Abstract
By 2050, there will be over 1.6 billion adults aged 65 years and older, making age-related diseases and conditions a growing public health concern. One of the leading causes of death in the aging population is pathogenic infections (e.g. influenza, S. pneumoniae). This age-dependent susceptibility to infection has been linked to a reduced ability of the aging immune system to mount protective responses against infectious pathogens, as well as to vaccines against these pathogens. The primary immune response that promotes protection is the production of antibodies by B cells – a response that is directly mediated by T follicular helper (TFH) cells within germinal centers in secondary lymphoid tissues. In this review, we will summarize the current knowledge on the development and functionality of TFH cells, the use of circulating TFH cells as vaccine biomarkers and the influence of age on these processes. Moreover, we will discuss the strategies for overcoming TFH cell dysfunction to improve protective antibody responses in the aging human population.
Keywords: Immunosenescence; T follicular regulatory cell; vaccine response; T cell differentiation, B cell antibody production, germinal center
Introduction.
Globally, human lifespan has been significantly increased – in part by medical advancement, improved sanitation and easier access to clean water. Indeed, it is predicted that by 2050, there will be over 1.6 billion (17% of the global population) adults over the age of 65 years. In the US alone, the older population is expected to double over the next 30 years – to 88 million people. As the population becomes older, age-related diseases and conditions also become a growing public health concern. A significant amount of aging research has shifted focus from extending lifespan to extending the length of disease-free living (termed “healthspan”). One of the major contributors to a reduced healthspan is increasing immune dysfunction with age. This immune aging phenomena results in a higher susceptibility to and mortality from pathogenic infections in older adults. Although vaccines have been developed to protect against many of these pathogens, older adults display diminished protective vaccine responses compared with younger adults. Thus, understanding why older adults have reduced protective immune responses to infection and vaccination is essential for designing more effective interventions to prevent infection-related morbidity and mortality in the older population.
The primary read-out for almost all vaccinations is the induction of protective antigen-specific antibodies. The induction of vaccine-specific antibodies can be mediated by follicular or extrafollicular B cell responses, which provide long-term or short-term protection, respectively. Although short-term responses provide rapid antigen-specific antibody production, the cells generated from these interactions display poor survival. Thus, the generation of long-lived antibody-producing cells is essential for an effective vaccine response.(1) In order to achieve long-lived protective antibody responses, B cells must undergo class switch recombination, somatic hypermutation and plasma cell differentiation, all of which require the help of a specialized T cell subset termed T follicular helper cells (TFH cells). Here, we discuss current understanding of TFH cell development, functionality and association with vaccine responses, how these processes are affected by aging and the clinical implications of age-dependent changes in TFH cells for immune modulation.
A specialized T cell subset to help B cell responses.
TFH cells are a subset of CD4+ T cells that are specialized in providing help to follicular B cells within germinal centers of secondary lymphoid tissues (i.e. lymph nodes, tonsils, spleen, Peyer’s patches). Discovered more than 18 years ago, TFH cells are uniquely delineated by the expression of CXC-chemokine receptor 5 (CXCR5).(2, 3) Functionally, CXCR5 binds to CXC-chemokine ligand 13 (CXCL13) secreted by follicular stromal cells present within the secondary lymphoid tissues and allows for the homing of TFH cells into follicles. Mature TFH cells within tissues are further distinguished by high co-expression of programmed death receptor 1 (PD-1).(4) Genetic mutations causing reduced TFH numbers, such as ICOS-deficient CVID (5, 6) and CD40 ligand-deficient hyper-IgM (7), lead to defects in humoral immunity; with major alterations in memory B cells and serum antibody levels.
The development of TFH cells.
The development of TFH cells is complex, involving multiple cell types and direct receptor and non-direct cytokine interactions.(8, 9) Classical differentiation of TFH cells occurs in three main phases: 1) extrafollicular priming, 2) follicular maturation and 3) germinal center development. During extrafollicular priming, naïve CD4 T cells interact with local dendritic cells in the extrafollicular space of secondary lymphoid tissue. This interaction requires T cell receptor engagement with antigen-MHC II complex as well as specific cognate and soluble factors. In mice, TFH cell priming is mediated by multiple cytokines including interleukin (IL)-6, IL-21 and IL-27.(10, 11) While TGF-β inhibits TFH generation in mice (12), TGF-β or activin A in combination with IL-12 and several other cytokines promotes strong polarization of human naïve CD4 T cells towards a TFH cell phenotype (13–15), demonstrating species-specific differences in TFH development. However, the overall outcome of naïve CD4 T cell priming in both species is the upregulation of the master transcription factor for TFH cells, BCL-6.(14, 16) BCL-6 expression also depends upon the engagement of the co-stimulatory surface receptor ICOS on the developing TFH cells by ICOS ligand.(17) Indeed, loss of ICOS in both mouse and man leads to significant reductions in TFH cells and generation of germinal centers.(5, 18) The expression of BCL-6, in turn, drives the upregulation of CXCR5 on naïve CD4 T cells and allows for subsequent migration of “primed” precursor TFH cells towards the B cell follicle border area.
The second phase of TFH development occurs at the T-B border of the follicle, where precursor TFH cells interact directly with antigen-specific B cells through their T cell receptor as well as via co-stimulatory (i.e. ICOS-ICOSL, OX40-OX40L) and co-inhibitory signals (i.e. PD-1-PD-L1). The intracellular adaptor protein, signal adaptor SLAM-associated protein (SAP), is critical for the development of a stable interaction between T and B cells during this second phase.(19) SAP-deficient mice and humans display normal frequencies of circulating memory TFH cells, however mature TFH cell frequencies and the formation of germinal centers is significantly reduced (20, 21), demonstrating that SAP is essential for transitioning from a precursor TFH to a mature GC TFH. During this second phase, B cells also receive the necessary help from maturing TFH cells in the form of secreted IL-21 and CD40L-CD40 engagement that initiate germinal center reactions.
