Abstract
In contrast to clathrin mediated endocytosis (CME) which is well characterized and understood, little is known about the regulation and machinery underlying clathrin independent endocytosis (CIE). There is also a wide variation in the requirements each individual CIE cargo has for its internalization. Recent studies have shown that CIE is affected by glycosylation and glycan interactions. We briefly review these studies and explore how these studies mesh with one another. We then discuss what this sensitivity to glycan interactions could indicate for the regulation of CIE. We address the spectrum of responses CIE has been shown to have with respect to changes in glycan interactions and attempt to reconcile disparate observations onto a shared conceptual landscape. We focus on the mechanisms by which cells can alter the glycan interactions at the plasma membrane and propose that glycosylation and glycan interactions could provide cells with a tool box with which cells can manipulate CIE. Altered glycosylation is often associated with a number of diseases and we discuss how under different disease settings, glycosylation-based modulation of CIE could play a role in disease progression.
Keywords: Endocytosis, membrane trafficking, glycosylation, galectin, galectin lattice, galectin 3, clathrin-independent endocytosis
Graphical Abstract

Introduction
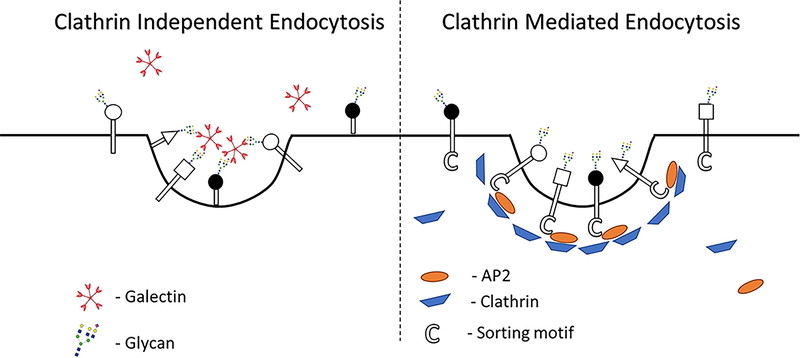
Endocytosis is known to play essential roles in numerous cell functions1. The best characterized form of endocytosis is clathrin mediated endocytosis(CME), which has well-defined machinery2, a visible coat protein (i.e. clathrin) and shared cytoplasmic sorting motifs with which cargo can bind specifically to adaptor proteins and clathrin. In contrast, there is little known about the machinery underlying clathrin independent endocytosis (CIE) and efforts to identify and characterize the common shared cellular machinery involved in CIE’s regulation have not been fruitful3. There is no apparent cytoplasmic coat protein in CIE analogous to clathrin in CME. The field has also been distracted by the heterogeneity of CIE observed in different cells types4,5. Recent studies have suggested that glycosylation and extracellular galectins can play a role in mediating CIE6–15. Our group has also recently published a study showing that two of the CIE cargo proteins we follow are differentially sensitive to changes in glycosylation and the presence and interactions with galectins, raising the possibility that glycosylation and galectins could modulate cargo entry by CIE15. These findings suggest that galectins could serve as extracellular machinery facilitating or inhibiting cargo entry from the extracellular/lumenal side of the membrane (Figure 1).
Figure 1:
Differences in mechanistic understanding of clathrin independent endocytosis (CIE) and clathrin mediated endocytosis (CME). CME has a well characterized mechanism with numerous components and their roles identified. AP2 subunits bind to motifs on CME cargo, they recruit clathrin which then helps to form a well-defined coat on the endosomes, dynamin then helps mediate scission of these endosomes. In contrast, there is little known about the machinery that drives CIE. Galectins and glycan interactions could serve as an extracellular coat and play a role in mediating CIE from the lumenal side of these endosomes.
One of the factors that has made finding shared machinery so challenging is that CIE is a bulk pathway and as such is responsible for the internalization of a diverse and varied assortment of proteins3,12,16. Unlike CME, where each cargo protein has cytoplasmic sorting motifs, CIE is responsible for internalizing proteins, from transmembrane proteins like the major histocompatibility complex Class I (MHCI) to GPI- anchored proteins like CD59, which don’t have a cytoplasmic domain let alone a sorting sequence.
