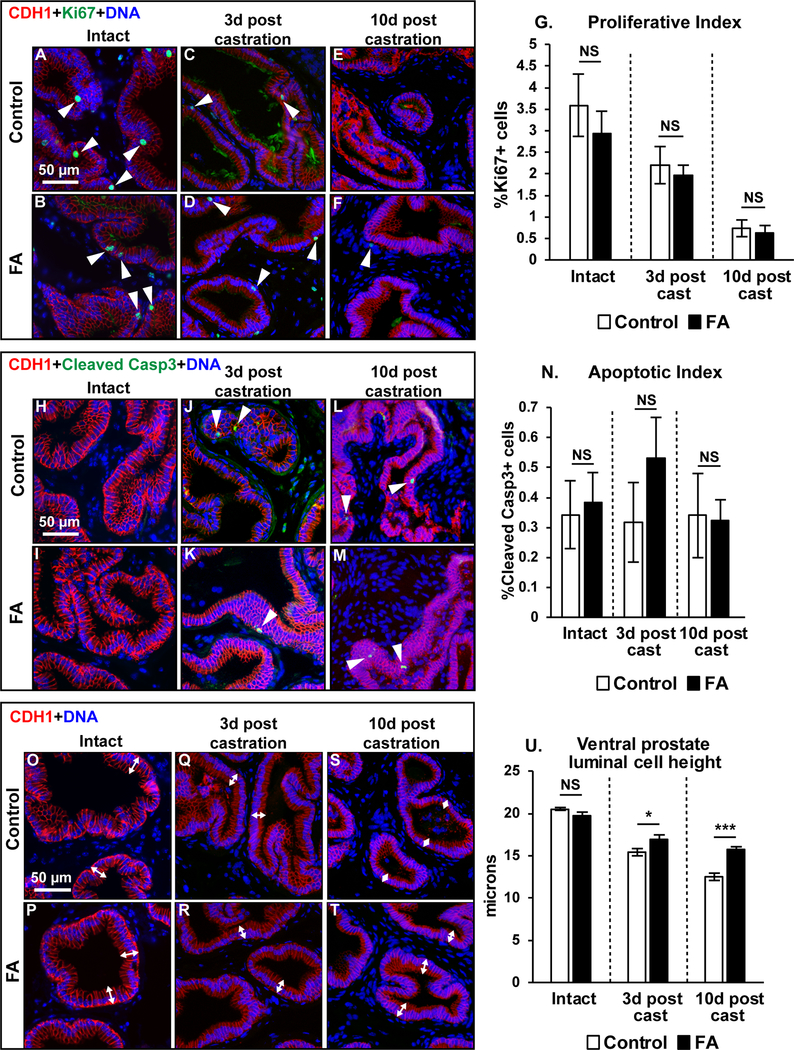
Castration causes prostate luminal epithelial cells to undergo apoptosis 48–51. Dietary modulation of prostatic apoptosis could underlie differences in the castration response. We tested whether there were also diet-mediated changes to prostatic cell proliferation for the same reason. Indices of Ki67 labeling (proliferation) and Cleaved Caspase 3 labeling (apoptosis) were very low in intact mouse ventral prostates and unaffected by diet. Likewise, ventral prostate Ki67 and Cleaved caspase 3 labeling indices did not differ significantly between control and FA enriched diet fed castrated mice (Figure 3A-N).
Figure 3: Greater luminal epithelial cell heights in castrated FA diet prostates compared to control diet mice but no differences in prostate cell proliferation and apoptosis between diet groups.
Mouse ventral prostate sections were labeled with antibodies to CDH1 (red) and Ki67 (green). Representative images are shown for (A-B) Intact mice or mice (C-D) 3 days (E-F) or 10 days post-castration. (G) Proliferation indices were compared between diet groups. Number of mice per group: Control-Intact N=7, FA-Intact N=8, Control-3d post cast N=7, FA-3d post cast N=7, Control-10d post cast N=6, FA-10d post cast N=5. Mouse ventral prostate sections were also labeled with antibodies to CDH1 (red) and Cleaved caspase 3 (green). Representative images are shown for (H-I) Intact mice or mice (J-K) at 3 days (L-M) or 10 days post-castration. (N) Apoptotic indices were compared between diet groups. Number of mice per group: Control-Intact N=8, FA-Intact N=10, Control-3d post cast N=8, FA-3d post cast N=6, Control-10d post cast N=6, FA-10d post cast N=4. Ventral prostate sections from both diet groups were labeled with antibodies to CDH1 (red). Representative images are shown for (O-P) Intact mice, (Q-R) mice at 3 days post- castration (S-T) and 10 days post castration. (U) Prostate luminal epithelial cell heights were compared between diet groups. N values for groups are as follows: Control-Intact N=4, FA-Intact N=5, Control-3d post cast N=4, FA-3d post cast N=3, Control-10d post cast N=6, FA-10d post cast N=5. DAPI staining is show in blue. Scale bar is 50 μm. White arrowheads indicate Ki67 or cleaved caspase 3 labeled cells. White double headed arrows depict cell height of selected luminal epithelial cells. Graphical results are presented as mean ± SEM. Homogeneity of variance was tested using Bartlett’s test. p-values represent results from unpaired Student’s t-test test between diet groups for each treatment. * p<0.05, ** p<0.01, ***p<0.001, NS: Not significant p>0.05.
