Abstract
Background:
Pain expectancies are associated with altered pain sensitivity in individuals with chronic pain. However, little is known about the processes by which pain expectancies impact pain processing. This study assessed the association between pain expectancies and temporal summation (TS) of pain, and examined whether pain catastrophizing mediated this association.
Methods:
In this cross-sectional study, participants (437 chronic low back pain (CLBP) patients, 115 controls) completed self-report measures of pain intensity, pain expectancies and pain catastrophizing before undergoing psychophysical pain-testing procedures designed to assess mechanical TS of mechanical pain. Pearson’s correlations examined the associations between study variables in CLBP patients and controls. Bootstrapping mediation analyses assessed the mediating role of pain catastrophizing on the association between pain expectancies and TS of pain.
Results:
TS of pain was significantly associated with pain expectancies (r = .113) and pain catastrophizing (r = .171) in CLBP patients. Results of mediation analyses revealed that pain catastrophizing mediated the relationship between pain expectancies and TS of pain in CLBP patients (ab = .309, 95% CI = .1222 - .5604), but not in healthy controls (ab = −.125, 95% CI = −.5864 - .0244).
Conclusions:
The findings from this study suggest that compared to controls, CLBP patients show increased sensitivity to mechanical pain procedures and enhanced pain facilitatory processing, proving further evidence for changes in central nervous system pain processing in CLBP patients. Our results also suggest that pain catastrophizing may be the mechanism by which pain expectancies are associated with TS of pain in CLBP patients.
Keywords: Pain expectancies, pain catastrophizing, pain sensitivity, temporal summation of pain, chronic pain
1. Introduction
Within the United States, low back pain is a leading cause of pain and disability and carries costs of over 300 billion dollars annually (Dagenais et al., 2008; Woolf and Pfleger, 2003). Although most individuals with acute low back pain recover within weeks, approximately 10% develop chronic low back pain (CLBP) and remain permanently disabled (Centers for Disease Control and Prevention (CDC), 2009). Over the past few decades, considerable efforts have been devoted towards the identification of factors that may be involved in the development and maintenance of chronic pain.
Pain expectancies have been discussed as an important determinant of the pain experience. In the context of chronic pain and during experimental pain procedures, individuals who expect higher pain report heightened pain sensitivity. Research has shown that higher pain expectancies are associated with reduced cold pressure pain tolerance and higher cold pain ratings (Hanssen et al., 2013; 2012). Similar findings have been reported in response to thermal pain (Larivière et al., 2007) and in response to mechanical high-frequency electrical stimulation (Van Den Broeke et al., 2013). Research also suggests that in chronic pain patients, higher pain expectancies are associated with deficits in conditioned pain modulation during cold and heat pain threshold tasks (France et al., 2016; Goffaux et al., 2007).
Currently lacking in the literature is an examination of the association between pain expectancies and temporal summation (TS) of pain. TS of pain refers to the progressive increase in pain severity despite constant peripheral afferent input (Vierck et al., 1997) and provides an indirect method of evaluating central nervous system sensitization (Edwards et al., 2006b; Goodin et al., 2013; Staud et al., 2003). There are indications that individuals who experience elevated pain sensitivity to repeated painful stimuli, such as TS, may be at greater risk for adverse pain outcomes (Edwards, 2005; George et al., 2006; Mankovsky-Arnold et al., 2014; Sullivan et al., 2010). It has been suggested TS may reflect a combination of neurophysiologic events, as well as psychological processes such as increased attention to pain (Schreiber et al., 2014), or reductions in the efficacy of pain-coping strategies with repeated stimulation (Edwards et al., 2006b).
Also lacking in the literature is an examination of the process by which pain expectancies influence pain sensitivity. There is a basis for proposing that pain catastrophizing might be the mechanism through which pain expectancies impact pain sensitivity and pain facilitatory processes, such as TS of pain. Pain catastrophizing has been described as an exaggerated negative response to actual or anticipated pain (Sullivan et al., 1995; Van Damme et al., 2002a) and has been associated with altered pain sensitivity and enhanced pain-facilitatory processes, including TS of pain (Edwards et al., 2004; 2006b; George et al., 2007). Moreover, several investigations have shown significant associations between expectancies and pain catastrophizing (Carriere et al., 2015a; Sullivan et al., 2011; 2001a; Van Damme et al., 2002b), which may suggest that more pessimistic expectancies about future pain experiences may lead to increases in ruminative thoughts, feelings of helplessness, and magnification of the threat value of pain (i.e., the dimensions of pain-related catastrophizing). There is also evidence that pain catastrophizing mediates the influence of dispositional optimism on TS of pain (Goodin et al., 2013), where dispositional optimism may be considered a proxy for expectancies.
This study examined the relationships between pain expectancies, pain catastrophizing and TS of pain in CLBP patients and healthy controls. Following theorized and demonstrated associations between pain expectancies and TS of pain, we examined whether pain catastrophizing mediates the influence of pain expectancies on TS of pain. From a theoretical perspective, an examination of the relationships between pain expectancies, pain catastrophizing and TS of pain will provide a better understanding of how model-relevant constructs summate or interact to give rise to changes in central nervous pain processing. Moreover, by comparing CLBP patients and healthy controls, we will be able to determine whether these processes are specific to chronic pain patients. From a clinical perspective, such research is important because it may help identify which factors should be key targets for psychosocial interventions designed to improve pain outcomes.
2. Methods
2.1. Participants
The study sample consisted of 552 participants (437 CLBP patients, 115 healthy controls) recruited from the Pain Management Center at Brigham and Women’s Hospital in Chestnut Hill, Massachusetts. Participants were eligible for inclusion if they were above 18 years of age and were able to speak, read, and write in English. CLBP patients had a diagnosis of back pain with or without radicular symptoms, and reported experiencing pain for at least 6 months. CLBP patients reported their use of prescription opioid medications. Patients’ reports of prescription opioids were verified by the research assistant using the electronic medical record system. Control subjects reported no history of persistent pain, no current acute pain and no current use of pain medications or recreational drugs. Participants were excluded if they had a diagnosis of cancer or other malignant diseases at the time of testing, or if they had cognitive limitations that precluded providing self-report data. All participants provided written consent prior to beginning the study procedures.
