Abstract
Objective:
We created normative growth charts of amygdala functional connectivity in typically developing youth and assessed age-associated deviations of these trajectories in psychosis spectrum youth, and explored how these disruptions are related to clinical symptomatology.
Methods:
Resting state functional neuroimaging data from four samples (3 cross-sectional, 1 longitudinal) was collected on 1062 participants (typically developing controls=622, psychosis spectrum=194, other psychopathology=246, ages 10–25 years). We assessed deviations in youth with psychosis spectrum and other psychopathology in age-related changes in resting state fMRI (rsfMRI) amygdala to whole brain connectivity from a normative range derived from control youth. We explored relationship between age-associated deviations in amygdala connectivity and positive symptoms in the patient group.
Results:
Normative trajectories demonstrated significant age-related decreases in centromedial amygdala connectivity with the rest of the brain. In contrast, psychosis spectrum youth failed to exhibit any significant age-associated changes between the centromedial amygdala and prefrontal cortices, striatum, occipital cortex, and thalamus (all q<.1). Age associated deviations in centromedial amygdala-striatum and centromedial amygdala-occipital connectivity were unique to psychosis spectrum youth and not observed in those with other psychopathology. Exploratory analyses revealed that greater age-related deviation in centromedial amygdala-thalamus connectivity was associated with increased severity of positive symptoms (r=0.19, p=0.01, q=0.05) in psychosis spectrum youth.
Conclusion:
Using neurodevelopmental growth charts to identify a lack of normative development of amygdala connectivity in psychosis spectrum youth may help us better understand the neural basis of affective impairments in psychosis, informing prediction models and interventions.
Keywords: resting state fMRI, schizophrenia, social cognition, adolescent development
Introduction
Affective dysfunction is a prominent feature of psychosis. Affective deficits are present prior to the onset of the full-blown illness (1, 2) and severity contributes to improved prediction of psychosis in high-risk samples (3, 4). Psychosis often develops during the transition from adolescence to adulthood, a time when significant specialization and strengthening in cognitive control of affective processes occurs (5). Additionally, adults with psychosis consistently exhibit structural and functional alterations in the amygdala (6–12), a brain structure that plays a key role in affective processes. Connectivity between the amygdala and brain regions supporting multiple cognitive and emotional functions undergo significant changes through adolescence (13–15). Thus, how amygdala connectivity is neurodevelopmentally affected in psychosis is critical to understanding the neural basis of affective impairment in psychosis.
We recently reported that two nuclei in the amygdala, the centromedial (CM) and basolateral (BL) amygdalae, exhibit differential developmental trajectories, with the majority of typical developmental decreases occurring between the CM amygdala and other brain regions (13). Here, we extend these findings by combining different developmental data sources to form a large dataset and construct a normative template of age related changes to be used as a growth chart for the development of amygdala connectivity. Growth charts, typically used as references for early identification of atypical development for metrics such a weight and head circumference (16), have recently been extended to assess how psychiatric disorders are related to deviations from normative development (17, 18). Multisite sample characterization of typical development of amygdala connectivity will provide a template to then assess abnormal development of brain function in those with psychosis spectrum. A growing body of literature has used resting state functional magnetic resonance imaging to identify amygdala connectivity disruptions in adults with psychosis (7, 19–21) and individuals with psychosis are impaired in social-cognitive processes that continue to develop during adolescence (18, 22–24). Thus, characterizing age-associated deviations in amygdala connectivity can inform affective dysregulation in psychosis and how it may underlie the development of psychotic symptoms and/or disruptions in social cognitive processes.
Importantly, unique neurodevelopmental trajectories of amygdala connectivity may distinguish psychosis spectrum disorders from other forms of psychopathology. Multiple social cognitive processes that involve the amygdala, including facial affect recognition, emotion regulation and theory of mind, are more impaired in schizophrenia compared to individuals with other psychiatric disorders, providing behavioral support for a differential deficit (25–27). Distinct patterns of amygdala-prefrontal connectivity differentiate individuals with psychosis from those without a psychosis history (19, 21). However, disruption of age-associated amygdala trajectories and their specificity to psychosis spectrum disorders has not been examined. Understanding the timing of disruption in psychosis in comparison to other psychopathology may help us identify individuals who are at greater risk for developing the disorder.
As such, the goals of this study were to 1) identify the strongest age-associated changes in CM and BL amygdala connectivity in typically developing youth across multiple samples, 2) characterize deviations from normative amygdala connectivity trajectories in psychosis, 3) determine the specificity of these abnormalities by comparing age-associated amygdala connectivity of youth with other psychopathologies, and 4) test if age-associated deviations in amygdala connectivity are related to psychotic symptoms and/or emotion recognition. We hypothesized that 1) consistent with our previous research, strongest age-associated decreases would be observed in CM amygdala connectivity in typically developing youth (13); 2) psychosis spectrum youth would fail to show age-associated decreases in CM amygdala-prefrontal connectivity, given that increased amygdala-prefrontal connectivity has previously been associated with psychosis (19, 21); and 3) distinct patterns of age-associated amygdala-prefrontal connectivity would differentiate psychosis spectrum youth from youth with other psychopathologies. Finally, we tested the exploratory hypothesis that age-associated deviations in amygdala connectivity would be associated with positive symptoms and emotion recognition performance.
Participants
The final neuroimaging data set consisted of 1062 10–25 year olds (typically developing controls=622, psychosis spectrum=194, other psychopathology=246) from four different samples. Three data sets were acquired at the University of Pittsburgh (Luna 1, Luna 2, Pitt) and one data set was acquired at the University of Pennsylvania (PNC, (28, 29). Participant information is presented in Table 1. Luna 1 was a longitudinal sample and the other three samples were cross-sectional. Details on participant recruitment and inclusion/exclusion criteria are in the Supplemental Text and Figure S1.
Table 1.
Demographic information for all samples.
| Cohort | Study Design | Age range (years) | Typically Developing | Psychosis Spectrum | Other Psychopathology | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean Age (SD) | M/F | N | Mean Age (SD) | M/F | N | Mean Age (SD) | M/F | |||
| Luna 1 | longitudinal (1–3 visits) | 10–25 | 213 | 16.7 (3.0) | 113/100 | 0 | NA | NA | 0 | NA | NA |
| Luna 2 | cross-sectional | 14–25 | 88 | 19.5 (3.6) | 45/43 | 0 | NA | NA | 0 | NA | NA |
| PNC | cross-sectional | 10–22 | 292 | 16.2 (3.4) | 151/141 | 162 | 16.0 (2.9) | 75/87 | 246 | 16.4 (3.3) | 96/150 |
| Pitt | cross-sectional | 12–25 | 29 | 21.0(2.7) | 18/11 | 32 | 20.8(3.1) | 19/13 | 0 | NA | NA |
Clinical Measures
PNC
Positive and negative symptoms were measured by summing the relevant SIPS/PS-R responses (0=definitely disagree, 1=somewhat disagree, 2=slightly disagree, 3=not sure, 4=slightly agree, 5=somewhat agree, 6=definitely agree). Table S1 reports included questions.
