Abstract
Background:
Brain-Machine Interfaces (BMIs) have been recently proposed as a new tool to induce functional recovery in stroke patients.
Objective.
Here we evaluated long-term effects of BMI training and physiotherapy in motor function of severely paralyzed chronic stroke patients 6 months after intervention.
Methods.
30 chronic stroke patients with severe hand paresis from our previous study were invited and 28 underwent follow-up assessments. BMI training included voluntary desynchronization of ipsilesional EEG-sensorimotor rhythms triggering paretic upper limb movements via robotic orthoses (experimental group, n=16), or random orthoses movements (Sham group, n=12). Both groups received identical physiotherapy following BMI sessions and a home based training program after intervention. Upper limb motor assessments scores, electromyography (EMG) and functional magnetic resonance imaging (fMRI) were assessed before (Pre), immediately after (Post1) and 6 months after intervention (Post2).
Results.
The experimental group presented upper limb Fugl-Meyer assessment (cFMA) scores significantly higher in Post2 (13.44±1.96) as compared to Pre session (11.16±1.73; p=0.015), and no significant changes between Post1 and Post2 sessions. The Sham group showed no significant changes on cFMA scores. Ashworth scores and EMG activity in both groups increased from Post1 to Post2. Moreover, fMRI-BOLD laterality index (LI) showed no significant difference from Pre or Post1 to Post2 sessions.
Conclusions.
BMI-based rehabilitation promotes long-lasting improvements in motor function of chronic stroke patients with severe paresis and represents a promising strategy in severe stroke neurorehabilitation.
Keywords: Brain-Machine Interface (BMI), Electrophysiology (EEG), Chronic stroke, Neurorehabilitation, Long-term effects
Introduction
Motor impairment after stroke is the leading cause of long-term disability in the adult population1. Approximately 30% of stroke survivors experience severe motor impairment and need assistance for daily living activities (Young & Forster, 2007; Langhorne, et al., 2009). While it was reported that current rehabilitation strategies or bilateral arm training improve motor function in chronic stroke patients with limited paresis (Luft, et al., 2004; Wolf, et al., 2006; Langhorne, et al., 2009; Langhorne, et al., 2011), patients with severe upper limb paresis are not eligible for these techniques as they are unable to perform the requested movements.
For those patients with severe paresis we demonstrated for the first time in a controlled randomized double-blind study (Ramos-Murguialday, et al., 2013) the clinical efficacy of Brain-Machine Interface (BMI) coupled with physiotherapy training to promote upper limb motor recovery. Posteriorly, these findings have been confirmed by other studies (Ang, Kai Keng Chua, Karen Sui Geok Phua, Kok Soon Wang, Chuanchu Chin, Zheng Yang Kuah and Low, Wilson Guan, 2014; Ono et al., 2014; Pichiorri et al., 2015). We used patients’ voluntary modulation of oscillatory brain activity associated with motor intention (sensorimotor rhythm, or SMR (Pfurtscheller & Aranibar, 1979; Pfurtscheller & Lopes da Silva, 1999)) to trigger a BMI-controlled orthotic device to move the paretic limb. This procedure created a contingency between the neural correlate of motor intention with sensory (visual and proprioceptive) feedback of the intended movement. We compared improvements in motor function between an experimental group receiving contingent BMI training and a control group receiving sham-BMI training over 20 training days. In both groups BMI sessions were followed by identical behaviorally oriented physiotherapy. Our previous results suggest that in chronic stroke patients with severe paresis a close associative connection between the neural correlate of motor intention and the feedback of the intended movement (established via a BMI) elicits: 1) superior associative learning of voluntary modulation of SMR, as demonstrated before in healthy participants (Ramos-Murguialday, et al., 2012); and 2) motor impairment reduction after four weeks of intervention, demonstrating previous theoretical predictions (Sirigu, et al., 1995; Birbaumer & Cohen, 2007; Daly & Wolpaw, 2008) and indicating that BMI has a beneficial effect on motor rehabilitation of severely paretic chronic stroke patients. Our results indicate that learning to control oscillatory brain activity through a BMI approach and the repetitive contingency between motor intention and sensory feedback constitute necessary therapeutic ingredients for motor recovery, and that behavioral physiotherapy allows generalization of re-learned motor skills to meaningful real life activities (Ramos-Murguialday, et al., 2013).