2.2. Procedures
All procedures were approved by the Partners Institutional Review Board at Brigham and Women’s Hospital. In line with the recommended minimal dataset for CLBP (Deyo et al., 2014), participants were invited to sign a consent form and to provide demographic information, which included information regarding age, gender, race, education level and medication use. Participants were asked to rate their current pain intensity and completed self-report questionnaires (see section 2.3) prior to undergoing standardized psychophysical pain-testing procedures designed to assess TS of mechanical pain. A measure of depressive symptoms was included because of its known associations with the study variables.
2.3. Measures
Pain Expectancies
Pain-specific expectancies were measured by asking participants “How much pain do you expect to experience from these testing procedures”. Participants indicated their responses on a scale with the end points “0” being no pain to “10” being the most pain imaginable. Higher scores indicate higher levels of expected pain. Similar measures of pain expectancies have been used in previous pain research (Arntz and Peters, 1995; Sullivan et al., 2001a).
Pain Catastrophizing
Pain catastrophizing was measured with the Pain Catastrophizing Scale (PCS) (Sullivan et al., 1995) a well-validated, widely-used self-report measure of catastrophic thinking associated with pain (Edwards et al., 2006a). The PCS consists of 13 items describing thoughts and feelings that individuals may experience when they are in pain, and consists of elements of rumination, magnification and helplessness. Total scores for the PCS range from 0 to 52; higher scores indicate a greater frequency of catastrophic thoughts. The PCS has good psychometric properties in pain patients and controls (Van Damme et al., 2002a). Cronbach’s a for the PCS has been shown to be above .9, indicating very high levels of internal consistency (Edwards et al., 2010).
Symptoms of Depression
The Beck Depression Inventory (BDI-II) was used as a measure of depressive symptom severity (Beck et al., 1996). The BDI-II consists of 21 items describing different symptoms of depression. Total scores on the BDI-II can range between 0 and 63 where higher scores reflect more severe symptoms of depression. The BDI-II has been shown to be a reliable and valid measure of depressive symptoms for individuals with pain (Harris and D’Eon, 2008), and has been frequently used in studies with patients with back and/or neck pain (Arnau et al., 2001; Bishop et al., 1993).
Temporal Summation of Pain
Participants underwent an assessment of mechanical TS of pain using weighted pinprick stimulators, as in previous studies (Edwards et al., 2016; Rhudy et al., 2011) . These punctuate mechanical probes have a flat contact area of .2 mm in diameter and exert forces between 8 and 512 mN. Participants rated the pain intensity produced by 64 mN, 128 mN, and 256 mN stimulators on a 0 to 100 verbal pain intensity scale used in previous studies (Edwards et al., 2009a; 2009b). The lowest-force stimulator that produced a sensation of discomfort (128 or 256mN for most subjects) was used to apply a train of 10 stimuli to the skin on the dorsum of the middle finger of the hand at the rate of 1 per second. Participants rated the painfulness of the first, fifth, and tenth stimulus using the same 0 to 100 pain verbal pain intensity scale previously described. TS was quantified as the difference in pain intensity ratings between the tenth and the first stimulus. This subtraction index is a well-accepted measure of TS of pain used in pain research (Cheng et al., 2015; Edwards et al., 2013; 2016; 2003; Goodin et al., 2013; Rhudy et al., 2011; Staud et al., 2014).
Pain Intensity
Participants rated their current pain intensity using a numeric rating scale with the end points “0” being no pain and “10” being the worst pain imaginable (Farrar et al., 2001). The NRS has been validated for specificity and use in chronic pain research (Farrar et al., 2001; Hjermstad et al., 2011).
2.4. Data analysis
Means, standard deviations and counts were calculated for relevant study variables. T-tests for independent samples were used to examine differences between males and females, as well as between CLBP patients and controls on measures of current pain intensity, depressive symptoms, pain expectancies, pain catastrophizing and TS of pain. Chi-square analyses were used to examine whether males and females differed in terms of prescription opioid use. Analyses of variance were then conducted to examine group differences on measures of race and education. Correlations were used to examine the associations between study variables in CLBP patients and controls.
Mediation analyses were conducted using the SPSS macro (PROCESS) developed by Preacher and Hayes (Preacher and Hayes, 2004; 2008). PROCESS uses bootstrapping, a nonparametric procedure that is increasingly being used to test mediation (i.e. indirect) effects. It provides a way of circumventing power deficiencies of normal theory tests (i.e. Sobel) typically introduced by the non-normality in the sampling distribution (Hayes, 2009; Mackinnon et al., 2002; Shrout and Bolger, 2002). Bias-corrected 95% confidence intervals were produced for each potential mediator and were used to test the significance of the total and indirect (i.e. mediation) effects. Estimates of indirect effects were considered significant in the case that zero was not included within the confidence intervals (Preacher and Hayes, 2004; 2008). Mediation analyses controlled for all study variables that were significantly associated with the primary study outcome (i.e. TS of pain).
3. RESULTS
3.1. Participant Characteristics
Participant demographic characteristics are presented in Table 1. Of the 552 participants who completed the self-report measures and the quantitative sensory testing procedures, 34.8% were male and 65.2% were female; 76.5% identified as White or Caucasian, 15.6% identified as Black or African American, 1.5% identified as Hispanic or Latino, and 4.7% identified as American Indian, Native Hawaiian or of more than one race. Participants ranged from 27 to 87 years of age (M = 55.87, SD = 12.17), and most had completed at least some college (81.6%). Gender differences were observed in CLBP patients, where female participants reported significantly higher levels of current pain intensity (t(2,549) = −2.620, p = .009). No other gender differences were observed.