In the third phase of TFH development, mature TFH cells move from the T-B border area into the germinal center of the follicle, becoming germinal center (GC) TFH cells. GC TFH cells again engage with GC B cells via antigenic signals and co-stimulatory signals, providing the necessary help to promote antigen-specific B cell proliferation and plasma cells or memory B cell differentiation. After GC TFH cells have provided help to GC B cells, they can exit the GC and migrate to a new GC, re-enter the original GC or downregulate BCL-6 for transition into a circulating memory TFH cell. Alternatively, GC TFH cells may stay within the germinal center until they encounter a secondary response.(22) Moreover, upon secondary antigen-exposure, circulating memory TFH cells are rapidly re-recruited to germinal centers to produce effector antibody responses, thereby providing better and more robust immune protection against antigenic re-challenges.
TFH cell functionality.
The main function of TFH cells is to provide help to antigen-specific B cells to promote proliferation, antibody class switching and somatic hypermutation for the generation of long-lived plasma cells and memory B cells. Within the follicles, TFH cells interact with B cells by secretion of soluble factors and direct cell-to-cell contact. IL-21 is the primary cytokine secreted by TFH cells and promotes B cell class switching and differentiation into antibody secreting cells.(23) Moreover, the development of memory B cells within the germinal center requires TFH-produced IL-9 secretion.(24) TFH cells also secrete other cytokines including, but not limited to, IL-10, IL-4, IL-17 and IFN-γ, which can alter the quality and quantity of subsequent B cell responses. Expression of CD40 ligand on the surface of TFH cells provides cognate signals to B cells required for B cell class switching and differentiation. A number of other co-stimulatory (ICOS, OX40) and co-inhibitory receptors (PD-1) also play important roles in T-B cell interactions and TFH development, which can affect downstream B cell responses.
Heterogeneity of TFH cells.
TFH cells are classically defined by the expression CXCR5. However, it is now clear that the TFH compartment is highly heterogeneous, consisting of different phenotypic and functional populations similar to TH1, TH2, TH17 and T regulatory CD4 T cells.(4) These subsets are distinguished by distinct patterns of surface receptor expression (e.g. CXCR3, CCR6, CCR4) that closely correlate with cytokine secretion profiles.(25) Moreover, these subsets can be further divided based on expression of CCR7, ICOS and PD-1, in which ICOS-PD-1-CCR7+ cells within the CXCR5+ compartment are most abundant in the circulation and consider to be quiescent TFH precursor or memory TFH subsets. ICOS+ TFH cells are a more activated TFH subset and PD-1low/+ TFH cells display features of an intermediate effector-like subset.(4, 26)
TFH cell subsets not only display different patterns of surface receptor expression and cytokine secretion, they also vary in their ability to provide help to B cells. TH2- and TH17-like TFH cells have the functional ability to help B cells produce antibodies whereas TH1-like TFH cells do not.(27, 28) Moreover, the secretion of different cytokines by these subsets can affect B cell class switching and thus the effectiveness of antibody responses. The specific requirements for TFH subset generation and tissue localization are currently unknown and warrant further investigation.
In addition to TFH cells, a subset of CXCR5-expressing regulatory CD4+ T cells can be found within secondary lymphoid tissues. These cells, termed T follicular regulatory cells (TFR cells), are related to thymus-derived Treg cells and are classically defined by the co-expression of FOXP3 and CD25.(29–31) These cells are further characterized by the expression of CTLA-4 (32) and surface receptors shared by TFH cells, such as CXCR5, ICOS and PD-1. TFR cells are most prevalent at the T-B borders and within B cell follicles with few TFR cells directly within the germinal centers in human lymph nodes.(33) However, recent studies have also shown a subset of CD25- TFR cells specially localize to germinal centers in mice and humans.(34, 35) Functionally, both CD25+ and CD25- TFR cells inhibit follicular B cell responses – most likely through direct suppression of TFH cell function.(31, 35, 36) Moreover, TFR cells are preferentially induced by exposure to self-antigen, however foreign antigens, such as those in vaccinations, can also induce their development.(37) Consistently, alterations in TFR to TFH cell ratios within secondary lymph nodes have been found in autoimmune diseases and chronic infections, such as rheumatoid arthritis, malaria and HIV (38–40), and can significantly influence antibody responses and subsequent humoral immune protection.
Circulating TFH cells in vaccine responses.
The induction of protective antibodies against infectious pathogens is the hallmark of almost all successful vaccines. With the discovery that TFH cells guide these responses, researchers have started to address whether this cellular subset could be used as a predictive marker of vaccine efficacy. As mentioned above, after the engagement in a germinal center response, TFH cells can exit the germinal center and become circulating memory TFH cells, thus the circulating subset potentially reflects the induction of antigen-specific immune responses and may be an accessible biomarker for determining the success of vaccinations. Indeed, many groups have now shown that the frequencies of cTFH cells, in particular with an activated (i.e. ICOS-expressing) phenotype, positively predicts the levels of antigen-specific responses to vaccination, including vaccines against influenza and S. pneumonia.(41–46) Activated cTFH frequencies are also predictive of mucosal antibody responses to oral vaccines (47) and recent functional studies show a dependence on ICOS, BCL-6 and IL-21 for effective TFH function in vaccine-specific mucosal antibody responses.(48) Moreover, mouse models demonstrate that activated cTFH cells elicit robust recall responses and antibody-mediated protection upon secondary exposure.(49) Thus, the frequency and activation status of cTFH cells may be useful biomarkers in blood to predict effectiveness of multiple types of vaccinations.
cTFH responses to vaccination in older individuals.
