Abstract
UVA irradiation is known to cause photoaging via production of reactive oxygen species (ROS) and activation of inflammatory processes. Previously, we have demonstrated that baicalin, a plant-derived flavonoid possessing both antioxidant and anti-inflammatory activity, protects mouse keratinocytes against damage from UVB irradiation. However, the role of baicalin in vivo has not been well studied, particularly in the setting of UVA irradiation. To explore the protective effects and mechanisms of baicalin treatment in mice after UVA irradiation, mice were exposed to acute and chronic doses of UVA irradiation with or without baicalin or vehicle. Skin samples were collected for histological staining, RNA isolation, flow cytometry, and protein extraction. Our results demonstrate the protective effect of baicalin against UVA-induced oxidative damage and inflammation in mouse skin. These effects are likely mediated via the TLR4 pathway, which may serve as a target for photochemoprevention against skin inflammation.
Keywords: baicalin, ultraviolet A radiation, inflammation, TLR4, immune system
Text for graphical abstract
The protective effect of baicalin treatment against UVA-induced oxidative damage and inflammation in mice skin via upregulation of IL-12 and IL-23 cytokines. These effects are likely mediated via inhibition of TLR4 pathway, which may serve as a target for prevention against skin inflammation by baicalin.
Introduction
Solar UV radiation can be categorized as UVA, -B, and –C; UVA represents the most abundant component, has the longest wavelength, and can penetrate the dermal layer of the skin. While UVB is known to directly damage DNA through the formation of cyclobutane pyrimidine dimers (CPD) and other bulky adducts, UVA is generally believed to cause DNA damage through more indirect methods (1, 2). Specifically, UVA induces the formation of reactive oxygen species (ROS), which target guanine residues in DNA, leading to additional mutated photoproducts. While these are thought to be the predominant mechanisms of DNA damage, respectively, both types of radiation can cause changes which ultimately lead to formation of reactive oxygen species, inflammatory products, and DNA mutations (1–3). In addition to these effects, UVA radiation is known to have immunosuppressive effects on multiple arms of the immune system. Interestingly, it has been shown to down-regulate activity of T cells in skin-draining lymph nodes, as well as the function of memory T cells (4, 5).
Toll-like receptors (TLR) are a family of 13 well-recognized ligand-binding receptors which initiate innate immune responses by recognizing pathogen-associated molecular patterns (PAMPs), ranging from bacterial and fungal cell wall components to microbial nucleic acids and lipoproteins (3, 6). These responses then further serve to prime adaptive immune responses through a variety of signaling and transcriptional mechanisms. Recent research has shown that TLRs are widely expressed on tumor cells and may be critical for the initiation and progression of malignancy. TLRs may be necessary to provide a stable microenvironment for cancer cell proliferation and evasion from immune responses, but they also are needed to launch an anti-tumor immune response (3). Our previous studies have shown that TLR4 mediates UVB-induced DNA damage and immunosuppression in a preclinical mouse model (7, 8). However, the effects of TLR4 in UVA-Induced inflammation requires further investigation.
Recent attention in treatment of photodamage and inflammation has shifted to naturally-occurring compounds as a less toxic and less invasive alternative to reduce the damage of UVA irradiation (9). One such naturally-occurring compound is baicalin, a plant-based flavonoid isolated from the roots of Scutellaria lateriflora (traditional name Huang-Qin), a common Chinese herbal medicine. Studies have shown that Baicalin is capable of reducing UV-mediated CPD formation and ROS production, both CPD and ROS result in DNA damage (10, 11). In previous studies, we have shown that baicalin effectively protected murine keratinocytes from UVB-induced oxidative stress, DNA damage, and inflammatory changes through modulation of the TLR4 pathway, specifically, by inhibiting TLR4 and its downstream signaling molecules, MyD88, TRIF, and TRAF6. Inhibiting this pathway also resulted in downregulation of NF-κB inactivation (3). However, the role of baicalin in protecting murine skin from UVA-induced photodamage remains to be investigated. The objective of the present study was to investigate the effects of baicalin in UVA-induced photodamage through its modulation of the TLR4 pathway in vivo.
Materials and Methods
Animals and reagents.
Female wild-type (WT) mice on a C3H/HeN background, 6–8 weeks of age, were purchased from Charles River laboratories (Wilmington, MA). All animal procedures were performed according to National Institutes of Health guidelines under protocols approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
UVA light source.
The dorsal skin of mice was shaved using clippers before exposure to UVA radiation. UVA was administered accordingly from a bank of four UVA lamps (Daavlin, UVA/UVB Research Irradiation Unit, Bryan, OH). The irradiation unit was equipped with an electronic controller to regulate UVA dosage at the fixed distance of 24 cm from the lamps to the dorsal skin surface of the mice. Wavelengths <290 nm were filtered out using Kodacel cellulose film (Eastman Kodak Co., Rochester, NY). The majority of the resulting wavelengths were in the UVA (320–400; 20%) range.