The extent of the diversity of CIE cargo is illustrated by the numerous pathways of CIE that have been described. These pathways can be constitutive or ligand stimulated, dynamin dependent or dynamin independent, associated with arf6 or independent. Fast endophilin mediate endocytosis (FEME) (17) and ultrafast endocytosis18 are examples of ligand stimulated CIE that are characteristically very rapid and require endophilin, dynamin, actin and synaptojanin. The FEME pathway is responsible for the uptake of cargo like the epidermal growth factor receptor (at high concentrations), interleukin 2 and a number of other G-Protein coupled receptors17,18. Constitutive processes like the Clathrin Independent carrier GPI enriched compartment (CLIC/GEEC) are responsible for the uptake of cargo like CD44 have been shown to be dependent on GRAF1, cdc42, Arf1 and actin polymerization but are independent of dynamin19. Cargo like Major Histocompatibility complex Class I and CD59 are also endocytosed constitutively in association with Arf6 and independent of dynamin20,21. More comprehensive overviews of the various types of CIE can be found in these reviews1–5,16. While each of these pathways has a number of unique components, they also share a few common requirements such as a need for cholesterol, lipid rafts and in many cases actin polymerization.
Another potential shared feature of all these different CIE cargo proteins is glycosylation. Almost every single protein at the cell surface is glycosylated. Recent studies from our group as well as others have highlighted how glycosylation can play important roles in CIE6–15.
Glycosylation of plasma membrane proteins (cargo) and interactions with cellular lectins
Glycosylation is an important post-translational modification. It has long been known that altered glycosylation is a hallmark of cancer22–24. This fact has been leveraged in the development of cancer biomarker like CA19–9 which is a carbohydrate antigen that is used as a diagnostic marker for pancreatic and gastrointestinal cancer25,26. While these changes were initially viewed as passive by products of abnormal cellular metabolism in cancer, a number of studies have highlighted important functional roles these changes in glycosylation can play in driving disease progression27–29. Glycosylation has also been shown to play numerous functional roles under normal physiological conditions from altering serum half-life of antibodies30 to orchestrating the leukocyte tethering and rolling required for their exiting the blood stream31.
N-linked glycosylation and O-linked glycosylation occur as proteins transit through the ER and the Golgi toward the cell surface. Unlike the biosynthetic processes of most complex macromolecules in cells like DNA, RNA and proteins, glycosylation is a complex, post-translation modification that is non-template driven32–38. As a result, the patterns of glycans produced on surface proteins are heterogenous and can shift based on changes in the availability of enzymes or their sugar substrates in the ER and Golgi. Glycans can be detected by a variety of lectins that bind to specific glycan linkages. For example, concanavalin A binds to high mannose structures and Ricinus Communis Agglutinin binds to β-galactoside39,40. The degree or extent of glycosylation on an individual protein (i.e. the number of glycans a protein displays) is also genetically encoded by the number of NXT/S motifs a protein has in its sequence8,41. A more comprehensive overview of glycosylation can be found in these reviews28,33,35,37,38,42.
Galectins are a family of to β-galactoside binding lectins43. They are synthesized in the cytoplasm where they are known to play a number of intracellular roles44. In addition, a proportion of these lectins are secreted by the cell by non-classical secretion45. Once outside the cell, galectins can bind to β-galactoside structures on cellular surfaces and mediate functional effects7,9,43,45–48. The galectins are the predominant class of mammalian cellular lectins that have been shown to influence CIE6–15. There are 3 main classes of galectins: 1) Prototypical galectins which have a single carbohydrate recognizing domain (CRD) and can homodimerize, 2) Tandem galectins which have 2 CRDs one at either end of the molecule and 3) Chimeric galectins like galectin 3 which have a single CRD but can multimerize to form a pentameric structure. Due to their multivalency, galectins can play important roles in clustering proteins and in forming large networks of interactions on the cell surface. A more comprehensive review of galectins is beyond the scope of this article and is undertaken in the following reviews47,48.
