Abstract
Background:
Colorectal cancer (CRC) is a leading cause of cancer death. Biomarkers to predict treatment outcomes are needed, as is evidence whether post-diagnosis diet and lifestyle can affect well-being and clinical outcomes. The international ColoCare Consortium aims to identify new biologic markers (e.g., metabolomic, transcriptomic, metagenomic, genetic, epigenetic, proteomic) that predict clinical outcomes, and to characterize associations between modifiable risk factors (e.g., diet, supplement use, physical activity) with short-term and long-term patient-reported and clinical outcomes among CRC patients.
Methods/Results:
ColoCare is recruiting newly diagnosed CRC patients across six sites in the U.S. and one in Germany. As of April 2018 we have recruited >2,000 patients across all sites. Our projected enrollment is >4,000 multiethnic CRC patients. The study includes uniformly collected, comprehensive sets of data and biospecimens at multiple time points up to 5 years after diagnosis. Treatment and clinical data are abstracted from medical records and centrally harmonized. Biospecimens are archived according to standardized procedures. Our initial studies demonstrated metabolic differences in adipose tissue types. We further reported on associations of biological factors (e.g., inflammation, DNA methylation, metabolomics) with lifestyle factors (e.g., adiposity, smoking, physical activity, dietary supplement use) or joint associations with clinical outcomes.
Conclusion:
ColoCare is a consortium for the investigation of multi-level factors relevant to CRC survivorship.
Impact:
The combination of a comprehensive set of biospecimens collected at multiple time points, jointly with detailed assessments of health behaviors and other prognostic factors, results in a unique resource that facilitates wide-ranging, innovative, and impactful research on CRC.
Trial registration:
Keywords: Colorectal cancer, epidemiology, biomarkers, lifestyle, survival
INTRODUCTION
Colorectal cancer is the second most frequent cancer globally (1,360,600 cases) and the third most common cause of cancer death (693,900 deaths) worldwide (1). The incidence and mortality rates among individuals below 50 years of age have increased in recent years (2). Concurrently, the number of survivors of colorectal cancer continues to increase (3) which is attributable at least in part to improved treatment regimens and to both improvements in and wider participation in colorectal cancer screening (4). However, recurrence affects nearly a third of colorectal cancer patients and is a major cause of morbidity and mortality (3,5). The discovery of biomarkers/risk prediction models with high sensitivity and specificity for recurrence remains a high research priority. At this time, the most accurate means for predicting prognosis for colorectal cancer remains pathological stage, despite the fact that significant clinical heterogeneity in treatment response exists among patients with the same stage of cancer (6). Blood, urine, or stool-based biomarkers have the advantage that they can be examined prior to and after surgery to monitor disease status non-invasively. Studies collecting detailed patient and treatment information as well as biospecimens prior to and post-diagnosis are rare. ColoCare is an innovative and transdisciplinary cohort specifically designed to discover and validate novel prognostic biomarkers and risk prediction models in a highly annotated set of colorectal cancer survivors, thus addressing a clearly defined clinical and public health need.
To date, still little is known about the effects of nutritional supplements, diet, physical activity, or use of hormones and other medications on cancer outcomes. The identification of behavioral factors affecting prognosis and response to treatment is a research area ColoCare is designed to address through a series of surveys that include key health behavior research questions related to potentially modifiable exposures such as physical activity, non-steroidal anti-inflammatory drug (NSAID) use, alcohol consumption, dietary factors, and many more. In particular, the topic of energy balance, adipose-tumor tissue interactions and physical activity is timely and important, considering the dramatic rise in obesity worldwide, the potential for direct growth-promoting effects of adipose tissue adjacent to colon tumors, and the accumulating evidence that physical activity can positively affect cancer outcomes (7–12).
Motivating the development of the ColoCare Study was the fact that very few prospectively collected colorectal cancer patient cohort studies currently exist, and those that do have multiple limitations related to sample size, comprehensiveness of exposure and outcome data, and collection of multiple types of longitudinal questionnaire data and biospecimens (13). The goal of the ColoCare Study is to provide the research infrastructure needed to identify new biologic markers (e.g., metabolomic, transcriptomic, metagenomic, genetic, epigenetic, and proteomic biomarkers) that predict clinical outcomes, and to characterize associations between potentially modifiable risk factors (e.g., diet, supplement use, NSAID use, physical activity, body composition, etc.) with short-term and long-term patient-reported and clinical outcomes (e.g., therapy-induced toxicities, recurrence, survival and quality of life, etc.) among patients with colorectal cancer. Although several of these factors have been studied in isolation, a unique feature of the ColoCare Study is its transdisciplinary approach, which enables concurrent, synergistic research on these factors within a single prospective cohort. Geographical and demographic diversity adds to the value of the study and increase generalizability. Study results leveraging ColoCare will inform potential approaches for optimizing therapies and treatment strategies for individual colorectal cancer patients and will provide evidence-based guidance for patients and clinicians on modifiable health behaviors that are associated with improved outcomes and quality of life. The collective results from the ColoCare Consortium have the potential to have an important impact on the clinical and survivorship management of this major disease.
MATERIALS AND METHODS
ColoCare (ClinicalTrials.gov Identifier: NCT02328677) is an international, multi-center prospective cohort study among women and men newly diagnosed with a primary invasive colorectal cancer of any stage. ColoCare goals are to investigate predictors of cancer recurrence, survival, treatment toxicities and health-related quality of life. The study was initially developed at the Fred Hutchinson Cancer Research Center (Seattle, Washington, USA) in 2007, with subsequent consortium sites at the H. Lee Moffitt Cancer Center and Research Institute (Tampa, Florida, USA) in 2009, National Center for Tumor Diseases and University Hospital of Heidelberg (Heidelberg, Germany) in 2010, Huntsman Cancer Institute (Salt Lake City, Utah, USA) in 2015, Cedars-Sinai Medical Center (Los Angeles, California, USA) in 2017, Washington University School of Medicine (WUSM) (St. Louis, Missouri, USA) in 2017, and the Center for Cancer Research (Memphis, Tennessee, USA) in 2017.
Eligible patients are defined as follows: newly diagnosed colon (ICD-10 C18), rectum, or rectosigmoid cancer (ICD-10 C19/C20); ≥18 years of age at the time of diagnosis, histopathologically confirmed invasive cancer of any stage of disease based on the American Joint Committee on Cancer (AJCC) classification, and able to provide informed consent. ColoCare has been approved by the local institutional review boards serving each site. This study enrolls English speaking patients at all U.S. sites, except for the Cedars-Sinai Medical Center who additionally enrolls non-English speaking individuals and the German site enrolls German-speaking patients. All study participants provide written informed consent (paper or electronically).
Participant Recruitment and follow-up
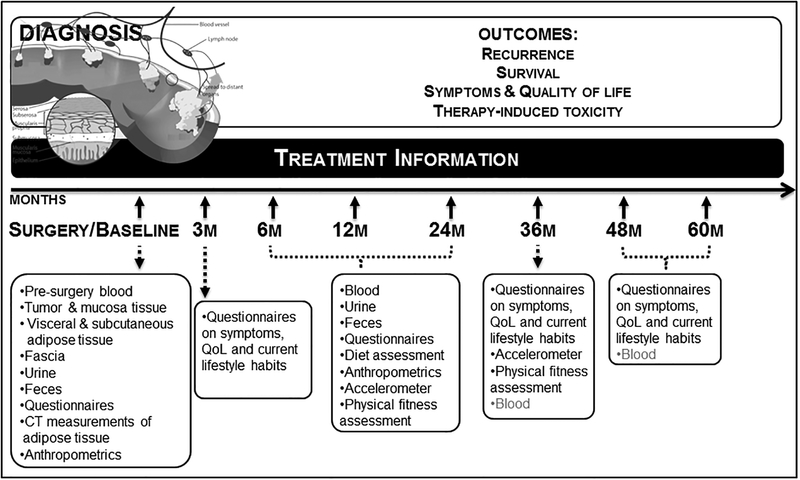
Mechanisms are in place at each site to identify newly diagnosed colorectal cancer patients and recruitment protocols are standardized across all sites. Patients are recruited before surgery (baseline). In brief, during a clinical appointment, preferably prior to their primary surgery to treat the cancer, patients are approached to participate in ColoCare and those willing to participate are consented. ColoCare Study participants agree to complete a baseline questionnaire and provide biological specimens at the time of enrollment and over the course of 5 years of follow-up, as outlined below. In addition, participants agree to provide access to their medical record. Figure 1 illustrates the ColoCare Study design and detailed data and sample collection procedures are described below and in Table 1. Of note, not all components are collected at each time point and across all centers.