Irradiation and treatment of mice.
Female C3H/HeN mice, 6 to 8 weeks old, were used for all experiments. A uniform formulation of baicalin with a hydrophilic cream (Fougera and Co., Melville, NY), which is prescribed for external use as an ointment or cosmetic base, was prepared. For experiments involving assessment of the effects of short-term UVA exposure on TLR4 based signaling, mice were first treated topically with baicalin formulation (4 mg baicalin per mouse) 3 days prior to UVA exposure. The mice were subsequently exposed to a single dose of UVA (25 Joules/m2). The mice were treated again with a single dose of baicalin formulation (4 mg baicalin per mouse) just after UVA exposure, and then sacrificed after 24 hours. For experiments involving chronic UVA exposure, mice were exposed to UVA (6 J/m2) for 25 weeks followed by treatment with baicalin formulation or vehicle (4 mg baicalin per mouse) every other day for 30 days.
Preparation of skin cell suspensions.
Single cell suspensions were prepared from dorsal skin as previously described (12), and skin tissue was sliced into smaller pieces and digested for 1.5 hours at 37 °C with a solution of RPMI + 10mM Hepes + 0.05% DNase I (Sigma-Aldrich) + 0.27% collagenase type XI (Sigma-Aldrich) + 0.027% hyaluronidase type IV-S (Sigma-Aldrich).
Antibodies and flow cytometry.
Fluorochrome-conjugated antibodies to CD11b, IA/IE Ab, Gr1(Ly6C), IL12p35, TLR4, IL23p19, and IL-12/23p40 were purchased from Thermofisher (Waltham, MA) or BioLegend (San Diego, CA).
Flow cytometric analysis of CD11b+Gr1+ myeloid cells and TLR4 expression on CD11b+MHCII+cells.
Skin cell suspension from baicalin and vehicle treated mice were prepared and CD11b+Gr1+ myeloid cells were analyzed using APC labeled CD11b and PE labeled Gr1 antibodies (BD Biosciences). The percentage of cells that expressing high levels of both CD11b and Gr1 were analyzed in a flow cytometer (BD Flow cytometer (San Diego, CA), and the data were analyzed using FlowJo software v10.5.0. Rat IgG2B-APC and rat IgG2A-PE antibodies were used as isotype controls (Thermofisher Scientific Waltham, MA). The skin cell suspensions were also stained with APC labeled CD11b, FITC labeled MHCII and PE labeled TLR4 and analyzed using Flow cytometry.
Flow cytometric analysis of IL12 and IL23 cytokines produced by CD11b+MHCii+ skin cells.
For analysis of cytokines produced by CD11b cells, mice were sacrificed 24 hr post baicalin treatment and digested using the protocol (12). Cells were counted and 2X106cells per group were stimulated with PMA (50ng/ml) and Ionomycin (250ng/ml) for 5 hr in the presence of brefeldin A (10ug/ml) for intracellular cytokine staining. Staining profiles were obtained using flow cytometer.
Preparation of tissues for western blotting and real time PCR.
Lysates were prepared from skin samples of mice as described earlier (Ahmad et al., 2014). For western blot analysis, 30μg protein was loaded in each well and was resolved on 10–12% SDS-polyacrylamide gel. The proteins were subsequently transferred to nitrocellulose membrane, which was then incubated in blocking buffer for 2h followed by incubation with primary antibodies in blocking buffer for 2h at room temperature or overnight at 4oC. The membrane was then washed with TBS-T and incubated with secondary antibody conjugated with horseradish peroxidase. Protein bands were visualized using an enhanced chemiluminescence detection system (Amersham Life Science, Inc., Piscataway, NJ). To verify equal protein loading and transfer of proteins from gel to membrane, the blots were stripped and re-probed for the loading control, β-actin.
Total RNA from skin and tumor samples was extracted by using Trizol reagent (Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. cDNA was synthesized from 1 μg RNA using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Using iQ™ SYBR Green Master Mix (Bio-Rad, Hercules, CA), cDNA was amplified by real-time PCR with a Bio-Rad MyiQ thermocycler and SYBR Green detection system (Applied Biosystem, Foster City, CA). The standard PCR conditions were 95°C for 10 min and then 40 cycles at 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. For mRNA analysis, the calculations for determining the relative level of gene expression were made using the cycle threshold (Ct) method. The mean Ct values from duplicate measurements were used to calculate the expression of the target gene (Table 1) with normalization to a housekeeping gene used as internal control and using the formula 2-ΔΔCT.