Table 1.
Demographic variables
| Total sample (N = 552) |
CLBP patients (n = 437) |
Controls (n = 115) |
||
|---|---|---|---|---|
| Variable | N (%) or Mean (SD) | |||
| Gender | Male | 192 (27.5) | 148 (33.9) | 44 (38.3) |
| Female | 360 (65.2) | 289 (66.1) | 71 (61.7) | |
| Age (years) | 55.87 (12.17) | 55.68 (12.4) | 56.61 (10.9) | |
| Race | White or Caucasian | 421 (76.5) | 326 (74.9) | 95 (82.6) |
| Black or African American | 86 (15.6) | 74 (17) | 12 (10.4) | |
| Hispanic or Latino | 8 (1.5) | 8 (1.8) | - | |
| Asian | 8 (1.5) | 3 (.7) | 5 (4.3) | |
| American Indian/Alaskan native | 4 (0.7) | 4 (.9) | - | |
| Native Hawaiian or other Pacific Islander | 1 (0.2) | 1 (.2) | - | |
| More than one race | 22 (4) | 19 (4.4) | 3 (2.6) | |
| Education Level | Some high school | 12 (2.2) | 10 (2.3) | 2 (1.8) |
| High school graduate/GED | 78 (14.3) | 66 (15.2) | 12 (10.6) | |
| Tech school graduate | 11 (2) | 10 (2.3) | 1 (.9) | |
| Some college | 137 (25.1) | 116 (26.8) | 21 (18.6) | |
| College graduate | 181 (33.2) | 133 (30.7) | 48 (42.5) | |
| Master’s degree | 103 (18.9) | 79 (18.2) | 24 (21.2) | |
| Doctoral degree | 24 (4.4) | 19 (4.4) | 5 (4.4) | |
| Prescription opioid use | 117 (16.8) | 117 (26.8) | - | |
3.2. Differences between CLBP patients and controls
Notable group differences were observed across study variables and are summarized in Table 2. CLBP patients reported significantly higher current pain intensity (t(2,549) = 31.121, p < .001), depressive symptoms (t(2,549) = 11.257, p < .001), pain catastrophizing (t(2,549) = 10.510, p < .001) and TS of pain (t(2,549) = 6.698, p < .001). Although CLBP patients (M = 3.84, SD = 2.54) reported higher pain expectancies than controls (M = 3.40, SD = 2.46), these differences were non-significant (t(2,549) = 1.682, p = .094). Analyses of variance revealed no group differences (between CLBP patients and controls) on measures of age, education and race.
Table 2.
Descriptive statistics for pain intensity, depressive symptoms, pain expectancies, pain catastrophizing and temporal summation of pain.
| Mean (SD) | Total Sample (N = 552) |
CLBP patients (n = 437) |
Controls (n = 115) |
|---|---|---|---|
| Pain Intensity | 3.27 (2.97) | 4.12 (2.76)* | .01 (.08) |
| Depressive symptoms | 11.09 (8.19) | 12.69 (7.91)* | 4.99 (6.12) |
| Pain expectancies | 3.75 (2.53) | 3.84 (2.54) | 3.40 (2.46) |
| Pain catastrophizing | 16.55 (12.10) | 18.55 (12.28)* | 8.96 (7.49) |
| Mechanical probe pain ratings | |||
| Probe 1 | 14.68 (14.50) | 15.10 (15.40) | 13.06 (10.27) |
| Probe 5 | 22.19 (20.12) | 23.62 (21.01)* | 16.76 (15.15) |
| Probe 10 | 28.98 (24.98) | 30.92 (26.05)* | 18.63 (17.26) |
| TS of pain | 13.68 (18.27) | 15.82 (18.80)* | 5.56 (13.28) |
Note. Pain intensity = current pain intensity; Depressive symptoms = Beck Depression Inventory-II; Pain catastrophizing = Pain Catastrophizing Scale, TS of pain = temporal summation of mechanical pain.
CLBP patients differ significantly from controls (p < .001)
3.3. Associations among study variables
Table 3 shows the bivariate correlations among study variables in each of the study groups. In CLBP patients, TS of pain was significantly positively associated with pain expectancies (r = .113, p = .018) and pain catastrophizing (r = .171, p < .001). In CLBP patients, TS of pain was not significantly associated with age, education, race, pain intensity and depressive symptoms. Prescription opioid use was significantly associated with age (t(2,549) = −7.619, p < .001), current pain intensity (t(2,549) = 10.82, p < .001), depressive symptoms (t(2,549) = 7.573, p < .001), pain expectancies (t(2,549) = 2.647, p = .009), and pain catastrophizing (t(2,549) = 5.935, p < .001). In the control group, only age was significantly positively associated with TS of pain (r = .208).
Table 3.
Associations between study variables
| 1. | 2. | 3. | 4. | 5. | 6. | ||
|---|---|---|---|---|---|---|---|
| CLBP patients (n = 437) | 1. Age | ||||||
| 2. Pain intensity | −.377** | ||||||
| 3. Depressive symptoms | −.159** | .289** | |||||
| 4. Pain expectancies | 0.006 | .080 | .150* | ||||
| 5. Pain catastrophizing | −.244** | .436** | .513** | .265** | |||
| 6. TS of pain | −.011 | .076 | .015 | .113* | .171** | ||
| 7. Opioid use | −7.62** | 10.82** | 7.57** | 2.65* | 5.94** | 1.62 | |
| Controls (n = 115) | 1. Age | ||||||
| 2. Pain intensity | .121 | ||||||
| 3. Depressive symptoms | −.059 | -.089 | |||||
| 4. Pain expectancies | −.209* | −.116 | −.209* | ||||
| 5. Pain catastrophizing | −.193* | −.065 | .348** | .230* | |||
| 6. TS of pain | .208* | .010 | −.050 | .053 | −.129 | ||
* p < .05 ,
p < .001.