Glycosylation and galectins play an important role in modulating CIE
One of the interesting aspects surrounding the role glycosylation and galectins play in CIE has been the differing reports that have arisen as to their effect. The Johannes lab has reported that galectin 3 can drive endocytic pit formation/entry of CD44 thus driving a stimulation in CIE12. In their study they show that galectin 3 can be observed in endocytic pits by electron microscopy, that addition of exogenous galectin 3 can stimulate the internalization of CD44 and further that these galectins can drive membrane bending in giant unilamellar vesicles. Their results suggest that the initial membrane curvature in CLIC/GEECs can depend on a mechanism termed the ‘GL-Lect’ hypothesis wherein extracellular clustering of cargo proteins mediated by galectin 3 or shiga and cholera toxins in conjunction with glycolipids drives the initial bending of the membrane6,7.
Work from the Nabi and Dennis labs focused on the CME of various receptors, but coincidentally suggested a role for galectin-glycan interactions in CIE as well. They altered glycosyltransferase expression levels to induce changes in glycan patterns. These altered glycan patterns increased galectin glycan interactions and resulted in a large network of interactions termed the galectin lattice that sequesters cargo like tumor growth factor β receptor and epidermal growth factor receptor (which internalizes by CIE at high concentrations49,50) at the cell surface thus inhibiting their internalization10,11. This inhibitory role of the galectin lattice on EGFR CIE was then directly characterized by the Yarema lab. 1,3,4-O-Bu3ManNAc (a sugar analog that increases the flux through the sialic acid biosynthetic pathway) was used as a means of increasing cell surface sialylation. Increased sialylation masked the epitopes for galectin binding and led to a disruption in the galectin lattice. This disruption of the galectin lattice stimulated CIE specifically13,14. The juxtaposition of these two contrasting modes of activity has made interpreting the role of galectins and glycosylation challenging.
Our recent study proposes a conceptual landscape that we believe can help reconcile these two contrasting views on the role of glycosylation in CIE 15. While it has been hinted at in previous studies that the 2 modes of action (stimulatory vs inhibitory) could be the 2 ends of a spectrum12,47, our study provides an in depth look at how proteins can transition between these two modes of action (Figure 2). We demonstrate that as the level of glycan interactions increases (from left to right in Figure 2), the internalization of CD59 is initially stimulated due to enhanced endocytic pit entry and as the level of glycan interactions continues to increase it then transitions into the cell sequestration mode of action and its internalization is inhibited. The Johannes lab also noticed this type of behavior for CD44, where if all glycan interaction were disrupted and then exogenous galectin 3 was reintroduced, initially there was a stimulation in CIE but at higher concentrations of exogenous galectin 3, they noticed a suppression in CIE12.
Figure 2:
Galectin-glycan interactions mediate a spectrum of response in clathrin independent endocytosis. Initial galectin-glycan interactions can stimulate CIE by stimulating pit formation or cargo entry into pits. Further increasing galectin-glycan interactions leads to the formation of large networks of interactions (i.e. the galectin lattice) which sequesters cargo at the cell surface thus inhibiting endocytosis. These galectin-glycan interactions can be tuned by changes in galectin secretion and by changes in the patterns of glycosylation. Glycan patterns depend on glycosyltransferase expression level and substrate availability. As a result, genetic variations in glycosyltransferase expression and nutrient sensing information via changes in substrate availability can be integrated into changes in glycan patterns on cargo protein. By tuning galectin-glycan interactions a cell may be able to modulate the CIE of various cargo proteins.
Our study also highlighted that within the same cell line distinct cargo could start at different positions on the spectrum. In untreated Hela cells while CD59 started on the inhibitory side of the spectrum major histocompatibility complex Class I was on the stimulatory side of the spectrum. Thus, increasing glycan interactions in these cells led to an increase in internalization of MHCI and a decrease in internalization of CD5915. This observation could also involve aspects of the GL-Lect hypothesis6,7 since an important difference between MHCI and CD59 is that one is an integral membrane protein and the other is a GPI-anchored protein and as such they occupy distinct positions in the membrane bending mechanism presented in that model. This intrinsic difference in these two cargos could account for the differences in sensitivity to changes in glycan interactions and the distinct response landscapes they are observed to have.