Table 1:
Primer sequences used in reverse transcription–polymerase chain reaction.
| Gene | Primer Sequence | References |
|---|---|---|
| GAPDH | 5′-AACTTTGGCATTGTGGAAGG-3′ 5′-ACACATTGGGGGTAGGAACA-3′ |
(29) |
| TLR4 | 5′-GCAATGTCTCTGGCAGGTGTA-3′ 5′-CAAGGGATAAGAACGCTGAGA-3′ |
(30) |
| MyD88 | 5′-ATCCGAGAGCTGGAAACG-3′ 5′-GCAAGGGTTGGTTAATC-3′ |
(31) |
| TIRAP | 5′-AGTGCTGTACCATCGACCTGCTG-3′ 5′-TTCCCCTTCTCCCTCCTGTAGTAG-3′ |
(32) |
| iNOS | 5′-TACTCCACCAACAATGGCAA-3′ 5′-ATAGCGGATGAGCTGAGCAT-3′ |
(33) |
| MMP1 | 5′-GCTAACCTTTGATGCTATAACTACGA-3′ 5′-TTTGTGCGCATGTAGAATCTG-3′ |
(25) |
| MMP9 | 5′-GGGAAGATGCTGTTCA-3′ 5′-TCAACTCACTCCGGGAACTC-3′ |
(25) |
| IL-1β | 5′-CCTTCCTACCCCAATTTCCA-3′ 5′-CGCACTAGGTTTGCCGAGTA-3′ |
(34) |
| IL12p35 | 5′-AAATGAAGCTCTGCATCCTGC-3′ 5′-TCACCCTGTTGATGGTCACG-3′ |
(35) |
| IL23p19 | 5′-CCAGCAGCTCTCTCGGAATC-3′ 5′-GATTCATATGTCCCGCTGGTG-3′ |
(36) |
| IL12p40 | 5′-GGAAGCACGGCAGCAGAATA-3′ 5′-AACTTGAGGGAGAAGTAGGAATGG-3′ |
(37) |
| IL10 | 5′-ATTTGAATTCCCTGGGTGAGAAG-3′ 5′-CACAGGGGAGAAATCGATGACA-3′ |
(38) |
| Cox-2 | 5′- AAGACTTGCCAGGCTGAACT-3′ 5′- CTTCTGCAGTCCAGGTTCAA-3′ |
(39) |
Histological staining.
The dorsal skin of mice was removed, fixed in 10% formalin and embedded in paraffin. Samples were cut into 4 micron sections and hematoxylin and eosin (H&E) staining was performed. Slides were imaged using a Keyence BZ-X710 microscope. Epidermal thickness was measured using ImageJ software.
Statistical analysis.
The differences between experimental groups were analyzed using the Student’s t-test. A simple one way ANOVA test was employed for analysis between the groups. In all cases, a p<0.05 was considered significant.
Results
Baicalin improves the immune response to UVA irradiation
To understand the role of baicalin following UVA exposure, C3H/HeN mice were first treated with baicalin (4mg/mouse) 3 days prior to UVA exposure. Mice were subsequently exposed to a one-time dose of UVA (25 Joules/m2), treated again with the same dose of baicalin, and then sacrificed after 24 hours. The skin of these animals was collected and H&E staining was performed (Figure 1A–E). Histological analysis showed that, while epidermal thickness was significantly increased after UV exposure, baicalin had no effect on epidermal thickness after a single dose of UVA (Figure 1F). While no morphologic changes were identified, flow cytometry was performed next to analyze baicalin’s effect on immune infiltrates.
Figure 1. Treatment with baicalin decreases skin thickness in mice exposed to acute UVA irradiation.
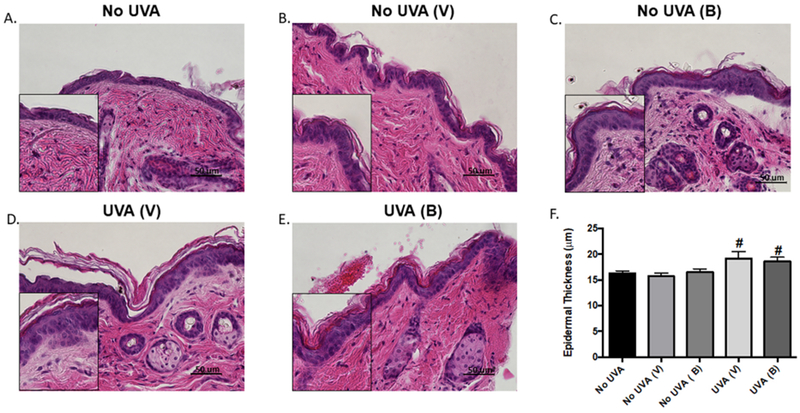
(A-E) Representative skin images (x400) of control, UVA-exposed, and baicalin-treated UVA exposed mice. (F) Quantification of epidermal thickness. Data are expressed as mean ± SEM. #P<0.05, compared to non-UV exposed control; *P<0.05, compared to UV-exposed, vehicle-treated group. N=5; experiment was repeated twice.