Because prostate cell cycling was unaffected by diet, we evaluated changes to prostate secretory protein synthesis as an alternative explanation for why prostates of FA enriched diet fed castrated mice are larger than those of control diet fed castrated mice. Androgen dependent secretory proteins are stored in apically localized granules within prostate luminal epithelial cells 52. Castration reduces secretory granule number and luminal epithelial cell height 53. Ventral prostate luminal epithelial cell height is progressively reduced by castration in both diet groups as expected. However, the FA enriched diet confers partial resistance and is associated with taller luminal epithelial cells at 3 and 10 days post-castration compared to control diet fed mice (Figure 3O-U).
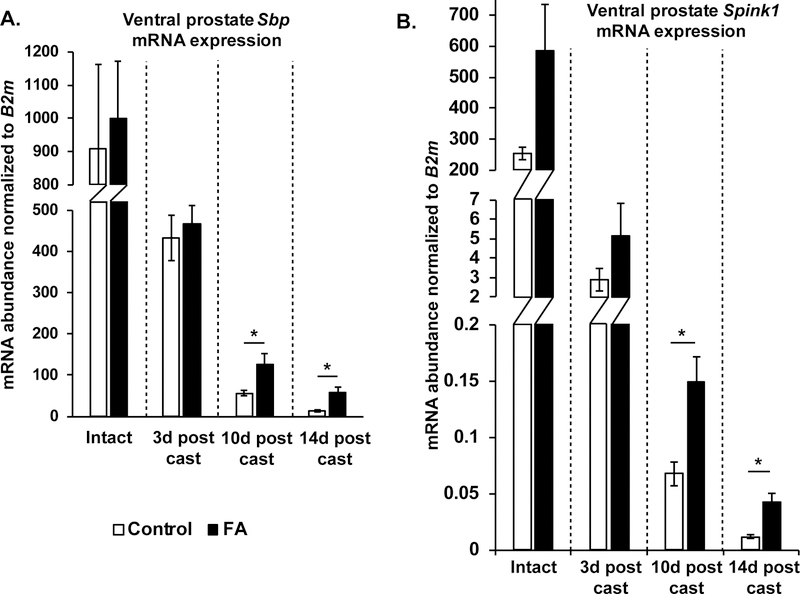
Castration reduces androgen dependent prostatic secretory gene transcription and secretory protein content, which is a major component of prostate weight 50,54,55. We focused on the ventral prostate and quantified mRNA abundance of spermine binding protein (Sbp), a ventral prostate-specific secretory protein 54. Castration reduces Sbp mRNA abundance but Sbp mRNA was significantly more abundant in castrated FA diet fed mice compared to control diet fed mice at 10 days and 14 days post-castration (Figure 4A). We further quantified abundance of another mRNA encoding a ventral prostate specific androgen dependent secreted protein, Serine protease inhibitor Kazal-type 1 (Spink1)56. Similar to Sbp, Castration reduces Spink1 mRNA expression but Spink1 mRNA was significantly more abundant in castrated FA diet fed mice compared to control diet fed mice at 10 days and 14 days post-castration (Figure 4B). Taken together, our results suggest that the increased prostate weights in castrated FA diet mice are a result of increased secretory activity and not increased proliferation or decreased apoptosis.
Figure 4: Mice fed a FA enriched diet and then castrated have relatively more ventral prostate specific secretory mRNAs Sbp and Spink1, than controls.
