Abstract
Background
Understanding how clinical stage and smoking history affect oncologic outcomes in human papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma (OPSCC) is critical for selecting patients for treatment de-intensification.
Methods
Kaplan-Meier and Cox-regression were used to evaluate overall survival (OS), locoregional (LRRFS), and distant recurrence-free survival (DRFS). Concordance statistics (C-indices) were used to compare discriminating ability.
Results
OS and DRFS, but not LRRFS, were significantly distributed using American Joint Committee on Cancer (AJCC) 7th and 8th Edition criteria. C-indices for OS, LRRFS, and DRFS were 0.57, 0.54, and 0.60, respectively, using the 7th Edition, and 0.63, 0.53, and 0.65 using the 8th. On multivariate analysis, 1+ pack-year smoking history correlated with OS (HR 1.96, 95%CI 1.2–3.1, p<0.01) but not LRRFS or DRFS.
Conclusions
These results support implementation of the AJCC 8th Edition for HPV-associated OPSCC. Clinical stage may be more important than smoking history in selection for de-intensification.
Keywords: Human papillomavirus (HPV), oropharyngeal squamous cell carcinoma, clinical staging, smoking, American Joint Committee on Cancer
1 INTRODUCTION
While OPSCC was historically associated with tobacco smoking and alcohol consumption, the incidence of HPV-associated OPSCC is increasing.1 Compared to smoking-related OPSCC, HPV-positivity is associated with distinct clinical behavior, including improved prognosis.2,3 It was therefore recognized that previous clinical staging systems, including the 7th Edition (Ed.) of the American Joint Committee on Cancer (AJCC) Staging Manual, were less applicable to HPV-associated OPSCC.4–6 In particular, HPV-positive OPSCC patients were observed to distribute nonuniformly among AJCC 7th Ed. clinical stages, which reduced prognostic utility of the system.5,7 With the goal to improve risk stratification and outcome prediction in HPV-associated OPSCC, the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S) developed a novel clinical staging system. This system was initially developed through a retrospective study of patients treated at Princess Margaret Hospital8 and was subsequently refined and validated in a larger, multi-institutional cohort.9 The ICON-S clinical staging system has been adapted for widespread implementation as part of the AJCC 8th Ed. Staging Manual.10 The ICON-S and AJCC 8th Ed. staging systems have been externally validated in two studies with cohorts of 150 and 279 patients each.11,12 As staging validation studies, these analyses appropriately investigated prognostication of OS only, and not disease-specific outcomes.
The negative effect of smoking on OS in patients with HPV-related OPSCC is well-recognized.9 However, it is not clear if this decreased OS is related directly to differences in cancer-specific outcomes as suggested by some studies,13–15 or merely the result of smoking-related comorbidities as supported by others.16,17 In the ICON-S report and the related preceding study, a 20 pack-year smoking history8 and pack-years as a continuous variable9 were associated with worse OS, although potential associations with disease-specific outcomes were not investigated. Importantly, smoking status was not incorporated into the AJCC 8th Ed.10
Due to the relatively good prognosis associated with HPV-positivity in OPSCC, efforts to de-intensify treatment in these patients have been proposed and are currently under clinical investigation. Appropriate selection of candidates for de-intensification relies on accurate and individualized risk stratification that considers not only OS, but disease-specific outcomes as well. We sought to describe the AJCC 8th Ed. staging system’s ability to predict multiple cancer-specific outcomes and to investigate the impact of smoking history in a robust cohort of patients with HPV-associated OPSCC.
2 MATERIALS AND METHODS
2.1 Patient population
Patients analyzed in this Institutional Review Board-approved study were seen at a single academic tertiary cancer center. Inclusion criteria stipulated adults with biopsy-proven OPSCC, AJCC 7th Ed. stage I-IVb, positive for p16 by immunohistochemical (IHC) staining or, in cases where p16 staining was not performed, HPV deoxyribonucleic acid (DNA) by polymerase chain reaction (PCR). Patients were treated definitively with surgery and/or radiotherapy (RT), with or without systemic therapy (chemotherapy or cetuximab) per institutional practices, which were consistent with National Comprehensive Cancer Network guidelines. Demographic, disease, and treatment data were collected prospectively in a password-protected epidemiology database. Patients provided informed consent to be included in this database. Smoking history was collected prospectively, with former smoking status defined as abstinence from tobacco use for at least 1 year prior to diagnosis. Patients who had quit smoking less than 1 year prior to diagnosis were classified as current-smokers. Patients were retrospectively restaged per the AJCC 8th Ed. guidelines.