Flow cytometry indicated that CD11b+Gr1+ myeloid-derived suppressor cells (MDSC) were significantly reduced in baicalin-treated mice after UVA exposure (Figure 2). The expression level of TLR4 on CD11b+ were also significantly reduced in baicalin treated mice followed by UVA exposure (Figure 2).
Figure 2. Baicalin decreases CD11b+ Gr1+ myeloid cell and TLR4 expression on CD11b population in UVA irradiated mice.
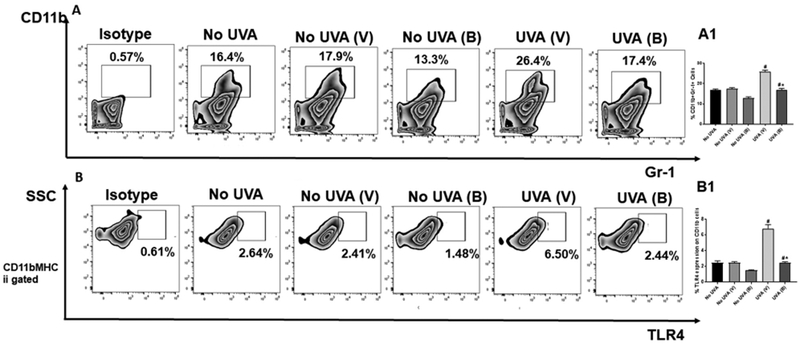
Mice were sacrificed 24hr post UVA exposure, single cell suspensions of skin (dermal/epidermal) were prepared from dorsal skin. A. Cells were stained with anti-mouse CD11b-APC and anti-mouse Gr-1-PE antibodies. The number of CD11b+Gr1+ cells, was analyzed by flow cytometry. A1. There were significantly less percentage of Gr1+CD11b+ myeloid cells (**p<0.01) in the skin of mice treated with baicalin than in vehicle treated mice. The histograms depict the mean ± SD of cell percentages per group. Mice not exposed to UVA radiation were used as controls (No UVA). B. The expression of TLR4 was also determined on the surface of CD11b+MHCII+ cells using flow cytometry. B1. There were significantly less percentage of TLR4 expression (**p<0.01) in the skin of mice treated with baicalin than in vehicle treated mice. The histograms depict the mean ± SD of cell percentages per group. Mice not exposed to UVA radiation were used as controls (No UVA). N=5; experiment was repeated twice.
In addition, flow cytometry showed that levels of IL12 p35, IL12 p40, and IL-23p19 subunits were significantly increased (Figure 3). These results suggest that baicalin upregulates the immune response and downregulates immune inhibition following UVA exposure. However, expression levels of inflammatory signals were also found to be reduced. Overall, the data show that, with single exposure of UVA, baicalin can improve the immune response, while reducing the inflammatory response of UV irradiation.
Figure 3. Treatment of mice irradiated with UVA with baicalin results in an increase in IL-12 and IL-23 cytokines.
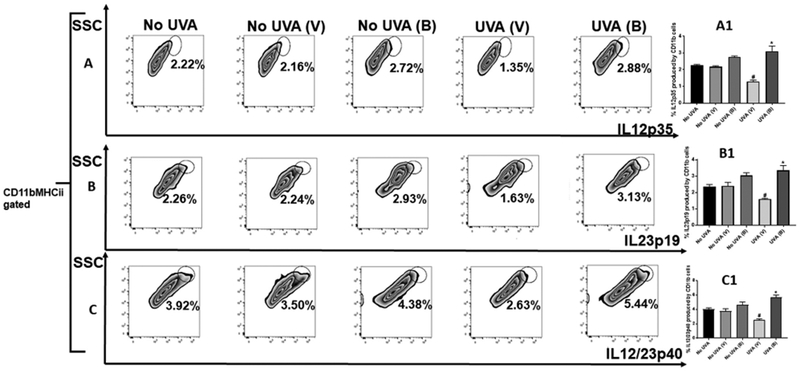
Mice were sacrificed after 24hr post UVA exposure, single cell suspensions of skin (dermal/epidermal) were prepared from dorsal skin. Cells were counted and 2X106 cells per group were stimulated with PMA (50ng/ml) and ionomycin (250ng/ml) for 5 hr in the presence of brefeldin A (10ug/ml) for intracellular cytokine staining 3A-C. Cells were stained with anti-CD11b, anti-MHCII and anti-IL12p35, IL23p19 and IL12/23 p40 antibodies and analyzed by flow cytometry. A1-C1. There were significantly higher percentage IL-12p35, IL-23p19 and IL-12/23p40 cytokines (**p<0.01) in the skin of mice treated with baicalin than in vehicle treated mice. The histograms depict the mean ± SD of cell percentages per group. N=5; experiment was repeated twice.
