Abstract
Lightheadedness after standing contributes to adverse clinical events, including falls. Recommendations for higher sodium intake to treat postural lightheadedness have not been evaluated in a trial setting. The Dietary Approaches to Stop Hypertension (DASH)‐Sodium trial (1998‐1999) tested the effects of the DASH diet and sodium reduction on blood pressure (BP). Participants were randomly assigned to DASH or a typical Western diet (control). During either diet, participants ate three sodium levels (50, 100, 150 meq/d at 2100 kcal) in random order for 30‐days, separated by 5‐day breaks. Participants reported the presence and severity of postural lightheadedness at baseline and after each feeding period. There were 412 participants (mean age 48 years; 57% women; 57% black). Mean baseline SBP/DBP was 135/86 mm Hg; 9.5% reported baseline lightheadedness. Among those consuming the DASH diet, high vs low sodium increased lightheadedness (OR 1.71; 95% CI: 1.01, 2.90; P = 0.047) and severity of lightheadedness (P = 0.02), but did not affect lightheadedness in those consuming the control diet (OR 0.77; 95% CI: 0.46, 1.29; P = 0.32). Among those consuming high vs low sodium in the context of the DASH diet, adults <60 vs ≥60 years old experienced more lightheadedness (P‐interaction = 0.04), along with obese vs non‐obese adults (P‐interaction = 0.01). In the context of the DASH diet, higher sodium intake was associated with more frequent and severe lightheadedness. These findings challenge traditional recommendations to increase sodium intake to prevent lightheadedness.
Keywords: DASH diet, orthostatic lightheadedness, sodium, trial
1. INTRODUCTION
Lightheadedness with standing (ie, postural lightheadedness) is a frequently encountered symptom among adults1 that results from a gravitational drop in blood pressure (BP) leading to transient cerebral hypoperfusion.2 While benign in many adults, postural lightheadedness has been cited as an important causal mediator for harmful clinical events, such as falls.3 As a result, treatments targeting postural lightheadedness include higher sodium intake with a goal of augmenting BP.4 However, contrary to these recommendations, some observational studies suggest that higher sodium intake may worsen orthostatic hypotension.5, 6, 7 In fact, there is limited evidence from clinical trials of the effects of higher sodium intake on postural lightheadedness.
The DASH‐Sodium trial was a controlled feeding study completed in 2001.8 In this study, adults consumed three levels of sodium (low, medium, or high) in the context of either a healthy diet (DASH Diet) or a typical American diet (control diet). At the end of each of the three sodium feeding periods, participants were asked about potential side effects including lightheadedness with standing. The trial documented that lower sodium intake, relative to higher sodium, and the DASH diet, relative to the control diet, significantly decreased blood pressure.8 Whether lower sodium also worsened postural lightheadedness has not been reported.
In this secondary analysis of the DASH‐Sodium trial, we examined the impact of increased sodium intake on postural lightheadedness. We hypothesized that higher sodium intake would be associated with fewer and less severe reports of postural lightheadedness than higher sodium intake.
2. METHODS
2.1. Trial overview
Dietary Approaches to Stop Hypertension‐Sodium was a multicenter, randomized clinical trial conducted from September 1998 through November 1999 with support from the National Heart, Lung, and Blood Institute. This study tested two diets, the DASH diet and a control diet, and measured the effects of three levels of sodium intake on blood pressure in adults with elevated blood pressure or hypertension and not taking hypertension medications. The three sodium intake levels based on 2100 kcal consumption were as follows: high (target of 150 mmol/d), intermediate (target of 100 mmol/d), and low (target of 50 mmol/d). Larger or more active individuals received more sodium and food than smaller and less active individuals, accounting for total energy requirements. There were five different caloric consumption levels: 1600, 2100, 2600, 3100, and 3600 kcal. The DASH diet consisted of fruits, vegetables, and low‐fat dairy products, and had low cholesterol, saturated fat, and total fat. It also had less red meat and sugary items than the typical Western diet, and more whole grains, poultry, fish, and nuts. A detailed description of the diets can be found in Table S1. The original trial along with secondary analyses was approved by the IRB at Johns Hopkins University.
2.2. Participants
There were 412 adults (age ≥22 years) enrolled at four clinical centers in the US. Each had an average systolic blood pressure between 120 and 159 mm Hg and diastolic blood pressure between 80 and 95 mm Hg, not on anti‐hypertensive medication. Persons with diabetes mellitus, pregnancy, inflammatory bowel disease, anemia, history of a cardiovascular event, renal insufficiency, poorly controlled dyslipidemia, use of insulin, and consumption of more than 14 alcoholic beverages per week were excluded. All study participants provided written informed consent.