MR Data Acquisition
For all samples, data were acquired using a Siemens 3 Tesla Tim Trio. Resting state data was collected using an echo-planar sequence sensitive to BOLD contrast (T2*). A magnetization-prepared rapid gradient-echo sequence (MPRAGE) was acquired to measure brain structure and for alignment of the rsfMRI images. Table S2 includes all scan instructions and parameters.
rsfMRI Processing
Supplemental Text reports details of rsfMRI data processing.
Statistical Analyses
rsfMRI First Level Statistical Analyses
We conducted voxelwise regressions on processed data using AFNI’s 3dDeconvolve with the average of each amygdala subregion ROI (CM, BL) time series as the seed. AFNI’s 3dREMLFIT program was applied to correct for temporal autocorrelation between voxels. These analyses resulted in voxelwise subject-level maps of Pearson correlations (r) between the average amygdala subregion ROI time course and each voxel’s time course. R-values were then normalized using Fisher r-to-z transformation.
Voxelwise Developmental Changes in Amygdala Subregion Connectivity
We used the 3dLME program in AFNI to examine voxelwise, developmental effects of age for each amygdala subregion in typically developing youth. 3dLME is a group analysis program in AFNI that computes linear mixed-models (31). Subject was included as a random effect, which allows us to model and account for the non-independence of data (multiple visits) in the longitudinal cohort (Luna 1,(32)). Age, sex, and site were included as fixed effects. Linear, inverse, and quadratic forms of age were examined. Results were corrected for multiple comparisons using a combination of cluster size and voxel probability, with parameters determined through a Monte Carlo simulation using AFNI’s 3dClustSim program (Supplemental Text for details).This implementation is the most current, stringent procedure recommended by the AFNI developers to prevent against obtaining false positive clusters of connectivity (33, 34).
For clusters where significant results obtained across multiple forms of age (linear, inverse, quadratic), we determined a conjunction cluster and extracted out the cluster’s mean ROI for each individual. We re-ran the above mixed effects models with linear, inverse, and quadratic forms of age on this data. The lowest combination of AIC and BIC was considered to be the best-fitting model.
To ensure that motion artifacts did not drive our results, we extracted out the mean ROI from the significant clusters, and re-ran the linear mixed effects models, and included average framewise displacement (FD) as an additional fixed effect. We also re-ran developmental analyses on subsets of “low motion” subjects: removing participants with average FD in upper 25th percentile (>0.17).
Disruption of age-associated amygdala connectivity in psychosis
To determine disruption of amygdala developmental connectivity changes in psychosis spectrum youth, we extracted out mean ROIs for clusters that exhibited significant developmental changes in typically developing youth and ran a linear mixed-model with each connectivity measure as the dependent variable. Fixed effects included an interaction term between age (inverse form, and group (control, psychosis spectrum), as well as the main effects of both variables. Subject was included as a random effect. Site, gender, and FD were included as fixed effects. False-discovery rate was used to correct for multiple comparisons (35). To determine the specificity of age-related deviations in psychosis spectrum youth in any clusters that exhibited significant age by group deviations, we added the other psychopathology group to the model and re-ran it. All significant interaction terms were further examined with the simple slopes of each group using least-squares means (lsmeans, (36)).
To explore whether there were any age-related alterations in psychosis and/or youth with other psychopathology that were not observed in the above analysis, we used 3dLME to run the above interaction model using a voxelwise approach (p<.001, cluster-wise p=0.05, 30 continuous voxels).
Relationships between age-associated deviations in amygdala connectivity, and positive symptoms
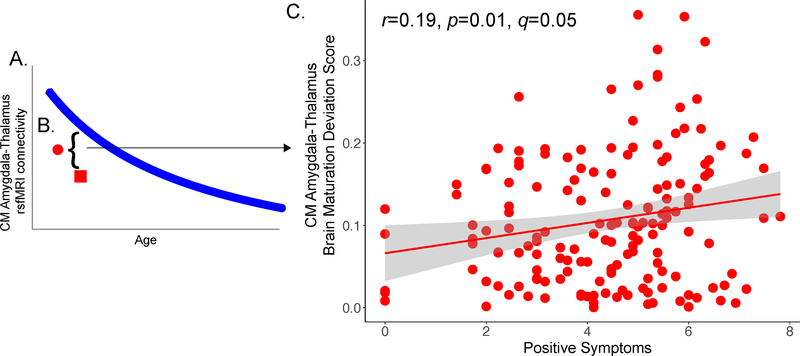
We took a two-step, developmentally-informed approach to examine relationships between amygdala connectivity and psychotic symptoms. In regions that we observed significant age-related alterations in psychosis, we first conducted analyses to characterize the extent to which age-associated deviations from normal development—independent of the directionality of the connectivity differences—were associated with psychotic symptoms. Using the model of best fit for developmental changes observed for amygdala connectivity in the typically developing youth, we predicted what the expected “normative” amygdala connectivity value would be for psychosis spectrum youth (Figure 2A). We then subtracted the predicted value from the actual amygdala connectivity value for each individual and took the absolute value of this score (Figure 2B). This created an amygdala connectivity maturation deviation score, a method that has been previously used to identify deviations from normative growth in brain connectivity metrics (17). We ran Pearson correlations between the amygdala brain maturation deviation score and positive symptoms in the psychosis spectrum youth. FDR was used to correct for multiple comparisons (q<.1).
Figure 2.