(A) mRNA abundance of Sbp was measured in Intact, 3 days, 10 days and 14 days post-castration ventral prostates from FA enriched diet or control diet fed mice. The abundance of Sbp was normalized to that of B2m. N values for groups are as follows: Control-Intact N=4, FA-Intact N=4, Control-3d post cast N=3, FA-3d post cast N=4, Control-10d post cast N=4, FA-10d post cast N=3, Control-14d post cast N=3, FA-14d post cast N=3. (B) mRNA abundance of Spink1 was measured in Intact, 3 days, 10 days and 14 days post-castration ventral prostates from FA enriched diet or control diet fed mice. The abundance of Spink1 was normalized to that of B2m. N values for groups are as follows: Control-Intact N=4, FA-Intact N=4, Control-3d post cast N=3, FA-3d post cast N=4, Control-10d post cast N=3, FA-10d post cast N=3, Control-14d post cast N=3, FA-14d post cast N=3. Graphical results are presented as mean ± SEM of mRNA abundance. p-values represent results from unpaired Student’s t-test between diet groups for each treatment, * p<0.05. Comparisons that did not produce significant p-values (>0.05) are not indicated.
3.3. FA enriched diet does not change circulating androgens or Androgen receptor expression
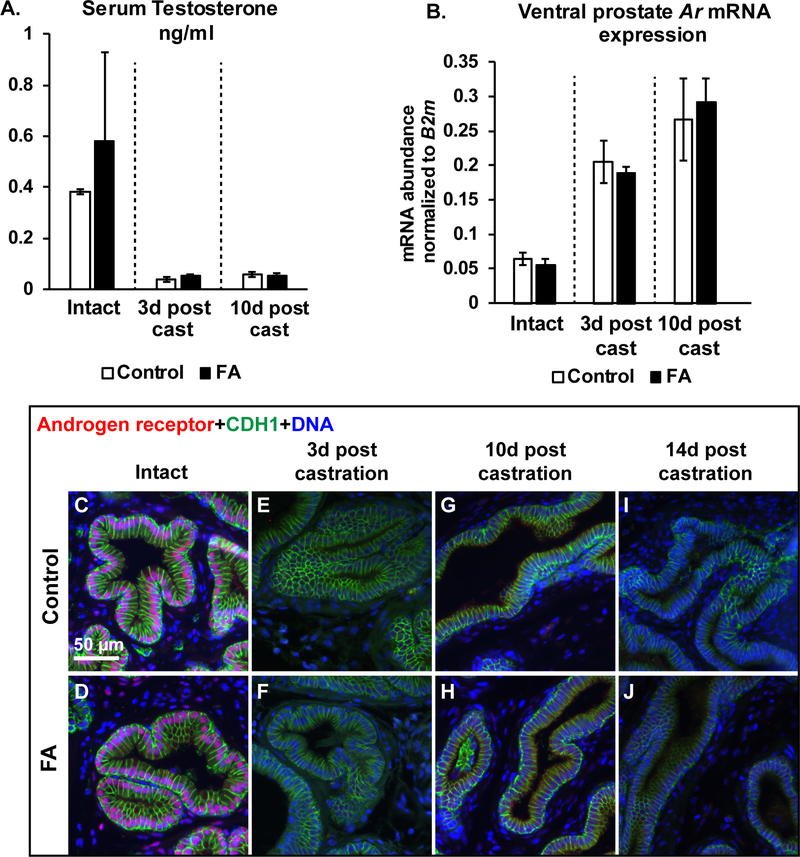
We hypothesized that the FA diet might impair testosterone metabolism, thereby prolonging its half-life in castrated mouse serum and slowing the rate of prostate gland involution. Such a mechanism would explain the taller luminal cells and more abundant secretory gene mRNA expression in FA enriched diet mice compared to control diet fed castrated mice. Serum testosterone concentrations were measured by ELISA. As expected, testosterone concentrations were reduced at 3 and 10 days post-castration compared to intact mice in both diet groups. Surprisingly, there were no diet group differences in serum testosterone concentrations among intact or castrated mice (Figure 5A).
Figure 5: The FA enriched diet does not change circulating androgens or prostatic Androgen receptor (Ar) abundance compared to controls.
(A) Serum testosterone concentrations were determined by ELISA. Serum from N=3 mice/group was used for analysis. (B) Ventral prostate Ar mRNA abundance normalized to B2m. N values for groups are as follows: Control-Intact N=4, FA-Intact N=4, Control-3d post cast N=3, FA-3d post cast N=4, Control-10d post cast N=4, FA-10d post cast N=4. Graphical results are presented as mean ± SEM. p-values represent results from unpaired Student’s t-test between diet groups for each treatment. Comparisons that did not produce significant p-values (>0.05) are not indicated. Mouse ventral prostate sections were also labeled with antibodies to Androgen receptor (AR, red) and CDH1 (green). Representative images are shown for (C-D) Intact mice or mice (E-F) at 3 days (G-H) or 10 days post-castration or (I-J) or 14 days post-castration. DAPI staining is show in blue. Scale bar is 50 μm.
