Abstract
To show the synthetic utility of palladium/norbornene (Pd/NBE) cooperative catalysis, here we report concise syntheses of indenone-based natural products, pauciflorol F and acredinone A, which are enabled by direct annulation between aryl iodides and unsaturated carboxylic acid anhydrides. Compared to the previous indenone-preparation approaches, this method allows simple aryl iodides to be used as substrates with complete control of the regioselectivity. The total synthesis of acredinone A features two different Pd/NBE-catalyzed ortho acylation reactions for constructing penta-substituted arene cores, including the development of a new ortho acylation/ipso borylation.
Keywords: acredinone A, pauciflorol F, indenone, norbornene, palladium
Graphical Abstract
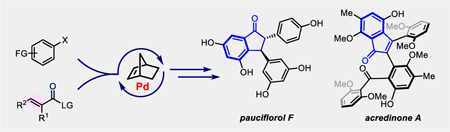
A straightforward method for indenone synthesis was achieved via Pd/NBE-catalyzed annulation between simple aryl iodides and unsaturated carboxylic acid anhydrides. This method enabled streamlined syntheses of pauciflorol F and acredinone A.
Indenones are commonly found in pharmaceuticals and bioactive natural products (Scheme 1a),[1] and often serve as versatile intermediates to access polysubstituted indanones.[2] While a number of methods have been developed for preparing indenones, conventional approaches usually take multiple steps and/or have limited scopes.[3] Recently, the transition metal-catalyzed cyclization with alkynes as coupling partners offers a broad and useful strategy to form various indenones (Scheme 1b).[4,5] However, it is not trivial to control regioselectivity when unsymmetrical alkynes are used.[2] In addition, vicinal difunctionalized aryl substrates [4] or those containing a directing group (DG)[5] are needed. Thus, it remains attractive to realize a general and regioselective indenone synthesis from simple readily available starting materials.
Scheme 1.
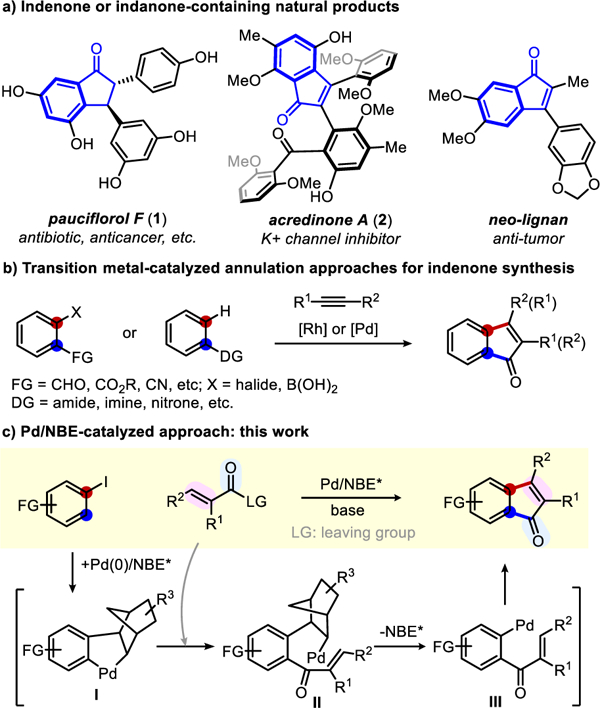
Indenone/indanone containing natural products and indenone synthesis
On the other hand, palladium/norbornene (Pd/NBE) cooperative catalysis,[6] originally discovered by Catellani,[7] has become an increasingly useful approach for preparing polysubstituted arenes via simultaneous functionalization of both ortho and ipso positions of aryl halides.[8] Notably, Lautens has pioneered in developing bifunctional reagents, which enabled forming various condensed rings via ipso-annulation reactions.[8a,9] While numerous Pd/NBE catalysis methods have been developed, only a few have been successfully applied to the total synthesis of natural products.[8p,10] Herein, we describe concise syntheses of pauciflorol F(1) and acredinone A (2), enabled by the development of a straightforward method to construct indenones from simple aryl iodides and unsaturated carboxylic anhydrides via Pd/NBE catalysis (Scheme 1c).