Histopathologic assessment of mice treated with baicalin after chronic UVA exposure
In order to assess the long-term effects of UVA irradiation, C3H/HeN mice were chronically exposed to UVA over 25 weeks. After UV exposure, mice were treated with baicalin or vehicle every other day for 30 days. H&E staining was performed on the skin of these mice and assessment of epidermal thickness showed a significant increase in UV-exposed mice. In addition, epidermal thickness was significantly reduced in the UV-exposed mice treated with baicalin compared with those treated with vehicle (Figure 4), suggesting baicalin protects against UV-induced skin thickening.
Figure 4. Treatment with baicalin decreases skin thickness in mice exposed to chronic UVA irradiation.
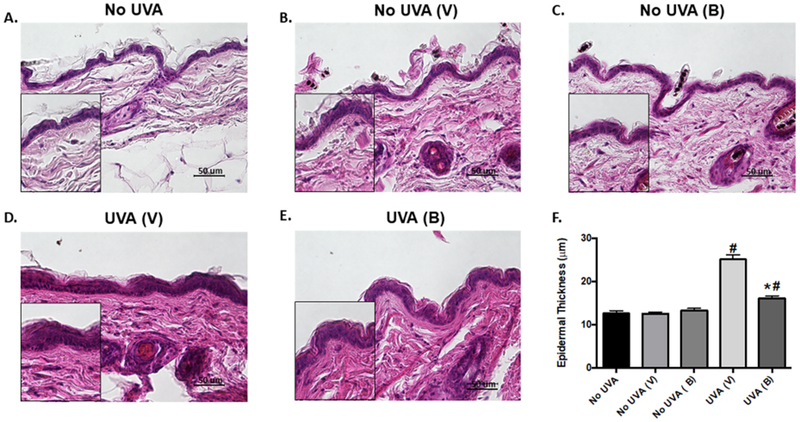
(A-E) Representative skin images (x400) of control, UVA-exposed, and baicalin-treated mice. (F) Quantification of epidermal thickness. Data are expressed as mean ± SEM. #P<0.05, compared to non-UV exposed control; *P<0.05, compared to UV-exposed, vehicle-treated group. N=5; experiment was repeated twice.
Baicalin promotes a protective response in chronically UVA-exposed mice
To elucidate the anti-inflammatory effects of baicalin, we analyzed the expression levels of TLR4 and subsequent pathway mediators via PCR and Western blot. Similar to earlier flow cytometry findings, the level of TLR4 was reduced significantly in chronically UVA-irradiated mice treated with baicalin versus their respective vehicle-treated controls. Further downstream, the protein expression of MyD88, IRAK4 and TRAF6 were all significantly downregulated in the irradiated baicalin-treated group compared to its respective vehicle-treated controls, while mRNA expression of MyD88 and TIRAP were also decreased (Figure 5). These results demonstrate that baicalin exerts its anti-inflammatory effects via modulation of TLR4 signaling. In addition, regulation of cyclooxygenase-2 (COX2) and IL1β were accordingly reduced as a result of the lower TLR4 expression, apparent by decreased expression levels of each (Figure 5). The expression of p47phox, a component of NADPH oxidase production of ROS that is regulated by TLR4, was also significantly decreased in UV-exposed mice after baicalin treatment. To determine the effects of baicalin on the immune response in mice after chronic UVA exposure, we assessed the expression level of various cytokines. Levels of IL12 p35 subunit and IL12 p40 subunit were increased, consistent with flow cytometry results from the short-term mice. In addition, the level of IL23 p19 subunit was also appropriately increased. These results are further suggestive that baicalin helps to stimulate the immune system in response to UVA irradiation.
Figure 5. Baicalin treatment post chronic UVA radiation causes decrease in expression of TLR4 pathway and inflammatory biomarkers.
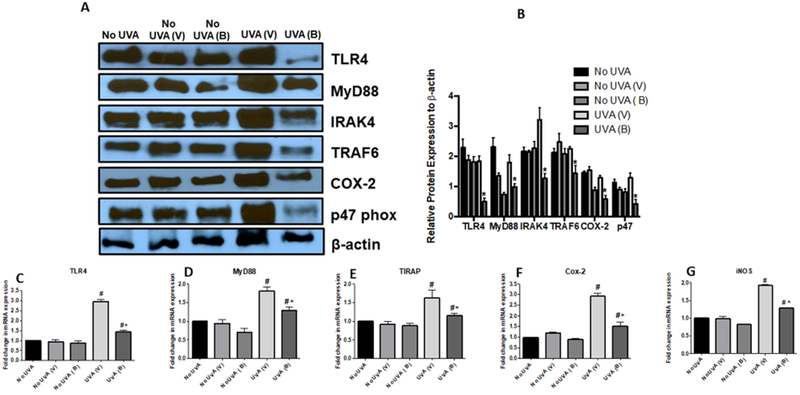
Skin lysates were prepared from mice sacrificed at the termination of the experiment as described in the Materials and Methods section. A. Expression levels of TLR4, MyD88, IRAK4, TRAF6, COX-2, p47 were determined by western blot analysis in the skin lysates of baicalin and vehicle treated, and from unexposed and exposed skin mice. B. Bar diagram shows densitometric analysis of each band in the western blot. C-G. Expression level of TLR4, MyD88, TIRAP, COX-2 and iNOS were also determined by RT PCR as described in Materials and Methods section. There was a significant decrease in expression levels of TLR4, MyD88, TIRAP, COX-2, and iNOS (*p<0.05), in the skin of baicalin treated mice compared to the vehicle treated mice. N=5; experiment was repeated twice.