We tested whether the FA diet changed ventral prostate Androgen receptor (Ar) mRNA abundance as an alternative mechanism to blunt glandular involution following castration. Relative Ar mRNA abundance was increased in both diet groups after castration, consistent with the previous observation that AR signaling negatively regulates Ar transcription 57. However, Ar mRNA abundance in castrated mice did not differ between control and FA enriched diet mice (Figure 5B). Nuclear localization of AR, an indicator of active androgen signaling, is detected in luminal epithelial cells and a subset of stromal cells in intact prostates from both diet groups. However, nuclear AR is undetectable at 3, 10 and 14 days post castration in both diet groups (Figure 5C-J).
3.4. FA enriched diet changes the mouse ventral prostate gene expression signature, affecting genes involved in nucleotide metabolism and DNA repair
To identify global changes in gene expression in FA enriched diet mice, we performed RNA-Seq analysis on RNA from intact, 3 and 10 days post-castration ventral prostates from both diet groups. Comparing Intact mice from both diet groups, we observed upregulation of 123 genes and downregulation of 193 genes in the FA enriched diet prostates (Table 1, Supplementary Table 2). Gene Ontology analysis revealed that genes upregulated in the FA enriched diet prostates were enriched for biological processes involved in DNA repair and cellular response to DNA damage stimulus. Downregulated genes in the FA enriched diet prostates were enriched for biological processes involved in muscle contraction and endoplasmic reticulum stress (Table 2, Supplementary Table 5). These results indicate that the FA enriched diet changes the gene expression program in ventral prostates from intact mice.
Table 1.
Summary of differential gene expression determined by RNA-Seq
| Comparison | Differentially expressed genes (FDR cutoff 0.05) | Upregulated in FA diet | Downregulated in FA diet |
|---|---|---|---|
| Intact: FA vs Control diet | n= 316 | n= 123 | n= 193 |
| 3d castration: FA vs Control diet | n= 244 | n= 113 | n= 131 |
| 10d castration: FA vs Control diet | n= 122 | n= 38 | n= 84 |
Table 2.
Enriched biological processes among upregulated or downregulated genes that were differentially expressed between treatment groups (tool: WebGestalt)
| Treatment | Differentially expressed gene list analyzed | GO ID Biological Process | Category | p-value | FDR (cutoff 0.05) | #genes in category |
|---|---|---|---|---|---|---|
| Intact | upregulated in FA | GO: 0006974 | cellular response to DNA damage stimulus | 2.85E-06 | 2.01E-02 | 14 |
| Intact | upregulated in FA | GO:0006281 | DNA repair | 4.82E-06 | 2.01E-02 | 11 |
| Intact | downregulated in FA | GO:0003012 | muscle system process | 5.37E-09 | 4.47E-05 | 16 |
| Intact | downregulated in FA | GO:0006936 | muscle contraction | 1.50E-08 | 6.25E-05 | 14 |
| Intact | downregulated in FA | GO:0034976 | response to endoplasmic reticulum stress | 1.41E-07 | 2.93E-04 | 12 |
| 3d castration | downregulated in FA | GO:0030334 | regulation of cell migration | 7.96E-07 | 4.31E-03 | 16 |
| 3d castration | downregulated in FA | GO:2000145 | regulation of cell motility | 1.69E-06 | 4.69E-03 | 16 |
| 3d castration | downregulated in FA | GO:0016477 | cell migration | 4.16E-06 | 6.71E-03 | 20 |
| 10d castration | upregulated in FA | GO:0009135 | purine nucleoside diphosphate metabolic process | 9.84E-06 | 3.31E-02 | 4 |
| 10d castration | upregulated in FA | GO:0009179 | purine ribonucleoside diphosphate metabolic process | 9.84E-06 | 3.31E-02 | 4 |
| 10d castration | upregulated in FA | GO:0009167 | purine ribonucleoside monophosphate metabolic process | 3.15E-05 | 4.47E-02 | 5 |
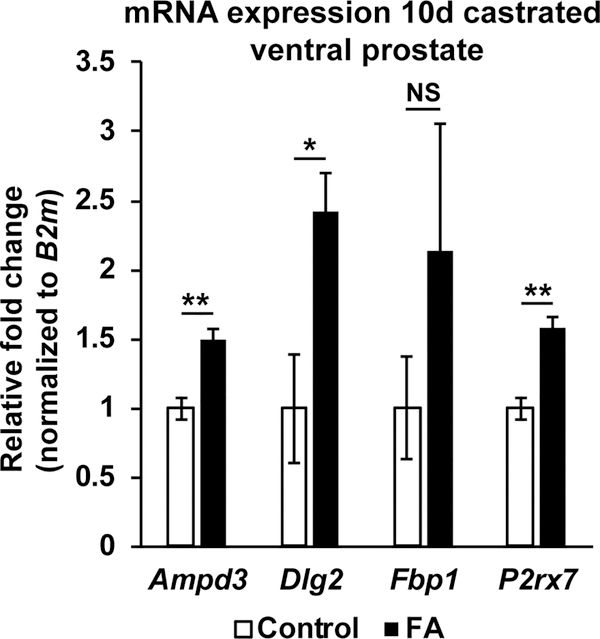