2.2 Outcome definitions and statistical methods
Overall survival was calculated from the date of diagnosis. Patients alive at last follow-up were censored at that date. Locoregional and distant recurrence-free survival (LRRFS and DRFS, respectively) were similarly calculated from the date of diagnosis, with patients alive without evidence of locoregional or distant recurrence, respectively, being censored at date of last follow-up. Kaplan Meier was used to compare estimated rates of OS, LRRFS, and DRFS. Log-rank test was used to evaluate the significance of outcome distribution. Concordance statistics (C-indices) were calculated to compare the discriminating ability of each staging system. Multivariate Cox-regression, accounting for AJCC 8th Ed. clinical group stage, was used to correlate age and smoking status with OS, LRRFS, and DRFS. Age was considered as a continuous variable and smoking history was analyzed using multiple cutoffs as detailed below.
3 RESULTS
3.1 Patient characteristics and staging
Five-hundred and thirty-one patients treated between 2003 and 2016 were identified and included in this analysis. Median follow-up was 48 months. Patient characteristics are shown in Table 1. Upon restaging from AJCC 7th Ed. to 8th Ed., all but 5 (0.9%) patients were assigned a new clinical group stage, with 13 (2.4%) changing from stage II to I, 25 (4.7%) from III to I, 28 (5.3%) from III to II, 224 (42.2%) from IVa to I, 80 (15.1%) from IVa to II, 110 (20.7%) from IVa to III, and 46 (8.7%) from IVb to III. No patient was assigned a higher clinical group stage upon reclassification.
Table 1.
Patient characteristics.
| Characteristic | |
|---|---|
|
| |
| Age | |
| Mean, years (SD) | 57.9 (9.1) |
| Median, years (range) | 57.1 (33 – 91) |
|
| |
| Female sex, no. of patients (%) | 69 (13.0%) |
|
| |
| Smoking status at time of diagnosis, no. of patients (%) | |
| Never | 214 (40.3%) |
| Former | 184 (34.6%) |
| Current | 133 (25.0%) |
| Mean pack years (SD) | 16.2 (23.2) |
|
| |
| p16 status, no. of patients (%) | |
| Positive | 316 (59.5%) |
| Unavailable | 215 (40.5%) |
|
| |
| HPV DNA status, no. of patients (%) | |
| Positive | 497 (93.6%) |
| Negative | 13 (2.4%) |
| Unavailable | 21 (4.0%) |
|
| |
| T classification, no. of patients (%) | |
| 1 | 139 (26.2%) |
| 2 | 187 (35.2%) |
| 3 | 78 (14.7%) |
| 4a | 113 (21.3%) |
| 4b | 14 (2.6%) |
|
| |
| AJCC 7th Ed. N classification, no. of patients (%) | |
| 0 | 46 (8.7%) |
| 1 | 49 (9.2%) |
| 2a | 70 (13.2%) |
| 2b | 238 (44.8%) |
| 2c | 90 (16.9%) |
| 3 | 38 (7.2%) |
|
| |
| AJCC 7th Ed. group stage, no. of patients (%) | |
| I | 5 (0.9%) |
| II | 13 (2.4%) |
| III | 53 (10.0%) |
| IVa | 414 (78.0%) |
| IVb | 46 (8.7%) |
|
| |
| AJCC 8th Ed. N classification, no. of patients (%) | |
| 0 | 46 (8.7%) |
| 1 | 357 (67.2%) |
| 2 | 90 (16.9%) |
| 3 | 38 (7.2%) |
|
| |
| AJCC 8th Ed. group stage, no. of patients (%) | |
| I | 269 (50.6%) |
| II | 108 (20.3%) |
| III | 154 (29.0%) |
|
| |
| Treatment modality*, no. of patients (%) | |
| Radiotherapy alone | 14 (2.6%) |
| Concurrent radiotherapy + systemic therapy** | 473 (89.1%) |
| Surgery followed by concurrent radiotherapy + systemic therapy | 21 (3.9%) |
| Surgery followed by radiotherapy | 10 (1.9%) |
| Surgery alone | 13 (2.4%) |
|
| |
| Treatment location, no. of patients (%) | |
| University of Michigan only | 462 (87.0%) |
| Potion of are at outside facility | 69 (13.0%) |
Treatment modality refers to initial, definitive treatment, excluding diagnostic procedures such as diagnostic tonsillectomy and excisional lymph node biopsy, as well as subsequent salvage and palliative therapies.