Older adults display diminished vaccine-specific antibody responses, including to vaccination against influenza, varicella zoster virus (VZV) and S. pneumonia. Thus, one could speculate that this reduction is linked with alterations in the cTFH compartment – in which the association between activated cTFH cells and vaccine-specific antibody titers would be lost with age. Indeed, studies have found that older adults fail to increase activated cTFH cells after vaccination with the influenza vaccine, whereas young adults have significant increases in this subset that directly correlates with the production of influenza-specific antibodies.(45) This was due to the fact that older individuals displayed higher frequencies of ICOS+ cTFH cells pre-vaccination compared with younger adults. Similarly, HIV-infected individuals, who display characteristics similar to that of immune aging within the CD4 T cell compartment, also had no upregulation of activated TFH cells after vaccination with the pneumococcal vaccine, in association with reduced titers of antigen-specific antibodies.(41) Notably, pre-vaccination levels of CD38+HLA-DR+ cTFH cells negatively correlated with influenza vaccine responses, suggesting that high baseline frequencies of activated cTFH cells may actually be inhibitory.(50) Thus, the magnitude of change in the activated cTFH compartment may be a more robust biomarker for monitoring vaccine responsiveness in the aging population. Moreover, the high basal frequencies of activated cTFH cells and the lack of expansion of activated cTFH populations post-vaccination in older individuals suggests that TFH development in the follicle or TFH activation within the germinal center is compromised during aging.
TFH cells dysfunction with age.
The diminished vaccine response in older individuals correlates with the loss of cTFH activation, suggesting declining functionality of memory TFH cells with age. Because there is limited information of TFH cells during human aging, we will discuss the current available knowledge of TFH cell aging from animal studies and how this is related to our current understanding of human TFH cells in the context of the aging CD4 T cell compartment. An overview of these concepts is provided in Figure 1.
Figure 1. Alterations in TFH cells during aging.
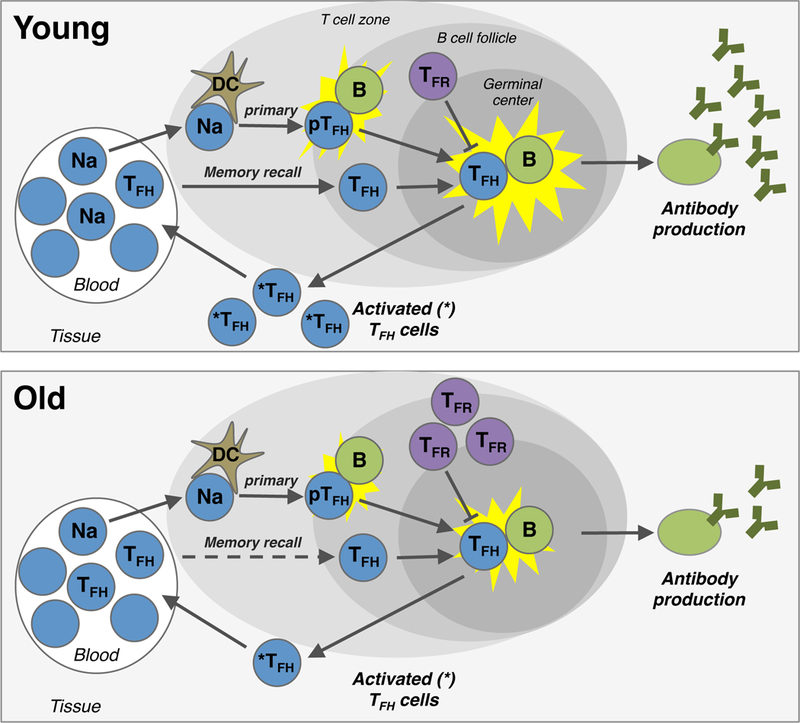
During a primary immune response, naïve CD4 T cells (Na) are recruited from the blood into the tissue, where they interaction with dendritic cells (DC). If activated via their T cell receptor in conjugation with the appropriate co-stimulation, naïve CD4 T cells upregulate CXCR5 and move into the B cell follicle. These precursor TFH cells (pTFH ) interact with local B cells to undergo full maturation into bona fide TFH cells, which again interact with B cells within germinal centers. This germinal center interaction induces the production of high affinity antibodies as well as the release of activated memory TFH cells (*TFH ) from the tissue back into the blood. Memory TFH cells in the blood can also be re-recruited into the follicle upon secondary exposure (i.e. memory recall) to rapidly promote the production of antibodies. TFH responses can be inhibited by T follicular regulatory cells (TFR) within follicles. During aging, multiple changes in this pathway occur, including alterations of naïve CD4 and TFH cell frequencies within the blood, reductions in TFH -B cell interactions and increases in TFR cells - which in turn lead to lower production of antigen-specific antibodies and activated TFH cells.