2.3. Controlled feeding
During run‐in and the three intervention periods, participants were provided with all of their meals and snacks. During a two‐week run‐in period, participants ate the high sodium, control diet. Afterward, using a parallel‐arm design, participants were randomly assigned to either the DASH diet or the control diet. On each diet, there were three 30‐day periods in which participants ate their assigned diet at each of the three sodium levels (a crossover design). Calorie content for each participant was adjusted to maintain weight constant throughout the study. Fluid intake was not restricted during the study.
2.4. Outcome measure: lightheadedness
Participants completed a questionnaire administered during the last 7 days of the run‐in period and each of the three feeding periods, which asked whether they felt “lightheadedness when standing up.” Participants could select: none (no experience of side effect), mild (symptom occurred but did not interfere with usual activities), moderate (occurrence of symptom somewhat interfered with usual activities), or severe (occurrence of symptom resulted in an inability to perform usual activities). Any lightheadedness was defined as having mild, moderate, or severe symptoms. Change in severity was the difference between severity scores (values 1 through 4) at the end of each feeding period minus scores after run‐in (baseline).
2.5. Other covariates
Data on other covariates were collected during screening or after the run‐in period (baseline) and each feeding period. Blood pressure was measured while participants were in a seated position, using their right arm both at baseline (the average of up to five measurements from screening and the run‐in period) and at the end of each sodium feeding period. Postural BP measurements were not obtained. Body mass index (BMI) was derived from height and weight measurements. Urine sodium levels were quantified in urine collected over a 24‐hour period.
2.6. Statistical analysis
The study population was characterized using proportions and means (SD). The distribution of severity score was characterized via histograms. The effect of sodium intake on lightheadedness was evaluated by comparing intermediate vs low sodium, high vs low sodium, and high vs intermediate sodium in strata of diet assignment (sodium effects). The effect of diet on lightheadedness was evaluated by comparing the DASH vs control diet in strata of sodium level (low, medium, and high). Generalized estimating equation (GEE) models with a logit link, binomial family, and an exchangeable covariance structure were used to model the odds of any lightheadedness. The difference in severity of lightheadedness at run‐in and lightheadedness at the end of each feeding period (end of period minus end of run‐in) was normally distributed and analyzed using GEE models with an identity link, normal family, and an exchangeable covariance structure. For diet effects in strata of sodium or combined diet‐sodium effects (high sodium‐control vs low sodium‐DASH), because only 1 measurement was compared between groups, we used simple logistic regression to model the odds of any lightheadedness and a simple linear regression to model the difference in severity of lightheadedness (end minus baseline).
A stratified analysis was performed to assess for the effect of baseline covariates on the relationship between sodium intake and lightheadedness in the following subgroups: age (<60, ≥60 years), sex (men, women), race (white, black), obesity (BMI ≥30, <30 kg/m2), and stage II hypertension (blood pressure ≥140/90 mm Hg, <140/90 mm Hg). Categories were chosen a priori based on established clinical cut points (BMI or hypertension) or on the distribution of baseline data (age). Differences across strata were evaluated using interaction terms. All analyses were performed using Stata/SE 14.0 (Stata Corporation LP, College Station, TX). A P value of <0.05 was considered statistically significant. Missing data were rare (19 of 809 visits or <3%) and evenly distributed across treatments.
3. RESULTS
3.1. Baseline characteristics
Of the 412 participants, 204 were assigned to the control diet and 208 to the DASH diet. Both diet groups had similar clinical and demographic characteristics (Table 1).
Table 1.
Baseline characteristics overall and by diet
| Overall (N = 412) | Control diet (N = 204) | DASH diet (N = 208) | |
|---|---|---|---|
| Age, y (SD) | 48.2 (10.0) | 49.1 (10.4) | 47.4 (9.6) |
| Women, % | 56.8 | 54.4 | 59.1 |
| Black, % | 56.8 | 56.4 | 57.2 |
| Stage II hypertensiona, % | 40.8 | 40.7 | 40.9 |
| Blood pressure, mm Hg | |||
| Systolic (SD) | 134.8 (9.5) | 135.4 (9.4) | 134.2 (9.6) |
| Diastolic (SD) | 85.7 (4.5) | 85.8 (4.1) | 85.6 (4.8) |
| BMI, kg/m2 (SD) | 29.2 (4.8) | 29.5 (5.0) | 28.8 (4.7) |
| BMI ≥30, % | 38.8 | 40.2 | 37.5 |
| Urinary sodiumb, mmol/d (SD) | 155.03 (75.4) | 152.5 (71.8) | 157.6 (78.9) |
| Any lightheadednessc, % | 9.5 | 9.8 | 9.1 |
BMI, body mass index; DASH, Dietary Approaches to Stop Hypertension.
Defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.
n = 408 (control diet, n = 204; DASH Diet, n = 204). This was measured after the run‐in period where all participants consumed a high sodium, control diet for an average of 2 wk.
Obtained at end of run‐in.
3.2. Diet effects on BP
As reported previously,8 BP was higher with higher sodium and the control diet (Table S2).
3.3. Sodium level and occurrence of lightheadedness
The distribution of lightheadedness by assigned diet and sodium intake level is in Figure 1. For the control diet, the highest occurrence of lightheadedness was in the low sodium group (11.8%), while the lowest occurrence of lightheadedness was in the intermediate and high sodium groups (9.3%). For the DASH diet, the highest occurrence of lightheadedness was in the high sodium group (15.4%), while the lowest occurrence of lightheadedness was in the low sodium group (9.6%).
Figure 1.
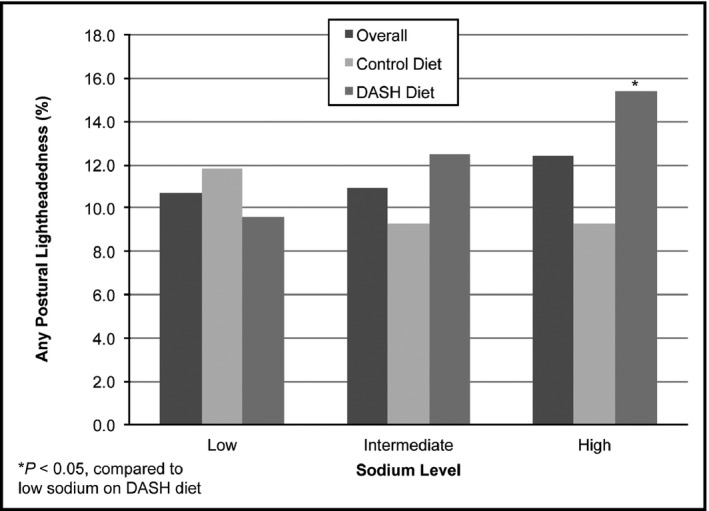
Frequency of postural lightheadedness by diet and sodium level
Table 2 details the occurrence and severity of lightheadedness by sodium level and diet. Among those assigned the control diet, severe lightheadedness was reported by two participants (1.0%) in the intermediate sodium group and by 1 participant (0.5%) in the high sodium intake group. Similarly, on the DASH diet, severe lightheadedness was only reported during the high sodium period by two participants (1.0%).
Table 2.
Occurrence and severity of postural lightheadedness by sodium diet and level, n (%)
| Mild | Moderate | Severe | |
|---|---|---|---|
| Level of sodium | |||
| Low | |||
| Control (n = 194) | 18 (9.3) | 6 (3.1) | 0 (0.0) |
| DASH (n = 200) | 16 (8.0) | 4 (2.0) | 0 (0.0) |
| Intermediate | |||
| Control (n = 197) | 15 (7.6) | 2 (1.0) | 2 (1.0) |
| DASH (n = 200) | 23 (11.5) | 3 (1.5) | 0 (0.0) |
| High | |||
| Control (n = 195) | 15 (7.7) | 3 (1.5) | 1 (0.5) |
| DASH (n = 201) | 23 (11.4) | 7 (3.5) | 2 (1.0) |
DASH, Dietary Approaches to Stop Hypertension.
3.4. Odds of lightheadedness
Because there was a significant interaction between diet and sodium intake with lightheadedness (P = 0.03), results are reported stratified by diet. Among those assigned the DASH diet, high vs low sodium intake increased one's odds of reporting any lightheadedness (OR 1.71; 95% CI: 1.01, 2.90; Table 3). Furthermore, high vs low sodium resulted in a statistically significant difference in change in lightheadedness severity from baseline (P = 0.02). In contrast, among those assigned the control diet, there was no significant effect of sodium on lightheadedness.
Table 3.