N = 552. Table presents results of correlational analyses between continuous study variables and independent samples t-tests for the categorical variable (prescription opioid use). CLBP patients = chronic low back pain patients; Pain intensity = current pain intensity; Depressive symptoms = Beck Depression Inventory-II; Pain catastrophizing = Pain Catastrophizing Scale, TS of pain = temporal summation of mechanical pain.
3.4. Mediation Analyses
The first mediation analysis was performed in the group of CLBP patients (Table 4; Figures 1-2). Results confirmed that pain catastrophizing mediates the influence of pain expectancies on TS of pain. All paths within the model were significant: pain expectancies were significantly positively associated with pain catastrophizing (path a = 1.281, p < .001), as well as with TS of pain (path c = .832, p = .018). Moreover, pain catastrophizing was significantly positively associated with TS of pain (path b = .241, p = .001). The bias-corrected bootstrap 95% confidence interval for the total indirect effect based on 5000 bootstrap samples did not include zero (BC 95% CI = .122 to .5604), confirming that pain catastrophizing mediates the influence of pain expectancies on TS of pain in CLBP patients.
Table 4.
Bootstrapped mediation analyses testing the indirect effect of pain expectancies on TS of pain through pain catastrophizing
|
Path coefficient |
Bootstrap SE |
t | p | BC 95% CI | ||
|---|---|---|---|---|---|---|
| CLBP patients (n = 437) | Path a | 1.2811 | .2238 | 5.7252 | .001 | |
| Path b | .2412 | .0745 | 3,2370 | .001 | ||
| Path c | .8320 | .3511 | 2.3695 | .018 | ||
| Path c1 | .5230 | .3602 | 1.4518 | .147 | ||
| Total indirect effect | ||||||
| a x b | .3090 | .1117 | LL=.1222 UL=.5604 | |||
| Controls (n = 115) | Path a | .6016 | .2827 | 2.1279 | .036 | |
| Path b | −.2076 | .1691 | −1.2278 | .222 | ||
| Path c | .5422 | .5071 | 1.0693 | .287 | ||
| Path c1 | .6671 | .5160 | 2.220 | .0282 | ||
| Total indirect effect | ||||||
| a x b | −.1249 | .1327 | LL=−.5864 UL=.0244 | |||
Note: SE, standard error; CI: Confidence intervals; LL, lower limit; UL, upper limit.Note. Table shows standardized path coefficients for the total and specific indirect effects. Path a, effect of pain expectancies on pain catastrophizing; Path b, effect of pain catastrophizing on TS of pain; Path c, total effect of pain expectancies on TS of pain; Path c1, direct effect of pain expectancies on TS of pain; Path ab, indirect effect of pain expectancies on TS of pain through pain catastrophizing. Path coefficients are based on 5,000 bootstraps for the indirect effect. Lower limit and upper limit confidence intervals were used to determine statistical significance of indirect effects.
Figure 1.
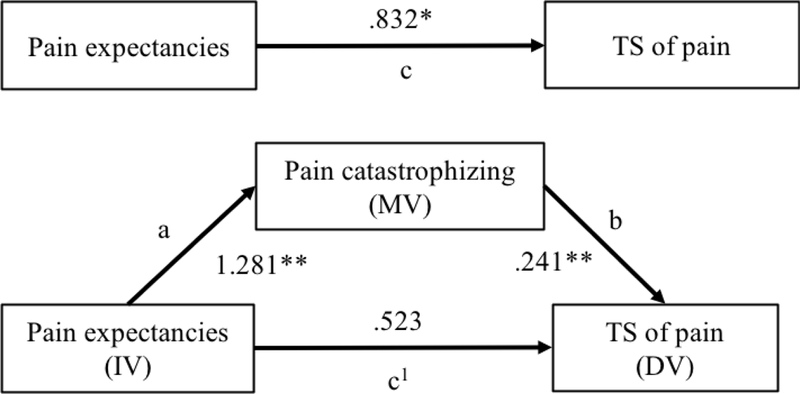
Mediation model illustrating the impact of pain expectancies on TS of pain through pain catastrophizing in CLBP patients. Path coefficients are presented. DV, dependent variable; IV, independent variable; MV, mediating variable. ** p < .001, * p < .05.
Figure 2.
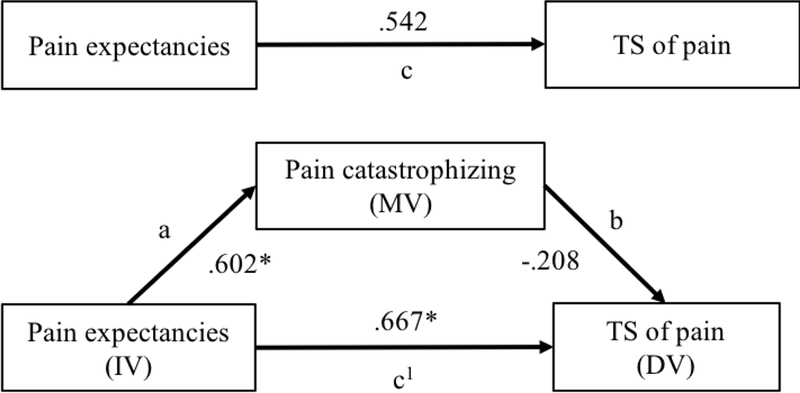
Mediation model illustrating the impact of pain expectancies on TS of pain through pain catastrophizing in healthy controls. Path coefficients are presented. DV, dependent variable; IV, independent variable; MV, mediating variable. * p < .05.
The second mediation analysis was performed in the group of healthy controls. Age was entered as a covariate because correlational analyses revealed it was significantly associated with TS of pain. Results revealed that although pain expectancies were significantly associated with pain catastrophizing (path a = .602, p = .036), none of the other paths within the model were significant. Moreover, the bias-corrected bootstrap 95% confidence interval for the total indirect effect based on 5000 bootstrap samples included zero (BC 95% CI = −.5864 to .0244), indicating that pain catastrophizing did not mediate the influence of pain expectancies on TS of pain in healthy controls.