In 2015, we reported an initial study on the ortho acylation/ipso hydrogenation using a mixed anhydride.[8i] Concurrently, the Liang[8k] and Gu[8j] groups independently developed the ortho acylation/ipso Heck reaction. Interestingly, when anhydrides derived from α,β-unsaturated carboxylic acids were employed, indenone products could be obtained (Scheme 1c). The reaction was likely initiated by oxidative addition of Pd(0) into the Ar−X bond, subsequent NBE migratory insertion and C−H metalation to generate an aryl-NBE-palladacycle (ANP) I,[11] which would then react with the anhydride to afford intermediate II. After NBE extrusion, the resulting intermediate III could further undergo intramolecular cyclization to give the indenone product (vide infra, Scheme 3).
Scheme 3.
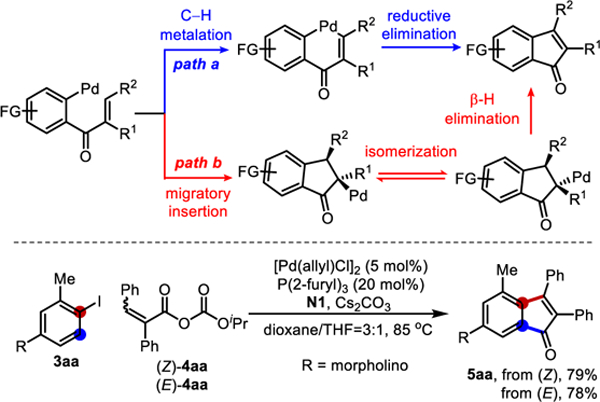
Mechanistic exploration
The reaction was further optimized using 2-iodotoluene (3a) as the substrate (see Supporting Information, Table S1). [Pd(allyl)Cl]2 and tri(2-furyl)phosphine still proved to be the best metal/ligand combination, and the amide-substituted NBE (N1) was also found more efficient than simple NBE. Under the optimal conditions, the scope of the reaction was examined (Table 1). Aryl iodides contain both electron-rich and deficient substituents were suitable substrates, and different substitution patterns on arenes were tolerated. Note that sterically hindered aryl iodides, such as 2,5-disubstituted ones (5q and 5r), were also competent substrates, which was the key requirement for the synthesis of acredinone A. Other unsaturated anhydrides were explored next as coupling partners. Anhydrides derived from alkyl (5t) and aryl-substituted acrylic acids (5u-5ac) could be successfully employed.[12] In particular, when α- or α,β-disubstituted acrylic anhydrides were used, 2-substituted and 2,3-disubstituted indenones (5aa-5ac) were still afforded in good yields. Finally, a number of functional groups were found compatible, such as tertiary amine (5c, 5j and 5k), silyl ether (5g), tertiary alcohol (5i), MOM-protected alcohol (5e), Weinreb amide (5n), ester (5f, 5m and 5p), Boc-protected amine (5l), trifluoromethyl (5w), aryl bromide (5v) and thiophene (5z), making this method attractive for complex molecule synthesis.
Table 1.
Substrate scope of Pd/NBE-catalyzed indenone synthesis.[a]
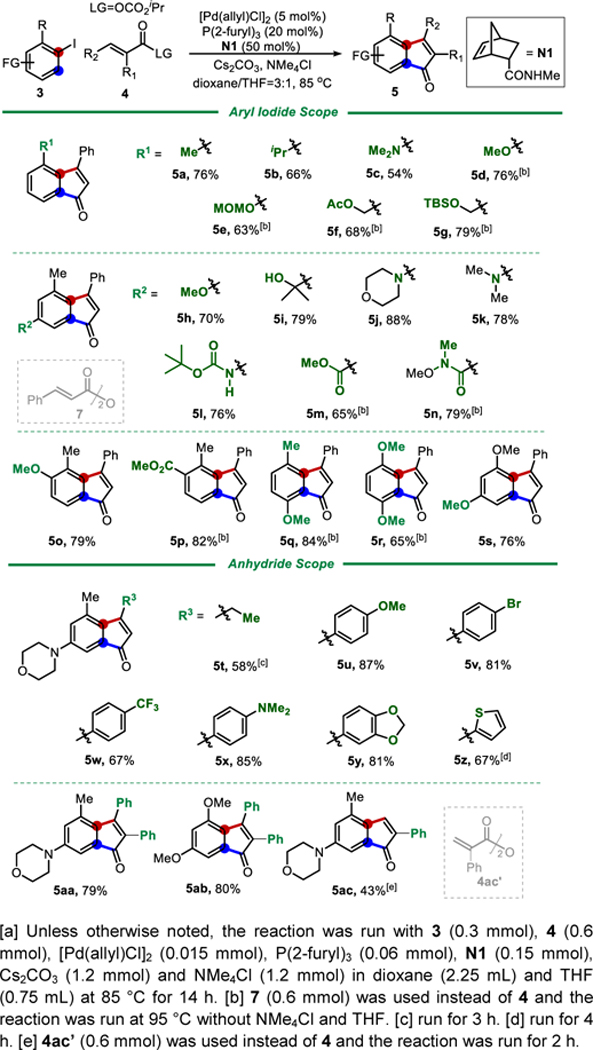 |
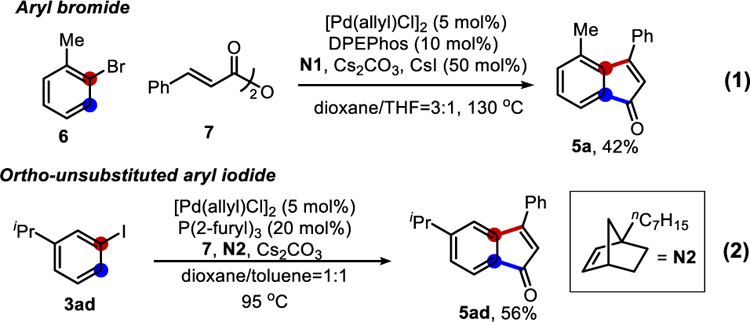