Previous studies evaluating long-term effects of rehabilitative interventions for stroke motor recovery reported contradictory findings regarding maintenance of improvements in motor outcomes. For example, while some studies indicated long-lasting improvements in motor function after distinct rehabilitative interventions (Taub, et al., 1993; Timmermans, et al., 2013) other studies reported improvements in motor function that were not preserved six months after intervention (Dahl, et al., 2008; Liang, et al., 2012). Here we analysed the long-term effects (six months after intervention) of the BMI-based rehabilitative intervention (Ramos-Murguialday, et al., 2013). In analogy to previous stroke motor rehabilitation studies (Taub, et al., 1993; Timmermans, et al., 2013) we hypothesized that if (4-weeks) BMI-based training promotes significant and stable motor functional gain, it should be maintained 6 months after intervention. The data presented in this manuscript were acquired between 2007 and 2011.
Methods
This is a follow-up study of the clinical and neurophysiological results obtained in the previous interventional study (Ramos-Murguialday, et al., 2013). Due to personnel, organizational and time constraints, the publication of these data and results has been delayed.
Patients
All patients who completed the previous interventional double-blinded controlled study were invited for the follow-up session, which ended in December 2011. Inclusion criteria for acceptance in the interventional study included: 1) paralysis of one hand with no active finger extension; 2) time since stroke of at least 10 months; 3) age between 18 and 80 years; 4) no psychiatric or neurological condition other than stroke; 5) no cerebellar lesion or bilateral motor deficit; 6) no pregnancy; 7) no claustrophobia; 8) no epilepsy or medication for epilepsy during the last 6 months; 9) eligibility to undergo magnetic resonance imaging (MRI); 10) ability to understand and follow instructions.
Patients were recruited via public information. From 504 potentially eligible patients, 32 were allocated to the intervention (See CONSORT Chart in the Supp. Material). Patients were matched for age, gender, paretic side and motor impairment scores at time of inclusion and were randomly assigned to experimental or control groups. Group assignment was blinded for all participants and for the scientific-clinical personnel; none of the patients or therapists was able to identify group assignment reflected in the results of placebo and motor function scales below. Two of the patients from the control group did not receive correct allocated intervention and 2 of the 30 patients, who received intervention had to be excluded from the follow-up analysis due to: 1) incidence of a second stroke; 2) economic and health related issues (See Figure 1). Both patients were from the control group and presented mixed lesions including cortical and subcortical structures (experimental group: n = 16 (7 females), age at study admittance = 49.3±12.5; sham group: n = 12 (5 females); age at study admittance = 50.3±12.2).
Figure 1.
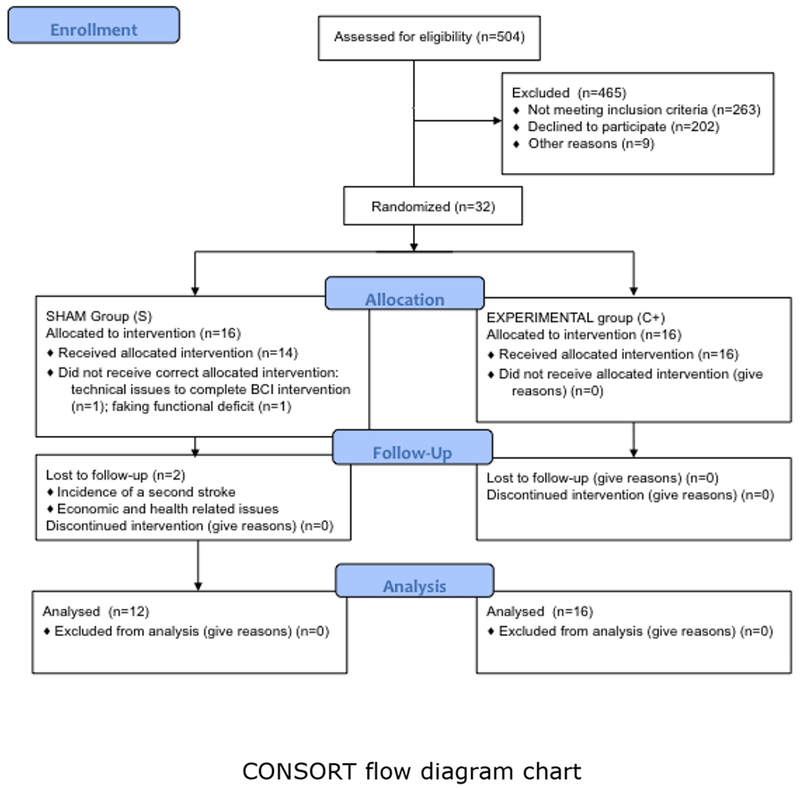
CONSORT flow diagram. Study enrollment diagram. 504 patients were screened to be eligible for the study and 465 were excluded. 32 underwent intervention and were randomly assigned to 2 groups depending on the BMI feedback received: a) Experimental group (brain activity was contingently and concurrently linked with orthoses movements) and b) control or sham feedback group (orthoses movements were random and not associated with brain activity). In the sham group 2 patients did not receive correct allocated intervention. Six months after the intervention patients were invited for follow up measurements. Two patients from the control group dropped out and did not come back for the final measurements.