Systemic therapy refers to cytotoxic chemotherapy and/or cetuximab.
HPV = human papillomavirus. DNA = deoxyribonucleic acid. AJCC = American Joint Committee on Cancer.
3.2 Prognostication of OS, LRRFS, and DRFS
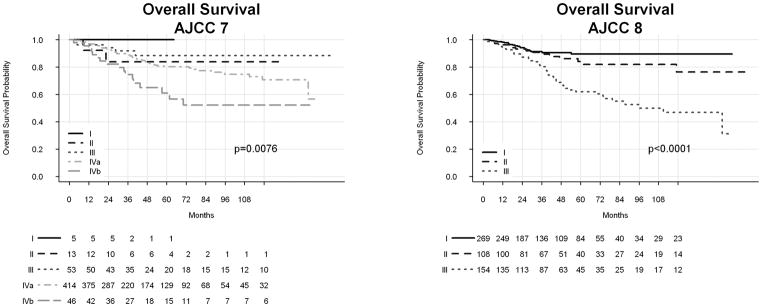
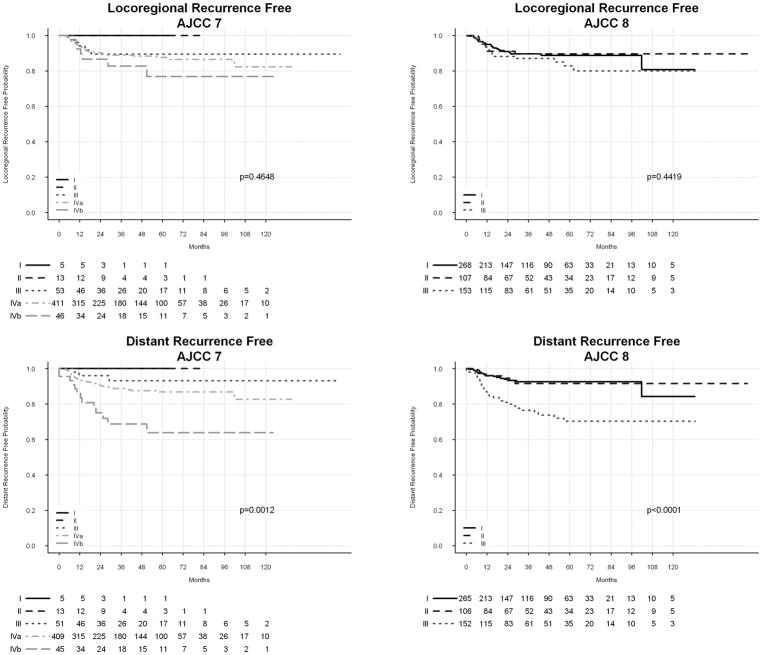
Kaplan-Meier estimates of OS by AJCC 7th and 8th Ed. clinical stage are shown in Figure 1. Log-rank test showed significant distribution of OS by AJCC 7th and 8th Ed. stages. Kaplan-Meier estimates of LRRFS and DRFS are shown in Figure 2. Prognostication of DRFS was significant by use of AJCC 7th and 8th Ed. staging, although neither system yielded significant distribution LRRFS. Actuarial rates of 5-year OS, LRRFS, and DRFS are shown in Supplemental Table 1. Table 2 shows hazard ratios (HRs) corresponding to AJCC 8th Ed. stage for each outcome. We also attempted to calculate HRs for AJCC 7th Ed. staging. Due to low numbers of patients and events, stages I and II were combined to create a reference category for OS. This approach yielded HRs of 0.50 (95% CI 0.1 – 2.6), 0.98 (0.2 – 4.0), and 2.21 (0.5 – 9.6) for AJCC 7th Ed. stages III, IVa, and IVb, respectively. However, there were too few recurrences in stages I and II to allow for estimation of HRs for LRRFS and DRFS.
Figure 1.
Kaplan Meier estimates of overall survival by AJCC 7th and 8th Editions Cancer Staging Manual.
AJCC = American Joint Committee on Cancer.
Figure 2.
Kaplan Meier estimates of locoregional (A, B) and distant (C, D) recurrence-free survival by AJCC 7th and 8th Ed. Cancer Staging Manual.
AJCC = American Joint Committee on Cancer.
Table 2.