Preliminary study showed that aryl bromides[13] could also be used as the substrate (eq. 1, Scheme 2) albeit in a lower efficiency; the use of large bite-angle (DPEPhos) ligand[13a] and additional CsI was important. Not surprisingly, ortho-unsubstituted aryl iodide 3ad was not a suitable substrate using N1 or simple NBE under the standard conditions due to the “ortho constraint”.[14] However, with the recently developed bridgehead-substituted norbornene (N2),[14b] the desired indenone product 5ad was successfully isolated in 56% yield.
Scheme 2.
Further reaction exploration
The mechanism regarding the conversion of intermediate III into indenone product was explored (Scheme 3). Two pathways are possible: path a involves activation of the vinyl β-C(sp2)−H bond of the enone intermediate followed by reductive elimination, while path b is through a Heck-like sequence: aryl migratory insertion into the olefin followed by β-H elimination. To differentiate these two pathways, both (Z) and (E)-anhydrides (4aa) were allowed to react with 3aa in parallel under the standard conditions. Interestingly, they gave almost identical yields of 5aa with a similar kinetic profile (see Supporting Information). Additionally, Z/E isomerization of the olefin moiety in 4aa was not observed during the reaction. Taken together, the observations are consistent with the Heck-like pathway (path b), though other pathways including a Nazarov-type cyclization cannot be excluded at the current stage (see Supporting Information).
The utility of the method was first demonstrated in the formal synthesis of pauciflorol F (1) (Scheme 4). As a polyphenolic natural product derived from resveratrol, pauciflorol F has been an attractive synthetic target since its discovery in 2004.[15] Several elegant routes have been developed to date.[2,16] Our approach started with a two-step preparation of mixed anhydride 10 from commercially available aldehyde 8.[17] As expected, the subsequent Pd/N1-catalyzed annulation between anhydride 10 and aryl iodide 3t provided indenone 11 in 82% yield, whose structure was confirmed by X-ray crystallography.[18] Note that the prior synthesis of indenone 11 via an alkyne insertion strategy generated a 1:1 mixture of regioisomers.[2] Demonstrated by Jeffery and Sarpong, indenone 11 can be converted to 1 in two steps;[2] thus, the synthesis of pauciflorol F can now be accomplished in 5 total steps and a high overall yield (~50%).
Scheme 4.