The effects of sodium intake and diet on the occurrence of postural lightheadedness and lightheadedness severity
| Lightheadedness | Difference in severity of lightheadedness (end‐baseline) | |||
|---|---|---|---|---|
| OR (95% CI) | P | Coefficient (95% CI) | P | |
| Sodium effects | ||||
| Sodium effects on the control dieta | ||||
| Intermediate vs low sodium | 0.77 (0.46, 1.29) | 0.32 | −0.03 (−0.09, 0.04) | 0.42 |
| High vs intermediate sodium | 1.00 (0.60, 1.67) | 1.00 | 0.00 (−0.05, 0.06) | 0.89 |
| High vs low sodium | 0.77 (0.46, 1.29) | 0.32 | −0.02 (−0.08, 0.04) | 0.48 |
| Sodium effects on the DASH dieta | ||||
| Intermediate vs low sodium | 1.34 (0.78, 2.32) | 0.29 | 0.02 (−0.04, 0.09) | 0.46 |
| High vs intermediate sodium | 1.28 (0.79, 2.04) | 0.32 | 0.07 (0.00, 0.15) | 0.06 |
| High vs low sodium | 1.71 (1.01, 2.90) | 0.047 | 0.10 (0.02, 0.18) | 0.02 |
| Diet effects (DASH vs control) | ||||
| Low sodiumb | 0.80 (0.43, 1.50) | 0.48 | −0.04 (−0.13, 0.06) | 0.51 |
| Medium sodiumb | 1.39 (0.74, 2.60) | 0.30 | 0.01 (−0.08, 0.10) | 0.84 |
| High sodiumb | 1.77 (0.97, 3.24) | 0.06 | 0.08 (−0.02, 0.19) | 0.11 |
| All sodium levels | 1.28 (0.81, 2.01) | 0.28 | 0.02 (−0.06, 0.10) | 0.59 |
| Combined effects | ||||
| High sodium on control vs low sodium on DASHb | 0.97 (0.50, 1.87) | 0.92 | 0.01 (−0.08, 0.10) | 0.82 |
CI, confidence interval; DASH, Dietary Approaches to Stop Hypertension.
A test for a diet‐sodium interaction was significant, P = 0.03.
Comparisons for diet effects at individual sodium levels (DASH vs control) and combined effects were performed using logistic regression (for lightheadedness) or linear regression (for difference in severity of lightheadedness) without generalized estimating equations.
Overall, the DASH diet compared to control did not significantly increase the odds of having lightheadedness (OR 1.28; 95% CI: 0.81, 1.81; P = 0.28) and did not significantly increase the severity of lightheadedness (β = 0.02; 95% CI: −0.06, 0.10; P = 0.59). Similarly, at each sodium level (low, medium, and high), the DASH diet did not significantly impact the odds of having lightheadedness or change in the severity of lightheadedness. In addition, high sodium intake on the control diet vs low sodium intake on the DASH diet did not significantly impact the odds of having lightheadedness (OR 0.97; 95% CI: 0.50, 1.87; P = 0.92) and did not significantly impact change in the severity of lightheadedness (P = 0.82).
3.5. Stratified analysis
We explored the effects of high vs low sodium intake on lightheadedness in strata of age, sex, race, BMI, and hypertension (Figure 2). Among those assigned the DASH diet, there were two significant sodium interactions: age and BMI. Participants <60 years of age experienced twice the odds of lightheadedness on high vs low sodium (OR 1.99; 95% CI: 1.13, 3.50), while participants aged 60 years or greater were not significantly affected by sodium (OR 0.30; 95% CI: 0.06, 1.68; P‐interaction = 0.04). With regard to BMI, there was no association of sodium intake with lightheadedness among participants with a BMI ≤30 kg/m2 (OR 0.93; 95% CI: 0.51, 1.68), but among those with a BMI >30 kg/m2, high vs low sodium increased their odds of lightheadedness by over five times (OR 5.16; 95% CI: 1.65, 16.1; P‐interaction = 0.01). There were no significant interactions among those assigned the control diet.
Figure 2.