Comparing mice from both diet groups at 3 days post-castration, we observed upregulation of 113 genes and downregulation of 131 genes in the FA enriched diet prostates (Table 1, Supplementary Table 3). Downregulated genes in the FA enriched diet prostates at 3 days post-castration were enriched for biological processes regulating cell motility and migration (Table 2, Supplementary Table 5). Comparing mice from both diet groups at 10 days post-castration, we observed upregulation of 38 genes and downregulation of 84 genes in the FA enriched diet prostates (Table 1, Supplementary Table 4). The lower number of differentially expressed genes at 10 days post-castration compared to intact mice could be due to the overall reduction in transcriptional activity in the prostate following androgen deprivation. Upregulated genes in the FA enriched diet prostates at 10 days post-castration were enriched for biological processes mediating purine nucleotide metabolism (Table 2, Supplementary Table 5). Significant upregulation of genes involved in the purine ribonucleoside diphosphate metabolic process (Ampd3, Dlg2 and P2rx7) were confirmed by quantitative PCR (Figure 6). In summary, the FA enriched diet changes the baseline transcriptional program of the intact mouse ventral prostate. The ventral prostate gene expression pattern of mice consuming a FA enriched diet was different than that of control diet fed mice at 3 and 10 days post-castration.
Figure 6: Increased expression of genes involved in purine ribonucleoside metabolism in FA enriched diet prostates after castration.
mRNA abundance of Ampd3 (N=4 mice/diet group), Dlg2 (N=4 mice/diet group), Fbp1 (N=3 mice/diet group) and P2rx7 (N=3 mice/diet group) were measured in 10 days post-castration ventral prostates from FA enriched diet or control diet fed mice. mRNA abundance was normalized to that of B2m and expressed as relative fold change to control diet samples. Graphical results are presented as mean ± SEM. p-values represent results from unpaired Student’s t-test between diet groups for each treatment. * p<0.05, ** p<0.01, NS: Not significant p>0.05.
4. Discussion
We showed that dietary folic acid supplementation changes mouse prostatic gene expression and blunts castration-mediated prostate involution. Mice fed a diet supplemented with folic acid at 12 times the daily requirement for mice, and examined 3, 10 and 14 days after castration had larger prostates, taller prostatic luminal epithelial cells and more abundant ventral prostate specific Sbp and Spink1 secretory gene mRNA than treatment matched controls. In addition, the FA enriched diet changed the prostatic gene expression profile, specifically affecting genes involved in nucleotide biosynthesis and DNA repair. Because androgens are required for prostate and seminal vesicle maintenance, attenuated involution of both organs after castration suggests that the FA diet affects these organs by the same mechanism. Taller prostatic luminal epithelial cells and more abundant secretory mRNAs are consistent with glandular secretory activity enduring longer after castration in FA enriched diet fed mice than in controls.
Folic acid is a synthetic and oxidized form of dietary folates and is more stable and bioavailable. Excessive folic acid consumption causes un-metabolized folic acid to accumulate in circulation. Unmetabolized folic acid is detectable in serum of 40% of older adults and is particularly abundant in folic acid supplement users 17. The consequences are not always beneficial 58–60. For example, excessive unmetabolized folic acid has been linked to impaired immune function in post-menopausal women 61. Folic acid, in the form of folate, also participates in several metabolic processes including nucleotide biosynthesis, polyamine synthesis and DNA methylation that influence cell proliferation and survival. Folic acid reduces efficacy of anti-proliferative cancer drugs in vitro 62 and supports cancer stem cell-like properties of human colorectal cancer cells in vitro 63. In our model, we do not observe any effect of the FA enriched diet on prostate cell proliferation. The impact of the FA enriched diet on castration-mediated prostate involution could be a consequence of a systemic shift in metabolism that favors cell survival, a notion supported by the altered prostatic gene expression profile resulting from continuous exposure to the FA enriched diet.