From a practical stand point, this highlights the care and consideration that needs to be exercised when studying glycosylation in the context of CIE. Depending on where on this spectrum a cargo protein starts, the effect of a particular change in glycan interactions could be very different. The understanding that cargo proteins can exist along this spectrum of behavior could also account for the contrasting observations by different groups. These observations may focus on and describe the immediate vicinity or the ‘local landscape’ these proteins exist in in different cell lines. This spectrum of behavior could also put into context some of the observed heterogeneity in CIE seen between cell lines and cargo. Each cell line often has a unique glycosylation pattern which could determine the baseline CIE activity of different cargo in individual cell lines51.
Changes in glycosylation alters activities of some plasma membrane receptors and may also influence their endocytosis. It was recently demonstrated that interferon γ receptor partitioning into lipid and actin nanodomains was dependent on glycosylation and that the conformational changes induced by shifting between these nanodomains was essential for JAK signaling52. These types of glycosylation site mutations could also affect endocytosis as changing the number of glycan sites could alter the position a protein starts on the conceptual landscape and thus alter its trafficking characteristics.
The possible scope of CIE regulation via glycan interactions
Glycosylation has an underappreciated ability to code information. N-linked glycosylation alone has a large amount of structural and pattern flexibility based on the non-template driven nature of its synthesis. As a result, the glycan patterns a cell produces can be shifted based on genetic changes (expression of glycosyltransferases, sugar transporters, etc.)32 and substrate availability (changes in nutrient availability)13–15. This allows glycan patterns to represent an integration of genetic and environmental information (Figure 2). In addition, the extent of glycosylation of individual proteins is subject to the number of available glycosylation sites on the protein and transit time through the ER and Golgi. As a result, different protein can also have subtly different information coded onto them based both on factors that are intrinsic to the proteins as well as extrinsic.
In addition to this, a cell can also control the degree to which these changes in glycosylation are transduced into functional effects by modulating the other side of the glycan interaction (i.e. the secreted galectins). The amount of galectin available to interact with glycans can be controlled by altering their expression level, secretion or which type of galectin is predominantly expressed.
This could provide the cell a unique tool box with which it could regulate CIE. While CIE is primarily thought of as a bulk pathway it is essential in numerous cellular functions such as plasma membrane turnover, recycling and repair, cell spreading, cell migration cell polarization and modulation of intercellular signaling1. Therefore, a means by which cells could regulate CIE could allow them to tune a number of essential cellular functions. By changing expression levels of glycosyltransferases or in response to a change in nutrient availability, a cell can shift its global glycan pattern landscape and by doing so drive changes in CIE. These changes in CIE would be cargo specific in so much as they would depend on where on the activity spectrum each protein begins (which could be influenced by the number of glycosylation sites, ER-Golgi transit time, lipid raft association, etc.). The cell can also tune its sensitivity to changes in glycosylation by altering galectin synthesis and secretion. The ability of glycosylation to orchestrate the trafficking of numerous cargo proteins simultaneously but in a targeted and cargo-specific manner could be especially important in numerous disease contexts where hallmark altered patterns of glycosylation are observed and could be affecting disease progression by driving widespread alterations in protein trafficking via CIE22,24,27,33,34,53,54.
Outside of diseases, cells from different lineages that exist in different biological microenvironments and niches also have distinct glycosylation patterns. This could suggest that cells in different settings may be able to perform complex regulation of their trafficking by using glycosylation to integrate and react to both genetic and environmental stimuli. Another setting that could result in alterations in glycosylation is nutrient stress often observed in cancer and during autophagy. A study in colon cancer cells showed that nutrient stress reduced the glycosylation of the G-protein coupled receptor (LGR5). This led to reduced protein stability and a decrease in cell surface localization55. Understanding how glycosylation changes under conditions of nutrient stress could allow us to characterize another avenue by which cells can transduce nutrient stress signals into functional effects.