Kaplan-Meier estimated HR of outcomes by AJCC 7th and 8th Ed. clinical stage.
| AJCC 8th Ed. | ||
|---|---|---|
| OS, HR (95% CI) | Stage I | Reference |
| Stage II | 1.54 (0.8 – 3.0) | |
| Stage III | 4.07 (2.5 – 6.8) | |
| LRRFS, HR (95% CI) | Stage I | Reference |
| Stage II | 0.95 (0.5 – 2.0) | |
| Stage III | 1.41 (0.8 – 2.6) | |
| DRFS, HR (95% CI) | Stage I | Reference |
| Stage II | 0.97 (0.4 – 2.3) | |
| Stage III | 3.70 (2.1 – 6.6) |
HR = hazard ratio. CI = confidence interval. OS = overall survival. LRRFS = locoregional recurrence-free survival. DRFS = distant recurrence-free survival.
As both AJCC 7th and 8th Ed. guidelines resulted in statistically significant distribution of OS and DRFS, we sought to compare the discriminating ability of these two systems by calculating C-indices. Table 3 shows these results, with the AJCC 8th Ed. yielding higher (better) C-indices for OS and DRFS compared to the AJCC 7th Ed.
Table 3.
C-indices calculated for OS, LRRFS, and DRFS using AJCC 7th vs. 8th Ed. clinical staging criteria.
| AJCC 7th Ed. | AJCC 8th Ed. | |
|---|---|---|
| OS | 0.57 | 0.63 |
| LRRFS | 0.54 | 0.53 |
| DRFS | 0.60 | 0.65 |
Higher C-index value indicates improved discriminating ability.
3.3 Impact of smoking history
In this cohort, there were no significant differences in the relative prevalence of AJCC 8th Ed. T classification, N classification, and group stages among never-, current-, and former-smokers (Supplemental Table 2). On multivariate analysis, accounting for clinical group stage (per AJCC 8th Ed.), age and any smoking history (1+ pack-year) were statistically significantly correlated with OS, while smoking history by other cutoffs, namely 10+ pack-years, 20+ pack-years, and current smoking status, were not (Table 4). No degree of smoking history was significantly associated with LRRFS or DRFS. Only age was significantly correlated with LRFS.
Table 4.
Multivariate analysis, accounting for group stage (per AJCC 8th Ed.), correlating smoking history and age with OS, LRRFS, and DRFS.
| OS | LRRFS | DRFS | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Any smoking | 1.96 (1.2 – 3.1) | <0.01 | 1.23 (0.7 – 2.2) | 0.42 | 1.32 (0.8 – 2.3) | 0.33 |
| 10+ pack years | 1.49 (1.0 – 2.3) | 0.05 | 1.21 (0.7 – 2.1) | 0.41 | 1.03 (0.6 – 1.7) | 0.95 |
| 20+ pack years | 1.26 (0.8 – 2.0) | 0.38 | 1.06 (0.6 – 1.9) | 0.93 | 1.02 (0.6 – 1.8) | 0.98 |
| Current smoking | 1.10 (0.7 – 1.8) | 0.72 | 0.78 (0.4 – 1.5) | 0.43 | 1.04 (0.6 – 1.9) | 0.88 |
| Age (per year increase) | 1.04 (1.0 – 1.1) | <0.01 | 1.03 (1.0 – 1.1) | 0.03 | 1.02 (1.0 – 1.1) | 0.14 |
Each row represents a separate Cox model. HR = hazard ratio. CI = confidence interval.
4 DISCUSSION
These data confirm the improved prognostication of OS in patients with HPV-associated OPSCC using AJCC 8th Ed. clinical staging compared to the AJCC 7th Ed. While both systems yielded significant distribution of OS, the C-index associated with the AJCC 8th Ed. was higher than that associated with the 7th, indicating superior performance. This is consistent with findings from the initial ICON-S studies8,9 as well as previously published validation reports.11,12 Compared to previous validation studies, this work analyzed a larger cohort of prospectively collected patients and investigated cancer-specific outcomes in addition to OS. While the primary goal of staging is prognostication of OS, understanding how stage predicts locoregional and distant control can guide clinical decision making. In this regard, we found that AJCC 8th Ed. clinical staging showed superior prognostication of DRFS compared to the 7th Ed., but that neither system resulted in significant LRRFS distribution. This observation is consistent with the understanding that in HPV-associated OPSCC, distant failure is a more important cause of cancer mortality than locoregional failure, likely due to the generally excellent locoregional control achieved in these patients.2,18–20 Interestingly, the rates of distant recurrence in patients with AJCC 8th Ed. stage I and II were relatively similar, while patients with stage III disease demonstrated a much higher risk.