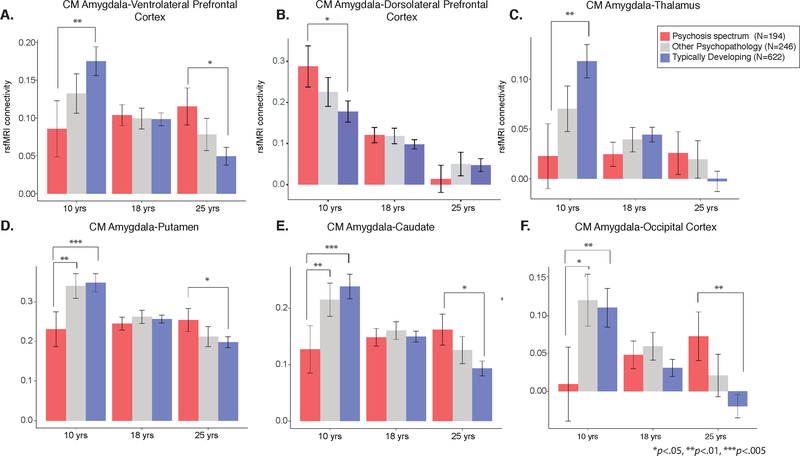
For visualization purposes of inverse age x group interactions of centromedial (CM) amygdala connectivity, we calculated least squared means for amygdala connectivity values at ages 10, 18, and 25 years for typically developing youth (blue), psychosis spectrum youth (red), and youth with other psychopathology (grey). A) In comparison to typically developing youth, psychosis spectrum youth exhibited reduced CM amygdala-ventrolateral prefrontal connectivity during late childhood/early adolescence, and increased connectivity during adulthood. B) In comparison to typically developing youth, psychosis spectrum youth exhibited increased CM amygdala-dorsolateral prefrontal connectivity during late childhood/early adolescence. C) In comparison to typically developing youth, psychosis spectrum youth exhibited reduced CM amygdala-thalamus connectivity during late childhood/early adolescence. D) In comparison to typically developing youth and youth with other psychopathology, psychosis spectrum youth exhibited reduced CM amygdala-putamen connectivity during late childhood/early adolescence. Psychosis spectrum youth exhibited greater connectivity between these two regions during adulthood. E) A similar pattern of developmental disruption was observed between CM amygdala-caudate connectivity. F) Psychosis spectrum youth exhibited reduced CM amygdala-occipital cortex connectivity when compared to typically developing youth and youth with other psychopathology.
In a post-hoc analysis, we wanted to determine the direction of any identified relationships and focus our analyses on the discrete developmental periods in which there were significant differences in amygdala connectivity between psychosis spectrum and controls (Table S5). After regressing out the effects of age, gender and motion covariates on the connectivity measure of interest, we conducted Pearson correlation analyses with the connectivity value and positive symptoms the during developmental period that was significantly different between psychosis spectrum and controls. We also conducted Pearson correlation between the connectivity value and positive symptoms in the developmental period in which amygdala connectivity values were not statistically different from each other.
Results
Typical age-associated development of amygdala subregion connectivity
Developmental effects were observed for functionl connectivity between the CM amygdala and 19 clusters (Table 2, Figure S2). These clusters included the following bilateral brain regions: posterior cingulate, insula, parahippocampal cortex, and precentral gyrus/frontal eye fields. Significant clusters were also observed for the left ventrolateral prefrontal cortex, left caudate, left dorsolateral prefrontal cortex, right thalamus, and right postcentral gyrus. We observed age-related decreases in connectivity strength between the CM amygdala and all clusters, with children exhibiting positive CM amygdala connectivity (mean = 0.19 at 10 years old) and adults exhibited near zero levels of connectivity (mean = 0.04 at 25 years old).
Table 2.
Nineteen clusters and associated brain regions and NI coordinates that exhibited significant age-associated changes in typically developing youth. On the right, the statistics for the age*group interactions between typically developing and psychosis spectrum youth are presented. Clusters that remained significant for the age*group interactions after FDR correction are highlighted in bold.
| 10 | R precentral/postcentral gyrus | 85 | 45 | −21 | 58 | 0.0 | 0.95 | 0.98 |
| 11 | L ventrolateral prefrontal cortex | 70 | −31 | 29 | −2 | 7.5 | 0.006 | 0.04 |
| 12 | L putamen | 61 | −22 | 2 | 8 | 8.2 | 0.004 | 0.04 |
| 13 | L BA 10/superior frontal gyrus | 57 | −13 | 71 | 1 | 2.6 | 0.11 | 0.23 |
| 14 | R thalamus | 57 | 20 | −33 | 10 | 6.7 | 0.009 | 0.05 |
| 15 | R insula | 51 | 38 | −28 | 15 | 3.6 | 0.05 | 0.15 |
| 16 | L caudate | 43 | −15 | 18 | 3 | 8.5 | 0.004 | 0.04 |
| 17 | L dorsolateral prefrontal cortex/BA 9 | 40 | −20 | 41 | 38 | 4.1 | 0.04 | 0.09 |
| 18 | L parahippocampal gyrus | 39 | −29 | −38 | −11 | 3.1 | 0.08 | 0.20 |
| 19 | R middle occipital cortex | 36 | 47 | −77 | 1 | 5.4 | 0.02 | 0.05 |
| Basolateral amygdala connectivity | ||||||||
| 1 | L uncus | 33 | −24 | −3 | −34 | 0.9 | 0.3400 | 0.35 |
Developmental effects were also observed for functional connectivity between the BL amygdala and one cluster, which encompassed the left uncus (Table 2). In this case, children exhibited positive CM amygdala connectivity (mean = 0.27 at 10 years old), while adults exhibited positive connectivity as well, albeit to a lesser extent (mean = 0.17 at 25 years old).
For all significant clusters, the inverse form of age was the best fit. All developmental effects remained significant when motion covariates (average FD) and MRI software version were included in the model and when high motion subjects were excluded from the analysis (Tables S3–4). Strikingly, age-associated changes were consistent across sites (Figure S3).
We also confirmed that site effects were appropriately accounted for by including the measure as a covariate. After regressing out the effects of site in each region, we then plotted the residuals (Figure S4). Because the residuals all clustered around zero, this provides evidence that we were able to effectively account for site in our model. Furthermore, when we conducted a mixed model linear regression to compare residuals between sites, there were no significant differences between sites for any of the ROIs (all |2=0, p=1), further solidifying evidence that ROI values were similar across sites once site was included in the model.