4. Discussion
The present study adds to a growing literature examining the contribution of psychological factors to the course of pain sensitization in individuals with CLBP. This investigation was the first to examine the relationship between pain expectancies and TS of pain. We used quantitative sensory testing to examine changes in central pain processing in CLBP patients and healthy controls. Results revealed that pain expectancies were significantly associated with TS of pain in CLBP patients, suggesting that pain expectancies may play a facilitatory role in the processing of pain-related information. In this study, we also sought to identify the mechanism by which pain expectancies are associated with TS of pain. The findings support the possibility that pain catastrophizing is the process by which pain expectancies are implicated in the process of sensitization in CLBP patients.
Pain expectancies have been associated with a number of indices of pain sensitivity in the context of experimental pain testing paradigms, both among healthy controls and individuals with chronic pain conditions (France et al., 2016; Goffaux et al., 2007; Hanssen et al., 2013; 2012; Van Damme et al., 2004), though the present findings are the first linking higher expectancies for pain with increased TS of pain. The literature also points to consistent associations between pain expectancies and numerous adverse pain-related outcomes, including self-reported pain intensity and disability (Boersma and Linton, 2006; Gandhi et al., 2009; Mun et al., 2017; Sullivan et al., 2001a), emotional distress (Carriere et al., 2015a; Sullivan et al., 2001a), and prolonged work disability (Carriere et al., 2017; 2015b; Cole et al., 2002; duBois and Donceel, 2008; Gross and Battié, 2010). This study extends previous findings by showing that pain expectancies are associated with elevated TS of pain, a marker of central pain-facilitatory processes, selectively in CLBP patients. This finding suggests that pain expectancies might be associated with alterations in the central processing of noxious stimuli, which may contribute to the development and maintenance of chronic pain (Edwards, 2005). The causal nature of these relationships remains to be verified, and will be an important area for future research.
The findings are consistent with previous research suggesting that CLBP patients exhibit differences in terms of psychosocial presentation and increased pain sensitivity compared to healthy controls (Giesecke et al., 2004; Jensen et al., 2010; O’Neill et al., 2007). In this study, CLBP patients reported marginally higher pain expectancies and significantly higher levels of pain catastrophizing. Moreover, the mean pain rating provided by CLBP patients during the TS task increased from 15.10 (Probe 1) to 30.92 (Probe 10), representing a 105% increase in pain intensity, compared to a 43% increase in pain intensity in the control group. These findings bring forward the possibility that the associations between pain expectancies, pain catastrophizing and increased pain sensitivity in CLBP patients are, in fact, driven by the condition itself. In this sense, pain expectancies and pain catastrophizing may more readily prime the central nervous system in CLBP patients relative to controls, leading to greater central nervous system sensitizability in CLBP patients. Alternatively, it is possible that pain expectancies and pain catastrophizing represent predisposing factors for the development of chronic pain and pain sensitization. In fact, In fact, pain catastrophizing and pain expectancies been shown to predict the development of chronic pain in previously pain-free individuals, as well as the maintenance and chronification of back pain and pain following a motor-vehicle accident (Edmed et al., 2017; Yarnitsky et al., 2008). It has also been suggested that pain catastrophizing and pain sensitization may be interrelated based on studies showing that catastrophizing contributes to increased pain sensitivity through aberrant central nervous system processing of pain-related information (O’Neill et al., 2007; Yarnitsky et al., 2008). As noted in previous research, our understanding of the influence of psychological factors on central pain processing is still limited and requires further investigation (Curatolo and Arendt-Nielsen, 2015).
A critical question in this area of research has been whether pain expectancies influence neurobiological processes or whether they simply reflect demand characteristics and report biases (Atlas and Wager, 2012). To answer these questions, researchers have used brain imaging techniques to test whether pain expectancies are associated with concomitant changes in nociceptive circuitry. Overall, studies have shown that pain expectancies have a strong effect on pain perception and pain-evoked responses (Atlas et al., 2010; Koyama et al., 2005; Lorenz et al., 2005). In one study, techniques such as conditioning and verbal information were used to induce expectancies about barely painful or highly painful thermal stimulation as a function of audio tones (Atlas et al., 2010). Using functional magnetic resonance imaging, the authors demonstrated that expectancy manipulations were associated with real changes in pain-related processing in the brain, confirming that expectancy effects likely cause changes in the underlying pain-related physiology. However, the brain regions that are often influenced by expectancies can also be shaped by emotion, attention and other processes (Atlas and Wager, 2012), highlighting the need for more research regarding the mediating mechanisms at play.
A central objective of this research was to address whether pain catastrophizing mediated the influence of pain expectancies on TS of pain. Results revealed that pain catastrophizing is a mechanism by which pain expectancies are associated with elevated TS of pain in CLBP patients. These findings are consistent with previous research demonstrating that pain catastrophizing mediates the influence of expectancies on functional disability in individuals with CLBP (Besen et al., 2017). Our findings are also in accordance with research showing that pain catastrophizing mediates the influence of dispositional optimism on TS of pain (Goodin et al., 2013). Although the pattern of findings that is emerging from the literature suggests that cognitive and emotional processes such as pain catastrophizing might be mechanisms underlying TS of pain, the manner in which pain catastrophizing contributes to pain summation effects is currently unclear.