Almost every protein on the cell surface is glycosylated. Endocytic pathways like CME are less sensitive to extracellular manipulation by glycan interactions because cargo proteins are efficiently sorted and concentrated into clathrin coated pits. In contrast, the various pathways that have been described as clathrin independent involve a variety of cargo proteins that have longer residency time at the plasma membrane and hence would have increased sensitivity to extracellular stimuli that arise via glycan interactions.
Galectin secretion could facilitate population regulation of CIE across different length scales
Apart from individual cellular regulation of CIE, glycans and galectins could provide interesting possibilities for population and microenvironment regulation. Galectins are cytoplasmic proteins that are secreted by the cell. As a result, changes in cellular secretion of galectins could affect not just the cell in question but also cells in the surrounding microenvironment. As a result, CIE across populations of cells could be regulated via galectins and glycans. This form of regulation could potentially provide cells a way to communicate and assert functional effects across populations. This could also be a means of communicating specific functional information over distance scales from micro to macro. For instance, galectin 3 serum levels are often observed to be elevated in cancer56–59, this could be a way in which a tumor alters or regulates CIE (among other things) at potential secondary sites for metastasis.
Elevated serum galectin 3 levels are also associated with almost all types of cardiovascular disease and is generally a prognostic marker for poorer outcomes60–62. High levels of circulating galectin 3 was found to be associated with depression in patients with type 1 diabetes63. Elevated serum levels of galectin 3 are also found to precede chronic kidney disease64 and are known to be associated with schizophrenia65. Aside from its dysregulation in these disease contexts, galectin 3 also plays important roles in proper bone cell differentiation and bone remodeling66, as well as in driving oligodendrocyte differentiation67. In all these various biological situations the correct balance of galectin 3 is important and shifting that balance has profound effects. Galectin 3 mediated modulation of CIE could potentially be a factor of the molecular mechanism underlying the effect of altered galectin 3 levels in these various contexts. The key role galectin 3 plays in the modulation of CIE also highlights the importance of a better understanding of the regulation of galectin synthesis, stability and how these proteins are secreted.
Conclusion
Galectin-glycan interactions organize the cell surface and can either promote or inhibit cargo entry by CIE. These interactions can also promote the formation of invaginations (pits). Our study along with a number of other recent studies suggest that CIE can be regulated by glycan interaction and that this regulation may be cargo protein specific. These galectin-glycan interactions could serve as extracellular machinery facilitating CIE and providing a unifying mode of regulation and control. Glycan interactions are a versatile tool that cells could use to regulate CIE in complex and nuanced ways ranging from the single cell level to large populations, and possibly even communicate some of this information across macroscale distances.
Synopsis:
Glycosylation and glycan interactions have been shown to affect clathrin independent endocytosis. We explore the implications of these findings and discuss how galectins and glycans can serve as extracellular machinery and may play a role in facilitating and modulating clathrin independent endocytosis.