Aging mice display many similar features of human immune aging - including reduced humoral immune responses to vaccination. Thus, mouse studies can provide some mechanistic insight into the role of TFH cells during aging. Humoral immune responses are recovered in old mice upon transfer of young T cells but not B cells, which demonstrates that the aging defects are specific to the T cell compartment.(51) Notably, studies on influenza vaccination in mice found that the ability of CD4 T cells to differentiate into precursor TFH cell was unaffected by age.(52) Instead, aged TFH cells demonstrate reduced activation, an inability to fully differentiate into bona fide GC TFH cells and a more regulatory phenotype. Moreover, although circulating TFH increase with age, the numbers and activation of TFH cells within germinal centers are reduced; leading to defective antigen-specific responses.(53) Defective TFH responses are also, in part, contributed to direct suppression by increased frequencies of TFR cells in older mice.(53)
Similar to mouse studies, the total frequency of cTFH cells increases with age in humans.(54) This phenomenon is also seen in HIV-infected individuals.(55) One possible explanation for the increase in cTFH cells in aged individuals is the loss of the naïve compartment during aging, which indirectly inflates the relative frequency of the memory compartment. As cTFH cells are composed primarily of memory cells, they would be indirectly expanded. However, the expansion of memory cTFH cells would, in theory, provide more immune protection, not less. Thus, these findings do not explain reduced humoral immunity during aging. An alternative hypothesis is that TFH cells lose functionality – such as observed in mouse models. It is still unclear how the findings in mice translate into TFH dysfunction in older humans and further studies of human TFH subsets and TFH cells within secondary lymphoid tissues during aging are required. Below, we detail how changes within the naïve and memory CD4 T cell compartments could affect TFH development and functionality during human aging.
Effect of aging on TFH development and memory recall responses.
In the context of TFH cells, there are two main possibilities for loss of functionality with age; defective TFH development or defective memory TFH recall responses. Immune protection from vaccination in older individuals relies mainly on recall responses by targeting and expanding already present memory T cells. However, naïve T cell responses play an important role in defense against new or forgotten pathogens and may also be affected by age. Indeed, our group has recently show that even recall responses in older individuals, as in the case of zoster vaccination, involves the recruitment of naïve T cells.(56) Thus, dysfunctions in both the naïve and memory CD4 T cell compartments may contribute to reductions in effective TFH responses during aging.
Naïve CD4 T cell priming during aging.
The naïve CD4 T cell compartment undergoes many cell-intrinsic changes that could affect TFH differentiation as well as functionality during aging. In particular, one hallmark of CD4 T cell aging is a reduction in T cell receptor signaling in naïve CD4 T cells caused by diminished expression of miR-181a in these cells with age.(57) Consistently, naïve CD4 T cells from older individuals show less activation than young adults after T cell receptor stimulation - exhibiting reduced expression of ICOS and CD40L on activated CD4 T cells.(58) Preliminary data from our group also demonstrates a bias of naïve CD4 T cells from older individuals to favor development into inflammatory effector T cells rather than into TFH cells upon T cell receptor stimulation under non-polarizing conditions, i.e. without adding T cell lineage determining cytokines. Similar shifts in naïve CD4 T cell differentiation, away from TFH development, have been found in mice and are linked with alterations in the aged tissue environment.(59) Thus, alterations in naïve CD4 T cell receptor signaling and local TFH priming may play an important role in the generation of TFH responses to new pathogenic infections or vaccines during aging.
Memory CD4 T cell recall responses during aging.
Most of the T cell responses in older individuals derive from memory recall responses. In particular, vaccine responses are highly dependent on pre-existing immunological memory. The link between decreased vaccine responses and poor upregulation of activated cTFH cells in older individuals is also indicative of a poor recall capacity of memory CD4 T cells. Such recall responses are classically derived from the central memory compartment, in which antigen-specific central memory CD4 T cells are recruited into secondary lymphoid organs and develop into CXCR5-expressing cells upon T cell receptor engagement. Although the repertoire diversity of the memory CD4 T cell compartment remains relatively unchanged with age(60), we do find distinct changes that may affect TFH cell function. Memory CD4 T cells from older individuals preferentially differentiate into DUSP4- and CD39-expressing cells upon T cell receptor stimulation, which lack the ability to provide help to B cells.(58, 61) Instead, they produce inflammatory cytokines, in particular IFN-γ and have an increased propensity to undergo apoptosis. A genetic polymorphism determining the ability to express CD39 directly correlates with influenza and VZV vaccine responses.(61) DUSP4 expression also inversely correlated with CD40L and ICOS expression after influenza vaccination. Together, altered recall responses of memory CD4 T cells with age include defects that specifically influence TFH functional ability to be activated and interact with B cells to promote humoral immune responses.
Targeting TFH cells to improve vaccine responses with older individuals.
Designing vaccines to specifically overcome age-related dysfunctions in TFH cells could provide a clinical intervention for improving immune protection in older individuals. Although more basic studies on TFH and TFR cells during human aging are needed, there are three main strategies one could employ for manipulating TFH cell responses (62); altering the frequency of TFH cells, changing the functionality of TFH cells or adjusting TFR cell suppression (outlined in Figure 2). The potential mechanisms and their caveats are detailed below.
Figure 2. Interventions for enhancing TFH-mediated vaccine responses during aging.