It is possible that pain catastrophizers’ previous experiences with painful situations serve to inform them that future pain-eliciting situations will lead to enhanced pain. As a function of their previous pain experiences, catastrophizers may develop pain expectancies about the high threat value of a painful stimuli or situation, or about their ability to effectively cope with pain. Once activated, these pain schemas may influence cognitive or emotional functioning and increase attention towards painful stimuli (Sullivan et al., 2001b). Research has shown that an excessive focus on pain is a central feature of pain catastrophizing (Crombez et al., 1998; Sullivan and Neish, 1998), and that catastrophizers are slower to disengage their attention from pain cues than non-catastrophizers (Van Damme et al., 2002b). Increased attention to pain has been associated with enhanced processing of nociceptive stimuli, suggesting the possibility that high catastrophizers’ attentional biases may directly or indirectly alter the processing of noxious stimuli (Ploghaus et al., 1999; Tracey et al., 2002). It is also possible that the repeated coping demands during a TS task may overwhelm catastrophizers’ coping resources, resulting in greater pain. It has been suggested that individuals who catastrophize show reduced effectiveness of pain coping strategies. For example, Sullivan and colleagues reported that high catastrophizers report using less active coping strategies during the experience of acute pain (Sullivan et al., 1995; 2004).
On the other hand, it has also been suggested that pain catastrophizers show a relative underprediction of expected pain (Sullivan et al., 2001a). This phenomenon may serve as a means of minimizing anticipatory distress, allowing catastrophizers to approach painful situations with less anxiety than if their expectancies were accurate. However, pain catastrophizers’ underprediction of pain may lead to unexpectedly high pain sensations, which might draw their attention to the painful stimuli. This may be particularly salient in the context of repeated painful stimulation, such as TS tasks. Research has shown that unexpected events are likely to receive more attention, particularly when the experience is negative (Pyszczynski and Greenberg, 1981; Taylor, 1991). Moreover, catastrophizers’ pain schemas may lead them to preferentially process pain-related information as being painful (Sullivan et al., 1997; 2001b). Attentional focus on pain may be a critical psychological process involved in the association between pain expectancies, pain catastrophizing and pain processing, and may warrant future research.
Given the weak association between pain expectancies and TS of pain, caution must be exercised prior to suggesting a therapeutic approach. However, the findings of this study may guide future research on psychological interventions for chronic pain. Pain expectancies and pain catastrophizing are generally considered modifiable factors and there is evidence that expectancy-change techniques such as verbal suggestion, conditioning and imagery lead to significant decreases in reported pain (for a review see Peerdeman and colleagues (Peerdeman et al., 2016)). Moreover, research has shown that Cognitive Behavior Therapy is effective at reducing catastrophizing and improving pain and function (Turner et al., 2007). To advance clinical practice in this area, more research is needed on the effectiveness of interventions aimed at modifying expectancies and pain catastrophizing on the development and maintenance of chronic pain.
Several limitations should be considered in the interpretations of these findings. First, we studied only a single type of laboratory pain stimulus (mechanical TS of pain); it is possible that the relationships between expectancies, catastrophizing and experimental pain responses vary as a function of the type of noxious stimulus. Second, other than depression, we did not specifically assess other psychosocial variables which have been shown to influence pain responses and interact with expectancies, such as fear of pain/movement (George et al., 2006), therefore we were unable to assess their potential contribution to pain sensitivity. Third, in this study, pain expectancies were measured with a single item that was task specific. Future studies may wish to include a broader measure of expectancies. Fourth, we do not have any data regarding other pain conditions (i.e. fibromyalgia) that may be associated with alterations in pain processing. Fifth, the nature of this study precludes any firm conclusions regarding the directionality of associations between pain expectancies and pain catastrophizing. Finally, this study used a trait measure of pain catastrophizing. Future studies may want to replicate our findings using task-specific measures of catastrophizing, which also have been shown to associate strongly with measures of pain sensitivity (Rhudy et al., 2011; Taub et al., 2017). Researchers may also want to consider measuring expectancies a various time-points, based on Edmed and colleagues’ recent findings showing the time-dependence of expectations after an initial pain episode, which influence pain reports and suggests that the time when expectancy is considered is not negligible (Edmed et al., 2018). We also urge replication of our findings within CLBP samples that may reflect greater racial and socioeconomic diversity, as well as extension of our findings in longitudinal and interventional studies.
Despite these limitations, this study provides evidence that high pain expectancies are associated with elevated TS of pain in CLBP patients. Moreover, the findings suggest that pain catastrophizing is an important mechanism by which pain expectancies are associated with elevated TS of pain. Elevated TS of pain is an important marker of central sensitization in both chronic and acute pain (Arendt-Nielsen, 2015; Woolf, 2011), and the finding of an association with pain expectancies, pain catastrophizing and TS of pain suggests that interventions aimed at reducing maladaptive cognitive-affective processes may help reduce detrimental alterations in pain processing.
Significance:
Individuals with chronic low back pain who expect higher levels of pain and catastrophize about their pain are more likely to experience altered pain sensitivity. Our results point to catastrophizing as a mechanism of action through which psychological factors may operate and lead to the development and maintenance of chronic low back pain.
Acknowledgments
This research was supported by National Institutes of Health (Grant numbers R21 DA041020, R01 AG034982; T32 AR055885; RRE).