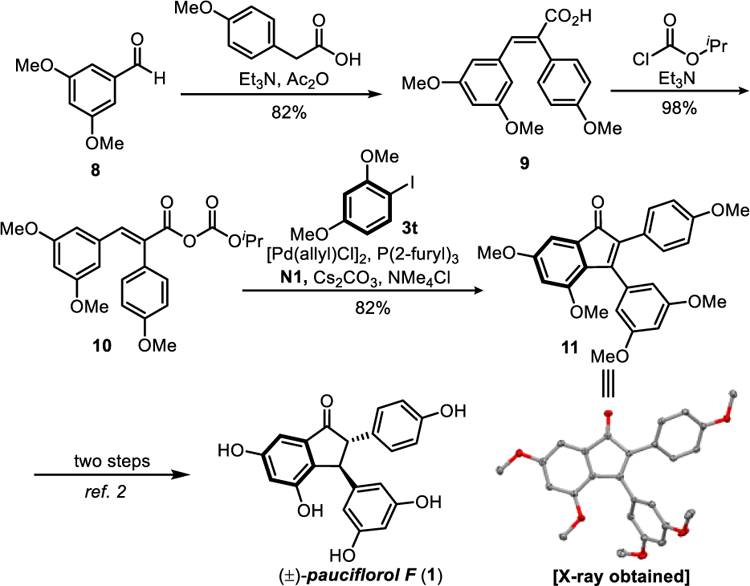
Formal synthesis of pauciflorol F.
We then focused on the total synthesis of acredinone A (2). Acredinone A was recently isolated in <5 mg (with [α]25D: 0 in CHCl3) from a marine-sponge-associated acremonium sp. Fungus, which represents the first nonpeptidic natural product that can inhibit the voltage-gated potassium channel.[19] Such an activity holds potential for developing inhibitors for treating type-II diabetes.[20] In addition, total synthesis of acredinone A has not been reported to date, and the structure-activity relationship (SAR) remains unknown. Given the pseudo-symmetry in 2, it might be possible to establish an efficient biomimetic strategy. However, to enable rapid access to structurally diversified analogues for future SAR studies, it could be more valuable to develop a modular and convergent approach. The challenges are how to construct the highly congested indenone core and how to prepare the two penta-substituted arenes efficiently. From a retrosynthetic viewpoint (Scheme 5), we envisioned that acredinone A could be constructed via a late-stage Suzuki−Miyaura coupling of two fragments (12 and 13) with similar complexity. Each fragment would be synthesized via a unique Pd/NBE catalysis. Ultimately, the natural product would be assembled using four arene building blocks (14-17).
Scheme 5.
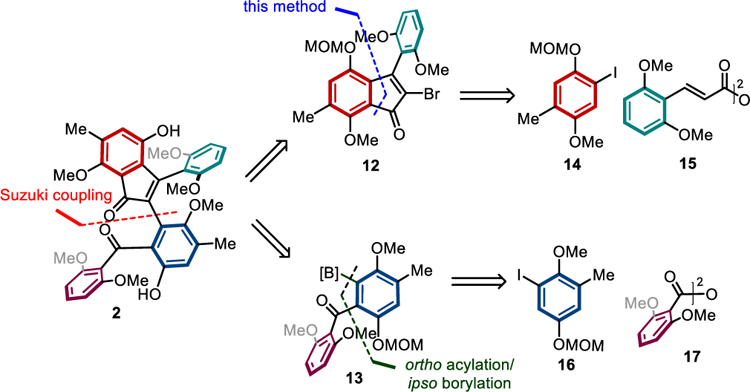
Retrosynthetic analysis of acredinone A.