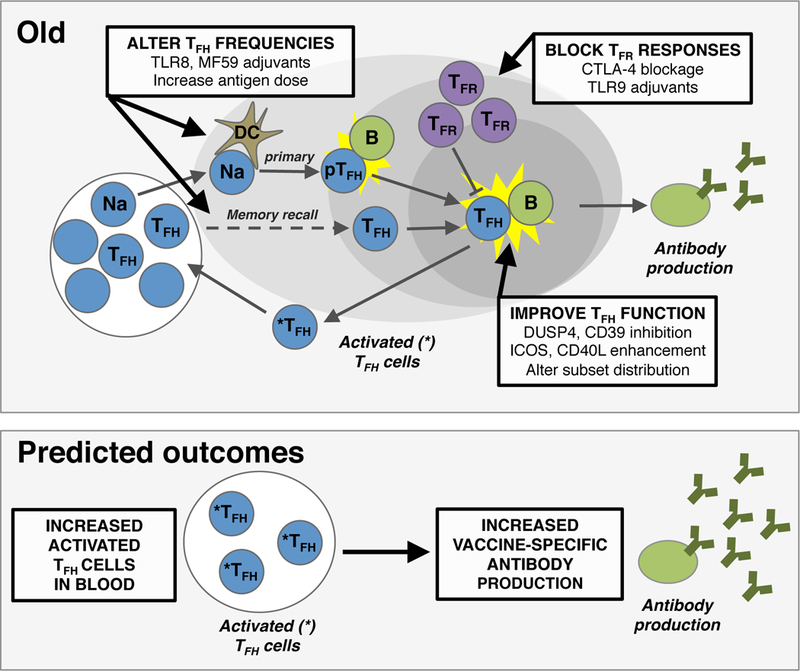
Multiple steps in the development of TFH responses with age are possible targets for therapeutic interventions. These targets include 1) altering the frequencies of naïve CD4 T cells (Na), precursor T follicular helper cells (pTFH) and T follicular helper cells (TFH) that participate in a vaccine response, 2) inhibition of T follicular regulatory cell (TFR) numbers and/or suppressive capacity, and 3) improving overall TFH functionality. The ultimate outcome of these interventions would be an increase in functional TFH cells indicated by higher frequencies of activated TFH (*TFH) within circulation and higher levels of vaccine-specific antibody production by B cells. Dendritic cell, DC.
Alter the frequency of TFH cells.
In most vaccine studies, increasing frequencies of cTFH cells correlate with the induction of protective humoral immunity.(41, 45, 47) Determining mechanisms for increasing cTFH cells is therefore thought to be an effective way to enhance humoral immune responses. Indeed, addition of liposome adjuvant and toll-like receptor 4 (TLR4) agonist to a malaria vaccine significantly enhanced TFH responses and subsequent antibody responses in mice.(63) Moreover, Ugolini et. al. recently discovered that TFH differentiation is driven by TLR8 engagement and the addition of TLR8 agonists enhanced TFH development and humoral immune responses in pigs.(64) These data suggest that the addition of specific adjuvants could increase TFH responses to vaccination and may be of utility for enhancing humoral immunity in older individuals.
The addition of adjuvants to enhance immunity in the elderly population is an interesting and relatively new area of study. The specific effects of adjuvants on immune responses in the elderly is nicely reviewed in Del Giudice et. al.(65) For example, an influenza vaccine adjuvanted with MF59, an oil-in-water emulsion of squalene oil, was recently approved by the FDA for individuals older than 65 years. While there is not much evidence for improved antibody responses, it appears to be more protective in the elderly. Moreover, a zoster vaccine using the AS01 adjuvant has also been proven to be highly efficacious in protecting against herpes zoster in older individuals and induces long-term increases in vaccine-specific antibodies and CD4 T cells. It is currently unclear whether either of these vaccines function by inducing TFH cells, however it highlights the idea that age-specific adjuvants may indeed be useful for enhancing TFH frequencies and subsequent antibody responses in the elderly population.
The idea of enhancing TFH frequencies is somewhat counterintuitive in the context of human aging, where elevated levels of cTFH cells but still significant reduction in humoral immunity are observed. However, it is important to note that in influenza vaccine trials, although older individuals had higher levels of cTFH cells prior to vaccination compared with young adults, there was no change in this frequency post-vaccination.(66) Thus, it is possible that current vaccines are not able to elicit antigen-specific TFH responses and the elevated levels of cTFH observed may simply be reflective of general accumulation of memory T cells with age. Indeed, a follow-up study revealed that increasing the antigen dose in the influenza vaccine elicited higher levels of activated cTFH cells compared with the standard vaccine and correlated with increased seroconversion in older individuals.(66) As T cell receptor stimulation thresholds in both naïve and memory CD4 T cells change with age, these data suggest that increasing antigenic stimulation of precursor or memory TFH cells may be an effective way to overcome age-dependent TFH dysfunctions and elicit more robust humoral immune response in the older population - an interesting new concept for age-specific vaccine design. Consistently, increasing antigen dose has been successfully used to improve efficacy to vaccines for trivalent influenza vaccine and live-attenuated VZV.(67, 68)
Change the functionality of TFH cells.
Besides altering the overall frequencies of TFH cells, another (not exclusive) strategy is to change TFH functionality by driving the development of specific TFH subsets. The importance of appropriate TFH subset development is clearly highlighted in the case of autoimmunity. Changes in TFH subset distribution are found in numerous autoimmune diseases, including rheumatoid arthritis (69) and juvenile dermatomyositis.(28) Moreover, in psoriasis vulgaris (70) and systemic lupus erthematosus (71), increased levels of TH17-like and TH2-like TFH cells, respectively, directly correlate with disease severity. In classical T helper cell differentiation, skewing of subsets is driven by cytokine exposure in the local environment.(72) Although currently unknown, TFH subsets may develop in a similar fashion, skewed by the local cytokine milieu. Better understanding of requirements for the development of individual subsets and the distribution of TFH subsets with aging are needed to better explore this strategy.