Age-associated disruptions in amygdala connectivity in psychosis spectrum youth
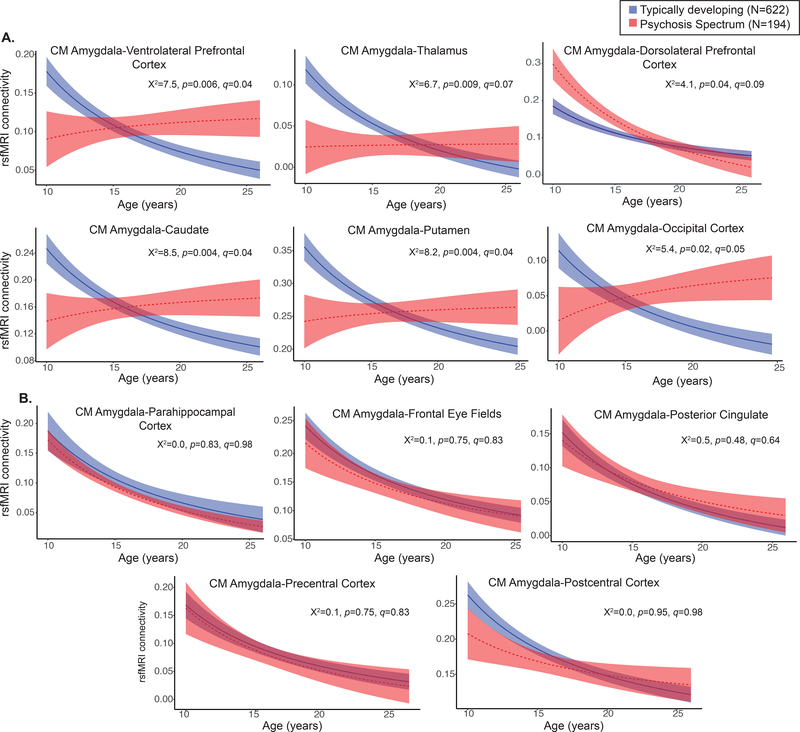
After correcting for multiple comparisons, significant age-associated deviations (inverse age*group interactions) were observed in the psychosis spectrum group for connectivity in the CM amygdala and five clusters in the following brain regions: L ventrolateral prefrontal cortex, L putamen, L caudate, R middle occipital gyrus, and R thalamus (Figure 1A). In all clusters, slope comparison analyses revealed that controls exhibited significant age-related decreases with increasing age, while psychosis spectrum youth failed to exhibit significant age-associated changes (Table S5). Pairwise contrasts revealed that during late childhood, in comparison to typically developing youth, psychosis spectrum exhibited significantly lower connectivity in the following pairs: CM amygdala-ventrolateral prefrontal, CM amygdala-putamen, and CM amygdala-caudate. In adulthood, psychosis spectrum youth exhibited higher connectivity in comparison to typically developing controls in CM amygdala-ventrolateral prefrontal cortex connectivity, CM amygdala-putamen connectivity, and CM amygdala-occipital cortex connectivity. These results are illustrated in Fig 1A, reflecting a lack of developmental decreases in psychosis emerging from underconnectivity in childhood. For specific time periods in which group differences were observed, please see Table S5. All significant age*group interactions remained when psychiatric medication status was included as a covariate. Amount of variance explained by the full model, the inverse age by group interactions, and the main effects of inverse age and group are reported in Table S7.
Figure 1.
A. In comparison to typically developing youth (blue), psychosis spectrum youth (red) exhibited significant deviations from typical centromedial amygdala connectivity development in the following regions: ventrolateral prefrontal cortex, thalamus, dorsolateral prefrontal cortex, caudate, putamen, and middle occipital gyrus. B. Psychosis spectrum and typically developing youth exhibit similar patterns of developmental decreases between the centromedial connectivity and the parahippocampal cortex, frontal eye fields, posterior cingulate, precentral, and postcentral cortices.
Similar to controls, psychosis spectrum youth exhibited a decline in CM amygdala connectivity with increases in age for clusters with the following regions: posterior cingulate, precentral, postcentral, and parahippocampal cortex (Figure 1B).
Specificity of age-associated disruptions in amygdala connectivity in psychosis spectrum youth
When youth with other psychopathology were added to the model, all five models maintained the significant inverse age*group interactions (Table S6). Like controls, youth with other psychopathology showed typical significant age-related decreases with increasing age in connectivity between CM amygdala in three regions: putamen, caudate, and occipital cortex (Figures S5 and S6, Table S7). However, like psychosis spectrum youth, the other psychopathology group failed to show age-associated changes in CM amygdala-ventrolateral prefrontal cortex connectivity, and CM amygdala-thalamus connectivity, though there were no significant inverse age by group interactions between typically developing and other psychopathology youth (p>0.07). Furthermore, the other psychopathology group and typically developing youth did not differ in amygdala connectivity levels at any point in development (Table S7). As seen in Figure S5, connectivity CM amygdala-VLPFC and CM amygdala-thalamus, the developmental trajectories for the other psychopathology youth fell in between the trajectories of the typically developing and psychosis spectrum youth. In comparison to the other psychopathology group, psychosis spectrum youth exhibited reduced connectivity during late childhood and early adolescence in the following connectivity pairs: CM amygdala-putamen and CM amygdala-occipital cortex (Figure 2).
Confirmatory analyses revealed that there were no significant interactions between 1) inverse age*group*gender, 3) inverse agegroup*site, 4) gender*group, or 5) group*site in psychosis spectrum and typically developing youth in these regions. Exploratory, voxelwise analyses of age*group interactions failed to find any other significant clusters.
Age-associated deviation in CM amygdala-thalamus connectivity is associated with increased positive symptoms
After calculating brain maturation deviation scores, we found that greater age-related deviation in CM amygdala-thalamus connectivity was associated with greater positive symptom severity in psychosis spectrum youth (r=0.19, p=0.01, q=0.05, Figure 2). Post hoc analyses revealed that increased severity of grandiose ideas (r=0.31, p=6-e05) and hallucinations (r=0.23, p=0.003) were related to CM amygdala-thalamus age-associated deviation, but not unusual thought content (r=0.08, p=0.32). This relationship was not present in the other psychopathology group (CM amygdala-thalamus: r=−0.02, p=0.89). This relationship was not observed between the CM amygdala-thalamus brain maturation deviation score and negative (r=0.02, p=0.8), depression (r=−0.06, p=0.42), or mania (r=−0.01, p=0.93) symptoms.
We next characterized where in the developmental trajectory amygdala connectivity related to positive symptoms. During late childhood and early adolescence, lower amygdala-thalamus connectivity was associated with greater positive symptoms (r=−0.27, p=0.01, q=0.05).
Discussion
We examined age-associated disruptions in amygdala functional connectivity in psychosis spectrum youth and explored how alterations in neurodevelopmental connectivity may be related to psychotic symptoms. First, we developed normative amygdala connectivity growth charts in typically developing youth and confirmed that strongest age-associated changes occur between centromedial (CM) amygdala and connectivity and multiple brain regions, with decreases in connectivity occurring as age increased, confirming our previous results (37). Then, we showed that psychosis spectrum youth failed to show typical age-associated decreases between CM amygdala connectivity and four distinct brain regions: the striatum, thalamus, ventrolateral prefrontal, and occipital cortex. Age-associated alterations in CM amygdala-putamen connectivity and CM amygdala-occipital cortex conenctivity were unique to psychosis spectrum youth, as youth with other psychopathology did not exhibit these age-associated deviations. Exploratory analyses revealed that greater age-related deviations in CM amygdala-thalamus functional maturation were associated with greater positive symptoms in psychosis spectrum youth. Our results provide a novel view of developmental alterations in functional connectivity in psychosis spectrum, implicating alterations during discrete developmental windows within neural circuits implicated in a wide array of cognitive and emotional processes.