References
- 1.Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Current Opinion in Cell Biology. 2010;22(4):519–527. [DOI] [PubMed] [Google Scholar]
- 2.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517–533. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson JG, Porat-Shliom N, Cohen LA. Clathrin-independent endocytosis: A unique platform for cell signaling and PM remodeling. Cellular Signalling. 2009;21(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayor S, Parton RG, Donaldson JG. Clathrin-Independent Pathways of Endocytosis. Cold Spring Harbor Perspectives in Biology. 2014;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandvig K, Kavaliauskiene S, Skotland T. Clathrin-independent endocytosis: an increasing degree of complexity. Histochemistry and Cell Biology. 2018;150(2):107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannes L, Parton RG, Bassereau P, Mayor S. Building endocytic pits without clathrin. Nat Rev Mol Cell Biol. 2015;16(5):311–321. [DOI] [PubMed] [Google Scholar]
- 7.Johannes L, Wunder C, Shafaq-Zadah M. Glycolipids and Lectins in Endocytic Uptake Processes. Journal of Molecular Biology. 2016;428(24, Part A):4792–4818. [DOI] [PubMed] [Google Scholar]
- 8.Dennis JW, Lau KS, Demetriou M, Nabi IR. Adaptive Regulation at the Cell Surface by N-Glycosylation. Traffic. 2009;10(11):1569–1578. [DOI] [PubMed] [Google Scholar]
- 9.Nabi IR, Shankar J, Dennis JW. The galectin lattice at a glance. Journal of Cell Science. 2015;128(13):2213–2219. [DOI] [PubMed] [Google Scholar]
- 10.Partridge EA, Le Roy C, Di Guglielmo GM, et al. Regulation of Cytokine Receptors by Golgi N-Glycan Processing and Endocytosis. Science. 2004;306(5693):120–124. [DOI] [PubMed] [Google Scholar]
- 11.Lajoie P, Partridge EA, Guay G, et al. Plasma membrane domain organization regulates EGFR signaling in tumor cells. The Journal of Cell Biology. 2007;179(2):341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakshminarayan R, Wunder C, Becken U, et al. Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nature cell biology. 2014;16(6):595. [DOI] [PubMed] [Google Scholar]
- 13.Mathew MP, Tan E, Saeui CT, et al. Metabolic flux-driven sialylation alters internalization, recycling, and drug sensitivity of the epidermal growth factor receptor (EGFR) in SW1990 pancreatic cancer cells. Oncotarget. 2016;7(41):66491–66511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathew MP, Tan E, Saeui CT, et al. Metabolic glycoengineering sensitizes drug-resistant pancreatic cancer cells to tyrosine kinase inhibitors erlotinib and gefitinib. Bioorganic & Medicinal Chemistry Letters. 2015;25(6):1223–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathew MP, Donaldson JG. Distinct cargo-specific response landscapes underpin the complex and nuanced role of galectin-glycan interactions in clathrin-independent endocytosis. Journal of Biological Chemistry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucrot E, Ferreira APA, Almeida-Souza L, et al. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2014;517:460. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe S, Rost BR, Camacho-Pérez M, et al. Ultrafast endocytosis at mouse hippocampal synapses. Nature. 2013;504:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundmark R, Doherty GJ, Howes MT, et al. The GTPase-Activating Protein GRAF1 Regulates the CLIC/GEEC Endocytic Pathway. Current Biology. 2008;18(22):1802–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Molecular biology of the cell. 2004;15(8):3542–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eyster CA, Higginson JD, Huebner R, et al. Discovery of New Cargo Proteins that Enter Cells through Clathrin-Independent Endocytosis. Traffic. 2009;10(5):590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakomori S-i. Tumor Malignancy Defined by Aberrant Glycosylation and Sphingo(glyco)lipid Metabolism. Cancer Research. 1996;56(23):5309–5318. [PubMed] [Google Scholar]
- 23.Hakomori S Glycosylation defining cancer malignancy: new wine in an old bottle. Proceedings of the National Academy of Sciences. 2002;99(16):10231–10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reis CA, Osorio H, Silva L, Gomes C, David L. Alterations in glycosylation as biomarkers for cancer detection. Journal of Clinical Pathology. 2010;63(4):322–329. [DOI] [PubMed] [Google Scholar]