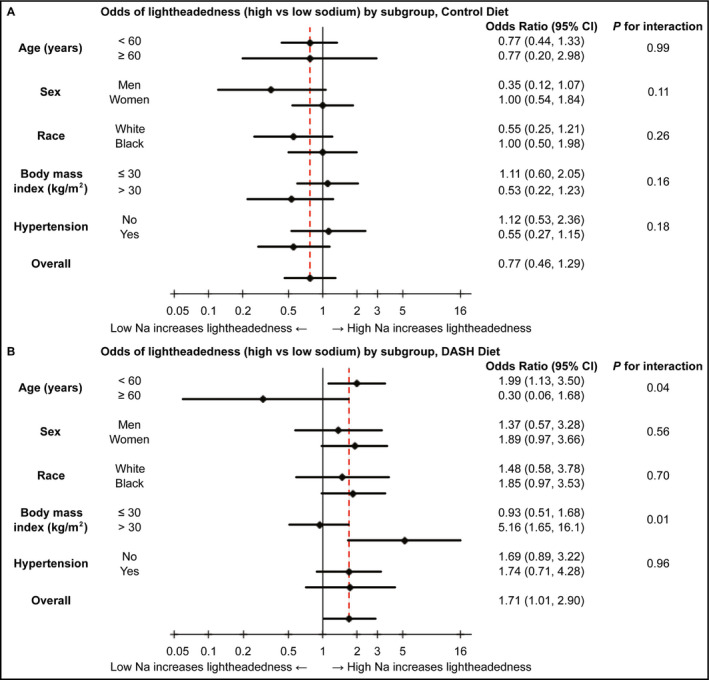
Odds are presented on a natural log scale. A, Odds of postural lightheadedness (high vs low sodium) by subgroup, in the control diet. B, Odds of postural lightheadedness (high vs low sodium) by subgroup, in the DASH diet. DASH, Dietary Approaches to Stop Hypertension
4. DISCUSSION
In this secondary analysis of the DASH‐sodium trial, dietary sodium intake affected the occurrence of postural lightheadedness, but the effects varied by diet. Among participants assigned to the DASH diet, higher sodium intake increased lightheadedness. However, higher sodium intake had no effect on lightheadedness in participants on the control diet. Among those assigned to the DASH diet, the association between sodium and lightheadedness was greater in younger (age <60 years) and obese adults. These findings suggest that higher sodium intake does not consistently improve postural lightheadedness and depending on the overall diet, age, or BMI could actually increase the risk of having symptoms.
Lightheadedness with standing is a common symptom1, 4, 9 associated with adverse clinical events such as falls, syncope, and stroke.1, 2, 3, 4 , 9, 10, 11 A frequent cause of postural lightheadedness is acute, gravitational shifts in blood pressure with standing called orthostatic hypotension.12 Several studies have demonstrated that higher sodium intake stabilizes blood pressure with standing9, 13 and reduces postural lightheadedness.10 This is thought to be related to increased intravascular volume that accompanies higher sodium intake.9 However, some studies report no effect from sodium restriction on orthostatic tolerance.14 Similarly, our study did not support this perspective and instead showed that higher sodium intake increased lightheadedness among those assigned the DASH diet.
Our observation that sodium increased lightheadedness was unexpected, but is supported by animal models that suggest greater orthostatic tolerance with long‐term sodium deprivation.15 This observation may be explained by the effects of sodium on hypertension. While higher sodium intake increases blood pressure16 and is strongly associated with hypertension,17, 18 sodium has been described to paradoxically suppress the sympathetic vasopressor response to standing16, 17 and contribute to syncope.18 In agreement with this literature, our study showed that higher sodium intake was associated with increased BP in both diets. Hypertension is further associated with endothelial dysfunction19, 20 and increased blood pressure variability.21 This variability may manifest as large drops in blood pressure with change in position, contributing to transient cerebral hypoperfusion and an ensuing sensation of lightheadedness.22 In fact, hypertension has been linked with orthostatic hypotension in several studies,23, 24, 25, 26 and more intensive treatment of hypertension has even been shown to lower orthostatic hypotension.27 We hypothesize that by increasing resting BP in adults with hypertension, sodium may cause more dramatic fluctuations in BP upon standing, resulting in both orthostatic hypotension and postural lightheadedness. However, further research is needed to confirm this hypothesis.
The effects of sodium on lightheadedness varied by assignment to either the DASH diet or a typical American control diet. This may be a result of higher potassium in the DASH diet. Serum potassium levels have been associated with postural intolerance in several studies,28 and low potassium levels have been associated with orthostatic hypotension.29, 30 In one small trial, potassium supplementation reduced drops in blood pressure with standing.31 However, we did not see an effect from diet (DASH vs control) on lightheadedness in our study. Rather, the combination of high sodium and high potassium (DASH diet) was associated with the highest prevalence of lightheadedness. This may be related in part to the vasodilatory effect of potassium on blood vessels.32, 33, 34 While unconfirmed by this study, it can be speculated that the combination of increased intravascular volume in the setting of vasodilation might promote greater variability in BP with change in position upon standing.
Among those assigned the DASH diet, the effects of sodium on lightheadedness were greater in younger participants (<60 years) than older participants (≥60 years) as well as in obese vs non‐obese participants. There is a higher prevalence of endothelial dysfunction and vascular stiffness among older adults.35, 36 As a result, the pathophysiology underlying high BP in this group may differ from that of younger adults. Age should be considered when applying the results of the current study in general practice. With regard to obese participants, prior studies have shown an association between postural symptoms and obesity.37, 38, 39 However, an interaction of BMI on the relationship between sodium and potassium with BP has not yet been reported.40 It is possible that the pronounced difference in effect observed according to obesity status is secondary to high total sodium consumption among the obese group, as total sodium consumption was proportional to energy consumption.41 If true, this would further reinforce our suggestion that higher sodium intake in the context of high potassium may contribute to lightheadedness, but further confirmatory evidence is needed.