Developmental Alterations in CM Amygdala Connectivity in Psychosis Spectrum Youth
In line with our previous findings, significant typical developmental function connectivity decreases occurred between the CM amygdala connectivity and multiple brain regions (13). These results are consistent with previous developmental neuroimaging rsfMRI studies reporting subcortical-cortical connectivity decreases into adulthood (38–41). In comparison to typically developing youth, psychosis spectrum youth exhibited reduced connectivity between the CM amygdala and the ventrolateral prefrontal cortex, striatum, thalamus, and occipital cortex, during late childhood and early adolescence with a lack of normative decreases from adolescence to adulthood. These findings suggest that either there is an accelerated developmental decrease in amygdala connectivity in psychosis preceding the normative timetable or that the earlier age of onset reflects deterioration of this circuitry as is evident later in adulthood. Normatively, decreases in connectivity can be seen as a period of specialization that occurs at a critical time when higher-level systems are becoming established to form adult trajectories. The lack of a marker of specialization through adolescence could reflect impairments in optimal specialization that could contribute to abnormal processing of executive affective information processing in psychosis.
Many of the regions that exhibited disrupted age-associated amygdala connectivity in psychosis spectrum youth are related to perception and salience (e.g., thalamus, striatum, and occipital cortex (45–52)). A primary function of the amygdala is to determine what is salient in one’s environment and facilitate learning for these items (53–55). Projections of the amygdala to the thalamus are thought to modulate attentional orientation and arousal, broadly speaking (56). Projections of the amygdala to visual cortex is known to enhance sensitivity, discrimination, and subjective vividness of perceived stimuli (57). Finally, projections of the amygdala to the striatum are thought to provide an interface between the amygdala and dopamine systems, which are known to regulate motivation, learning, and behavioral activation (58). Thus, abnormal connectivity of the amygdala with the thalamus, visual cortex, and striatum could reflect processes that result in the misattribution of salience to stimuli in psychosis as well as a facilitation of learning associations about threat-related items. Critically, this type of aberrant salience processing may provide the foundation for higher-order features of positive symptoms, such as delusions (59).
Connections between the CM amygdala and lateral prefrontal regions also exhibited a disruption in age associated changes in psychosis spectrum youth. During late childhood and early adolescence, psychosis spectrum youth exhibited reduced amygdala-VLPFC connectivity; however, during adulthood psychosis spectrum had increased connectivity between these two regions. Amygdala-VLPFC connectivity is necessary during the reappraisal phase of regulating one’s emotions (60–64) and how these two structures interact during emotion regulation changes during adolescent development (65). Indeed, impairments in lateral prefrontal-mediated circuitry are related to emotional deficits typically observed in psychosis (66–68) and age-associated amygdala-VLPFC functional connectivity alterations during an affective labeling task have been observed in youth at clinical high risk for developing psychosis (69). Thus, CM amygdala-VLPFC age-associated disruptions may underlie the emotional dysregulation that often precedes and predicts increased psychotic symptomatology (70–72). Experience sampling studies show that adults with schizophrenia report more intense negative emotions and greater social stress than healthy controls (73–75). Heightened amygdala-VLPFC connectivity in adults experiencing psychosis spectrum symptoms that we observed may reflect underlying biological vulnerability to psychosis onset, which interacts with these environmental stressors. Future studies integrating experience-sampling methods (76) with neuroimaging in youth at high risk for developing psychosis are necessary to test this hypothesis.
We also observed a unique, altered age-associated pattern of CM amygdala-DLPFC connectivity in psychosis spectrum youth compared to typically developing youth. During late childhood and early adolescence, psychosis spectrum youth exhibited increased CM amygdala-DLPFC coupling compared to typically developing youth. This group difference was no longer present in adulthood, with both typically developing and psychosis spectrum youth exhibiting similar levels of connectivity. Previously, in adults with schizophrenia, absent or reduced amygdala-DLPFC functional connectivity has been observed during emotional distraction during a working memory task (77) and at rest (7, 21). Our developmentally-sensitive results of increased CM amygdala-DLPFC connectivity in psychosis spectrum youth during late childhood and early adolescence contrast with these findings and highlight the importance of examining how one’s developmental stage may affect the directionality and interpretation of brain connectivity (78). Reduced GABA levels are consistently observed in the prefrontal cortex in schizophrenia. While GABAergic deficits have not been identified in the amygdala in schizophrenia, the majority of neurons projecting from the CM amygdala are GABAergic (79). These generate down-regulation of GABA interneuron activity in the prefrontal cortex and amygdala, resulting in a lack of inhibition, which may be responsible for the increased connectivity observed between the amygdala and DLPFC during late childhood and early adolescence in psychosis spectrum youth.
In summary, we identified developmentally sensitive alterations in cortico-limbic and intralimbic rsfMRI connectivity in psychosis spectrum youth. These neurodevelopmental alterations provide support for multiple theories associated with schizophrenia and complement the building body of literature showing that which shows progressive maturational disturbances in those who go on to develop psychosis (80–83).”
Specificity of Age-Associated Amygdala Connectivity Alterations to Psychosis Spectrum
Two amygdala connectivity age-associated alterations were distinct to psychosis spectrum youth. In comparison to both the typically developing and other psychopathology group, psychosis spectrum youth exhibited reduced connectivity during late childhood and early adolescence in the following connectivity pairs: CM amygdala-putamen and CM amygdala-occipital cortex. Though psychotic symptoms rarely separate clinical samples into discrete groups, our results suggest that these brain abnormalities are unique to psychosis spectrum youth, and in the future, could potentially differentiate psychotic disorders from other psychiatric disorders. Alternatively, in other amygdala connectivity measures, the other psychopathology developmental trajectories fell in between typically developing and psychosis spectrum youth. These findings suggest that there is a less severe neurobiological impact on other psychopathology youth in these connectivity metrics.
Amygdala-Thalamus Brain Maturation Deviations & Positive Symptoms
The growth charting methods employed in this study establish a novel connection between deviations from amygdala-thalamus connectivity development and increased positive symptom severity. In our exploratory analyses, we found that deviation from the normative trajectory of neurodevelopment is relevant to positive symptoms. Though this relationship was statistically significant after multiple comparisons (q<.1), it is a small effect and should be replicated in future studies. These findings, along with others (17, 18, 78), highlight the importance of examining the role that (dys)maturation patterns play in the development of psychiatric disorders. While small effect sizes may not indicate a direct intervention, it is important to identify these deviations to fully characterizing the pathophysiology of psychosis risk
Use of “Big Data” to Create Neurodevelopmental Growth Charts
This study also represents a proof of principle approach for merging multiple rsfMRI data sets to inform normative developmental trajectories and identify aberrant trajectories in psychosis spectrum youth. Despite the samples having multiple sites, protocols, and recruitment methods, the age-associated changes were remarkably consistent across the different samples of typically developing youth (Figure S3) and including sample as a covariate effectively removed any site differences (Figure S4). In conjunction with recent work (10, 17, 92–94), these results support using publicly available data to assess developmental changes in brain function and relevance to psychiatric disorders. Given that age-associated changes can be small albeit significant, an approach that takes advantage of large sample size will be necessary for identifying distinct periods of development in which there are disruptions related to psychiatric disorders or symptoms.