- 25.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 Update of Recommendations for the Use of Tumor Markers in Gastrointestinal Cancer. Journal of Clinical Oncology. 2006;24(33):5313–5327. [DOI] [PubMed] [Google Scholar]
- 26.Wong D, Ko AH, Hwang J, Venook AP, Bergsland EK, Tempero MA. Serum CA19–9 Decline Compared to Radiographic Response as a Surrogate for Clinical Outcomes in Patients With Metastatic Pancreatic Cancer Receiving Chemotherapy. Pancreas. 2008;37(3):269–274. [DOI] [PubMed] [Google Scholar]
- 27.Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconjugate Journal. 1997;14(5):569–576. [DOI] [PubMed] [Google Scholar]
- 28.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochimica et Biophysica Acta (BBA) - General Subjects. 1999;1473(1):21–34. [DOI] [PubMed] [Google Scholar]
- 29.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nature Reviews Cancer. 2015;15:540. [DOI] [PubMed] [Google Scholar]
- 30.Goetze AM, Liu YD, Zhang Z, et al. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology. 2011;21(7):949–959. [DOI] [PubMed] [Google Scholar]
- 31.Almaraz RT, Mathew MP, Tan E, Yarema KJ. Metabolic Oligosaccharide Engineering: Implications for Selectin-Mediated Adhesion and Leukocyte Extravasation. Annals of Biomedical Engineering. 2012;40(4):806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD. Dietary and Genetic Control of Glucose Transporter 2 Glycosylation Promotes Insulin Secretion in Suppressing Diabetes. Cell. 2005;123(7):1307–1321. [DOI] [PubMed] [Google Scholar]
- 33.Ohtsubo K, Marth JD. Glycosylation in Cellular Mechanisms of Health and Disease. Cell. 2006;126(5):855–867. [DOI] [PubMed] [Google Scholar]
- 34.Almaraz RT, Tian Y, Bhattarcharya R, et al. Metabolic Flux Increases Glycoprotein Sialylation: Implications for Cell Adhesion and Cancer Metastasis. Molecular & Cellular Proteomics. 2012;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulson JC, Colley KJ. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. Journal of Biological Chemistry. 1989;264(30):17615–17618. [PubMed] [Google Scholar]
- 36.Burda P, Aebi M. The dolichol pathway of N-linked glycosylation. Biochimica et Biophysica Acta (BBA)-General Subjects. 1999;1426(2):239–257. [DOI] [PubMed] [Google Scholar]
- 37.Imperiali B, Hendrickson TL. Asparagine-linked glycosylation: specificity and function of oligosaccharyl transferase. Bioorganic & medicinal chemistry. 1995;3(12):1565–1578. [DOI] [PubMed] [Google Scholar]
- 38.Steen PVd, Rudd PM, Dwek RA, Opdenakker G. Concepts and Principles of O-Linked Glycosylation. Critical Reviews in Biochemistry and Molecular Biology. 1998;33(3):151–208. [DOI] [PubMed] [Google Scholar]
- 39.Lis H, Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. [DOI] [PubMed] [Google Scholar]
- 40.Rüdiger H, Gabius H-J. Plant lectins: Occurrence, biochemistry, functions and applications. Glycoconjugate Journal. 2001;18(8):589–613. [DOI] [PubMed] [Google Scholar]
- 41.Lau KS, Partridge EA, Grigorian A, et al. Complex N-Glycan Number and Degree of Branching Cooperate to Regulate Cell Proliferation and Differentiation. Cell. 2007;129(1):123–134. [DOI] [PubMed] [Google Scholar]
- 42.Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12(4):43R–56R. [DOI] [PubMed] [Google Scholar]
- 43.Hirabayashi J, Hashidate T, Arata Y, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochimica et Biophysica Acta (BBA) - General Subjects. 2002;1572(2):232–254. [DOI] [PubMed] [Google Scholar]
- 44.Liu F-T, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochimica et Biophysica Acta (BBA) - General Subjects. 2002;1572(2):263–273. [DOI] [PubMed] [Google Scholar]
- 45.Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconjugate Journal. 2002;19(7):433–440. [DOI] [PubMed] [Google Scholar]
- 46.Patterson RJ, Wang W, Wang JL. Understanding the biochemical activities of galectin-1 and galectin-3 in the nucleus. Glycoconjugate Journal. 2002;19(7):499–506. [DOI] [PubMed] [Google Scholar]
- 47.Johannes L, Jacob R, Leffler H. Galectins at a glance. Journal of Cell Science. 2018;131(9). [DOI] [PubMed] [Google Scholar]