Figure 1:
ColoCare Study design. Not all components are collected at each time point and across all centers.
Table 1:
ColoCare Study – Overview of questionnaires, measurements and biospecimen collection at each time point
| Baseline | 3 months | 6 months | 12 months | 24 months | 36 months | 48 months | 60 months | |
|---|---|---|---|---|---|---|---|---|
| Questionnaires | ||||||||
| Demographic information | X | X | X | X | ||||
| Smoking | X | X | X | X | ||||
| Alcohol consumption | X | |||||||
| Medical history | X | |||||||
| Family history | X | |||||||
| Screening assessment | X | |||||||
| Therapy and follow-up of the disease | ||||||||
| Hormone replacement therapy | X | X | X | X | ||||
| Dietary supplement use | X | X | X | X | ||||
| Medication use | X | X | X | X | ||||
| Physical activity | X | X | X | X | X | X | X | X |
| Quality of life | X | X | X | X | X | X | X | X |
| Symptoms | X | X | X | X | X | X | X | X |
| Social network | X | X | X | X | ||||
| Sleep rhythm and quality | X | X | X | X | X | X | X | X |
| Work limitation assessment | X | X | X | |||||
| Dietary behavior assessment | X | X | X | |||||
| Measurements | ||||||||
| Height | X | |||||||
| Weight | X | X | X | X | X | X | X | |
| Body Mass Index | X | X | X | X | X | X | X | |
| Waist to hip ratio | X | X | X | X | X | X | X | |
| Waist circumference | X | X | X | X | X | X | X | |
| Accelerometer | X | X | X | |||||
| Physical fitness testing | X | X | X | |||||
| Computed tomography scan analyses | X | |||||||
| Biospecimens | ||||||||
| Blood | X | X | X | X | (X) | (X) | (X) | |
| Stool | X | X | X | X | ||||
| Urine | X | X | X | X | ||||
| Saliva | X | X | X | X | ||||
| Tumor and mucosa tissue | X | |||||||
| Metastases | X | |||||||
| Visceral fat tissue | X | |||||||
| Subcutaneous fat tissue | X | |||||||
| Fascia tissue | X |
Patients are followed up both actively and passively by study staff (in-person or remote and through medical record reviews), as well as via linkages to cancer registry and vital status records. We conduct a brief telephone assessment of physical activity and symptom measures 3 months after surgery, which for patients undergoing adjuvant therapy is approximately half-way through this treatment. Follow-up questionnaires on health behaviors, symptoms and quality of life are administered at 6, 12, 24, 36, 48 and 60 months (details below). For patients undergoing neoadjuvant and/or adjuvant chemotherapy assessments are performed at least two weeks after completing a cycle.
Data collection
All questionnaires used in ColoCare are self-administered and can be completed on paper or electronically. The baseline questionnaire includes assessments of demographics (e.g., education, ethnicity, marital status), lifestyle factors known/suspected to be associated with colorectal cancer (e.g., smoking, alcohol use, and physical activity), hormone replacement therapy for women, self-reported anthropometric measures (e.g., height and weight), medical history, family history of cancer, dietary supplement use, and prescription and non-prescription medication use. General and colorectal cancer specific quality of life questionnaires (e.g., SF-12, MDASI, EORTC QLQ-C30) are also included (see section Patient reported outcomes for details on quality of life assessments). We use both standardized instruments and adapted questions from ongoing studies, such as the Women’s Health Initiative or the Colorectal Cancer Family Registry (14,15). The baseline questionnaire has been designed to be relatively brief to reduce patient burden and improve completion rates.
The 6- to 60-month questionnaires include demographics, physical activity, cancer and treatment distress, work limitations and work productivity and activity impairment questionnaires; and re-administration of the general and colorectal cancer specific quality of life and symptom measures. From the 6 month questionnaire assessment, dietary habits are measured using a food frequency questionnaire (FFQ) (16,17).
Specific assessment of dimensions of energy balance
In light of the obesity epidemic worldwide – and in lieu of the increasingly strong evidence that physical activity reduces the risk of colorectal cancer recurrence – this study includes state-of-the-art measures of physical activity and adiposity, that capture multiple dimensions of energy balance, from (1) the tumor microenvironment of tumor-adjacent adipose tissue to (2) adipose tissue distribution and (3) physical activity during key phases of colorectal cancer treatment and survivorship.
Physical activity measurement by accelerometry:
Study participants may wear ActiGraph GT3X+ (ActiGraph, Pensacola, FL) accelerometers for a state-of-the-art, objective assessment of physical activity (18) at 6, 12, and 24 months. We have successfully tested the implementation of accelerometers and demonstrated feasibility at the German ColoCare Study site as part of a pilot study (18). Based on this initial research, study participants wear accelerometers for four full days. In our pilot we have used accelerometers that have been fastened around the chest with a band. Subsequently we have implemented accelerometers that are worn around the wrist, as they reduce participant burden. In our initial studies we successfully demonstrated patient compliance at ColoCare Heidelberg: 59% of patients contacted agreed to wear accelerometers and, of these, 83% completed the assessment with ≥4 days of valid data. The implementation of accelerometers for physical activity measurement is currently ongoing at other ColoCare sites.
Physical fitness test:
Participants are asked to perform a physical fitness assessment at the 12, 24 and 36 month time point. This is an optional component of the study. All exercises are conducted, monitored and recorded by a certified exercise physiologist or trained study staff. The assessment consists of 9 different parts: six-minute walk, hand grip, 30-seconds chair stand, the timed up and go, core endurance, leg and chest strength tests, and active range of motion testing in shoulders and hips. Pilot testing of the physical fitness testing protocol at the Wellness Center at the Huntsman Cancer Institute is currently underway, and thus far n=8 patients have completed the physical fitness test. Once this pilot evaluation is complete, we will expand the physical fitness test to additional ColoCare sites.
Physical activity measurement by questionnaires:
All ColoCare questionnaires assess physical activity using the VITAL questionnaire, which contains questions on light, moderate and vigorous physical activities (19). Starting with the 6-month follow-up, the ColoCare questionnaires also contain a physical activity instrument adapted from the CHAMPS questionnaire (20). As of April 2018, 80% (range: 64% to 91% across sites) of our participants have competed baseline questionnaires. These proportions continue to increase.
Anthropometric assessment:
All ColoCare sites have implemented anthropometric measurements at baseline, 6, 12, and 24 months, technician-measured height, weight, waist and hip circumferences are obtained at an in person visit. If this is not possible, height and weight are abstracted from patients’ medical records or anesthesiology protocols; alternatively, self-measurement per procedures from the Iowa Women’s cohort is performed (21). The overall completion rate of anthropometric assessments at baseline is 80% (August, 2018).
Adipose tissue collection:
Visceral and subcutaneous adipose tissue are collected as described in section “Biospecimen collection and processing” and available for research on adipose tissue metabolomics, gene expression, and proteomics.