A major criticism of AJCC 7th Ed. staging for HPV-associated OPSCC is that patients demonstrate uneven stage distribution.5–7 Specifically, a disproportionately high number of patients are diagnosed with higher stage disease, which results in poor differentiation of outcomes. In this analysis, distribution of patients by clinical stage was markedly different using the AJCC 7th versus 8th Ed. guidelines. We found that most patients in our cohort were initially diagnosed with stage IV disease using the AJCC 7th Ed. criteria, but when retrospectively reassessed using the AJCC 8th Ed., a majority were assigned an early stage. This redistribution was associated with improved stratification of OS and DRFS.
Since the earliest studies describing HPV-associated OPSCC, smoking has been consistently associated with worse OS.2 Of particular relevance to our present work are studies that included redefined staging for HPV-associated disease. In the staging reclassification work preceding the ICON-S study, Huang et al. identified a 20 pack-year smoking history as being associated with worse OS, particularly in patients with early stage disease.8 In the subsequent ICON-S report, O’Sullivan et al. identified smoking pack-years as a significant correlate with OS on multivariate analysis.9 However, neither of the two published validation studies found smoking to be independently correlated with OS when using updated staging.11,12 Results from the present study demonstrate a statistically significant association between OS and a 1+ pack-year smoking history, but no significant correlation using other cutoffs, including 20 pack-years.
While the impact of smoking on OS is relatively well-established, it remains uncertain if this is related to mortality from non-cancer smoking-related health effects or is directly related to OPSCC outcomes. Certain studies have shown higher rates of treatment failure or disease progression in HPV-positive smokers than non-smokers,13–15 although others have shown no impact of smoking history on disease-specific outcomes.16,17 In the work presented here, we found that when accounting for stage and age, neither current- nor former-smokers were at increased risk for locoregional or distant recurrence when compared to non-smokers. These findings suggest that in patients with HPV-positive disease, smoking may impact OS primarily through mechanisms not directly related to disease recurrence.
In the era of treatment de-intensification for HPV-associated OPSCC, the identification of truly “low-risk” patients who may be eligible for such strategies is critical. The most important factors considered in reported and ongoing de-intensification trials have been stage and smoking history. In this setting, the impact of these variables on disease-specific outcomes, and not solely on OS, is particularly relevant. In Eastern Cooperative Oncology Group (ECOG) study 1308, a trial of reduced-dose RT and cetuximab in patients with complete clinical response to induction chemotherapy, patients with nodal classification < N2c (< N2 by AJCC 8th Ed. criteria), T classification < T4, and a ≤ 10 pack-year smoking history showed substantially better OS and progression-free survival than patients with more advanced disease or a more significant smoking history.21 Some have interpreted these results to indicate that an extensive smoking history should exclude patients from de-intensified treatment. Conversely, in an analysis of patients treated for HPV-associated OPSCC with RT alone or concurrent chemo-RT (CRT), O’Sullivan et al. found that for patients with a > 10 pack-year smoking history, only OS, and not cancer-specific outcomes, was decreased in patients treated with RT alone compared to CRT.16 Some have suggested that these results indicate that smoking history should not disqualify patients from de-intensification. Following these results, some subsequent studies have excluded patients with a significant smoking history, including Trans-Tasman Radiation Oncology Group (TROG) 12.01 and National Research Group (NRG) Oncology HN002, while others have not, such as Radiation Therapy Oncology Group (RTOG) 1016 and ECOG 3311 (reviewed in22). This lack of consistency regarding smoking history as an exclusion criterion is not surprising given the conflicting nature of the available data. Our work indicates that smoking history may be less important than stage in relation to disease-specific outcomes, suggesting that treatment de-intensification may be appropriate for otherwise low-risk patients with a significant smoking history.