The effects of sodium on lightheadedness did not differ by hypertension status. Given the strong association between hypertension and postural hypotension,26, 42, 43, 44, 45, 46, 47 we expected that higher sodium intake might worsen lightheadedness among this group. However, there was no evidence for an interaction by hypertension status. This may reflect our study population, which did not include adults on medications for hypertension.
This study has limitations. First, the exclusion of individuals with prior cardiovascular disease, diabetes, and renal insufficiency affects the generalizability of our findings. Second, the feeding periods lasted 4 weeks. Hence, long‐term effects are projected, rather than supported by empiric evidence. Third, the study protocol did not include a measure of standing blood pressure. As a result, we are unable to determine the effects of sodium intake on orthostatic hypotension. Although there is some debate as to whether orthostatic symptoms are more important for long‐term events than direct measures of orthostatic hypotension. Fourth, mild lightheadedness was more common than moderate or severe lightheadedness. As a result, conclusions from this study are more applicable to adults with mild lightheadedness. Fifth, the range of sodium intake in this study may not reflect interventions used to treat orthostatic lightheadedness, which tend to involve substantially greater sodium consumption. Last, a lack of statistical power may be a limitation for our study, particularly when assessing for interactions across subgroups.
This study has several strengths. First, the randomized design of DASH‐Sodium allowed for unbiased comparisons of both sodium intake and diet on lightheadedness. Second, data were collected according to a standardized protocol by data collection and intervention staff who were trained and certified. Third, the study participants were demographically diverse. Fourth, there were few drop outs, and data collection rates were high, close to 100%.
Our study has clinical and research implications. Greater sodium intake is widely viewed as an intervention for postural symptoms, like lightheadedness.9 In contrast, our analysis showed that higher sodium intake in the context of the DASH diet actually increased lightheadedness and had a variable effect in subgroups based on age or BMI. Hence, our results serve to caution health practitioners against recommending increased sodium intake as a universal treatment for lightheadedness. Additionally, our results demonstrate an important need for research to understand the role of sodium, and more broadly of diet, on lightheadedness.
In conclusion, increased sodium intake was associated with a significantly higher risk of lightheadedness in the context of the DASH diet. These effects were greater among young adults and obese adults. Mechanisms for these heterogeneous effects and the impact of sodium and potassium ratio on clinical events like falls, syncope, and CVD represent important questions for subsequent research.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
Supporting information
ACKNOWLEDGMENTS
We are indebted to the study participants for their sustained commitment to the DASH‐Sodium Trial; to the Almond Board of California, Beatrice Foods, Bestfoods, Cabot Creamery, C.B. Foods, Dannon, Diamond Crystal Specialty Foods, Elwood International, Hershey Foods, Hormel Foods, Kellogg, Lipton, McCormick, Nabisco U.S. Foods Group, Procter & Gamble, Quaker Oats, and Sun‐Maid Growers for donating food; to Frost Cold Storage for food storage. Supported by cooperative agreements and grants from the National Heart, Lung, and Blood Institute (U01‐HL57173, to Brigham and Women's Hospital; U01‐HL57114, to Duke University; U01‐HL57190, to Pennington Biomedical Research Institute; U01‐HL57139 and K08 HL03857‐01, to Johns Hopkins University; and U01‐HL57156, to Kaiser Permanente Center for Health Research) and by the General Clinical Research Center Program of the National Center for Research Resources (M01‐RR02635, to Brigham and Women's Hospital, and M01‐RR00722, to Johns Hopkins University). SPJ is supported by a NIH/NHLBI K23HL135273 and NIH/NHLBI R21HL144876. NTM is supported by a NIH/NHLBI K01HL141589. This trial is registered at clinicaltrials.gov, number: NCT00000608.
Peng AW, Appel LJ, Mueller NT, Tang O, Miller ER III, Juraschek SP. Effects of sodium intake on postural lightheadedness: Results from the DASH‐sodium trial. J Clin Hypertens. 2019;21:355–362. 10.1111/jch.13487
This trial is registered at clinicaltrials.gov, number: NCT00000608.