Radiological measures of abdominal fat deposition:
Abdominal fat deposition (visceral and subcutaneous fat distribution) is assessed by using multi-detector CT (computed tomography) imaging collected as part of standard of care for diagnostic purposes. The basic analysis of abdominal CT images is performed on a digital radiology workstation. Compartmental densitometry is used to quantify the total, visceral and subcutaneous fat area (TFA, VFA and SFA), and functional muscle mass on single axial slice of intervertebral space levels L3/4 and L4/5 by setting the CT attenuation level between 40 to 100 Hounsfield units (HU). These area-based assessments of VFA and SFA are strongly associated (r>0.93) with volumetric measurements of the entire abdominal adipose compartments (22).
Biospecimen collection and processing
Biospecimens are collected and stored at each ColoCare site to facilitate future biomarker analyses, such as (i) gene expression and methylation profiling in tumor, uninvolved mucosa and fat tissue, (ii) plasma/serum biomarkers that may be associated with colorectal cancer prognosis (e.g., 25-hydroxyvitamin D3 (25(OH)D3) and folate-dependent one-carbon metabolites), and (iii) urinary markers of oxidative stress (e.g., 7,8-dihydro-8-oxo-2’-deoxyguanosine). Collection of stool samples at multiple time points allows studying changes in gut microbiota before and after treatment. Standardized protocols for biospecimen collection are used at all sites.
All samples are processed according to harmonized protocols and standard operating procedures across all institutions. Blood, stool, urine and tissue samples [fresh frozen and formalin fixed (FFPE)] are collected at all sites. At the German site additionally fascia tissue from the incisional site is collected. During the process of sample collection information on sample acquisition, processing and storage, time of sample excision, and time of freezing are documented.
Blood:
Blood draws are performed at baseline as well as at 6, 12, 24, and at select sites also at 36, 48 and 60 months post-surgery. The serum, plasma and buffy coat (or cell rich interface) are divided into aliquots and stored at −80°C. We have successfully implemented remote collection procedures for study participants who are not able to come to the study site cancer centers for their follow-up visit.
Urine:
Spot urine is obtained at each study time point during a clinic visit. Urine is immediately aliquoted (1/2 of samples with vitamin C added) and stored at −80°C.
Stool:
Stool samples are collected at baseline, and from the 6 month follow-up time point. At the baseline time point, stool collection occurs 2–3 days prior to bowel preparation for surgery. A plain stool specimen, as well as a specimen in RNAlater is collected at the participant’s home, immediately frozen by the patient and brought to a visit or sent frozen to the lab with provided freeze packs. The stool samples are stored at −80°C.
Saliva:
Saliva samples are collected for microbial studies from all participants and serves as a source of germline DNA from participants who are not willing to provide blood samples for genetic studies. Saliva is collected in RNAlater and aliquoted and stored at −80°C.
Tissue:
Tumor, normal mucosa (adjacent and distant to tumor), visceral and subcutaneous adipose tissue are collected during surgery following the standard operating procedures across all ColoCare sites. At the German site, fascia tissue is additionally collected. All tissues are collected as fresh frozen and FFPE. Fresh frozen samples are collected, processed and frozen within 60 minutes of excision (cold ischemia). During the process of tissue collection, the following parameters are documented for each sample: Dates of sample acquisition, processing and storage, time of sample excision, time of freezing, length of fixation, surgical procedure, facility where performed, surgeon and staff involved. For normal tissue: distance from tumor and location in relation to the tumor (proximal or distal, adjacent or at resection margin) are recorded.
Biospecimen collection rates differ by time point and study center. This is due to the fact, that collection of urine, stool and saliva was implemented largely after U01 funding was received (FHCRC, Moffitt Cancer Center, Cedars-Sinai, Washington University in St. Louis and the University of Tennessee). Blood samples are currently available for 82% of patients at baseline, 61% of patients at the 6 month time point, and 65% of patients at the 12 month time point. Fresh frozen tissue is collected from about 50% of colorectal cancer patients undergoing surgery, while FFPE tissue is generally available.
Clinical outcomes
We have implemented standardized medical record abstraction forms to obtain detailed information on surgical procedures, treatments (e.g., adjuvant chemotherapy), toxicities, recurrences and second primaries. Information collected as part of these reviews includes detailed data on all colorectal cancer treatments (modality, timing and dose), pathologic features, tumor molecular testing and follow-up testing results to monitor for disease recurrence (e.g., CEA test and imaging).
Toxicity: The National Cancer Institute’s Common Toxicity Criteria for Adverse Events (CTCAE) methodology is used to grade treatment-related toxicity, using specific adverse events classified in broad categories and grading the severity of the adverse event (Grades 0 through 5). While clinicians themselves do not generally use the CTCAE grading to characterize toxicity, the definitions of CTCAE Grades 0–5 are specific enough to enable our abstractors to translate information available in the medical record into these categories.
Colorectal cancer recurrence and survival: We focus on three important clinical outcomes: (i) 2 and (ii) 5-year overall survival - which considers time of death, irrespective of cause, with appropriate censoring of loss to follow-up, (iii) recurrence loco-regional or distant metastases related to the same tumor, with censoring of deaths and loss to follow-up, and disease-free survival – which considers time to event (recurrence or death) with appropriate censoring for loss of follow-up. Detailed information on colorectal cancer recurrence is ascertained through reviews of patient medical records, pathology and imaging reports documenting the diagnosis of a recurrence. Medical record reviews are conducted at least two years post-diagnosis on all patients to ensure that any recurrences diagnosed within two years of diagnosis – the time frame over which the vast majority of colorectal cancer recurrences present (~80%) – are captured. Patients’ vital status, or survival, is obtained through local medical records, routine follow-up mailings, periodic requests for outside medical records, and state or national cancer and death registries. We initially review primary medical records for any signs that a patient is deceased, followed by review of outside medical records during follow-up, and any information received from routine follow-up mailings. Any informal reports of a patient’s status (e.g., family member reports) are confirmed through other data sources and additional data on date and cause of death obtained. Finally, we use patient information to search national and local data sources for vital status. In the U.S. these include the Social Security Death Index (SSDI), National Death Index (NDI), Cancer Surveillance System (CSS) Death Tapes in the state of Washington, state obituaries, and the Florida Cancer Data System (FCDS). In Germany, every person is registered and vital status information including date of death can be reliably obtained at no cost from the Registration Office.
Patient reported outcomes
Health-related quality of life is assessed by the validated and widely used core questionnaire QLQ-C30 and the colorectal cancer module QLQ-CR29, developed by the European Organization for Research and Treatment of Cancer (EORTC) (23)) at each study time point. Moreover, at baseline and all follow-up time points, we perform the MD Anderson Symptom Inventory (MDASI), a multi-symptom patient-reported outcome measure for clinical and research use (24). Chemotherapy-induced peripheral neuropathy is measured by the EORTC QLQ-CIPN20 (25). For measurement of patients’ physical and mental health the 12-item Short-Form (SF-12) health survey is used (26).
To assess the productivity impact of on-the-job work limitations due to employees’ physical or mental health problems from the 6 month follow-up the Work Limitation Questionnaire (WLQ) are performed (27). Additionally, the Work Productivity and Activity Impairment General Health (WPAI: GH) Questionnaire is used to ask about the effect of patients’ health problems on their ability to work and perform regular activities (28). To assess distress specific to cancer and its treatment the Cancer and Treatment Distress (CTXD) questionnaire are provided to patients (29) starting at 6 months after enrolment.
Power consideration and data analysis
The ColoCare Study provides a biorepository and resource for a multitude of research questions, including in collaboration with the other ColoCare sites, with at least 4,000 patients enrolled across the ColoCare Consortium by 2020. The study power will differ depending on the research question and the size of the study population included.
Future analyses will include cross sectional analyses of associations between biomarkers and health behaviors, longitudinal descriptive analyses of health behaviors over time. Repeated measures analyses will be applied to assess changes over time in lifestyle factors and molecular biomarkers in relation to recurrence and survival. Hazard ratios will be calculated for lifestyle factors and molecular characteristics at recruitment in relation to clinical outcomes.