Study design
Intervention involved daily training for 4 weeks (excluding weekends) including one hour of BMI training followed by one hour of behavioral physiotherapy. There was no difference in time of training (BMI + Physiotherapy) between groups (unpaired t-test for BMI runs between experimental and Sham groups resulted in not significant differences p=0.42; experimental (mean ± SE) 281±10.03; sham 290.29±5.13 runs).
In the experimental group patients’ successful control of ipsilesional SMR desynchronization was translated concurrently into movement of the orthosis attached to the paretic limb, while in the Sham group movements of the orthosis occurred randomly, i.e., unrelated to patients’ SMR desynchronization (for methodological details please see Ramos-Murguialday, et al., 2013 ). During BMI-training patients were instructed to try to move their paretic upper limb. Two movements were practiced, each of them associated with a specific robotic orthosis: (1) open and close the fingers, or (2) move the paretic upper limb forwards and backwards. Only one type of movement was practiced in any given day. The level of paresis determined the kind of movement to be performed during BMI-training but all patients performed the movement of opening and closing the fingers. The amount of sessions with hand or arm movements were balanced between groups. (More details in Ramos-Murguialday, et al., 2013).
After each intervention session, physiotherapists taught and demonstrated to the patients several exercises comprising functional movements of their affected limb acquired during BMI training and mainly daily live activities for home exercise (e.g. to use a toothpaste tube (Broetz, et al., 2014)). These exercises were also provided to the patients in a handbook (Broetz & Augustinski, 2010). The primary outcome measure used was the combined hand and arm scores (motor part) from the modified upper limb Fugl-Meyer-Assessment (cFMA) (mean ± standard deviation, maximal score is 54 points (More details in Ramos-Murguialday, et al., 2013)), being of 14,75±2,71 for all patients, who underwent BMI and behavioral physiotherapy intervention.
The study was conducted at the University of Tübingen, Germany. Informed consent was obtained from all patients involved. The study was approved by the ethics committee of the Faculty of Medicine of the University of Tübingen (Germany).
Assessments
To evaluate the long-term BMI-based rehabilitation effects on motor function, the identical comprehensive battery of assessment instruments given twice before (eight weeks and one day before the first training session) and once immediately after treatment (Post 1) described in our previous study6 and reported below, was repeated 6 months after the 4-weeks intervention period (Post 2). Scores from the two pre-intervention assessments were averaged (Pre) to reduce variability of the data (Whitall, et al., 2011) and increase reliability of pre-intervention assessment.
Primary behavioral outcome measure: combined hand and arm scores (motor part) from the modified upper limb Fugl-Meyer Assessment (cFMA)
We used the combined hand and arm scores (motor part) from the modified upper limb Fugl-Meyer-Assessment scale (cFMA) of our previous study (with a maximum score of 54) as primary behavioral outcome measure (Fugl-Meyer, et al., 1975), because they are related to the two body parts trained during the BMI (hand and arm), reflect motor recovery and measure motor impairment that may limit but is unrelated to task accomplishment (e.g. joint motion). We excluded upper limb FMA scores related to i) coordination and speed and ii) reflexes because: a) before intervention patients in this study could not touch their noses with the index finger fully extended and had no remaining finger extension (no active finger extension was an inclusion criteria); and b) reflex scores add uncertainty to the measurement (Crow & Harmeling-van der Wel, 2008).
Secondary outcome measures: GAS, MAL and Ashworth scores
Goal Attainment Scale (GAS) (Hurn, et al., 2006), Motor Activity Log (MAL) (Uswatte, et al., 2005) and Ashworth Scale were used as secondary behavioral measures. It should be mentioned that during treatment phase placebo questionnaires demonstrated no difference between the two groups in placebo responding (Ramos-Murguialday, et al., 2013).