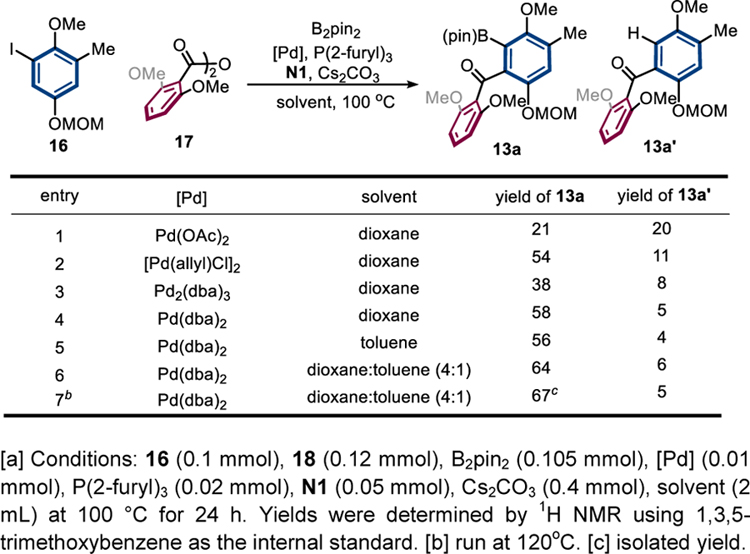
In a forward manner, aryl iodides 14 and 16 were synthesized from the same commercially available 3-methyl-4-anisaldehyde 18 in 4 steps (Scheme 6). The Pd/NBE-catalyzed indenone synthesis proceeded in a good yield on gram scales despite the bulkiness of the substrates. Bromination of indenone 19 with NBS afforded fragment 12. Meanwhile, fragment 13 was proposed to be prepared via Pd/NBE-catalyzed ortho acylation/ipso borylation, which has been an unknown transformation. Encouraged by Ritter’s ortho amination/ipso borylation,[21] the three-component coupling of aryl iodide 16, anhydride 17 and B2pin2 was carefully studied (Table 2). To our delight, the desired product 13a was obtained in 21% yield in the first attempt using Pd(OAc)2 as the catalyst, and the major side-reaction was the ipso-hydrogenation (13a’) (entry 1). A quick condition screening revealed that the use of Pd(dba)2 could suppress the formation of side-product 13a’ (entries 1–4), though the exact reason is unclear. Further tuning the solvent and reaction temperature finally afforded the desired borylation product 13a in 67% isolation yield (entries 5–7).
Scheme 6.
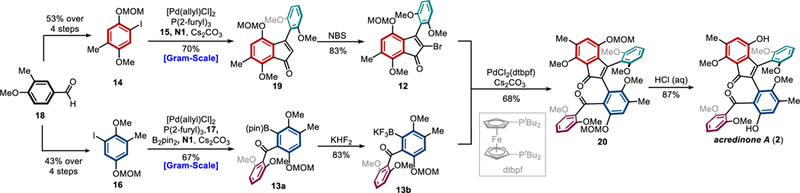
Total synthesis of acredinone A.
Table 2.
Optimization for ortho acylation/ipso borylation.[a]
 |
The Suzuki−Miyaura coupling between aryl bromide 12 and aryl boronic ester 13a turned out to be extremely challenging due to the large steric hindrance in both coupling partners. The commonly employed monodentate or bidentate ligands, such as PPh3, PtBu3, Buchwald’s biarylphosphines, Hartwig’s Q-Phos and NHC ligands, gave trace or no desired product. After extensive studies, gratifyingly, the bulky and electron-rich bidentate dtbpf ligand afforded the desired coupling product 20 albeit in a low yield.[22] The major side-reaction was the protonation of boronic ester 13a, suggesting low stability of this compound under the reaction conditions. Considering that Molander salts generally exhibit enhanced stability than the corresponding boronic esters in cross couplings,[23] the corresponding potassium aryltrifluoroborate 13b was then prepared. Indeed, the coupling between 12 and 13b proceeded smoothly to afford product 20 in 68% yield, which was subsequently deprotected to give acredinone A (2). The spectroscopic data of synthetic 2 matched those of the reported natural sample in all respects. Hence, the first total synthesis of acredinone A was accomplished in eight steps in the longest linear sequence (LLS) from commercial chemicals.
In summary, a Pd/NBE-catalyzed annulation between aryl iodides and unsaturated carboxylic acid anhydrides has been developed, which provides a simple and straightforward approach for preparing indenones in a regio- and chemoselective manner. Using this method, concise syntheses of pauciflorol F and acredinone A have been achieved. The utility of Pd/NBE catalysis for streamlined synthesis of tetra- and penta-substituted arenes could have broad implications beyond this work. Efforts on preparing analogues of acredinone A for future SAR studies are ongoing.
Supplementary Material
Acknowledgements
Financial supports from the University of Chicago and NIGMS (1R01GM124414-01A1) are acknowledged. F.L. is supported by a CSC fellowship. Mr. Ki-Young Yoon is thanked for checking the experiment and for X-ray crystallography.
Contributor Information
Feipeng Liu, Department of Applied Chemistry, China Agricultural University, Beijing 100193, China, Department of Chemistry, University of Chicago, Chicago, IL, 60637 (USA).
Zhe Dong, Department of Chemistry, University of Chicago, Chicago, IL, 60637 (USA).
Jianchun Wang, Department of Chemistry, University of Chicago, Chicago, IL, 60637 (USA).