RESULTS
To date in total we recruited >2,000 colorectal cancer patients across all ColoCare sites. Baseline characteristics of recruited ColoCare patients are given in Table 2.
Table 2:
Patient characteristics of the ColoCare Consortium as of April 30, 2018
| n=2,031 | Total % | ||
|---|---|---|---|
| Gender | |||
| Male | 1,197 | 59 | |
| Female | 834 | 41 | |
| Age | |||
| <50 | 358 | 18 | |
| 50–59 | 516 | 25 | |
| 60–69 | 603 | 30 | |
| ≥70 | 554 | 27 | |
| Race* | |||
| American Indian/Alaska Native | 16 | 1 | |
| Asian | 52 | 3 | |
| Native Hawaiian/Pacific Islander | 8 | 0 | |
| Black or African American | 96 | 5 | |
| White | 1,796 | 88 | |
| More than one race | 12 | 1 | |
| Other | 28 | 1 | |
| Unknown | 23 | 1 | |
| Ethnicity* | |||
| Not Hispanic | 1,918 | 94 | |
| Hispanic or Latino | 70 | 3 | |
| Unknown | 43 | 2 | |
| AJCC stage | |||
| I | 340 | 17 | |
| II | 482 | 24 | |
| III | 645 | 32 | |
| IV | 386 | 19 | |
| Missing** | 178 | 8 | |
| Tumor site | |||
| Colon | 1,014 | 50 | |
| Rectum | 955 | 47 | |
| Synchronous | 36 | 2 | |
| Missing** | 26 | 1 |
All ColoCare patients from Heidelberg are Caucasian. Racial composition of the cohort is expected to change due to increasing enrollment at ColoCare sites with a specific focus on minorities.
not abstracted yet.
AJCC=American Joint Committee on Cancer
Components of the ColoCare Cohort have been leveraged to support initial research; including on biologic determinants of colorectal cancer (e.g., tumor immunity, gut microbiome, epigenome), health behaviors (e.g., physical activity or dietary patterns), components of energy balance (e.g., adipose tissue distribution, accelerometry), clinical endpoints (e.g., surgical complications, quality of life), and novel biomarkers (proteomics, metabolomics, metagenomics). Furthermore, we have published on the interrelations between these factors, specifically in the context of colorectal cancer prognosis (18,30–48). Table 3 highlights research contributions using the growing ColoCare Study cohort.
Table 3:
Selected ColoCare publications
| Manuscript title | Year | Full author block | Journal | PMID/PMCID |
|---|---|---|---|---|
| Altered RECQ helicase expression in sporadic primary colorectal cancers | 2013 | Lao VV, Welsch P, Luo Y, Carter KT, Dzieciatkowski S, Dintzis S, Meza J, Sarvetnick NE, Monnat RJ, Loeb LA, Grady WM | Translational Oncology | 23908689/3730021 |
| NTRK3 is a potential tumor suppressor gene commonly inactivated by epigenetic mechanisms in colorectal cancer | 2013 | Luo Y, Kaz AM, Kanngurn S, Welsch P, Morris SM, Wang J, Lutterbaugh JD, Markowitz SD, Grady WM | PLoS Genetics | 23874207/3708790 |
| RET is a potential tumor suppressor gene in colorectal cancer | 2013 | Luo Y, Tsuchiya KD, Il Park D, Fausel R, Dannagurn S, Welsch P, Dzieciatkowski S, Wang J, Grady WM | Oncogene | 22751117/3465636 |
| Tumor-infiltrating lymphocytes in colorectal tumors display a diversity of T cell receptor sequences that differ from the T cells in adjacent mucosal tissue | 2013 | Sherwood A, Emerson R, Scherer D, Habermann N, Buck K, Staffa J, Desmarais C, Halama N, Jaeger D, Schirmacher P, Herpel E, Kloor M, Ulrich A, Schneider M, Ulrich C, Robins H | Cancer Immunotherapy and Immunology | 23771160/5714563 |
| Plasma 25-hydroxyvitamin D3, folate and vitamin B12 biomarkers among international colorectal cancer patients: a pilot study | 2013 | Ulrich CM, Toriola AT, Siegel E, Brenner H, Chang-Claude J, Abbenhardt C, Kotzmann J, Song X, Owen RW, Hoffmeister M, Becher H, Shibata D, Vickers K, Rush SK, Makar K, Würtele G, Haubner R, Sellers TA, Grady W | Journal of Nutritional Science | 25191595/4153124 |
| Differences in DNA methylation signatures reveal multiple pathways of progression from adenoma to colorectal cancer | 2014 | Luo Y, Wong CJ, Kaz AM, Dzieciatkowski S, Carter KT, Morris SM, Wang J, Willis JE, Makar KW, Ulrich CM, Lutterbaugh JD, Shrubsole MJ, Zheng W, Markowitz SD, Grady WM | Gastroenterology | 24793120/4107146 |
| Suitability of circulating miRNAs as potential prognostic markers in colorectal cancer | 2014 | Ristau J, Staffa J, Schrotz-King P, Gigic B, Makar KW, Hoffmeister M, Brenner H, Ulrich A, Schneider M, Ulrich CM, Habermann N | Cancer Epidemiology Biomarkers and Prevention | 25472670/5699859 |
| Potential of fecal microbiota for early-stage detection of colorectal cancer. | 2014 | Zeller G, Tap J, Voigt AY, Sunagawa S, Kultima JR, Amiot A, Brunetti F, Costea PI, Habermann N, Hercog R, Koch M, Luciani A, Mende DR, Schneider MA, Schrotz-King P, Staffa J, Tournigand C, Van Nhieu JT, Yamada T, Zimmermann J, Benes V, Kloor M, Ulrich CM, von Knebel Doeberitz M, Sobhani I, Bork P | Molecular Systems Biology | 25432777/4299606 |
| Changes in urinary metabolic profiles of colorectal cancer patients enrolled in a prospective cohort study (ColoCare) | 2015 | Liesenfeld D, Habermann N, Toth R, Owen R, Frei E, Böhm J, Schrotz-King P, Klika K, Ulrich CM | Metabolomics | 29250455/5730072 |
| Repeat physical activity measurement by accelerometry among colorectal cancer patients-feasibility and minimal number of days of monitoring | 2015 | Skender S, Schrotz-King P, Boehm J, Abbenhardt C, Gigic B, Chang-Claude J, Siegel EM, Steindorf K, Ulrich CM | BMC Research Notes | 26048683/4456792 |
| Methylight digital droplet PCR for detection and absolute quantification of infrequent methylated alleles | 2015 | Yu M, Carter KT, Makar KW, Vickers K, Ulrich CM, Schoen RE, Markowitz SD, Grady WM | Epigenetics | 26186366/4623055 |
| Discovery of novel plasma proteins as biomarkers for the development of incisional hernias after midline incision in patients with colorectal cancer: The ColoCare study | 2016 | Böhm J, Pianka F, Stüttgen N, Rho J, Gigic B, Zhang Y, Habermann N, Schrotz-King P, Abbenhardt-Martin C, Zielske L, Lampe PD, Ulrich A, Diener MK, Ulrich CM | Surgery | 27745870/5560863 |
| Genome-wide interaction analyses between genetic variants and alcohol consumption and smoking for risk of colorectal cancer | 2016 | Gong J, Hutter CM, Newcomb PA, Ulrich CM, Bien SA, Campbell PT, Baron JA, Berndt SI, Bezieau S, Brenner H, Casey G, Chan AT, Chang-Claude J, Du M, Duggan D, Figueiredo JC, Schrotz-King P, Seminara D, Slattery ML, Thibodeau SN, Thornquist M, Toth R, Wallace R, White E, Jiao S, Lemire M, Hsu L, Peters U; CCFR and GECCO | PLoS Genetics | 27723779/5065124 |
| CT-based compartmental quantification of adipose tissue versus body metrics in colorectal cancer patients | 2016 | Nattenmuller J, Hoegenauer H, Boehm J, Scherer D, Paskow M, Gigic B, Schrotz-King P, Grenacher L, Ulrich CM, Kauczor HU | European Radiology | 26852215/5587125 |