Limitations
We are limited by the fact that there was only cross-sectional data available for psychosis spectrum youth. Thus, the neurodevelopmental trajectories in psychosis spectrum do not reflect within person change but rather cross-sectional associations with age. Our cross-sectional sample cannot definitively show if our results are due to altered development or abnormalities due to time of onset of psychosis. Thus, Longitudinal studies of psychosis spectrum youth, with multiple visits per individual, can extend our understanding of psychosis by identifying how the shape and rate of maturation of subject-specific developmental trajectories in psychosis spectrum youth diverge, converge, or remain stable in comparison typical development (95–97). Furthermore, many developmental changes that occur during adolescence are non-linear and these patterns are most accurately captured with longitudinal analyses (98). Additionally, the presence of psychotic symptoms are dynamic and change over time (99, 100), these changes need to be taken into account when characterizing neurodevelopmental change. Recently, using novel time-varying analytic approaches (101, 102), we found that connectivity measures were differentially related to individual differences in anxiety and depression at different points in adolescent development (13). A similar approach could be applied to longitudinal neuroimaging and psychotic symptom data, to identify particular periods of development in which psychic symptoms are linked to rsfMRI connectivity metrics.
Finally, while we are fairly confident that we were able to appropriately account for site in our analyses (Figure S4), we observed a statistically significant effect of site in many of the ROIs (Table S4). Despite all scans being performed on the same scanner model, there were still differences in task instructions, MRI resolution, and length of scan. Site effects may have obscured our ability to identify smaller developmental changes in normative amygdala connectivity developmental trajectories. It is also possible that we failed to identify more subtle age-related deviations in amygdala connectivity between psychosis spectrum and typically developing youth due to site effects. Recently, methodology from genetics has been used for to harmonize structural MRI data across sites (103, 104); modifying and applying this method to resting state connectivity data is a logical next step. Despite these site differences, we still see a significant interaction between group and age; these findings suggest that multi-site neuroimaging datasets will be important for understanding how biomarkers may be sensitive or specific to developmental stage.
Conclusions
Taken together our results provide compelling novel evidence for developmental disruptions of age-associated trajectories of amygdala connectivity in psychosis specific to circuitry underlying salience and cognitive control of affect. Importantly, this disruption appears early in development with evidence for a subsequent lack of normative refinement. In the future, we hope to build upon these finding and examine how metrics of affective brain dysmaturation may serve as predictors for identification of youth at risk for developing psychosis and other severe psychiatric disorders and/or impairments in functioning. In addition, our approach adds to the relevance of using Big Data to establish a growth chart to discern impairment and its potential to inform clinical trajectories.
Supplementary Material
Figure 3.
Age-associated brain maturation deviation scores were calculated by A) determining the developmental line of best fit for amygdala connectivity in typically developing youth, B) subtracting a psychosis spectrum youth’s individual score from the line of best fit, and taking the absolute value of this measure, and calculating Pearson correlations between the brain maturation deviation scores. C) Greater deviations from centromedial amygdala-thalamus connectivity are associated with increased positive symptoms in psychosis spectrum youth.
References
- 1.Walker EF, Grimes KE, Davis DM, et al. : Childhood precursors of schizophrenia: facial expressions of emotion. Am J Psychiatry 1993; 150:1654–1660 [DOI] [PubMed] [Google Scholar]
- 2.Knight RA, Roff JD: Childhood and young adult predictors of schizophrenic outcome. Origins of Psychopathology: Problems in Research and Public Policy 1983; 129–153 [Google Scholar]
- 3.Corcoran CM, Keilp JG, Kayser J, et al. : Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychol Med 2015; 45:2959–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allott KA, Schäfer MR, Thompson A, et al. : Emotion recognition as a predictor of transition to a psychotic disorder in ultra-high risk participants. Schizophr Res 2014; 153:25–31 [DOI] [PubMed] [Google Scholar]
- 5.Luna B, Marek S, Larsen B, et al. : An Integrative Model of the Maturation of Cognitive Control. Annu Rev Neurosci 2015; 38:151–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee P, Whalley HC, McKirdy JW, et al. : Altered amygdala connectivity within the social brain in schizophrenia. Schizophr Bull 2014; 40:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anticevic A, Tang Y, Cho YT, et al. : Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophr Bull 2014; 40:1105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velakoulis D, Wood SJ, Wong MTH, et al. : Hippocampal and amygdala volumes according to psychosis stage and diagnosis: A magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra--high-risk individuals. Arch Gen Psychiatry 2006; 63:139–149 [DOI] [PubMed] [Google Scholar]
- 9.van Erp TGM, Hibar DP, Rasmussen JM, et al. : Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 2016; 21:547–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf DH, Satterthwaite TD, Calkins ME, et al. : Functional neuroimaging abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry 2015; 72:456–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anticevic A, Repovs G, Krystal JH, et al. : A broken filter: prefrontal functional connectivity abnormalities in schizophrenia during working memory interference. Schizophr Res 2012; 141:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee P, Sabharwal A, Kotov R, et al. : Disconnection Between Amygdala and Medial Prefrontal Cortex in Psychotic Disorders. Schizophr Bull 2016; 42:1056–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalbrzikowski M, Larsen B, Hallquist MN, et al. : Development of White Matter Microstructure and Intrinsic Functional Connectivity Between the Amygdala and Ventromedial Prefrontal Cortex: Associations With Anxiety and Depression. Biol Psychiatry 2017; 82:511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin S, Young CB, Supekar K, et al. : Immature integration and segregation of emotion-related brain circuitry in young children. Proceedings of the National Academy of Sciences 2012; 109:7941–7946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabard-Durnam LJ, Flannery J, Goff B, et al. : The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage 2014; 95:193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole TJ: The development of growth references and growth charts. Ann Hum Biol 2012; 39:382–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessler D, Angstadt M, Sripada C: Growth Charting of Brain Connectivity Networks and the Identification of Attention Impairment in Youth. JAMA Psychiatry 2016; 73:481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gur RC, Calkins ME, Satterthwaite TD, et al. : Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry 2014; 71:366–374 [DOI] [PubMed] [Google Scholar]