Footnotes
Disclosures:
Conflicts of interest: None declared
References
- Arnau RC, Meagher MW, Health MN, Bramson R (2001). Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. J Pers Soc Psychol 20, 112–119. [DOI] [PubMed] [Google Scholar]
- Arntz A, Peters M (1995). Chronic low back pain and inaccurate predictions of pain: is being too tough a risk factor for the development and maintenance of chronic pain? Behav Res Ther 33, 49–53. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Wager TD (2012). How expectations shape pain. Neuroscience Letters 520, 140–148. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996). Manual for the Beck Depression Inventory-II (San Antonio). [Google Scholar]
- Besen E, Gaines B, Linton SJ, Shaw WS (2017). The role of pain catastrophizing as a mediator in the work disability process following acute low back pain. J Appl Biobehav Res 22, e12085. [Google Scholar]
- Bishop SR, Edgley K, Fisher R, Sullivan MJL (1993). Screening for depression in chronic low back pain with the Beck Depression Inventory. Can J Rehabil 7, 143–148. [Google Scholar]
- Boersma K, Linton SJ (2006). Expectancy, fear and pain in the prediction of chronic pain and disability: a prospective analysis. Eur J Pain 10, 551–557. [DOI] [PubMed] [Google Scholar]
- Carriere JS, Thibault P, Adams H, Milioto M, Ditto B, Sullivan MJL (2017). Expectancies mediate the relationship between perceived injustice and return to work following whiplash injury: A 1‐year prospective study. Eur J Pain 21, 1234–1242. [DOI] [PubMed] [Google Scholar]
- Carriere JS, Thibault P, Milioto M, Sullivan MJL (2015a). Expectancies mediate the relations among pain catastrophizing, fear of movement, and return to work outcomes after whiplash injury. J Pain 16, 1280–1287. [DOI] [PubMed] [Google Scholar]
- Carriere JS, Thibault P, Sullivan MJL (2015b). The mediating role of recovery expectancies on the relation between depression and return-to-work. J Occup Rehabil 25, 348–356. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2009). Prevalence and most common causes of disability among adults--United States, 2005. Morb Mortal Wkly Rep 58, 421–426. [PubMed] [Google Scholar]
- Cheng JC, Erpelding N, Kucyi A, DeSouza DD, Davis KD (2015). Individual differences in temporal summation of pain reflect pronociceptive and antinociceptive brain structure and function. J Neurosci 35, 9689–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DC, Mondloch MV, Hogg-Johnson S, Early Claimant Cohort Prognostic Modelling Group (2002). Listening to injured workers: how recovery expectations predict outcomes--a prospective study. Can Med Assoc J 166, 749–754. [PMC free article] [PubMed] [Google Scholar]
- Crombez G, Eccleston C, Baeyens F, Eelen P (1998). When somatic information threatens, catastrophic thinking enhances attentional interference. Pain 75, 187–198. [DOI] [PubMed] [Google Scholar]
- Dagenais S, Caro J, Haldeman S (2008). A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 8, 8–20. [DOI] [PubMed] [Google Scholar]
- duBois M, Donceel P (2008). A screening questionnaire to predict no return to work within 3 months for low back pain claimants. Eur Spine J 17, 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmed SL, Moss KM, Warren J, Kenardy J (2017). The effect of changes in pain expectations on persistent pain following a road traffic crash. Eur J Pain 22, 426–436. [DOI] [PubMed] [Google Scholar]
- Edwards RR (2005). Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology 65, 437–443. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Bingham CO, Bathon J, Haythornthwaite JA (2006a). Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis & Rheum 55, 325–332. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Dolman AJ, Michna E, Katz JN, Nedeljkovic SS, Janfaza D, Isaac Z, Martel MO, Jamison RN, Wasan AD (2016). Changes in pain sensitivity and pain modulation during oral opioid treatment: The impact of negative affect. Pain Med 17, 1882–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB, Maixner W, Sigurdsson A, Haythornthwaite J (2004). Catastrophizing predicts changes in thermal pain responses after resolution of acute dental pain. J Pain 5, 164–170. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB, Ness TJ (2003). Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain 101, 155–165. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Giles J, Bingham CO III, Campbell C, Haythornthwaite JA, Bathon J (2010). Moderators of the negative effects of catastrophizing in arthritis. Pain Med 11, 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT (2009a). Sleep continuity and architecture: Associations with pain‐inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain 13, 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Mensing G, Cahalan C, Greenbaum S, Narang S, Belfer I, Schreiber KL, Campbell C, Wasan AD, Jamison RN (2013). Alteration in pain modulation in women with persistent pain after lumpectomy: influence of catastrophizing. J Pain Symptom Manage 46, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA (2006b). Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain 22, 730–737. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Wasan AD, Bingham CO, Bathon J, Haythornthwaite JA, Smith MT, Page GG (2009b). Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res Ther 11, R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM (2001). Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94, 149–158. [DOI] [PubMed] [Google Scholar]
- France CR, Burns JW, Gupta RK, Buvanendran A, Chont M, Schuster E, Orlowska D, Bruehl S (2016). Expectancy effects on conditioned pain modulation are not influenced by naloxone or morphine. Ann Behav Med 50, 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R, Davey JR, Mahomed N (2009). Patient expectations predict greater pain relief with joint arthroplasty. J Arthroplasty 24, 716–721. [DOI] [PubMed] [Google Scholar]
- George SZ, Wittmer VT, Fillingim RB, Robinson ME (2006). Fear-avoidance beliefs and temporal summation of evoked thermal pain influence self-report of disability in patients with chronic low back pain. J Occup Rehabil 16, 92–105. [DOI] [PubMed] [Google Scholar]
- George SZ, Wittmer VT, Fillingim RB, Robinson ME (2007). Sex and pain-related psychological variables are associated with thermal pain sensitivity for patients with chronic low back pain. J Pain 8, 2–10. [DOI] [PubMed] [Google Scholar]
- Goffaux P, Redmond WJ, Rainville P, Marchand S (2007). Descending analgesia--when the spine echoes what the brain expects. J Occup Rehabil 130, 137–143. [DOI] [PubMed] [Google Scholar]
- Goodin BR, Glover TL, Sotolongo A, King CD, Sibille KT, Herbert MS, Cruz-Almeida Y, Sanden SH, Staud R, Redden DT, Bradley LA, Fillingim RB (2013). The association of greater dispositional optimism with less endogenous pain facilitation is indirectly transmitted through lower levels of pain catastrophizing. J Pain 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DP, Battié MC (2010). Recovery expectations predict recovery in workers with back pain but not other musculoskeletal conditions. J Spinal Disord Tech 23, 451–456. [DOI] [PubMed] [Google Scholar]