- 48.Demetriou M, Nabi IR, Dennis JW. Galectins as Adaptors: Linking Glycosylation and Metabolism with Extracellular Cues. Trends in Glycoscience and Glycotechnology. 2018;30(172):SE167–SE177. [Google Scholar]
- 49.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-Mediated Internalization Is Essential for Sustained EGFR Signaling but Dispensable for Degradation. Developmental Cell. 2008;15(2):209–219. [DOI] [PubMed] [Google Scholar]
- 50.Sigismund S, Woelk T, Puri C, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(8):2760–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nature Reviews Molecular Cell Biology. 2012;13:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blouin CM, Hamon Y, Gonnord P, et al. Glycosylation-Dependent IFN-γR Partitioning in Lipid and Actin Nanodomains Is Critical for JAK Activation. Cell. 2016;166(4):920–934. [DOI] [PubMed] [Google Scholar]
- 53.Mechref Y, Hu Y, Garcia A, Zhou S, Desantos-Garcia JL, Hussein A. Defining putative glycan cancer biomarkers by MS. Bioanalysis. 2012;4(20):2457–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adamczyk B, Tharmalingam T, Rudd PM. Glycans as cancer biomarkers. Biochimica et Biophysica Acta (BBA) - General Subjects. 2012;1820(9):1347–1353. [DOI] [PubMed] [Google Scholar]
- 55.Morgan RG, Molnár E, Jones RF, et al. Nutrient stress alters the glycosylation status of LGR5 resulting in reduced protein stability and membrane localisation in colorectal tumour cells: implications for targeting cancer stem cells. British Journal Of Cancer. 2015;112:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bresalier RS, Mazurek N, Sternberg LR, et al. Metastasis of human colon cancer is altered by modifying expression of the β-galactoside-binding protein galectin 3. Gastroenterology. 1998;115(2):287–296. [DOI] [PubMed] [Google Scholar]
- 57.Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconjugate Journal. 2002;19(7):543–549. [DOI] [PubMed] [Google Scholar]
- 58.Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of Galectin-3 in the Sera of Normal Controls and Cancer Patients. Clinical Cancer Research. 2000;6(4):1389–1393. [PubMed] [Google Scholar]
- 59.Sakaki M, Oka N, Nakanishi R, Yamaguchi K, Fukumori T, Kanayama H-o. Serum level of galectin-3 in human bladder cancer. The Journal of Medical Investigation. 2008;55(1,2):127–132. [DOI] [PubMed] [Google Scholar]
- 60.Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P, Cohn JN. Baseline and serial measurements of galectin-3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. European Journal of Heart Failure. 2013;15(5):511–518. [DOI] [PubMed] [Google Scholar]
- 61.Takemoto Y, Ramirez RJ, Yokokawa M, et al. Galectin-3 Regulates Atrial Fibrillation Remodeling and Predicts Catheter Ablation Outcomes. JACC Basic to translational science. 2016;1(3):143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piper SE, de Courcey J, Sherwood RA, Amin-Youssef GF, McDonagh TA. Serial galectin-3 for the monitoring of optimally treated stable chronic heart failure: A pilot study. International Journal of Cardiology. 2016;207:279–281. [DOI] [PubMed] [Google Scholar]
- 63.Melin EO, Dereke J, Thunander M, Hillman M. Depression in type 1 diabetes was associated with high levels of circulating galectin-3. 2018;7(6):819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Seaghdha CM, Hwang S-J, Ho JE, Vasan RS, Levy D, Fox CS. Elevated galectin-3 precedes the development of CKD. Journal of the American Society of Nephrology : JASN. 2013;24(9):1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kajitani K, Yanagimoto K, Nakabeppu Y. Serum galectin-3, but not galectin-1, levels are elevated in schizophrenia: implications for the role of inflammation. Psychopharmacology. 2017;234(19):2919–2927. [DOI] [PubMed] [Google Scholar]
- 66.Iacobini C, Blasetti Fantauzzi C, Bedini R, et al. Galectin-3 is essential for proper bone cell differentiation and activity, bone remodeling and biomechanical competence in mice. Metabolism. 2018;83:149–158. [DOI] [PubMed] [Google Scholar]
- 67.Pasquini LA, Millet V, Hoyos HC, et al. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell death and differentiation. 2011;18(11):1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]