| Smoking is associated with hypermethylation of the APC 1A promoter in colorectal cancer: the ColoCare Study | 2017 | Barrow TM, Klett H, Toth R, Bohm J, Gigic B, Habermann N, Scherer D, Schrotz-King P, Skender S, Abbenhardt-Martin C, Zielskie L, Schneider M, Ulrich A, Schirmacher P, Herpel E, Brenner H, Busch H, Boerries M, Ulrich CM, Michels KB | Journal of Pathology | 28791728/5647242 |
| Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: the ColoCare Study | 2017 | Liesenfeld DB, Grapov D, Fahrmann JF, Salou M, Scherer D, Toth R, Habermann N, Böhm J, Schrotz-King P, Gigic B, Schneider M, Ulrich A, Herpel E, Schirmacher P, Fiehn O, Lampe JW, Ulrich CM | American Journal of Clinical Nutrition | 26156741/4515859 |
| Plasma 25-hydroxyvitamin D3 levels in colorectal cancer patients and associations with physical activity. | 2017 | Skender S, Böhm J, Schrotz-King P, Chang-Claude J, Siegel EM, Steindorf K, Owen RW, Ose J, Hoffmeister M, Brenner H, Ulrich CM | Nutrition and Cancer: An International Journal | 28094599/5587127 |
| Dynamic plasma microRNAs are biomarkers for prognosis and early detection of recurrence in colorectal cancer | 2017 | Yuan Z, Baker K, Redman MW, Wang L, Adams SV, Yu M, Dickinson B, Makar K, Ulrich N, Böhm J, Wurscher M, Westerhoff M, Medwell S, Moonka R, Sinanan M, Fichera A, Vickers K, Grady WM | British Journal of Cancer | 28809863/5674097 |
| Quantitative assessment of visceral obesity and postoperative colon cancer outcomes | 2017 | Ozoya OO, Siegel EM, Srikumar T, Bloomer AM, DeRenzis A, Shibata D | Journal of Gastrointestinal Surgery | 28101721/5560865 |
| Associations between dietary patterns and longitudinal quality of life changes in colorectal cancer patients – the ColoCare Study | 2017 | Gigic B, Boeing H, Toth T, Böhm J, Habermann N, Scherer D, Schrotz-King P, Abbenhardt-Martin C, Skender S, Brenner H, Chang-Claude J, Hoffmeister M, Syrjala S, Jacobsen PB, Schneider M, Ulrich A, Ulrich CM | Nutrition and Cancer: An International Journal | 1397707/5867188 |
| Associations of branched-chain amino acids with parameters of energy balance and survival in colorectal cancer patients: Results from the ColoCare Study | 2018 | Delphan M, Lin T, Liesenfeld DB, Nattenmüller J, Böhm JT, Gigic B, Habermann N, Zielske L, Schrotz-King P, Schneider M, Ulrich A, Kauczor HU, Ulrich CM, Ose J | Metabolomics | 29706852/5922458 |
| Robust prediction of gene regulation in colorectal cancer tissues from DNA methylation profiles | 2018 | Klett H, Balavarca Y, Toth R, Gigic B, Habermann N. Scherer D, Schrotz-King P, Ulrich A, Schirmacher P, Herpel E, Brenner H, Ulrich CM, Michels KB, Busch H, Boerries M | Epigenetics | 29697014 |
| Body fatness, adipose tissue compartments and biomarkers of inflammation and angiogenesis in colorectal cancer: the ColoCare Study | 2018 | Himbert C, Ose J, Nattenmueller J, Warby CA, Holowatyj AN, Böhm J, Lin T, Haffa M, Gigic B, Hardikar S, Scherer D, Zielske L, Schrotz-King P, Koelsch T, Siegel EM, Shibata D, Ulrich A, Schneider M, Hursting SD, Kauczor HU, Ulrich CM | Cancer Epidemiology Biomarkers and Prevention | Accepted September, 2018, in press |
| Implications of epigenetic drift in colorectal neoplasia | 2018 | Luebeck G, Hazelton W, Curtius K, Maden S, Yu M, Carter K, Burke W, Lampe P, Li C, Ulrich CM, Newcomb P, Westerhoff M, Kaz K, Luo Y, Inadomi J, Grady W | Cancer Research | Accepted September, 2018, in press |
| Evidence for association of NTHL1, BRCA2 and BRIP1 with the development of colorectal cancer and polyps | 2018 | Rosenthal EA, Shirts BH, Amendola LM, Horike-Pyne M, Robertson PD, Hisama FM, Bennett RL, Dorschner MO, Nickerson, DA, Stanaway IB, Nassir, R, Vickers KA, Li C, Grady WM, Peters U, Jarvik GP on behalf of the NHLBI GO Exome Sequencing Project | Human Genetics | Accepted September, 2018, in press |
To illustrate the breadth of research supported by ColoCare, we briefly describe some key findings. For example, Skender et al. showed that accelerometry is a feasible method to assess physical activity in colorectal cancer patients with three valid days of physical activity measurement sufficient for an accurate assessment; further, accelerometry-based vigorous and moderate-to-vigorous physical activities are positively associated with 25(OH)D3 levels (18,43). Nattenmüller et al. reported that the densitometric quantification of adipose tissue on CT is highly reproducible, visceral obesity is associated with metabolic comorbidities and with increased risk of recurrence in colon and rectal cancer (41,42,45). As one of the first studies, Liesenfeld et al. comprehensively assessed differences in metabolic, lipidomic, and transcriptomic profiles between paired human VAT and SAT and their association with colorectal tumor stage. They identified markers of inflammation in VAT, which supports prior evidence regarding the role of visceral adiposity and cancer (31). Ristau and Yuan et al. have also investigated miRNAs as prognostic biomarkers (33,49). We evaluated DNA methylation patterns, for example in relation to health behaviors (30,50) and contributed to a study that showed the potential of fecal microbiota for early-stage detection of colorectal cancer (34). Recently, Gigic et al. showed that patients following a “Western” diet had lower chances to improve in physical functioning, constipation and diarrhea after surgery, whereas patients following a “fruit & vegetable” diet showed improving diarrhea scores (4).
Additionally, the ColoCare Cohort participant’s data and samples have contributed to multiple international consortia, such as CORECT (ColoRectal Transdisciplinary Study), GAME-ON (Genetic Associations and Mechanisms in Oncology), GECCO (Genetics and Epidemiology of Colorectal Cancer Consortium), ISACC (The International Survival Analysis in Colorectal Cancer Consortium), COMETS (Consortium of Metabolomics Studies), the EDRN (Early Detection Research Network), and several European Consortia, e.g., MetaboCCC (Metabolomic profiles throughout the continuum of colorectal carcinogenesis) and FOCUS (Biomarkers related to folate-dependent metabolism in colorectal cancer recurrence and survival).
DISCUSSION
The ColoCare Study is unique in that it brings together a transdisciplinary team of clinicians, epidemiologists and laboratory scientists to address novel questions of colorectal cancer prognosis. Multiple promising biologic and epidemiologic characteristics can be studied with cutting-edge approaches that predict outcomes among colorectal cancer patients. Within the ColoCare Consortium at least 4,000 newly-diagnosed colorectal cancer patients will be recruited and followed over a period of 5 years.