REFERENCES
- 1. Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epidemiology, prognosis, and treatment. J Am Coll Cardiol. 2015;66:848‐860. [DOI] [PubMed] [Google Scholar]
- 2. Bradley JG, Davis KA. Orthostatic hypotension. Am Fam Physician. 2003;68:2393‐2398. [PubMed] [Google Scholar]
- 3. Naschitz JE, Rosner I. Orthostatic hypotension: framework of the syndrome. Postgrad Med J. 2007;83:568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Low PA, Tomalia VA. Orthostatic hypotension: mechanisms, causes, management. J Clin Neurol Seoul Korea. 2015;11:220‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raj SR, Biaggioni I, Black BK, et al. Sodium paradoxically reduces the gastropressor response in patients with orthostatic hypotension. Hypertens Dallas Tex. 2006;1979(48):329‐334. [DOI] [PubMed] [Google Scholar]
- 6. Sharma AM, Schorr U, Oelkers W, Distler A. Effects of sodium salts on plasma renin activity and norepinephrine response to orthostasis in salt‐sensitive normotensive subjects. Am J Hypertens. 1993;6:780‐785. [DOI] [PubMed] [Google Scholar]
- 7. Vernikos J, Convertino VA. Advantages and disadvantages of fludrocortisone or saline load in preventing post‐spaceflight orthostatic hypotension. Acta Astronaut. 1994;33:259‐266. [DOI] [PubMed] [Google Scholar]
- 8. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3‐10. [DOI] [PubMed] [Google Scholar]
- 9. Figueroa JJ, Basford JR, Low PA. Preventing and treating orthostatic hypotension: as easy as A, B, C. Cleve Clin J Med. 2010;77:298‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stewart JM, Clarke D. “He’s Dizzy when he Stands Up”. An introduction to initial orthostatic hypotension. J. Pediatr. 2011;158:499‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benvenuto LJ, Krakoff LR. Morbidity and mortality of orthostatic hypotension: implications for management of cardiovascular disease. Am J Hypertens. 2011;24:135‐144. [DOI] [PubMed] [Google Scholar]
- 12. Juraschek SP, Miller ER, Appel LJ. Orthostatic hypotension and symptoms in the AASK trial. Am J Hypertens. 2018;31:665‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mtinangi BL, Hainsworth R. Early effects of oral salt on plasma volume, orthostatic tolerance, and baroreceptor sensitivity in patients with syncope. Clin Auton Res. 1998;8:231‐235. [DOI] [PubMed] [Google Scholar]
- 14. Davrath LR, Gotshall RW, Tucker A, et al. Moderate sodium restriction does not alter lower body negative pressure tolerance. Aviat Space Environ Med. 1999;70:577‐582. [PubMed] [Google Scholar]
- 15. Wilke WL, Marley WS, Gotshall RW. Preconditioning with sodium deficits to improve orthostatic tolerance in rats. Aviat Space Environ Med. 1995;66:757‐762. [PubMed] [Google Scholar]
- 16. Chrysant SG. Effects of high salt intake on blood pressure and cardiovascular disease: the role of COX inhibitors. Clin Cardiol. 2016;39:240‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haddy FJ, Pamnani MB. Role of dietary salt in hypertension. J Am Coll Nutr. 1995;14:428‐438. [DOI] [PubMed] [Google Scholar]
- 18. Rust P, Ekmekcioglu C. Impact of salt intake on the pathogenesis and treatment of hypertension. In: Islam MS, ed. Hypertension: From Basic Research to Clinical Practice. Cham, Switzerland: Springer;2016:61‐84. [DOI] [PubMed] [Google Scholar]
- 19. Higashi Y, Kihara Y, Noma K. Endothelial dysfunction and hypertension in aging. Hypertens Res. 2012;35:1039‐1047. [DOI] [PubMed] [Google Scholar]
- 20. Quyyumi AA, Patel RS. Endothelial dysfunction and hypertension: cause or effect? Hypertension. 2010;55:1092‐1094. [DOI] [PubMed] [Google Scholar]
- 21. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood‐pressure variability. Nat Rev Cardiol. 2013;10:143‐155. [DOI] [PubMed] [Google Scholar]
- 22. Novak P. Orthostatic cerebral hypoperfusion syndrome. Front Aging Neurosci. 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gangavati A, Hajjar I, Quach L, et al. Hypertension, orthostatic hypotension, and the risk of falls in a community‐dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kearney F, Moore A. Treatment of combined hypertension and orthostatic hypotension in older adults: more questions than answers still remain. Expert Rev Cardiovasc Ther. 2009;7:557‐560. [DOI] [PubMed] [Google Scholar]