Guangbin Dong, Department of Chemistry, University of Chicago, Chicago, IL, 60637 (USA).
References
- [1].(a) Morrell A, Placzek M, Parmley S, Grella B, Antony S, Pommier Y, Cushman M, J. Med. Chem 2007, 50, 4388; [DOI] [PubMed] [Google Scholar]; (b) Ahn JH, Shin MS, Jung SH, Kang SK, Kim KR, Rhee SD, Jung WH, Yang SD, Kim SJ, Woo JR, Lee JH, Cheon HG, Kim SS, J. Med. Chem 2006, 49, 4781. [DOI] [PubMed] [Google Scholar]
- [2].Jeffrey JL, Sarpong R, Org. Lett 2009, 11, 5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].(a) Marvel CS, Hinman CW, J. Am. Chem. Soc 1954, 76, 5435; [Google Scholar]; (b) Martens H, Hoornaer G, Tetrahedron 1974, 30, 3641; [Google Scholar]; (c) Vasilyev AV, Walspurger S, Haouas M, Sommer J, Pale P, Rudenko AP, Org. Biomol. Chem 2004, 2, 3483; [DOI] [PubMed] [Google Scholar]; (d) Vasilyev AV, Walspurger S, Pale P, Sommer J, Tetrahedron Lett 2004, 45, 3379; [Google Scholar]; (e) Pan C, Huang B, Hu W, Feng X, Yu J, J. Org. Chem 2016, 81, 2087; [DOI] [PubMed] [Google Scholar]; (f) Zhang X-S, Jiao J-Y, Zhang X-H, Hu B-L, Zhang X-G, J. Org. Chem 2016, 81, 5710; [DOI] [PubMed] [Google Scholar]; (g) Suchand B, Satyanarayana G, J. Org. Chem 2017, 82, 372. [DOI] [PubMed] [Google Scholar]
- [4].(a) Larock RC, Doty MJ, Cacchi S, J. Org. Chem 1993, 58, 4579; [Google Scholar]; (b) Alonso DA, Najera C, Pacheco MC, Adv. Synth. Catal 2002, 344, 172; [Google Scholar]; (c) Pletnev AA, Tian Q, Larock RC, J. Org. Chem 2002, 67, 9276; [DOI] [PubMed] [Google Scholar]; (d) Miura T, Murakami M, Org. Lett 2005, 7, 3339; [DOI] [PubMed] [Google Scholar]; (e) Harada Y, Nakanishi J, Fujihara H, Tobisu M, Fukumoto Y, Chatani N, J. Am. Chem. Soc 2007, 129, 5766; [DOI] [PubMed] [Google Scholar]; (f) Tsukamoto H, Kondo Y, Org. Lett 2007, 9, 4227; [DOI] [PubMed] [Google Scholar]; (g) Liu CC, Korivi RP, Cheng CH, Chem. Eur. J 2008, 14, 9503; [DOI] [PubMed] [Google Scholar]; (h) Morimoto T, Yamasaki K, Hirano A, Tsutsumi K, Kagawa N, Kakiuchi K, Harada Y, Fukumoto Y, Chatani N, Nishioka T, Org. Lett 2009, 11, 1777; [DOI] [PubMed] [Google Scholar]; (i) Yan X, Zou S, Zhao P, Xi C, Chem. Commun 2014, 50, 2775. [DOI] [PubMed] [Google Scholar]
- [5].(a) Li B-J, Wang H-Y, Zhu Q-L, Shi Z-J, Angew. Chem. Int. Ed 2012, 51, 3948; Angew. Chem. 2012, 124, 4014; [DOI] [PubMed] [Google Scholar]; (b) Chen S, Yu J, Jiang Y, Chen F, Cheng J, Org. Lett 2013, 15, 4754; [DOI] [PubMed] [Google Scholar]; (c) Qi Z, Wang M, Li X, Org. Lett 2013, 15, 5440. [DOI] [PubMed] [Google Scholar]
- [6].(a) Catellani M, Synlett 2003, 298–313;; (b) Catellani M, Top. Organomet. Chem 2005, 14, 21; [Google Scholar]; (c) Catellani M, Motti E, Della Ca’ N, Acc. Chem. Res 2008, 41, 1512; [DOI] [PubMed] [Google Scholar]; (d) Martins A, Mariampillai B, Lautens M, Top. Curr. Chem 2010, 292, 1; [DOI] [PubMed] [Google Scholar]; (e) Ye J, Lautens M, Nat. Chem 2015, 7, 863; [DOI] [PubMed] [Google Scholar]; (f) Della Ca’ N, Fontana M, Motti E, Catellani M, Acc. Chem. Res 2016, 49, 1389. [DOI] [PubMed] [Google Scholar]