- 19.Anticevic A, Brumbaugh MS, Winkler AM, et al. : Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry 2013; 73:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai XJ, Whitfield-Gabrieli S, Shinn AK, et al. : Abnormal Medial Prefrontal Cortex RestingState Connectivity in Bipolar Disorder and Schizophrenia. Neuropsychopharmacology 2011; 36:2009–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Tang Y, Womer F, et al. : Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophr Bull 2014; 40:469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler CG, Walker JB, Martin EA, et al. : Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull 2010; 36:1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry JD, Rendell PG, Green MJ, et al. : Emotion regulation in schizophrenia: affective, social, and clinical correlates of suppression and reappraisal. J Abnorm Psychol 2008; 117:473–478 [DOI] [PubMed] [Google Scholar]
- 24.Langdon R, Coltheart M, Ward PB: Empathetic perspective-taking is impaired in schizophrenia: evidence from a study of emotion attribution and theory of mind. Cogn Neuropsychiatry 2006; 11:133–155 [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Altshuler L, Glahn DC, et al. : Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry 2013; 170:334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goghari VM, Sponheim SR: More pronounced deficits in facial emotion recognition for schizophrenia than bipolar disorder. Compr Psychiatry 2013; 54:388–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowland JE, Hamilton MK, Vella N, et al. : Adaptive Associations between Social Cognition and Emotion Regulation are Absent in Schizophrenia and Bipolar Disorder. Front Psychol 2012; 3:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calkins ME, Merikangas KR, Moore TM, et al. : The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry 2015; 56:1356–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satterthwaite TD, Elliott MA, Ruparel K, et al. : Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage 2014; 86:544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohler CG, Bilker W, Hagendoorn M, et al. : Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biol Psychiatry 2000; 48:127–136 [DOI] [PubMed] [Google Scholar]
- 31.Bates D, Maechler M, Bolker B, et al. : lme4: Linear mixed-effects models using Eigen and S4 [Internet]. R package version 2014; Available from: http://keziamanlove.com/wp-content/uploads/2015/04/StatsInRTutorial.pdf [Google Scholar]
- 32.Singer JD, Willett JB, Eliot Charles William Willett John B et al. : Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press, USA, 2003 [Google Scholar]
- 33.Cox RW, Chen G, Glen DR, et al. : FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect 2017; 7:152–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eklund A, Nichols TE, Knutsson H: Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A 2016; 113:7900–7905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y: Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 289–300 [Google Scholar]
- 36.Lenth RV, Others: Least-squares means: the R package lsmeans. J Stat Softw 2016; 69:1–33 [Google Scholar]
- 37.Jalbrzikowski M, Larsen B, Hallquist MN, et al. : Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry 2017; 82:511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Supekar K, Musen M, Menon V: Development of large-scale functional brain networks in children. PLoS Biol 2009; 7:e1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dosenbach NUF, Nardos B, Cohen AL, et al. : Prediction of individual brain maturity using fMRI. Science 2010; 329:1358–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padmanabhan A, Lynn A, Foran W, et al. : Age related changes in striatal resting state functional connectivity in autism. Front Hum Neurosci 2013; 7:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alarcón G, Cservenka A, Rudolph MD, et al. : Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage 2015; 115:235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murty VP, Calabro F, Luna B: The role of experience in adolescent cognitive development: Integration of executive, memory, and mesolimbic systems. Neurosci Biobehav Rev 2016; 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapoport JL, Giedd JN, Gogtay N: Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry 2012; 17:1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forsyth JK, Lewis DA: Mapping the Consequences of Impaired Synaptic Plasticity in Schizophrenia through Development: An Integrative Model for Diverse Clinical Features. Trends Cogn Sci 2017; 21:760–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z: A saliency map in primary visual cortex. Trends Cogn Sci 2002; 6:9–16 [DOI] [PubMed] [Google Scholar]
- 46.Amassian VE, Cracco RQ, Maccabee PJ, et al. : Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol 1989; 74:458–462 [DOI] [PubMed] [Google Scholar]
- 47.Cooper JC, Knutson B: Valence and salience contribute to nucleus accumbens activation. Neuroimage 2008; 39:538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zink CF, Pagnoni G, Martin-Skurski ME, et al. : Human Striatal Responses to Monetary Reward Depend On Saliency. Neuron 2004; 42:509–517 [DOI] [PubMed] [Google Scholar]
- 49.Zink CF, Pagnoni G, Martin ME, et al. : Human Striatal Response to Salient Nonrewarding Stimuli. J Neurosci 2003; 23:8092–8097[cited 2018 Feb 11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris JS, Friston KJ, Dolan RJ: Neural responses to salient visual stimuli. Proc Biol Sci 1997; 264:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saalmann YB, Kastner S: Gain control in the visual thalamus during perception and cognition. Curr Opin Neurobiol 2009; 19:408–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunsperger RW, Roman D: The integrative role of the intralaminar system of the thalamus in visual orientation and perception in the cat. Exp Brain Res 1976; 25:231–246 [DOI] [PubMed] [Google Scholar]
- 53.Santos A, Mier D, Kirsch P, et al. : Evidence for a general face salience signal in human amygdala. Neuroimage 2011; 54:3111–3116 [DOI] [PubMed] [Google Scholar]
- 54.Cunningham WA, Brosch T: Motivational Salience: Amygdala Tuning From Traits, Needs, Values, and Goals. Curr Dir Psychol Sci 2012; 21:54–59 [Google Scholar]
- 55.Anderson AK, Phelps EA: Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 2001; 411:305–309 [DOI] [PubMed] [Google Scholar]
- 56.Hermans EJ, Henckens MJAG, Joëls M, et al. : Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 2014; 37:304–314 [DOI] [PubMed] [Google Scholar]
- 57.Pessoa L, Adolphs R: Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci 2010; 11:773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fudge JL, Kelly EA, Pal R, et al. : Beyond the Classic VTA: Extended Amygdala Projections to DA-Striatal Paths in the Primate. Neuropsychopharmacology 2017; 42:1563–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kapur S: Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 2003; 160:13–23 [DOI] [PubMed] [Google Scholar]
- 60.Diekhof EK, Geier K, Falkai P, et al. : Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage 2011; 58:275–285 [DOI] [PubMed] [Google Scholar]
- 61.Buhle JT, Silvers JA, Wager TD, et al. : Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb Cortex 2014; 24:2981–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ochsner KN, Ray RD, Cooper JC, et al. : For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 2004; 23:483–499 [DOI] [PubMed] [Google Scholar]
- 63.Silvers JA, Weber J, Wager TD, et al. : Bad and worse: neural systems underlying reappraisal of high- and low-intensity negative emotions. Soc Cogn Affect Neurosci 2015; 10:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ochsner KN, Silvers JA, Buhle JT: Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 2012; 1251:E1–E24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silvers JA, Insel C, Powers A, et al. : vlPFC-vmPFC-Amygdala Interactions Underlie Age-Related Differences in Cognitive Regulation of Emotion [Internet]. Cereb Cortex 2016; Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=27341851&retmode=ref&cmd=prlinks [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooker CI, Benson TL, Gyurak A, et al. : Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia. J Abnorm Psychol 2014; 123:190–204 [DOI] [PubMed] [Google Scholar]