Assessments associated with the primary behavioral outcome measure
We measured electromyography (EMG) to document muscle activity and innervation (Ramos-Murguialday, et al., 2015) and blood-oxygen level dependent (BOLD) signal from functional magnetic resonance imaging (fMRI) (Sehm, et al., 2010) to identify changes in brain function associated with changes in motor outcomes.
Electromyography (EMG)
EMG activity was recorded from 16 bipolar electrodes placed at 8 different locations obtaining data from muscles in both paretic and healthy upper limbs: 1) extensor carpi ulnaris; 2) extensor digitorum; 3) flexor carpi radialis, palmaris longus, flexor carpi; 4) biceps; 5) triceps; 6) anterior deltoid; 7) deltoid; 8) posterior deltoid. Patients were asked to perform 6 concurrent bilateral movements from the upper limb arm and hand FMA: 1) shoulder flexion; 2) shoulder abduction; 3) elbow extension; 4) supination; 5) wrist extension; and 6) fingers extension. In another task, patients were asked to perform continuous opening and closing of the paralyzed hand at a comfortable speed and pace (fingers extension and flexion).
Acquired changes in EMG amplitude during muscle contraction provided indicators for EMG signal amplitude and frequency (Ramos-Murguialday, et al., 2013) and can be used to decode motor intention (Ramos-Murguialday, et al., 2015).
Functional magnetic resonance imaging (fMRI)
Inside the scanner, patients were asked to perform three different tasks: (1) to perform (try to perform) hand closing and opening (2), to imagine hand closing and opening and (3) to remain motionless. Conditions and movement pace (every 1.5 seconds) were cued by auditory-visual signals. A lateralization index (LI) was calculated to assess changes in cortical lateralization (Stinear, 2010; Caria, et al., 2011). The LI, computed as the normalized difference between the number of all active voxels in the ipsilesional and contralesional areas (anatomically defined regions of interest according to MNI-space) was assessed separately for motor and premotor cortices, and for the somatosensory cortex of the paretic and healthy hand (Wilke & Lidzba, 2007). All patients underwent fMRI but only those who underwent the follow-up measurement and presenting subcortical lesions only (not directly affecting sensorimotor and premotor areas) were considered for LI assessment (More information about fMRI data acquisition and processing can be found in our previous study (Ramos-Murguialday, et al., 2013)).
Post-Intervention
Patients were allowed to be involved in any other rehabilitation intervention. A battery of exercises comprising functional movements performed during intervention physiotherapy sessions were explained, trained and summarized in a handbook, which was given to the patients for home training. Exercises for home training were individually adapted based on each patient’s goals and residual or regained motor capacity, but the expected frequency of home training was the same for all patients comprising 2 sessions of 30 to 45 minutes of exercises per day. These movements were designed to maintain and improve motor function. In the follow-up measurement, patients were required to fill out questionnaires regarding the use and practice of the aforementioned home training. A value ranging from 3 to 1 was calculated for each person to evaluate his/her frequency of exercising at home, on which 3 = exercises were regularly done (maximal score) and 1 = exercises were not done (minimal score).
Results
Correlation of motor assessment scores with frequency of home and BMI training
Self-reported frequency of home training presented no significant difference between C+ and Sham groups (C+: 2.6±0,09; Sham: 2.27±0,17; p=0.08). We found no significant correlation between frequency/intensity of home training and changes in motor function or impairment (as evaluated by cFMA scores, EMG activity, MAL, GAS, and Ashworth scores) between Post1 and Post2 (Supplementary Table 1). Correlation between number of BMI runs (i.e. BMI-training frequency) and changes in motor function or impairment indicated a significant correlation with changes in cFMA scores from Pre to Post2 (p=0.001, r=0.761) and near significance correlation with changes in cFMA scores from Pre to Post1 (Post1 – Pre, p=0.03, r=0.543; significant values were Bonferroni corrected for multiple (12) comparisons (p=0.004), see Supplementary Table 2). Only seven patients did not visit a therapist and 12 and 11 patients from the experimental sham group respectively visited a therapist between Post1 and the follow-up. All of them but 1patient from the sham group (who trained the home exercises with the therapist) did the physiotherapy that they used to do before the intervention and none of them was involved in any other therapy not being the standard physiotherapy treatment they got before the intervention.