- 25. Lee T, Donegan C, Moore A. Combined hypertension and orthostatic hypotension in older patients: a treatment dilemma for clinicians. Expert Rev Cardiovasc Ther. 2005;3:433‐440. [DOI] [PubMed] [Google Scholar]
- 26. Naschitz JE, Slobodin G, Elias N, Rosner I. The patient with supine hypertension and orthostatic hypotension: a clinical dilemma. Postgrad Med J. 2006;82:246‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Randomized A. Trial of intensive versus standard blood‐pressure. Control N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gratze G, Mayer H, Skrabal F. Sympathetic reserve, serum potassium, and orthostatic intolerance after endurance exercise and implications for neurocardiogenic syncope. Eur Heart J. 2008;29:1531‐1541. [DOI] [PubMed] [Google Scholar]
- 29. Luutonen S, Neuvonen P, Ruskoaho H, et al. The role of potassium in postural hypotension: electrolytes and neurohumoral factors in elderly hypertensive patients using diuretics. J Intern Med. 1995;237:375‐380. [DOI] [PubMed] [Google Scholar]
- 30. Slama R, Guedon J, Foucault J. [Orthostatic hypotension and chronic potassium depletion]. Coeur Med Interne. 1963;2:105‐111. [PubMed] [Google Scholar]
- 31. Heseltine D, Thomas T, Wilkinson R, James OF, Potter JF. Potassium supplementation in the treatment of idiopathic postural hypotension. Age Ageing. 1990;19:409‐414. [DOI] [PubMed] [Google Scholar]
- 32. Gijsbers L, Dower JI, Schalkwijk CG, et al. Effects of sodium and potassium supplementation on endothelial function: a fully controlled dietary intervention study. Br J Nutr. 2015;114:1419‐1426. [DOI] [PubMed] [Google Scholar]
- 33. Bekar LK, Nedergaard M. Is potassium a ubiquitous mediator of vasodilation in the central nervous system? Biophys J. 2013;105:2238‐2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Treasure J, Ploth D. Role of dietary potassium in the treatment of hypertension. Hypertension. 1983;5:864‐872. [DOI] [PubMed] [Google Scholar]
- 35. Herrera MD, Mingorance C, Rodríguez‐Rodríguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev. 2010;9:142‐152. [DOI] [PubMed] [Google Scholar]
- 36. Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corna S, Aspesi V, Cau N, et al. Dizziness and falls in obese in patients undergoing metabolic rehabilitation. PLoS ONE. 2017;12:e0169322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fjeldstad C, Fjeldstad AS, Acree LS, Nickel KJ, Gardner AW. The influence of obesity on falls and quality of life. Dyn Med. 2008;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee JF, Harrison ML, Christmas KM, Kim K, Hurr C, Brothers RM. Elevated resting heart rate and reduced orthostatic tolerance in obese humans. Clin Auton Res. 2014;24:39‐46. [DOI] [PubMed] [Google Scholar]
- 40. Dyer AR, Elliott P, Shipley M, Stamler R, Stamler J. Body mass index and associations of sodium and potassium with blood pressure in INTERSALT. Hypertens Dallas Tex. 1994;1979(23):729‐736. [DOI] [PubMed] [Google Scholar]
- 41. Murtaugh MA, Beasley JM, Appel LJ, et al. Relationship of sodium intake and blood pressure varies with energy intake. Hypertension. 2018;71(5):858‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Valbusa F, Labat C, Salvi P, Vivian ME, Hanon O, Benetos A. Orthostatic hypotension in very old individuals living in nursing homes: the PARTAGE study. J Hypertens. 2012;30:53‐60. [DOI] [PubMed] [Google Scholar]
- 43. Hiitola P, Enlund H, Kettunen R, Sulkava R, Hartikainen S. Postural changes in blood pressure and the prevalence of orthostatic hypotension among home‐dwelling elderly aged 75 years or older. J Hum Hypertens. 2009;23:33‐39. [DOI] [PubMed] [Google Scholar]
- 44. Lagi A, Rossi A, Comelli A, Rosati E, Cencetti S. Postural hypotension in hypertensive patients. Blood Press. 2003;12:340‐344. [DOI] [PubMed] [Google Scholar]
- 45. Applegate WB, Davis BR, Black HR, Smith WM, Miller ST, Burlando AJ. Prevalence of postural hypotension at baseline in the Systolic Hypertension in the Elderly Program (SHEP) cohort. J Am Geriatr Soc. 1991;39:1057‐1064. [DOI] [PubMed] [Google Scholar]
- 46. Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertens Dallas Tex. 1992;1979(19):508‐519. [DOI] [PubMed] [Google Scholar]
- 47. Strogatz DS, Keenan NL, Barnett EM, Wagner EH. Correlates of postural hypotension in a community sample of elderly blacks and whites. J Am Geriatr Soc. 1991;39:562‐566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