UVA damage additionally causes destruction of collagen in the skin, as mediated by matrix metalloproteinases (MMPs). To further investigate this, we assessed expression levels of MMP-1 and MMP-9, two enzymes that are stimulated by UVA exposure, and found that the levels of each were reduced in UV-exposed mice treated with baicalin compared to those with vehicle (Figure 6).
Figure 6. Baicalin treatment results in an increase of IL-12 and IL-23 cytokines followed by decrease in proinflammatory markers.
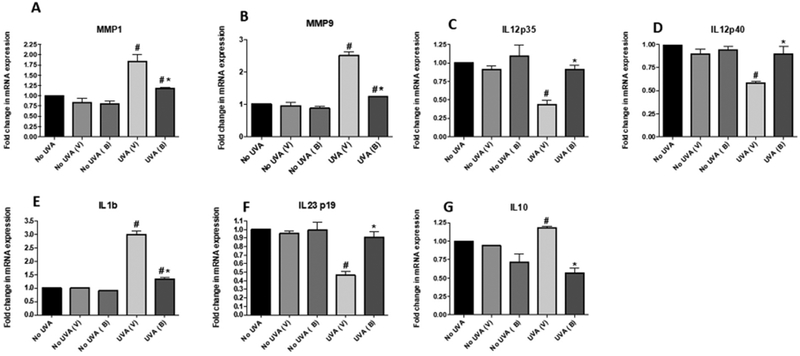
Mice were sacrificed at the termination of the experiment and skin and tissue lysates were prepared from mice. The expression levels (A) MMP1, (B) MMP9, (C) IL-12p35, (D) IL-12/23 p40, (E) IL-1β, (F) IL-23p19 and IL10 were determined using RT PCR as described in Materials and Methods sections. Data are shown as the means ± SD from experiments with 5 animals in each group (**p < 0.01). N=5; experiment was repeated twice.
Discussion
UVA irradiation is known to cause harmful effects in skin (13, 14) notably leading to photoaging and skin cancer. Various mechanisms of damage have been reported, including ROS production and activation of inflammatory processes (15). These processes are also key events in the progression of skin carcinogenesis. Among these mechanisms, TLR signaling has been reported to be important to the cellular response to UV exposure, as well as cancer progression. Previously, we have shown that the TLR pathway is involved in the inflammatory process mediated by UVB irradiation (3). However, little evidence exists regarding the mechanisms in UVA exposure, particularly in vivo.
Baicalin is a plant-derived flavonoid that has been widely reported to possess antioxidant and anti-inflammatory activity, especially after UVB exposure. We previously showed that baicalin treatment in mouse keratinocytes significantly protected against inflammation and cell death after UVB irradiation (3, 16, 17). In the current study, we found that baicalin exerted these protective effects by promoting the immune response and reducing the anti-inflammatory response. IL12 is an important cytokine involved in activation of Th1 cells and has been shown to be protective against UV-induced damage (18–20). In addition, IL12 is notable for reducing the amount of MDSCs and thereby further promoting the immune response (21). Our study illustrates how baicalin may act to modulate the immune response via increased IL-12.
The activation of inflammatory processes by UVA irradiation is a result of oxidative damage in the cell and is regarded as a crucial mediator of progression to skin cancer. Of particular note are the TLRs which are important regulators of this process (22, 23). Upon stimulation, TLR4 recruits adaptor proteins starting from MyD88 down through IRAK4 and TRAF6. Eventually, TLR4 signaling induces the activation of COX2, which is a key regulator of inflammation. In our study, we discovered that baicalin likely targets the TLR4 pathway following UVA irradiation, leading to an inhibition of the inflammatory cascade and thus many of the subsequent damages to the cell. Clearly, TLR4 represents a vital target and elucidation of baicalin targeting to this pathway may be important to prevent tumorigenesis.
In addition, TLR4 is significant beyond its role in inflammation. UVA irradiation induces indirect DNA damage via the generation of ROS. It has been reported that UVA irradiation may induce ROS generation via activation of TLR4 and its downstream regulation of NADPH oxidase (24, 25). Our results in fact showed downregulation of p47phox, a key subunit of NADPH oxidase, after baicalin treatment. Furthermore, UVA activation of MMPs and subsequent degradation of collagen is a major mechanism of photoaging (26). Our study showed that baicalin was associated reduced levels of two particular enzymes, MMP1 and MMP9; however, the mechanism behind these decreases remain unclear. One possibility is regulation by TLR4, which has been shown to upregulate the levels of MMPs (27–29).