The impact of folates on prostate cancer risk is currently inconclusive. A 5-year clinic trial involving folic acid supplementation found no correlation between folic acid supplementation and cancer risk 64. However, in another study, men given folic acid supplements had an increased risk of prostate cancer compared to the placebo group although baseline folate levels in non-supplement users were inversely associated with prostate cancer risk 65. Additional studies report that high serum folate levels modestly increase prostate cancer risk 66,67 and prostate cell proliferation in prostate cancer patients 68. Further research on the impact of folates on prostate biology is important due to widespread fortification and supplement use.
Serum folate levels have risen in all age groups in the last few decades, with aging men experiencing a substantial increase 45. We found that a FA enriched diet attenuates the prostate involution response to castration. Because drugs that reduce or prevent androgen synthesis are widely used for treating BPH and prostate cancer, our study identify a new need to address interactions between dietary folate and androgen-reducing therapy efficacy.
5. Conclusions
We identified an interaction between dietary folic acid supplementation and tissue involution in response to androgen deprivation. Dietary folic acid supplementation attenuated the involution of two androgen dependent organs, the prostate and seminal vesicle, after castration. In the prostate, this was accompanied by greater luminal epithelial cell secretory activity and secretory gene expression compared to castrated controls. Given the widespread use of folic acid in dietary supplements and fortified food stuffs, our conclusions warrant further research into the interaction between folic acid supplementation and anti-androgen therapies used to treat prostate disease.
Supplementary Material
Acknowledgements
We thank members of the Vezina laboratory for technical assistance and Scott Swanson (Morgridge Institute) for help with RNA-Seq data submission. This work is supported by National Institutes of Health R01 DK099328 and U54 DK104310. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest
Disclosures: None
References
- 1.Rosen RC, Giuliano F, Carson CC. Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). Eur Urol. 2005;47(6):824–837. [DOI] [PubMed] [Google Scholar]
- 2.McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(5 Suppl):S122–128. [PubMed] [Google Scholar]
- 3.Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189(1 Suppl):S102–106. [DOI] [PubMed] [Google Scholar]
- 4.Parikesit D, Mochtar CA, Umbas R, Hamid A. The impact of obesity towards prostate diseases. Prostate International. 2016;4(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarma AV, Kellogg Parsons J. Diabetes and benign prostatic hyperplasia: emerging clinical connections. Current urology reports. 2009;10(4):267–275. [DOI] [PubMed] [Google Scholar]
- 6.Breyer BN, Sarma AV. Hyperglycemia and Insulin Resistance and the Risk of BPH/LUTS: an Update of Recent Literature. Current urology reports. 2014;15(12):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinosa G. Nutrition and benign prostatic hyperplasia. Current opinion in urology. 2013;23(1):38–41. [DOI] [PubMed] [Google Scholar]
- 8.Ma RW, Chapman K. A systematic review of the effect of diet in prostate cancer prevention and treatment. J Hum Nutr Diet. 2009;22(3):187–199; quiz 200–182. [DOI] [PubMed] [Google Scholar]
- 9.Stacewicz-Sapuntzakis M, Borthakur G, Burns JL, Bowen PE. Correlations of dietary patterns with prostate health. Mol Nutr Food Res. 2008;52(1):114–130. [DOI] [PubMed] [Google Scholar]
- 10.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3(1):21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey RL, McDowell MA, Dodd KW, Gahche JJ, Dwyer JT, Picciano MF. Total folate and folic acid intakes from foods and dietary supplements of US children aged 1–13 y. Am J Clin Nutr. 2010;92(2):353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey RL, Dodd KW, Gahche JJ, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am J Clin Nutr. 2010;91(1):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141(2):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MRC. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338(8760):131–137. [PubMed] [Google Scholar]
- 15.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981–2986. [DOI] [PubMed] [Google Scholar]
- 16.Jagerstad M Folic acid fortification prevents neural tube defects and may also reduce cancer risks. Acta paediatrica. 2012;101(10):1007–1012. [DOI] [PubMed] [Google Scholar]
- 17.Bailey RL, Mills JL, Yetley EA, et al. Unmetabolized serum folic acid and its relation to folic acid intake from diet and supplements in a nationally representative sample of adults aged > or =60 y in the United States. Am J Clin Nutr. 2010;92(2):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troen AM, Mitchell B, Sorensen B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136(1):189–194. [DOI] [PubMed] [Google Scholar]