There are important limitations of this study that deserve mention. While patients were accrued prospectively, abstraction of certain data was conducted retrospectively. This raises issues of bias as well as data integrity and accuracy, in addition to concerns regarding patients who were lost to follow-up. More robust prospective data that uses the 8th Ed. a priori is needed to more definitively address questions of selection criteria for treatment de-intensification. Statistical analysis was also complicated by the low number of patients and events within certain group stages. This resulted from nonuniform distribution of patients among AJCC 7th Ed. clinical stages, which highlights a significant shortcoming of that system. Another limitation to this study is the inclusion of patients whose specimens were positive for HPV DNA but for whom p16 status was unavailable. The AJCC 8th Ed. stipulates that p16 staining should be used to determine the HPV-relatedness of oropharynx cancers. However, a significant number of patients in this analysis were diagnosed before p16 staining was routinely performed at our institution. While HPV DNA detection and p16 positivity are highly correlated,23 this deviation from current guidelines should be recognized. It is also important to note that this study analyzed the impact of clinical stage only, and not pathologic. The AJCC 8th Ed. has a separate pathologic staging schema, which has been a source of early controversy. However, as questions regarding clinical stage and smoking history also apply to patients treated with surgery, we elected to include these patients without focusing on their pathologic stage. As a result, of course, we cannot use these data to comment on the AJCC 8th Ed. surgical staging system.
5 CONCLUSIONS
The AJCC 8th Ed. clinical staging system appropriately stratifies outcomes for HPV-associated OPSCC in terms of OS and DRFS, but not LRRFS. While smoking history was associated with worse OS in this cohort, it did not correlate with LRRFS or DRFS. These results support implementation of the AJCC 8th Ed. Cancer Staging Manual for HPV-associated OPSCC and suggest that smoking history should not inherently exclude patients from treatment de-intensification paradigms.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by the Newman Family Professorship Fund and RO1 CA184153 (Eisbruch)
Footnotes
This work will be presented at the Multidisciplinary Head and Neck Cancers Symposium in February 2018.
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. Journal of the National Cancer Institute. 2008;100(4):261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB American Joint Committee on Cancer. AJCC cancer staging manual. 7. New York: Springer; 2010. p. xiv.p. 648. [DOI] [PubMed] [Google Scholar]
- 5.Ward MJ, Mellows T, Harris S, et al. Staging and treatment of oropharyngeal cancer in the human papillomavirus era. Head Neck. 2015;37(7):1002–13. doi: 10.1002/hed.23697. [DOI] [PubMed] [Google Scholar]
- 6.Spector ME, Gallagher KK, Bellile E, et al. Patterns of nodal metastasis and prognosis in human papillomavirus-positive oropharyngeal squamous cell carcinoma. Head Neck. 2014;36(9):1233–40. doi: 10.1002/hed.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119(1):81–9. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(8):836–45. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 9.O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. The Lancet Oncology. 2016;17(4):440–51. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- 10.Amin MB, Edge SB. AJCC cancer staging manual. Springer; 2017. [Google Scholar]
- 11.Wurdemann N, Wagner S, Sharma SJ, et al. Prognostic Impact of AJCC/UICC 8th Edition New Staging Rules in Oropharyngeal Squamous Cell Carcinoma. Frontiers in oncology. 2017;7:129. doi: 10.3389/fonc.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porceddu SV, Milne R, Brown E, et al. Validation of the ICON-S staging for HPV-associated oropharyngeal carcinoma using a pre-defined treatment policy. Oral oncology. 2017;66:81–86. doi: 10.1016/j.oraloncology.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(4):1226–35. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(17):2102–11. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vainshtein JM, Spector ME, McHugh JB, et al. Refining risk stratification for locoregional failure after chemoradiotherapy in human papillomavirus-associated oropharyngeal cancer. Oral oncology. 2014;50(5):513–9. doi: 10.1016/j.oraloncology.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Sullivan B, Huang SH, Perez-Ordonez B, et al. Outcomes of HPV-related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;103(1):49–56. doi: 10.1016/j.radonc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 17.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(5):543–50. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 18.Trosman SJ, Koyfman SA, Ward MC, et al. Effect of human papillomavirus on patterns of distant metastatic failure in oropharyngeal squamous cell carcinoma treated with chemoradiotherapy. JAMA otolaryngology-- head & neck surgery. 2015;141(5):457–62. doi: 10.1001/jamaoto.2015.136. [DOI] [PubMed] [Google Scholar]
- 19.Huang SH, Perez-Ordonez B, Weinreb I, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral oncology. 2013;49(1):79–85. doi: 10.1016/j.oraloncology.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Huang SH, Perez-Ordonez B, Liu FF, et al. Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. International journal of radiation oncology, biology, physics. 2012;82(1):276–83. doi: 10.1016/j.ijrobp.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Marur S, Li S, Cmelak AJ, et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016 doi: 10.1200/JCO.2016.68.3300. JCO2016683300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. European journal of cancer. 2014;50(15):2636–48. doi: 10.1016/j.ejca.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120(8):1731–8. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.