Strengths and limitations
Unique and innovative aspects of the ColoCare cohort include: (a) multi-center standardized recruitment of patients at the time of surgery, enabling the collection of fresh-frozen specimens and pre-treatment blood samples, (b) standardized, harmonized and comprehensive clinical annotation, (c) uniform protocols for collection and processing of numerous biospecimens, including tumor tissue, mucosa, fat tissue, blood, urine, stool and saliva, (d) serial collection of specimens at defined post-diagnosis intervals to enable assessment of changes in biomarkers in prognosis, (e) detailed measurement of health behaviors, quality of life, and symptoms at defined intervals after surgery, (f) geographically and ethnically diverse population to enhance the applicability of the findings generated, and (g) minority research (18,51,52).
Nevertheless, some limitations should be noted. The gold standard method to evaluate the effects of factors on clinical outcomes is the randomized controlled trial (RCT). ColoCare is an observational, non-randomized study, which cannot proof causality. Still, the ColoCare Study design addresses important clinical questions in absence of RCT data as well as research questions not suitable for RCTs (e.g., on alcohol consumption, smoking, and other health behaviors) (53). While an RCT can generally only test one or few specific hypotheses, the multidimensional design of ColoCare allows for the simultaneous assessment of multiple factors relevant to colorectal cancer prognosis. We acknowledge the study population may not be fully representative of the general population of CRC patients in the U.S., however, at nearly all sites our participation rates are ranging between 70–94%, which illustrates that we capture a representative proportion of our cancer center patients. A limitation is that due to the extensive data and biospecimen collection over multiple time points, retention of participants is a challenge, the per-participant-investment is large and study-staff time required is substantial. Therefore, a variety of retention strategies have been implemented across ColoCare sites. These include 2–3 newsletters to the participants each year, birthday and holiday cards, raffle of gift certificates, invitations to cancer center events, as well as providing participants with a summary of their physical activity testing. A further challenge in long-term follow-up of cancer patients is their post-cancer identity. Colorectal cancer patients predominantly identify themselves as survivors and with growing time since diagnosis this patient group is less likely to participate in surveys/biospecimens collections that re-label them as cancer patients (54). The consortium is currently working on tailoring strategies to ensure follow-up rates for long-term survivors. Thus, the sample size is not as large as for other consortia that have focused on lower-involvement assessments (e.g., genetic testing) or are pooling existing data. Moreover, the consistency in protocols and assessments results in fully comparable data from a diverse set of recruitment sites.
Priorities for future use and research
The ColoCare design is observational, which is a critical first step in colorectal cancer prognosis research, in order to inform clinical trials, each of which can focus on only a single, primary factor at considerable expense. Strong, well-designed observational studies are essential to provide information on the best intervention to test in any trial. There are many strong hypotheses tested in this transdisciplinary research, most of which have not been/cannot be addressed in trials. The ColoCare Study design enables the analysis of novel, integrated research questions, novel components of energy balance in relation to colorectal cancer outcomes, the role of dietary supplements and integrative health, the role of the gut microbiome in colorectal cancer health and prognosis, as influenced by health behaviors, and the discovery of novel biomarkers for clinical outcomes to advance precision medicine. In all these areas, we will investigate differences by race/ethnicity as we will also be positioned to assess contributors to known colorectal cancer disparities.
The epidemiologic factors being assessed are highly relevant to Western populations since they are focused on drug and supplement use and obesity and energy metabolism. Likewise the molecular studies currently funded or planned will use cutting-edge technology to assess the cancer proteome and methylome and will integrate these results to determine functionally-relevant patterns of molecular changes. The incorporation of the molecular results with the epidemiologic studies will permit an understanding of how specific molecular biomarkers can be employed in relation to the clinical management of colorectal cancer patients to predict and minimize the likelihood of developing recurrent cancer.
Thus, the ColoCare Study will provide valuable insight into the effects of common, modifiable health behaviors on colorectal cancer biology (gene expression, epigenetic profile, proteomics, gut microbiome), patient well-being and clinical outcomes and unify the predictive and prognostic potential from cutting-edge molecular tumor characterization, gut microbiome, blood biomarkers, and epidemiology in one large patient cohort and risk model. We will focus on three important clinical outcomes: (i) 2 and (ii) 5-year overall survival, and (iii) disease recurrence. We also recognize, that follow-up beyond 5 years would be beneficial especially for younger patients. Further funding will be sought to achieve this. Together, the integrative nature of the ColoCare Study will provide a critical understanding of key factors that affect response to treatment, colorectal cancer recurrence and outcomes.
Ultimately, ColoCare Study investigators aim to identify key epidemiologic risk factors, methylation and proteomic signatures, as well as microbial patterns that influence patient survival and well-being. This research promises to lead to the discovery of cancer biomarkers that will help to optimize and personalize medical and surgical treatment of colorectal cancer and improve patient survival rates. In addition, we aim to discern new interconnections between health behaviors and molecular characteristics that will provide insight into cancer biology. We expect this study to contribute substantially to the discovery of cancer biomarkers that will help to optimize and personalize medical and surgical treatment of colorectal cancer and improve patients’ clinical outcomes. Together, this information will provide guidance for clinicians and patients on how to increase survival rates, manage treatment toxicities, and improve quality of life after colorectal cancer diagnosis.
ACKNOWLEDGEMENT
The authors thank all ColoCare Study participants and the ColoCare Study teams as multiple locations, specifically:
Fred Hutchinson Cancer Research Center: Drs. George McDonald, Johanna Lampe, Karen Makar, Meredith Hullar, Chris Velicer, and also Kathy Vickers, and Shannon Rush.
National Center for Tumor Diseases and University Hospital of Heidelberg: Drs. Petra Schrotz-King, Clare Abbenhardt-Martin, Nina Habermann, Dominique Scherer, Robert W. Owen, Romy Kirsten, Peter Schirmacher, Esther Herpel, Magnus von Knebel Doeberitz, Matthias Kloor, Hans-Ulrich Kauczor, Johanna Nattenmüller, Nikolaus Becker, Hermann Brenner, Jenny Chang-Claude, Michael Hoffmeister, Heiner Boeing, Stephanie Skender, Werner Diehl, and also Susanne Jakob, Judith Kammer, Lin Zielske, Anett Brendel, Marita Wenzel, Renate Skatula, Rifraz Farook, and Torsten Kölsch.
Huntsman Cancer Institute: Drs. Bartley Pickron, Lyen Huang, Sam Finlayson, John Swettenham, Courtney Scaife, Laura Lambert, Ignacio Garrido-Laguna, Mary Bronner, Eric Swanson, James Whisenant, John Weis, Ute Gawlick, Mark Lewis, June Round, Zac Stephenson, James Cox, Chris Fillmore, Dominik Ose, Kenneth Boucher, and also Debra Ma, Samir Courdy, Therese Berry, Tengda Lin, Caroline Himbert, Christy Warby, Elisa Santori, Candice Garcia, Bailee Rushton, Tyler Farr, Darren Walker, and Marissa Grande.
Moffitt Cancer Center: Drs. Stephanie Schmit, Paul Jacobsen, Domenico Coppola, Seth Felder, Julian Sanchez, Christine Pierce, Heather Jim and also Gazelle Rouhani, Maria Gomez, Kristen Maddox, Amanda DeRenzis, Gillian Trujillo, Amanda Bloomer, Paige Parkinson, Bianca Nguyen, Alina Hoehn, Bridget Riggs.
Cedars-Sinai Medical Center: Drs. Karen Zaghiyan, Zuri Murrell, Yosef Nasseri, Andrew Hendifar, David Hoffman, Beth Moore, Phillip Fleshner, Richard Tuli, Robert Decker, Solomon Hamburg, David Magner, Sepehr Rokhsar, Samuel Klempner, Joshua Ellenhorn, Thomas Sokol, Alexandra Gangi, Maryliza El-Masry, Eiman Firoozmand, and also Julissa Ramirez, Sarah Villeda, Cindy Miao, Blair Carnes, Dogra Khushi, Carissa Huynh, and Nathalie Nguyen.