- 19.Ly A, Lee H, Chen J, et al. Effect of maternal and postweaning folic acid supplementation on mammary tumor risk in the offspring. Cancer Res. 2011;71(3):988–997. [DOI] [PubMed] [Google Scholar]
- 20.Barua S, Chadman KK, Kuizon S, et al. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PLoS One. 2014;9(7):e101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sie KK, Medline A, van Weel J, et al. Effect of maternal and postweaning folic acid supplementation on colorectal cancer risk in the offspring. Gut. 2011;60(12):1687–1694. [DOI] [PubMed] [Google Scholar]
- 22.Barua S, Kuizon S, Chadman KK, Flory MJ, Brown WT, Junaid MA. Single-base resolution of mouse offspring brain methylome reveals epigenome modifications caused by gestational folic acid. Epigenetics Chromatin. 2014;7(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barua S, Kuizon S, Brown WT, Junaid MA. DNA Methylation Profiling at Single-Base Resolution Reveals Gestational Folic Acid Supplementation Influences the Epigenome of Mouse Offspring Cerebellum. Front Neurosci. 2016;10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barua S, Kuizon S, Brown WT, Junaid MA. High Gestational Folic Acid Supplementation Alters Expression of Imprinted and Candidate Autism Susceptibility Genes in a sex-Specific Manner in Mouse Offspring. J Mol Neurosci. 2016;58(2):277–286. [DOI] [PubMed] [Google Scholar]
- 25.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, et al. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4(11):e7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bistulfi G, Diegelman P, Foster BA, Kramer DL, Porter CW, Smiraglia DJ. Polyamine biosynthesis impacts cellular folate requirements necessary to maintain S-adenosylmethionine and nucleotide pools. FASEB J. 2009;23(9):2888–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun D, Wollin A, Stephen AM. Moderate folate deficiency influences polyamine synthesis in rats. J Nutr. 2002;132(9):2632–2637. [DOI] [PubMed] [Google Scholar]
- 28.Devens BH, Weeks RS, Burns MR, Carlson CL, Brawer MK. Polyamine depletion therapy in prostate cancer. Prostate Cancer Prostatic Dis. 2000;3(4):275–279. [DOI] [PubMed] [Google Scholar]
- 29.Keil KP, Abler LL, Altmann HM, et al. Impact of a folic acid-enriched diet on urinary tract function in mice treated with testosterone and estradiol. Am J Physiol Renal Physiol. 2015;308(12):F1431–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82(4–5):184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McConnell JD. Androgen ablation and blockade in the treatment of benign prostatic hyperplasia. Urol Clin North Am. 1990;17(3):661–670. [PubMed] [Google Scholar]
- 32.Perlmutter MA, Lepor H. Androgen Deprivation Therapy in the Treatment of Advanced Prostate Cancer. Reviews in Urology. 2007;9(Suppl 1):S3–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Meadows DN, Bahous RH, Best AF, Rozen R. High Dietary Folate in Mice Alters Immune Response and Reduces Survival after Malarial Infection. PLoS One. 2015;10(11):e0143738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meadows DN. High Dietary Folate in Mice Alters Immune Response and Reduces Survival after Malarial Infection. 2015;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CDC. Use of folic acid for prevention of spina bifida and other neural tube defects−−1983–1991. MMWR Morb Mortal Wkly Rep. 1991;40(30):513–516. [PubMed] [Google Scholar]
- 36.Oh KH, Lee JH, Kim KN, Chang N. Effects of folic acid intake on plasma folate, vitamin B12 and homocysteine levels, hepatic SAM/SAH ratio and the expression of cerebral myelin basic protein in pregnant and lactating rats. The FASEB Journal. 2009;23(1_supplement):904.905–904.905. [Google Scholar]
- 37.Abler LL, Keil KP, Mehta V, Joshi PS, Schmitz CT, Vezina CM. A high-resolution molecular atlas of the fetal mouse lower urogenital tract. Dev Dyn. 2011;240(10):2364–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keil KP, Mehta V, Branam AM, et al. Wnt inhibitory factor 1 (Wif1) is regulated by androgens and enhances androgen-dependent prostate development. Endocrinology. 2012;153(12):6091–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan JS, Reed A, Chen F, Stewart CN. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou Z, Jiang P, Swanson SA, et al. A cost-effective RNA sequencing protocol for large-scale gene expression studies. Sci Rep. 2015;5:9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leng N, Dawson JA, Thomson JA, et al. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics. 2013;29(8):1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Vasaikar S, Shi Z, Greer M, Zhang B. WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017;45(W1):W130–W137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfeiffer CM, Hughes JP, Lacher DA, et al. Estimation of Trends in Serum and RBC Folate in the U.S. Population from Pre- to Postfortification Using Assay-Adjusted Data from the NHANES 1988–2010. J Nutr. 2012;142(5):886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeiffer CM, Johnson CL, Jain RB, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988 2004. Am J Clin Nutr. 2007;86(3):718–727. [DOI] [PubMed] [Google Scholar]