- Hanssen MM, Peters ML, Vlaeyen JW, Meevissen YM, Vancleef L (2013). Optimism lowers pain: evidence of the causal status and underlying mechanisms. Pain 154, 53–58. [DOI] [PubMed] [Google Scholar]
- Hanssen MM, Vancleef LMG, Vlaeyen JWS, Peters ML (2012). More optimism, less pain! The influence of generalized and pain-specific expectations on experienced cold-pressor pain. J Behav Med 37, 47–58. [DOI] [PubMed] [Google Scholar]
- Harris CA, D’Eon JL (2008). Psychometric properties of the Beck Depression Inventory-Second Edition (BDI-II) in individuals with chronic pain. Pain 137, 609–622. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2009). Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Commun Monogr 76, 408–420. [Google Scholar]
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S, European Palliative Care Research Collaborative EPCRC (2011). Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manag 41, 1073–1093. [DOI] [PubMed] [Google Scholar]
- Mackinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V (2002). A comparison of methods to test mediation and other intervening variable effects. Psychol Methods 7, 83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankovsky-Arnold T, Wideman TH, Larivière C, Sullivan MJL (2014). Measures of spontaneous and movement-evoked pain are associated with disability in patients with whiplash injuries. J Pain 15, 967–975. [DOI] [PubMed] [Google Scholar]
- Mun CJ, Thummala K, Davis MC, Karoly P, Tennen H, Zautra AJ (2017). Predictors and social consequences of daily pain expectancy among adults with chronic pain. Pain 158, 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill S, Manniche C, Nielsen TG, Nielsen LA (2007). Generalized deep‐tissue hyperalgesia in patients with chronic low‐back pain. Eur J Pain 11, 415–420. [DOI] [PubMed] [Google Scholar]
- Peerdeman KJ, van Laarhoven AIM, Keij SM, Vase L, Rovers MM, Peters ML, Evers AWM (2016). Relieving patients’ pain with expectation interventions: a meta-analysis. Pain 157, 1179–1191. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JNP (1999). Dissociating pain from its anticipation in the human brain. Science 284, 1979–1981. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Meth Ins C 36, 717–731. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40, 879–891. [DOI] [PubMed] [Google Scholar]
- Pyszczynski TA, Greenberg JJ (1981). Role of disconfirmed expectancies in the instigation of attributional processing. J Pers Soc Psychol 40, 31–38. [Google Scholar]
- Rhudy JL, Martin SL, Terry EL, France CR, Bartley EJ, DelVentura JL, Kerr KL (2011). Pain catastrophizing is related to temporal summation of pain but not temporal summation of the nociceptive flexion reflex. Pain 152, 794–801. [DOI] [PubMed] [Google Scholar]
- Schreiber KL, Campbell C, Martel MO, Greenbaum S, Wasan AD, Borsook D, Jamison RN, Edwards RR (2014). Distraction analgesia in chronic pain patients: the impact of catastrophizing. Anesthesiology 121, 1292–1301. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N (2002). Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods 7, 422–445. [PubMed] [Google Scholar]
- Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ Jr, (2003). Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain 102, 87–95. [DOI] [PubMed] [Google Scholar]
- Staud R, Weyl EE, Riley JL III, Fillingim RB (2014). Slow temporal summation of pain for assessment of central pain sensitivity and clinical pain of fibromyalgia patients. PLoS One 9, e89086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M, Tanzer M, Reardon G, Amirault D, Dunbar M, Stanish W (2011). The role of presurgical expectancies in predicting pain and function one year following total knee arthroplasty. Pain 152, 2287–2293. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Adams H, Sullivan ME (2004). Communicative dimensions of pain catastrophizing: social cueing effects on pain behaviour and coping. Pain 107, 220–226. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Bishop SR, Pivik J (1995). The Pain Catastrophizing Scale: Development and validation. Psychol Assess 7, 524–532. [Google Scholar]
- Sullivan MJL, Larivière C, Simmonds M (2010). Activity-related summation of pain and functional disability in patients with whiplash injuries. Pain 151, 440–446. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Neish NR (1998). Catastrophizing, anxiety and pain during dental hygiene treatment. Community Dent Oral Epidemiol 26, 344–349. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Rodgers WM, Kirsch I (2001a). Catastrophizing, depression and expectancies for pain and emotional distress. Pain 91, 147–154. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Rouse D, Bishop SR, Johnston S (1997). Thought suppression, catastrophizing, and pain. Cog Ther Res 21, 555–568. [Google Scholar]
- Sullivan MJL, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC (2001b). Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 17, 52–64. [DOI] [PubMed] [Google Scholar]
- Taub CJ, Sturgeon JA, Johnson KA, Mackey SC, Darnall BD (2017). Effects of a pain catastrophizing induction on sensory testing in women with chronic low back pain: A pilot study. Pain Res Manag 2017, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE (1991). Asymmetrical effects of positive and negative events: the mobilization-minimization hypothesis. J Pers Soc Psychol 110, 67–85. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM (2002). Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci 22, 2748–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Holtzman S, Mancl L (2007). Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain 127, 276–286. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B (2002a). A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain 96, 319–324. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Eccleston C (2002b). Retarded disengagement from pain cues: the effects of pain catastrophizing and pain expectancy. Pain 100, 111–118. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Eccleston C (2004). Disengagement from pain: the role of catastrophic thinking about pain. Pain 107, 70–76. [DOI] [PubMed] [Google Scholar]
- Van Den Broeke EN, Geene N, van Rijn CM, Wilder-Smith OHG, Oosterman J (2013). Negative expectations facilitate mechanical hyperalgesia after high-frequency electrical stimulation of human skin. Eur J Pain 18, 86–91. [DOI] [PubMed] [Google Scholar]
- Vierck CJJ, Cannon RL, Fry G, Maixner W, Whitsel BL (1997). Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol 78, 992–1002. [DOI] [PubMed] [Google Scholar]
- Woolf AD, Pfleger B (2003). Burden of major musculoskeletal conditions. Bull World Health Organ 81, 646–656. [PMC free article] [PubMed] [Google Scholar]
- Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best L-A, Granot M (2008). Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 138, 22–28. [DOI] [PubMed] [Google Scholar]