Washington University School of Medicine: Drs. Matthew G. Mutch, Cory Bernadt, and also Sonya Izadi, Michelle Sperry, June Smith, and Esinam Wash.
Center for Cancer Research, Memphis: Drs. Justin Monroe, Scott Daugherty, Holly Hilsenbeck, Thomas O’Brien, Leah Hendrick, and also Nuzhat Ali, Meghana Karchi, and Demi Ajidhahun.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE AND FOR PUBLICATION
The ColoCare Study has been approved by the local institutional review boards serving each site. All participants signed a written informed consent form. This study was also registered to ClinicalTrials.gov (Identifier: NCT02328677, https://www.clinicaltrials.gov/).
Financial support
Cornelia M. Ulrich and Jennifer Ose are funded by the Huntsman Cancer Foundation and the National Cancer Institute projects R01 CA189184, R01 CA207371 and U01 CA206110. Dr. Ulrich is also funded by P30 CA042014. Biljana Gigic is funded by the Lackas Foundation, the ERA-NET on Translational Cancer Research (TRANSCAN) project 01KT1503 (Federal Ministry of Education and Research, Germany), the National Cancer Institute project R01 CA189184, and the Stiftung Lebensblicke. William M. Grady is funded by the Fred Hutchinson Cancer Research Center, the Seattle Translational Tumor Research Program, the Cottrell Family, and is supported by following grants: NIH grants P30CA015704, U01CA152756, R01CA194663, and R01CA220004. Dr. Siegel and ColoCare Moffitt were funded by the Florida Department of Health Bankhead Coley New Investigator Award (09BN-13) and the National Cancer Institute projects R01 CA189184, R01 CA207371 and U01 CA206110. ColoCare Moffitt has been supported in part by the Tissue Core Facility and the Survey Methods Core at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30 CA076292). Adetunji T. Toriola and Graham A. Colditz are supported by U01 CA206110 and the Alvin J. Siteman Cancer Center, supported in part by the NCI Cancer Center Support Grant P30 CA091842.
LIST OF ABBREVIATIONS
- AJCC
American Joint Committee on Cancer
- CHAMPS
Community Healthy Activities Model Program for Seniors
- COMETS
Consortium of Metabolomics Studies
- CORECT
ColoRectal Transdisciplinary Study
- CRC
Colorectal cancer
- CSS
Cancer Surveillance System
- CT
Computed tomography
- CTCAE
Common Toxicity Criteria for Adverse Events
- CTXD
Cancer and Treatment Distress
- DNA
Deoxyribonucleic acid
- EORTC QLQ
European Organization for Research and Treatment of Cancer Quality of Life Questionnaires
- FCDS
Florida Cancer Data System
- FFPE
Formalin-fixed paraffin-embedded
- FFQ
Food Frequency Questionnaire
- FOCUS
Biomarkers related to folate-dependent metabolism in colorectal cancer recurrence and survival
- GAME-ON
Genetic Associations and Mechanisms in Oncology
- GECCO
Genetics and Epidemiology of Colorectal Cancer Consortium
- HU
Hounsfield unit
- ICD
International Statistical Classification of Disease and Related Health Problems
- ISACC
International Survival Analysis in Colorectal Cancer Consortium
- MDASI
MD Anderson Symptom Inventory
- MetaboCCC
Metabolomic profiles throughout the continuum of colorectal carcinogenesis
- NDI
National Death Index
- NSAID
Non-steroidal anti-inflammatory drug
- RCT
Randomized controlled trial
- SAT
Subcutaneous adipose tissue
- SFA
Subcutaneous fat area
- SF-12
12-item Short-Form
- SSDI
Social Security Death Index
- TFA
Total fat area
- 25(OH)D3
25-hydroxyvitamin D3
- VAT
Visceral adipose tissue
- VFA
Visceral fat area
- VITAL
VITamin D and OmegA-3 TriaL
- WLQ
Work Limitation Questionnaire
- WPAI: GH
Work Productivity and Activity Impairment General Health
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
AVAILABILITY OF DATA AND MATERIALS
ColoCare Study data is available from the corresponding author on reasonable request and as described on the ColoCare website (https://uofuhealth.utah.edu/huntsman/labs/colocare-consortium/). Our data sharing procedures are evolving and will be updated online.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359–86 doi 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67(3):177–93 doi 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66(4):271–89 doi 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 4.Gigic B, Boeing H, Toth R, Bohm J, Habermann N, Scherer D, et al. Associations Between Dietary Patterns and Longitudinal Quality of Life Changes in Colorectal Cancer Patients: The ColoCare Study. Nutr Cancer 2017:1–10 doi 10.1080/01635581.2018.1397707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2005;23(34):8664–70 doi 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 6.Chien C, Morimoto LM, Tom J, Li CI. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer 2005;104(3):629–39 doi 10.1002/cncr.21204. [DOI] [PubMed] [Google Scholar]
- 7.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307(5):491–7 doi 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 8.Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev 2011;32(4):550–70 doi 10.1210/er.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardikar S, Newcomb PA, Campbell PT, Win AK, Lindor NM, Buchanan DD, et al. Prediagnostic Physical Activity and Colorectal Cancer Survival: Overall and Stratified by Tumor Characteristics. Cancer Epidemiol Biomarkers Prev 2015;24(7):1130–7 doi 10.1158/1055-9965.EPI-15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Blarigan EL, Meyerhardt JA. Role of Physical Activity and Diet After Colorectal Cancer Diagnosis. J Clin Oncol 2015;33(16):1825–34 doi 10.1200/JCO.2014.59.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arem H, Pfeiffer RM, Engels EA, Alfano CM, Hollenbeck A, Park Y, et al. Pre- and postdiagnosis physical activity, television viewing, and mortality among patients with colorectal cancer in the National Institutes of Health-AARP Diet and Health Study. J Clin Oncol 2015;33(2):180–8 doi 10.1200/JCO.2014.58.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romaguera D, Ward H, Wark PA, Vergnaud AC, Peeters PH, van Gils CH, et al. Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: a cohort study. BMC Med 2015;13:107 doi 10.1186/s12916-015-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elena JW, Travis LB, Simonds NI, Ambrosone CB, Ballard-Barbash R, Bhatia S, et al. Leveraging epidemiology and clinical studies of cancer outcomes: recommendations and opportunities for translational research. J Natl Cancer Inst 2013;105(2):85–94 doi 10.1093/jnci/djs473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 15.Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev 2007;16(11):2331–43 doi 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 16.Nothlings U, Hoffmann K, Bergmann MM, Boeing H. Fitting portion sizes in a self-administered food frequency questionnaire. J Nutr 2007;137(12):2781–6. [DOI] [PubMed] [Google Scholar]
- 17.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9(3):178–87. [DOI] [PubMed] [Google Scholar]
- 18.Skender S, Schrotz-King P, Bohm J, Abbenhardt C, Gigic B, Chang-Claude J, et al. Repeat physical activity measurement by accelerometry among colorectal cancer patients--feasibility and minimal number of days of monitoring. BMC Res Notes 2015;8:222 doi 10.1186/s13104-015-1168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White E, Patterson RE, Kristal AR, Thornquist M, King I, Shattuck AL, et al. VITamins And Lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol 2004;159(1):83–93. [DOI] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(9 Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 21.Folsom AR, Kaye SA, Prineas RJ, Potter JD, Gapstur SM, Wallace RB. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol 1990;131(5):794–803. [DOI] [PubMed] [Google Scholar]
- 22.Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond) 2010;34(4):781–7 doi 10.1038/ijo.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fayers P, Bottomley A, Group EQoL, Quality of Life U. Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer 2002;38 Suppl 4:S125–33. [DOI] [PubMed] [Google Scholar]