- 47.CDC. Folate status in women of childbearing age, by race/ethnicity--United States, 1999–2000, 2001–2002, and 2003–2004. MMWR Morb Mortal Wkly Rep. 2007;55(51–52):1377–1380. [PubMed] [Google Scholar]
- 48.Lee C Physiology of castration-induced regression in rat prostate. Prog Clin Biol Res. 1981;75a:145–159. [PubMed] [Google Scholar]
- 49.Wang XD, Wang BE, Soriano R, et al. Expression profiling of the mouse prostate after castration and hormone replacement: implication of H-cadherin in prostate tumorigenesis. Differentiation. 2007;75(3):219–234. [DOI] [PubMed] [Google Scholar]
- 50.Isaacs JT, Furuya Y, Berges R. The role of androgen in the regulation of programmed cell death/apoptosis in normal and malignant prostatic tissue. Semin Cancer Biol. 1994;5(5):391–400. [PubMed] [Google Scholar]
- 51.English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11(3):229–242. [DOI] [PubMed] [Google Scholar]
- 52.Cohen RJ, McNeal JE, Edgar SG, Robertson T, Dawkins HJ. Characterization of cytoplasmic secretory granules (PSG), in prostatic epithelium and their transformation-induced loss in dysplasia and adenocarcinoma. Hum Pathol. 1998;29(12):1488–1494. [DOI] [PubMed] [Google Scholar]
- 53.Kawamura H, Kimura M, Ichihara I. The effect of androgen and estrogen on secretory epithelial cells and basal cells of the rat ventral prostate after long-term castration. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 1993;175(6):569–575. [DOI] [PubMed] [Google Scholar]
- 54.Mills JS, Needham M, Parker MG. Androgen regulated expression of a spermine binding protein gene in mouse ventral prostate. Nucleic Acids Res. 1987;15(19):7709–7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z, Tufts R, Haleem R, Cai X. Genes regulated by androgen in the rat ventral prostate. Proc Natl Acad Sci U S A. 1997;94(24):12999–13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berquin IM, Min Y, Wu R, Wu H, Chen YQ. Expression signature of the mouse prostate. J Biol Chem. 2005;280(43):36442–36451. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee PP, Banerjee S, Brown TR. Increased Androgen Receptor Expression Correlates with Development of Age-Dependent, Lobe-Specific Spontaneous Hyperplasia of the Brown Norway Rat Prostate. Endocrinology. 2001;142(9):4066–4075. [DOI] [PubMed] [Google Scholar]
- 58.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87(3):517–533. [DOI] [PubMed] [Google Scholar]
- 59.Caudill MA. Folate bioavailability: implications for establishing dietary recommendations and optimizing status. Am J Clin Nutr. 2010;91(5):1455s–1460s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crider KS, Bailey LB, Berry RJ. Folic Acid Food Fortification—Its History, Effect, Concerns, and Future Directions. Nutrients. 2011;3(3):370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Troen AM, Mitchell B, Sorensen B, et al. Unmetabolized Folic Acid in Plasma Is Associated with Reduced Natural Killer Cell Cytotoxicity among Postmenopausal Women. The Journal of Nutrition. 2006;136(1):189–194. [DOI] [PubMed] [Google Scholar]
- 62.Xavier MA, de Oliveira MT, Baranoski A, Mantovani MS. Effects of folic acid on the antiproliferative efficiency of doxorubicin, camptothecin and methyl methanesulfonate in MCF-7 cells by mRNA endpoints. Saudi J Biol Sci. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farias N, Ho N, Butler S, et al. The effects of folic acid on global DNA methylation and colonosphere formation in colon cancer cell lines. J Nutr Biochem. 2015;26(8):818–826. [DOI] [PubMed] [Google Scholar]
- 64.Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50 000 individuals. Lancet. 2013;381(9871). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Figueiredo JC, Grau MV, Haile RW, et al. Folic Acid and Risk of Prostate Cancer: Results From a Randomized Clinical Trial. J Natl Cancer Inst. 2009;101(6):432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collin SM, Metcalfe C, Refsum H, et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Vogel S, Meyer K, Fredriksen A, et al. Serum folate and vitamin B12 concentrations in relation to prostate cancer risk--a Norwegian population-based nested case-control study of 3000 cases and 3000 controls within the JANUS cohort. Int J Epidemiol. 2013;42(1):201–210. [DOI] [PubMed] [Google Scholar]
- 68.Tomaszewski JJ, Cummings JL, Parwani AV, et al. Increased cancer cell proliferation in prostate cancer patients with high levels of serum folate. Prostate. 2011;71(12):1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.