- [7].Catellani M, Frignani F, Rangoni A, Angew. Chem. Int. Ed 1997, 36, 119; Angew. Chem. 1997, 109, 142. [Google Scholar]
- [8].For selected examples of Pd/NBE catalysis: a)Lautens M, Piguel S, Angew. Chem. Int. Ed 2000, 39, 1045; Angew. Chem. 2000, 112, 1087; [DOI] [PubMed] [Google Scholar]; (b) Catellani M, Motti E, Baratta S, Org. Lett 2001, 3, 3611; [DOI] [PubMed] [Google Scholar]; (c) Faccini F, Motti E, Catellani M, J. Am. Chem. Soc 2004, 126, 78; [DOI] [PubMed] [Google Scholar]; (d) Bressy C, Alberico D, Lautens M, J. Am. Chem. Soc 2005, 127, 13148; [DOI] [PubMed] [Google Scholar]; (e) Mariampillai B, Alberico D, Bidau V, Lautens M, J. Am. Chem. Soc 2006, 128, 14436; [DOI] [PubMed] [Google Scholar]; (f) Maestri G, Motti E, Della Ca’ N, Malacria M, Derat E, Catellani M, J. Am. Chem. Soc 2011, 133, 8574; [DOI] [PubMed] [Google Scholar]; (g) Dong Z, Dong G, J. Am. Chem. Soc 2013, 135, 18350; [DOI] [PubMed] [Google Scholar]; (h) Zhang H, Chen P, Liu G, Angew. Chem. Int. Ed 2014, 53, 10174; Angew. Chem. 2014, 126, 10338; [DOI] [PubMed] [Google Scholar]; (i) Dong Z, Wang J, Ren Z, Dong G, Angew. Chem. Int. Ed 2015, 54, 12664; Angew. Chem. 2015, 127, 12855; [DOI] [PubMed] [Google Scholar]; (j) Huang YZ, Zhu R, Zhao K, Gu ZH, Angew. Chem. Int. Ed 2015, 54, 12669; Angew. Chem. 2015, 127, 12860. [DOI] [PubMed] [Google Scholar]; (k) Zhou P-X, Ye Y-Y, Liu C, Zhao L-B, Hou J-Y, Chen D-Q, Tang Q, Wang A-Q, Zhang J-Y, Huang Q-X, Xu P-F, Liang Y-M, ACS Catal 2015, 5, 4927; [Google Scholar]; (l) Wang J, Zhang L, Dong Z, Dong G, Chem 2016, 1, 581; [Google Scholar]; (m) Cheng H-G, Wu C, Chen H, Chen R, Qian G, Geng Z, Wei Q, Xia Y, Zhang J, Zhang Y, Zhou Q, Angew. Chem. Int. Ed 2018, 57, 3444; Angew. Chem. 2018, 130, 3502; [DOI] [PubMed] [Google Scholar]; (n) Li R, Dong G, Angew. Chem. Int. Ed 2018, 57, 1697; Angew. Chem. 2018, 130, 1713; [DOI] [PubMed] [Google Scholar]; (o) for Pd(II)-initiated Pd/NBE catalysis, see:Jiao L, Bach T, J. Am. Chem. Soc 2011, 133, 12990; [DOI] [PubMed] [Google Scholar]; (p) Jiao L, Herdtweck E, Bach T, J. Am. Chem. Soc 2012, 134, 14563; [DOI] [PubMed] [Google Scholar]; (q) Wang X-C, Gong W, Fang L-Z, Zhu R-Y, Li SH, Engle KM, Yu J-Q, Nature 2015, 519, 334; [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Dong Z, Wang J, Dong G, J. Am. Chem. Soc 2015, 137, 5887; [DOI] [PubMed] [Google Scholar]; (s) Shen P-X, Wang X-C, Wang P, Zhu RY, Yu J-Q, J. Am. Chem. Soc 2015, 137, 11574; [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Shi H, Herron AN, Shao Y, Shao Q, Yu J-Q, Nature 2018, 558, 581; [DOI] [PMC free article] [PubMed] [Google Scholar]; (v) Zhang H, Wang H-Y, Luo Y, Chen C, Cao Y, Chen P, Guo Y-L, Lan Y, Liu G, ACS Catal 2018, 8, 2173. [Google Scholar]
- [9].For the seminal use of bifunctional reagent in Pd/NBE catalysis, see ref 8a;for the fluorenone synthesis via Pd/NBE catalysis, see Zhao Y-B, Mariampillai B, Candito DA, Laleu B, Li M, Lautens M, Angew. Chem. Int. Ed 2009, 48, 1849; Angew. Chem. 2009, 121, 1881.