- 24.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 2000;89(7):1634–46. [DOI] [PubMed] [Google Scholar]
- 25.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer 2005;41(8):1135–9 doi 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- 27.Lerner D, Amick BC 3rd, Lee JC, Rooney T, Rogers WH, Chang H, et al. Relationship of employee-reported work limitations to work productivity. Med Care 2003;41(5):649–59 doi 10.1097/01.MLR.0000062551.76504.A9. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Bansback N, Boonen A, Young A, Singh A, Anis AH. Validity of the work productivity and activity impairment questionnaire--general health version in patients with rheumatoid arthritis. Arthritis Res Ther 2010;12(5):R177 doi 10.1186/ar3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syrjala KL, Yi JC, Langer SL. Psychometric properties of the Cancer and Treatment Distress (CTXD) measure in hematopoietic cell transplantation patients. Psychooncology 2016;25(5):529–35 doi 10.1002/pon.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu M, Carter KT, Makar KW, Vickers K, Ulrich CM, Schoen RE, et al. MethyLight droplet digital PCR for detection and absolute quantification of infrequently methylated alleles. Epigenetics 2015;10(9):803–9 doi 10.1080/15592294.2015.1068490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liesenfeld DB, Grapov D, Fahrmann JF, Salou M, Scherer D, Toth R, et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: the ColoCare study. Am J Clin Nutr 2015;102(2):433–43 doi 10.3945/ajcn.114.103804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo Y, Wong CJ, Kaz AM, Dzieciatkowski S, Carter KT, Morris SM, et al. Differences in DNA methylation signatures reveal multiple pathways of progression from adenoma to colorectal cancer. Gastroenterology 2014;147(2):418–29 e8 doi 10.1053/j.gastro.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ristau J, Staffa J, Schrotz-King P, Gigic B, Makar KW, Hoffmeister M, et al. Suitability of circulating miRNAs as potential prognostic markers in colorectal cancer. Cancer Epidemiol Biomarkers Prev 2014;23(12):2632–7 doi 10.1158/1055-9965.EPI-14-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeller G, Tap J, Voigt AY, Sunagawa S, Kultima JR, Costea PI, et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol Syst Biol 2014;10:766 doi 10.15252/msb.20145645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu JJ, Druta M, Shibata D, Coppola D, Boler I, Elahi A, et al. Metabolic syndrome and colorectal cancer: is hyperinsulinemia/insulin receptor-mediated angiogenesis a critical process? J Geriatr Oncol 2014;5(1):40–8 doi 10.1016/j.jgo.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lao VV, Welcsh P, Luo Y, Carter KT, Dzieciatkowski S, Dintzis S, et al. Altered RECQ Helicase Expression in Sporadic Primary Colorectal Cancers. Transl Oncol 2013;6(4):458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulrich CM, Toriola AT, Siegel EM, Brenner H, Chang-Claude J, Abbenhardt C, et al. Plasma 25-hydroxyvitamin D3, folate and vitamin B12 biomarkers among international colorectal cancer patients: a pilot study. J Nutr Sci 2013;2:e9 doi 10.1017/jns.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherwood AM, Emerson RO, Scherer D, Habermann N, Buck K, Staffa J, et al. Tumor-infiltrating lymphocytes in colorectal tumors display a diversity of T cell receptor sequences that differ from the T cells in adjacent mucosal tissue. Cancer Immunol Immunother 2013;62(9):1453–61 doi 10.1007/s00262-013-1446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo Y, Tsuchiya KD, Il Park D, Fausel R, Kanngurn S, Welcsh P, et al. RET is a potential tumor suppressor gene in colorectal cancer. Oncogene 2013;32(16):2037–47 doi 10.1038/onc.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo Y, Kaz AM, Kanngurn S, Welsch P, Morris SM, Wang J, et al. NTRK3 is a potential tumor suppressor gene commonly inactivated by epigenetic mechanisms in colorectal cancer. PLoS Genet 2013;9(7):e1003552 doi 10.1371/journal.pgen.1003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark W, Siegel EM, Chen YA, Zhao X, Parsons CM, Hernandez JM, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg 2013;216(6):1070–81 doi 10.1016/j.jamcollsurg.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nattenmueller J, Hoegenauer H, Boehm J, Scherer D, Paskow M, Gigic B, et al. CT-based compartmental quantification of adipose tissue versus body metrics in colorectal cancer patients. Eur Radiol 2016;26(11):4131–40 doi 10.1007/s00330-016-4231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skender S, Bohm J, Schrotz-King P, Chang-Claude J, Siegel EM, Steindorf K, et al. Plasma 25-Hydroxyvitamin D3 Levels in Colorectal Cancer Patients and Associations with Physical Activity. Nutr Cancer 2017;69(2):229–37 doi 10.1080/01635581.2017.1265131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohm J, Pianka F, Stuttgen N, Rho J, Gigic B, Zhang Y, et al. Discovery of novel plasma proteins as biomarkers for the development of incisional hernias after midline incision in patients with colorectal cancer: The ColoCare study. Surgery 2017;161(3):808–17 doi 10.1016/j.surg.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozoya OO, Siegel EM, Srikumar T, Bloomer AM, DeRenzis A, Shibata D. Quantitative Assessment of Visceral Obesity and Postoperative Colon Cancer Outcomes. J Gastrointest Surg 2017;21(3):534–42 doi 10.1007/s11605-017-3362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenthal EA, Shirts BH, Amendola LM, Horike-Pyne M, Robertson PD, Hisama FM, Bennett RL, Dorschner MO, Nickerson DA, Stanaway IB, Nassir R, Vickers KA, Li C, Grady WM, Peters U, Jarvik GP on behalf of the NHLBI GO Exome Sequencing Project. (in press) Evidence for association of NTHL1, BRCA2 and BRIP1 with the development of colorectal cancer and polyps. Human Genetics 2018. [Google Scholar]
- 47.Luebeck G, Hazelton W, Curtius K, Maden S, Yu M, Carter K, Burke W, Lampe P, Li C, Ulrich CM, Newcomb P, Westerhoff M, Kaz K, Luo Y, Inadomi J, Grady WM. (in press) Implications of epigenetic drift in colorectal neoplasia. Cancer Research 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Himbert C, Ose J, Nattenmueller J, Warby CA, Holowatyj AN, Böhm J, Lin T, Haffa M, Gigic B, Hardikar S, Scherer D, Zielske L, Schrotz-King P, Koelsch T, Siegel EM, Shibata D, Ulrich A, Schneider M, Hursting SD, Kauczor HU, Ulrich CM. (in press) Body fatness, adipose tissue compartments and biomarkers of inflammation and angiogenesis in colorectal cancer: the ColoCare Study. Cancer Epidemiology Biomarkers & Prevention 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan Z, Baker K, Redman MW, Wang L, Adams SV, Yu M, et al. Dynamic plasma microRNAs are biomarkers for prognosis and early detection of recurrence in colorectal cancer. Br J Cancer 2017;117(8):1202–10 doi 10.1038/bjc.2017.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrow TM, Klett H, Toth R, Bohm J, Gigic B, Habermann N, et al. Smoking is associated with hypermethylation of the APC 1A promoter in colorectal cancer: the ColoCare Study. J Pathol 2017;243(3):366–75 doi 10.1002/path.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benson AB 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al. Localized colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11(5):519–28. [DOI] [PubMed] [Google Scholar]
- 52.Benson AB 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11(2):141–52; quiz 52. [DOI] [PubMed] [Google Scholar]
- 53.Boyko EJ. Observational research--opportunities and limitations. J Diabetes Complications 2013;27(6):642–8 doi 10.1016/j.jdiacomp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park CL, Zlateva I, Blank TO. Self-identity after cancer: “survivor”, “victim”, “patient”, and “person with cancer”. J Gen Intern Med 2009;24 Suppl 2:S430–5 doi 10.1007/s11606-009-0993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]