- 67.Yin H, Tully LM, Lincoln SH, et al. : Adults with high social anhedonia have altered neural connectivity with ventral lateral prefrontal cortex when processing positive social signals. Front Hum Neurosci 2015; 9:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tully LM, Lincoln SH, Hooker CI: Lateral prefrontal cortex activity during cognitive control of emotion predicts response to social stress in schizophrenia. Neuroimage Clin 2014; 6:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gee DG, Karlsgodt KH, van Erp TGM, et al. : Altered age-related trajectories of amygdalaprefrontal circuitry in adolescents at clinical high risk for psychosis: A preliminary study. Schizophr Res 2012; 134:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fowler D, Hodgekins J, Garety P, et al. : Negative Cognition, Depressed Mood, and Paranoia: A Longitudinal Pathway Analysis Using Structural Equation Modeling. Schizophr Bull 2012; 38:1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freeman D, McManus S, Brugha T, et al. : Concomitants of paranoia in the general population. Psychol Med 2011; 41:923–936 [DOI] [PubMed] [Google Scholar]
- 72.Marwaha S, Parsons N, Flanagan S, et al. : The prevalence and clinical associations of mood instability in adults living in England: results from the Adult Psychiatric Morbidity Survey 2007. Psychiatry Res 2013; 205:262–268 [DOI] [PubMed] [Google Scholar]
- 73.Myin-Germeys I, Peeters F, Havermans R, et al. : Emotional reactivity to daily life stress in psychosis and affective disorder: an experience sampling study. Acta Psychiatr Scand 2003; 107:124–131 [DOI] [PubMed] [Google Scholar]
- 74.Myin-Germeys I, van Os J, Schwartz JE, et al. : Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry 2001; 58:1137–1144 [DOI] [PubMed] [Google Scholar]
- 75.Myin-Germeys I, Delespaul PA, deVries MW: Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr Bull 2000; 26:847–854 [DOI] [PubMed] [Google Scholar]
- 76.Kumar D, Tully LM, Iosif A-M, et al. : A Mobile Health Platform for Clinical Monitoring in Early Psychosis: Implementation in Community-Based Outpatient Early Psychosis Care. JMIR Ment Health 2018; 5:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anticevic A, Repovs G, Corlett PR, et al. : Negative and nonemotional interference with visual working memory in schizophrenia. Biol Psychiatry 2011; 70:1159–1168 [DOI] [PubMed] [Google Scholar]
- 78.Di Martino A, Fair DA, Kelly C, et al. : Unraveling the miswired connectome: a developmental perspective. Neuron 2014; 83:1335–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sah P, Faber ESL, Lopez De Armentia M, et al. : The amygdaloid complex: anatomy and physiology. Physiol Rev 2003; 83:803–834 [DOI] [PubMed] [Google Scholar]
- 80.Sun D, Phillips L, Velakoulis D, et al. : Progressive brain structural changes mapped as psychosis develops in “at risk” individuals. Schizophr Res 2009; 108:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cannon TD, Chung Y, He G, et al. : Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry 2015; 77:147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pantelis C, Velakoulis D, Mcgorry PD, et al. : Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 2003; 361:281–288 [DOI] [PubMed] [Google Scholar]
- 83.Job DE, Whalley HC, Johnstone EC, et al. : Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage 2005; 25:1023–1030 [DOI] [PubMed] [Google Scholar]
- 84.Van Winkel R, Stefanis NC, Myin-Germeys I: Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull 2008; 34:1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kapur S: Psychosis as a State of Aberrant Salience: A Framework Linking Biology, Phenomenology, and Pharmacology in Schizophrenia. AJP 2003; 160:13–23 [DOI] [PubMed] [Google Scholar]
- 86.van Os J: A salience dysregulation syndrome. Br J Psychiatry 2009; 194:101–103 [DOI] [PubMed] [Google Scholar]
- 87.Roiser JP, Stephan KE, den Ouden HEM, et al. : Do patients with schizophrenia exhibit aberrant salience? Psychol Med 2009; 39:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fletcher PC, Frith CD: Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci 2009; 10:48–58 [DOI] [PubMed] [Google Scholar]
- 89.Mattar MG, Wymbs NF, Bock AS, et al. : Predicting future learning from baseline network architecture. Neuroimage 2018; 172:107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fusar-Poli P, Bonoldi I, Yung AR, et al. : Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry 2012; 69:220–229 [DOI] [PubMed] [Google Scholar]
- 91.Cannon TD, Yu C, Addington J, et al. : An Individualized Risk Calculator for Research in Prodromal Psychosis. Am J Psychiatry 2016; 173:980–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Satterthwaite TD, Wolf DH, Calkins ME, et al. : Structural Brain Abnormalities in Youth With Psychosis Spectrum Symptoms. JAMA Psychiatry 2016; 73:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alnæs D, Kaufmann T, Doan NT, et al. : Association of Heritable Cognitive Ability and Psychopathology With White Matter Properties in Children and Adolescents [Internet]. JAMA Psychiatry 2018; [cited 2018 Feb 2] Available from: https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2670370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corcoran CM, Carrillo F, Fernández-Slezak D, et al. : Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry 2018; 17:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ordaz SJ, Foran W, Velanova K, et al. : Longitudinal Growth Curves of Brain Function Underlying Inhibitory Control through Adolescence. Journal of Neuroscience 2013; 33:18109–18124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simmonds DJ, Hallquist MN, Asato M, et al. : Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. Neuroimage 2014; 92:356–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Montez DF, Calabro FJ, Luna B: The expression of established cognitive brain states stabilizes with working memory development [Internet]. Elife 2017; 6Available from: 10.7554/eLife.25606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grimm KJ, Ram N, Hamagami F: Nonlinear growth curves in developmental research. Child Dev 2011; 82:1357–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calkins ME, Moore TM, Satterthwaite TD, et al. : Persistence of psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort: a prospective two-year followup. World Psychiatry 2017; 16:62–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schlosser DA, Jacobson S, Chen Q, et al. : Recovery From an At-Risk State: Clinical and Functional Outcomes of Putatively Prodromal Youth Who Do Not Develop Psychosis. Schizophr Bull 2012; 38:1225–1233[cited 2018 Jul 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tan X, Shiyko MP, Li R, et al. : A time-varying effect model for intensive longitudinal data. Psychol Methods 2012; 17:61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shiyko MP, Burkhalter J, Li R, et al. : Modeling nonlinear time-dependent treatment effects: An application of the generalized time-varying effect model (TVEM). J Consult Clin Psychol 2014; 82:760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fortin J-P, Parker D, Tunç B, et al. : Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017; 161:149–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fortin J-P, Cullen N, Sheline YI, et al. : Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 2018; 167:104–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.