In conclusion, our results demonstrate the protective effect of baicalin treatment against UVA-induced oxidative damage and inflammation in mice skin. These effects are likely mediated via the TLR4 pathway, which may serve as a target for photochemoprevention against skin inflammation.
Acknowledgements
This work was partially supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases 1R01AR071157–01A1 to NY.
Footnotes
Conflict of Interest Statement
The authors do not have any potential conflict of interest with this submission.
References
- 1.D’Orazio J, Jarrett S, Amaro-Ortiz A and Scott T (2013) UV radiation and the skin. International journal of molecular sciences 14, 12222–12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anna B, Blazej Z, Jacqueline G, Andrew CJ, Jeffrey R and Andrzej S (2007) Mechanism of UV-related carcinogenesis and its contribution to nevi/melanoma. Expert review of dermatology 2, 451–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min W, Ahmad I, Chang ME, Burns EM, Qian Q and Yusuf N (2015) Baicalin Protects Keratinocytes from Toll-like Receptor-4 Mediated DNA Damage and Inflammation Following Ultraviolet Irradiation. Photochemistry and photobiology 91, 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norval M and Halliday GM (2011) The consequences of UV-induced immunosuppression for human health. Photochemistry and photobiology 87, 965–977. [DOI] [PubMed] [Google Scholar]
- 5.Halliday GM, Byrne SN and Damian DL (2011) Ultraviolet A radiation: its role in immunosuppression and carcinogenesis. Seminars in cutaneous medicine and surgery 30, 214–221. [DOI] [PubMed] [Google Scholar]
- 6.Barton GM and Kagan JC (2009) A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nature reviews. Immunology 9, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis W, Simanyi E, Li H, Thompson CA, Nasti TH, Jaleel T, Xu H and Yusuf N (2011) Regulation of ultraviolet radiation induced cutaneous photoimmunosuppression by toll-like receptor-4. Arch Biochem Biophys 508, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad I, Simanyi E, Guroji P, Tamimi IA, delaRosa HJ, Nagar A, Nagar P, Katiyar SK, Elmets CA and Yusuf N (2014) Toll-like receptor-4 deficiency enhances repair of UVR-induced cutaneous DNA damage by nucleotide excision repair mechanism. The Journal of investigative dermatology 134, 1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen AC, Halliday GM and Damian DL (2013) Non-melanoma skin cancer: carcinogenesis and chemoprevention. Pathology 45, 331–341. [DOI] [PubMed] [Google Scholar]
- 10.Wang SC, Chen SF, Lee YM, Chuang CL, Bau DT and Lin SS (2013) Baicalin scavenges reactive oxygen species and protects human keratinocytes against UVC-induced cytotoxicity. In vivo (Athens, Greece) 27, 707–714. [PubMed] [Google Scholar]
- 11.Bing-Rong Z, Song-Liang J, Xiao EC, Xiang-Fei L, Bao-Xiang C, Jie G and Dan L (2008) Protective effect of the Baicalin against DNA damage induced by ultraviolet B irradiation to mouse epidermis. Photodermatology, photoimmunology & photomedicine 24, 175–182. [DOI] [PubMed] [Google Scholar]
- 12.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH and Hogquist KA (2007) Identification of a novel population of Langerin+ dendritic cells. The Journal of Experimental Medicine 204, 3147–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krutmann J (2000) Ultraviolet A radiation-induced biological effects in human skin: relevance for photoaging and photodermatosis. Journal of dermatological science 23 Suppl 1, S22–26. [DOI] [PubMed] [Google Scholar]
- 14.Min W, Liu X, Qian Q, Lin B, Wu D, Wang M, Ahmad I, Yusuf N and Luo D (2014) Effects of baicalin against UVA-induced photoaging in skin fibroblasts. The American journal of Chinese medicine 42, 709–727. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Xu Q, Peng Y, Gong Z, Chen H, Lai W and Maibach HI (2017) Expression Profiles of Long Noncoding RNA in UVA-Induced Human Skin Fibroblasts. Skin pharmacology and physiology 30, 315–323. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Zhou B, Wu D, Yin Z and Luo D (2012) Baicalin modulates microRNA expression in UVB irradiated mouse skin. Journal of biomedical research 26, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiping Z, Hua T, Hanqing C, Li C, Binyan Y and Jing M (2009) Effects of Baicalin on inflammatory mediators and pancreatic acinar cell apoptosis in rats with sever acute pancreatitis. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences 14, 19–27. [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz A (2005) Prevention of UV radiation–induced immunosuppression by IL-12 is dependent on DNA repair. 201, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meeran SM, Punathil T and Katiyar SK (2008) Interleukin-12-Deficiency Exacerbates Inflammatory Responses in UV-Irradiated Skin and Skin Tumors. The Journal of investigative dermatology 128, 2716–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werth VP, Bashir MM and Zhang W (2003) IL-12 Completely Blocks Ultraviolet-Induced Secretion of Tumor Necrosis Factor α from Cultured Skin Fibroblasts and Keratinocytes. Journal of Investigative Dermatology 120, 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD and Kao C (2011) The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology 133, 221–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harberts E and Gaspari AA (2013) TLR signaling and DNA repair: are they associated? The Journal of investigative dermatology 133, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harberts E, Fishelevich R, Liu J, Atamas SP and Gaspari AA (2014) MyD88 mediates the decision to die by apoptosis or necroptosis after UV irradiation. Innate immunity 20, 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valencia A and Kochevar IE (2008) Nox1-based NADPH oxidase is the major source of UVA-induced reactive oxygen species in human keratinocytes. The Journal of investigative dermatology 128, 214–222. [DOI] [PubMed] [Google Scholar]
- 25.Park HS, Jung HY, Park EY, Kim J, Lee WJ and Bae YS (2004) Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. Journal of immunology (Baltimore, Md. : 1950) 173, 3589–3593. [DOI] [PubMed] [Google Scholar]
- 26.Ujfaludi Z, Tuzesi A, Majoros H, Rothler B, Pankotai T and Boros IM (2018) Coordinated activation of a cluster of MMP genes in response to UVB radiation. Scientific Reports 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho JS, Kang JH, Um JY, Han IH, Park IH and Lee HM (2014) Lipopolysaccharide induces pro-inflammatory cytokines and MMP production via TLR4 in nasal polyp-derived fibroblast and organ culture. PLoS One 9, e90683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Xu H and Liu S (2011) Toll-like receptors 4 induces expression of matrix metalloproteinase-9 in human aortic smooth muscle cells. Molecular biology reports 38, 1419–1423. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Xu H and Sun B (2012) Lipopolysaccharide regulates MMP-9 expression through TLR4/NF-kappaB signaling in human arterial smooth muscle cells. Molecular medicine reports 6, 774–778. [DOI] [PubMed] [Google Scholar]
- 30.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS and van ’t Veer C (2002) In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. Journal of immunology (Baltimore, Md. : 1950) 168, 1286–1293. [DOI] [PubMed] [Google Scholar]
- 31.Applequist SE, Wallin RP and Ljunggren HG (2002) Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. International immunology 14, 1065–1074. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Yang J, Xu X, Liang L, Sun H, Liu G, Zhang L and Su Y (2016) The influence of genetic polymorphisms in TLR4 and TIRAP, and their expression levels in peripheral blood, on susceptibility to sepsis. Experimental and Therapeutic Medicine 11, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kostyuk V, Potapovich A, Stancato A, De Luca C, Lulli D, Pastore S and Korkina L (2012) Photo-oxidation products of skin surface squalene mediate metabolic and inflammatory responses to solar UV in human keratinocytes. PLoS One 7, e44472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen TTN, Kim YM, Kim TD, Le OTT, Kim JJ, Kang HC, Hasegawa H, Kanaho Y, Jou I and Lee SY (2013) Phosphatidylinositol 4-Phosphate 5-Kinase α Facilitates Toll-like Receptor 4-mediated Microglial Inflammation through Regulation of the Toll/Interleukin-1 Receptor Domain-containing Adaptor Protein (TIRAP) Location. J Biol Chem 288, 5645–5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Guan X, Tamura T, Ozato K and Ma X (2004) Synergistic activation of interleukin-12 p35 gene transcription by interferon regulatory factor-1 and interferon consensus sequence-binding protein. J Biol Chem 279, 55609–55617. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Ouyang X, Yang J, Liu J, Li Q, Gu Y, Fukata M, Lin T, He JC, Abreu M, Unkeless JC, Mayer L and Xiong H (2009) AP-1 Activated by Toll-like Receptors Regulates Expression of IL-23 p19. J Biol Chem 284, 24006–24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gounder AP, Yokoyama CC, Jarjour NN, Bricker TL, Edelson BT and Boon ACM (2018) Interferon induced protein 35 exacerbates H5N1 influenza disease through the expression of IL-12p40 homodimer. PLoS Pathogens 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yee CS, Yao Y, Xu Q, McCarthy B, Sun-Lin D, Tone M, Waldmann H and Chang CH (2005) Enhanced production of IL-10 by dendritic cells deficient in CIITA. Journal of immunology (Baltimore, Md. : 1950) 174, 1222–1229. [DOI] [PubMed] [Google Scholar]
- 39.Lakhan R, Baylink DJ, Lau KH, Tang X, Sheng MH, Rundle CH and Qin X (2015) Local administration of AAV-DJ pseudoserotype expressing COX2 provided early onset of transgene expression and promoted bone fracture healing in mice. Gene therapy 22, 721–728. [DOI] [PubMed] [Google Scholar]