Reduce TFR cell suppression.
In the context of aging, TFR cells increase, at least in mouse models. Thus, decreasing the number of TFR cells could be an avenue to enhance TFH responses. Specific vaccine adjuvants can favor the development of TFH cells over TFR cells in an immune response. Immunization with TLR9 adjuvants significantly enhances the number of TFH cells, reduces the number of TFR cells and increases antigen-specific IgG2 production in mice.(73) TLR9 adjuvants also induce GC TFH cells with increased ICOS expression and IL-21 secretion in neonatal mice.(74) In addition to making less TFR cells during an immune response, the suppressive capacity of TFR cell can be blocked or diminished by targeting the inhibitory molecules on TFR cells. Inhibition of the inhibitory molecule cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) significantly increases TFH function.(32, 75) Although the CTLA-4 inhibitor ipilmumab was the first immune checkpoint inhibitor approved for use in cancer patients and has been used in clinical studies, CTLA-4 blockade can cause significant side effect in patients including the development of autoimmunity. Likewise, recent evidence demonstrates that TFR cells are critical to prevent autoimmune responses, where diminished TFR responses cause high induction of auto-antibodies.(76) Of note, the T cell receptor repertoires of TFR cells differs from that of TFH cells,(77) suggesting that these cells may actually be blocking off-target (i.e. self) responses during the development of germinal center responses to foreign antigen. Thus, strategies to block the development or function of TFR cells in older individuals could potentially drive the development of autoimmunity – of which older individuals are already at higher risk of developing. Although the risk of autoimmunity in young children is unacceptable, the trade-off between immune protection and development of autoimmunity in older individuals needs to be better assessed to determine whether the benefit outweighs the risk.
Concluding remarks.
Mounting evidence suggests that TFH dysfunction causes, at least in part, reductions in protective antibody responses against infection and to vaccination during aging. Although we have a good understanding of TFH development and function from mouse models, these processes differ in humans. Moreover, we have limited knowledge on the cellular and molecular effect of age, and the aging tissue environments, on TFH and TFR subsets. Understanding these changes will be crucial for developing clinical interventions that will improve protective immunity, in particular vaccine responses, in older individuals.
Acknowledgements.
This work was supported by the National Institutes of Health (R01 AR042527, R01 HL117913, R01 AI108906 and P01 HL129941 to CMW; R01 AI108891, R01 AG045779, U19 AI057266, R01 AI129191, I01 BX001669 to JJG; and T32 AG047126 to CEG).
Abbreviations:
- CTLA
cytotoxic T-lymphocyte-associated protein
- CVID
common variable immunodeficiency
- CXCR
CXC receptor
- CXCL
CXC ligand
- GC
germinal center
- ICOS
inducible T cell co-stimulator
- IFNγ
interferon-gamma
- IL
interleukin
- MHC
major histocompatibility complex
- SAP
signal adaptor SLAM-associated protein
- TFH cell
T follicular helper cell
- cTFH
circulating TFH
- TFR cell
T follicular regulatory cell
- TLR
toll-like receptor
- VZV
varicella zoster virus
References.
- 1.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nature reviews Immunology. 2006;6(10):741–50. 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 2.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine. 2000;192(11):1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. The Journal of experimental medicine. 2000;192(11):1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong MT, Chen J, Narayanan S, Lin W, Anicete R, Kiaang HT, et al. Mapping the Diversity of Follicular Helper T Cells in Human Blood and Tonsils Using High-Dimensional Mass Cytometry Analysis. Cell reports. 2015;11(11):1822–33. 10.1016/j.celrep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. Journal of immunology. 2006;177(7):4927–32. [DOI] [PubMed] [Google Scholar]
- 6.Warnatz K, Bossaller L, Salzer U, Skrabl-Baumgartner A, Schwinger W, van der Burg M, et al. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 2006;107(8):3045–52. 10.1182/blood-2005-07-2955. [DOI] [PubMed] [Google Scholar]
- 7.Agematsu K, Nagumo H, Shinozaki K, Hokibara S, Yasui K, Terada K, et al. Absence of IgD-CD27(+) memory B cell population in X-linked hyper-IgM syndrome. The Journal of clinical investigation. 1998;102(4):853–60. 10.1172/JCI3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jogdand GM, Mohanty S, Devadas S. Regulators of Tfh Cell Differentiation. Frontiers in immunology. 2016;7:520 10.3389/fimmu.2016.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb LMC, Linterman MA. Signals that drive T follicular helper cell formation. Immunology. 2017;152(2):185–94. 10.1111/imm.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. The Journal of experimental medicine. 2010;207(13):2895–906. 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PloS one. 2011;6(3):e17739 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarron MJ, Marie JC. TGF-beta prevents T follicular helper cell accumulation and B cell autoreactivity. The Journal of clinical investigation. 2014;124(10):4375–86. 10.1172/JCI76179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locci M, Wu JE, Arumemi F, Mikulski Z, Dahlberg C, Miller AT, et al. Activin A programs the differentiation of human TFH cells. Nature immunology. 2016;17(8):976–84. 10.1038/ni.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunology and cell biology. 2009;87(8):590–600. 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, et al. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nature immunology. 2014;15(9):856–65. 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–5. 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–46. 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. Journal of immunology. 2005;175(4):2340–8. [DOI] [PubMed] [Google Scholar]