- [10].(a) Sui X, Zhu R, Li G, Ma X, Gu Z, J. Am. Chem. Soc 2013, 135, 9318; [DOI] [PubMed] [Google Scholar]; (b) Weinstabl H, Suhartono M, Qureshi Z, Lautens M, Angew. Chem. Int. Ed 2013, 52, 5305; Angew. Chem. 2013, 125, 5413 [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu Z-S, Qian G, Gao Q, Wang P, Cheng H-G, Wei Q, Liu Q, Zhou Q, ACS Catal 2018, 8, 4783. [Google Scholar]
- [11].Catellani M, Chiusoli GP, J. Organomet. Chem 1988, 346, C27. [Google Scholar]
- [12].The anhydride derived from unsubstituted acrylic acid did not yield the desired product, due to its instability under the reaction conditions.
- [13].(a) Dong Z, Lu G, Wang JC, Liu P, Dong G, J. Am. Chem. Soc 2018, 140, 8551; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Della Ca N, Casnati A, Fontana M, Coruzzi G, Aresta BM, Corriero N, Maggi R, Maestri G, Motti E, ChemCatChem 2018, 10, 4346; [Google Scholar]; (c) Elsayed MSA, Griggs B, Cushman M, Org. Lett 2018, 20, 5228. [DOI] [PubMed] [Google Scholar]
- [14].(a) Catellani M, Fagnola MC, Angew. Chem. Int. Ed. Engl 1994, 33, 2421; Angew. Chem. 1994, 106, 2559. [Google Scholar]; (b) Wang J, Li R, Dong Z, Liu P, Dong G, Nat. Chem 2018, 10, 866. [DOI] [PubMed] [Google Scholar]
- [15].Ito T, Tanaka T, Iinuma M, Nakaya K, Takahashi Y, Sawa R, Murata J, Darnaedi D, J. Nat. Prod 2004, 67, 932. [DOI] [PubMed] [Google Scholar]
- [16].(a) Snyder SA, Zografos AL, Lin Y, Angew. Chem. Int. Ed 2007, 46, 8186; Angew. Chem. 2007, 119, 8334; [DOI] [PubMed] [Google Scholar]; (b) Lee BH, Choi YL, Shin S, Heo JN, J. Org. Chem 2011, 76, 6611; [DOI] [PubMed] [Google Scholar]; (c) Yang Y, Philips D, Pan SF, J. Org. Chem 2011, 76, 1902; [DOI] [PubMed] [Google Scholar]; (d) Kerr DJ, Miletic M, Manchala N, White JM, Flynn BL, Org. Lett 2013, 15, 4118. [DOI] [PubMed] [Google Scholar]
- [17].Xiao C-F, Zou Y, Du J-L, Sun H-Y, Liu X-K, Synth. Commun 2012, 42, 1243. [Google Scholar]
- [18].CCDC 1881705 (11) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre.
- [19].Kim H, Yang I, Ryu SY, Won DH, Giri AG, Wang W, Choi H, Chin J, Hahn D, Kim E, Han C, Lee J, Nam SJ, Ho WK, Kang H, J. Nat. Prod 2015, 78, 363. [DOI] [PubMed] [Google Scholar]
- [20].Wulff H, Castle NA, Pardo LA, Nat. Rev. Drug Discov 2009, 8, 982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shi H, Babinski DJ, Ritter T, J. Am. Chem. Soc 2015, 137, 3775. [DOI] [PubMed] [Google Scholar]
- [22].Colacot TJ, Shea HA, Org. Lett 2004, 6, 3731. [DOI] [PubMed] [Google Scholar]
- [23].Molander GA, Bernardi CR, J. Org. Chem 2002, 67, 8424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