- 19.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455(7214):764–9. 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33(2):241–53. 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39(4):770–81. 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Moysi E, Pallikkuth S, De Armas LR, Gonzalez LE, Ambrozak D, George V, et al. Altered immune cell follicular dynamics in HIV infection following influenza vaccination. The Journal of clinical investigation. 2018. 10.1172/JCI99884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. Journal of immunology. 2007;179(12):8180–90. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Shi J, Yan J, Xiao Z, Hou X, Lu P, et al. Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nature immunology. 2017;18(8):921–30. 10.1038/ni.3788. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Feng J, Hou Z, Wang XM, Yu D. Flow cytometric analysis of circulating follicular helper T (Tfh) and follicular regulatory T (Tfr) populations in human blood. Methods in molecular biology. 2015;1291:199–207. 10.1007/978-1-4939-2498-1_17. [DOI] [PubMed] [Google Scholar]
- 26.Trub M, Barr TA, Morrison VL, Brown S, Caserta S, Rixon J, et al. Heterogeneity of Phenotype and Function Reflects the Multistage Development of T Follicular Helper Cells. Frontiers in immunology. 2017;8:489 10.3389/fimmu.2017.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39(4):758–69. 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–21. 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 30.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. Journal of immunology. 2005;175(7):4180–3. [DOI] [PubMed] [Google Scholar]
- 31.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nature medicine. 2011;17(8):975–82. 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41(6):1013–25. 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Sayin I, Radtke AJ, Vella LA, Jin W, Wherry EJ, Buggert M, et al. Spatial distribution and function of T follicular regulatory cells in human lymph nodes. The Journal of experimental medicine. 2018;215(6):1531–42. 10.1084/jem.20171940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritvo PG, Churlaud G, Quiniou V, Florez L, Brimaud F, Fourcade G, et al. Tfr cells lack IL-2Ralpha but express decoy IL-1R2 and IL-1Ra and suppress the IL-1-dependent activation of Tfh cells. Science immunology. 2017;2(15). 10.1126/sciimmunol.aan0368. [DOI] [PubMed] [Google Scholar]
- 35.Wing JB, Kitagawa Y, Locci M, Hume H, Tay C, Morita T, et al. A distinct subpopulation of CD25(−) T-follicular regulatory cells localizes in the germinal centers. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(31):E6400–E9. 10.1073/pnas.1705551114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature medicine. 2011;17(8):983–8. 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aloulou M, Carr EJ, Gador M, Bignon A, Liblau RS, Fazilleau N, et al. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nature communications. 2016;7:10579 10.1038/ncomms10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurup SP, Obeng-Adjei N, Anthony SM, Traore B, Doumbo OK, Butler NS, et al. Regulatory T cells impede acute and long-term immunity to blood-stage malaria through CTLA-4. Nature medicine. 2017;23(10):1220–5. 10.1038/nm.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Wang D, Song Y, Lu S, Zhao J, Wang H. Increased circulating CD4(+)CXCR5(+)FoxP3(+) follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. International immunopharmacology. 2018;56:261–8. 10.1016/j.intimp.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 40.Miles B, Miller SM, Folkvord JM, Kimball A, Chamanian M, Meditz AL, et al. Follicular regulatory T cells impair follicular T helper cells in HIV and SIV infection. Nature communications. 2015;6:8608 10.1038/ncomms9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abudulai LN, Fernandez S, Corscadden K, Burrows SA, Hunter M, Tjiam MC, et al. Production of IgG antibodies to pneumococcal polysaccharides is associated with expansion of ICOS+ circulating memory T follicular-helper cells which is impaired by HIV infection. PloS one. 2017;12(5):e0176641 10.1371/journal.pone.0176641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Science translational medicine. 2013;5(176):176ra–32. 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Armas LR, Cotugno N, Pallikkuth S, Pan L, Rinaldi S, Sanchez MC, et al. Induction of IL21 in Peripheral T Follicular Helper Cells Is an Indicator of Influenza Vaccine Response in a Previously Vaccinated HIV-Infected Pediatric Cohort. Journal of immunology. 2017;198(5):1995–2005. 10.4049/jimmunol.1601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Armas LR, Pallikkuth S, George V, Rinaldi S, Pahwa R, Arheart KL, et al. Reevaluation of immune activation in the era of cART and an aging HIV-infected population. JCI insight. 2017;2(20). 10.1172/jci.insight.95726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herati RS, Reuter MA, Dolfi DV, Mansfield KD, Aung H, Badwan OZ, et al. Circulating CXCR5+PD-1+ response predicts influenza vaccine antibody responses in young adults but not elderly adults. Journal of immunology. 2014;193(7):3528–37. 10.4049/jimmunol.1302503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spensieri F, Siena E, Borgogni E, Zedda L, Cantisani R, Chiappini N, et al. Early Rise of Blood T Follicular Helper Cell Subsets and Baseline Immunity as Predictors of Persisting Late Functional Antibody Responses to Vaccination in Humans. PloS one. 2016;11(6):e0157066 10.1371/journal.pone.0157066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardeno A, Magnusson MK, Quiding-Jarbrink M, Lundgren A. Activated T follicular helper-like cells are released into blood after oral vaccination and correlate with vaccine specific mucosal B-cell memory. Scientific reports. 2018;8(1):2729 10.1038/s41598-018-20740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aljurayyan A, Puksuriwong S, Ahmed M, Sharma R, Krishnan M, Sood S, et al. Activation and Induction of Antigen-Specific T Follicular Helper Cells Play a Critical Role in Live-Attenuated Influenza Vaccine-Induced Human Mucosal Anti-influenza Antibody Response. Journal of virology. 2018;92(11). 10.1128/JVI.00114-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38(4):805–17. 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parmigiani A, Alcaide ML, Freguja R, Pallikkuth S, Frasca D, Fischl MA, et al. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PloS one. 2013;8(11):e79816 10.1371/journal.pone.0079816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X, Stedra J, Cerny J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. The Journal of experimental medicine. 1996;183(3):959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lefebvre JS, Masters AR, Hopkins JW, Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Scientific reports. 2016;6:25051 10.1038/srep25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sage PT, Tan CL, Freeman GJ, Haigis M, Sharpe AH. Defective TFH Cell Function and Increased TFR Cells Contribute to Defective Antibody Production in Aging. Cell reports. 2015;12(2):163–71. 10.1016/j.celrep.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou M, Zou R, Gan H, Liang Z, Li F, Lin T, et al. The effect of aging on the frequency, phenotype and cytokine production of human blood CD4 + CXCR5 + T follicular helper cells: comparison of aged and young subjects. Immunity & ageing : I & A. 2014;11:12 10.1186/1742-4933-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miles B, Miller SM, Connick E. CD4 T Follicular Helper and Regulatory Cell Dynamics and Function in HIV Infection. Frontiers in immunology. 2016;7:659 10.3389/fimmu.2016.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi Q, Cavanagh MM, Le Saux S, NamKoong H, Kim C, Turgano E, et al. Diversification of the antigen-specific T cell receptor repertoire after varicella zoster vaccination. Science translational medicine. 2016;8(332):332ra–46. 10.1126/scitranslmed.aaf1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nature medicine. 2012;18(10):1518–24. 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu M, Li G, Lee WW, Yuan M, Cui D, Weyand CM, et al. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(15):E879–88. 10.1073/pnas.1109797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, Haynes L. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging cell. 2012;11(5):732–40. 10.1111/j.1474-9726.2012.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, et al. Diversity and clonal selection in the human T-cell repertoire. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(36):13139–44. 10.1073/pnas.1409155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang F, Yu M, Cavanagh MM, Hutter Saunders J, Qi Q, Ye Z, et al. Expression of CD39 on Activated T Cells Impairs their Survival in Older Individuals. Cell reports. 2016;14(5):1218–31. 10.1016/j.celrep.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linterman MA, Hill DL. Can follicular helper T cells be targeted to improve vaccine efficacy? F1000Research. 2016;5 10.12688/f1000research.7388.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radtke AJ, Anderson CF, Riteau N, Rausch K, Scaria P, Kelnhofer ER, et al. Adjuvant and carrier protein-dependent T-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate. Scientific reports. 2017;7:40312 10.1038/srep40312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ugolini M, Gerhard J, Burkert S, Jensen KJ, Georg P, Ebner F, et al. Recognition of microbial viability via TLR8 drives TFH cell differentiation and vaccine responses. Nature immunology. 2018;19(4):386–96. 10.1038/s41590-018-0068-4. [DOI] [PubMed] [Google Scholar]
- 65.Del Giudice G, Goronzy JJ, Grubeck-Loebenstein B, Lambert PH, Mrkvan T, Stoddard JJ, et al. Fighting against a protean enemy: immunosenescence, vaccines, and healthy aging. NPJ aging and mechanisms of disease. 2018;4:1 10.1038/s41514-017-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pilkinton MA, Nicholas KJ, Warren CM, Smith RM, Yoder SM, Talbot HK, et al. Greater activation of peripheral T follicular helper cells following high dose influenza vaccine in older adults forecasts seroconversion. Vaccine. 2017;35(2):329–36. 10.1016/j.vaccine.2016.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. The New England journal of medicine. 2014;371(7):635–45. 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 68.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. The New England journal of medicine. 2005;352(22):2271–84. 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 69.Arroyo-Villa I, Bautista-Caro MB, Balsa A, Aguado-Acin P, Bonilla-Hernan MG, Plasencia C, et al. Constitutively altered frequencies of circulating follicullar helper T cell counterparts and their subsets in rheumatoid arthritis. Arthritis research & therapy. 2014;16(6):500 10.1186/s13075-014-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Wang L, Shi Y, Wang F, Yang H, Han S, et al. Altered circulating T follicular helper cell subsets in patients with psoriasis vulgaris. Immunology letters. 2017;181:101–8. 10.1016/j.imlet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Le Coz C, Joublin A, Pasquali JL, Korganow AS, Dumortier H, Monneaux F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PloS one. 2013;8(9):e75319 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmitt N, Ueno H. Regulation of human helper T cell subset differentiation by cytokines. Current opinion in immunology. 2015;34:130–6. 10.1016/j.coi.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rookhuizen DC, DeFranco AL. Toll-like receptor 9 signaling acts on multiple elements of the germinal center to enhance antibody responses. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(31):E3224–33. 10.1073/pnas.1323985111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mastelic B, Kamath AT, Fontannaz P, Tougne C, Rochat AF, Belnoue E, et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. Journal of immunology. 2012;189(12):5764–72. 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- 75.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41(6):1026–39. 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu W, Liu X, Lin X, Feng H, Sun L, Li S, et al. Deficiency in T follicular regulatory cells promotes autoimmunity. The Journal of experimental medicine. 2018;215(3):815–25. 10.1084/jem.20170901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maceiras AR, Almeida SCP, Mariotti-Ferrandiz E, Chaara W, Jebbawi F, Six A, et al. T follicular helper and T follicular regulatory cells have different TCR specificity. Nature communications. 2017;8:15067 10.1038/ncomms15067. [DOI] [PMC free article] [PubMed] [Google Scholar]