Primary behavioral outcome measure: combined hand and arm scores (motor part) from the modified upper limb Fugl-Meyer Motor Assessment (cFMA)
In our previous study, analyses of cFMA scores (2-way mixed model ANOVA) indicated a significant group (C+ and Sham) × session (Pre and Post1) interaction, and post hoc tests indicated that cFMA changed significantly over time in the experimental group only6. Here we assessed the maintenance of this effect after six months comparing Pre to Post2 and Post1 to Post2 measurements. Paired-samples t-test revealed a significant increase in cFMA scores in the C+ group when comparing Pre (11.16±1.73) and Post2 sessions (13.44±1.96, p=0.015) and no significant changes between Post1 (14.56±1.95) and Post2 sessions (p=0.2). Paired samples t-tests showed the same long-term effects in the arm scores but not in the hand FMA scores (See Figure 2 and Table 1). Analyses of cFMA scores in the Sham group indicated no significant changes between Pre (13.29±2.86) and Post2 (14.75±2.71; p=0.07) sessions nor between Post1 (13.64±2.91) and Post2 (p=0.52) sessions.
Figure 2.
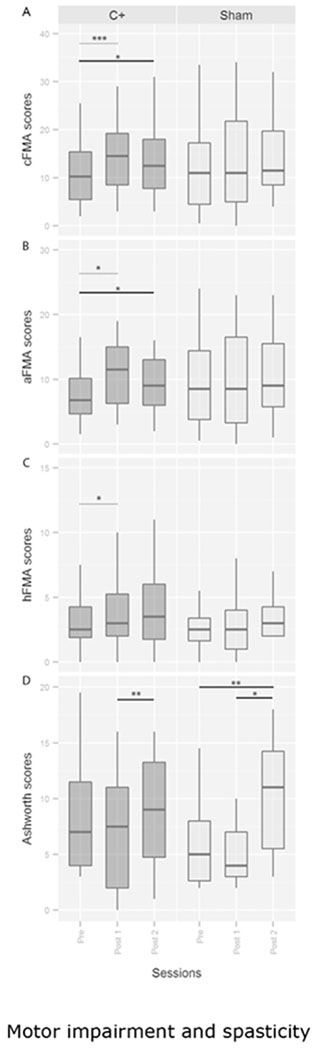
Motor impairment and spasticity. FMA and Ashworth scores before, immediately after, and 6 months after BMI intervention. Values are presented as medians and quartiles. cFMA = Fugl Meyer scores combining hand and arm upper limb motor scores excluding reflexes and speed scores. aFMA = arm part of the cFMA. hFMA = hand part of the cFMA scores. * = p<0.05, ** = p<0.01, *** = p<0.001 respectively. Dashed line refers to the significant effects published in the previous manuscript and continuous line to the significant differences observed with respect to the follow-up measurement.
Table 1.
Fugl-Meyer Assessment scores changes in hand, arm and upper limb
| Group | Session | handFMA | armFMA | cFMA |
|---|---|---|---|---|
| C+ | Post2 | 3.87±0,74 | 9.56±1.42 | 13.44±1.96 |
| Post1 | 4.06±0,73 | 10.05±1.34 | 14.56±1.95 | |
| Pre | 3.25±0,61 | 7.91±1.28 | 11.16±1.73 | |
| Post2 vs. Post1 (p value) | 0.670 | 0.220 | 0.200 | |
| Post2 vs. Pre (p value) | 0.093 | 0.039 | 0.015 | |
| Sham | Post2 | 4.17±0.99 | 10.58±1.95 | 14.75±2.71 |
| Post1 | 3.71±0.98 | 9.93±2.12 | 13.64±2.91 | |
| Pre | 3.32±0.88 | 9.96±2.19 | 13.29±2.86 | |
| Post2 vs. Post1 (p value) | 0.900 | 0.540 | 0.520 | |
| Post2 vs. Pre (p value) | 0.250 | 0.230 | 0.070 |
FMA = Fugl Meyer Assessment scale. Values are given as mean ± standard error.
Primary behavioral outcome measure: analyses of motor scores from the modified upper limb Fugl-Meyer Assessment (cFMA) in patients with mixed or subcortical lesions
While we found a significant increase in cFMA scores only in the experimental group immediately after intervention and six months after intervention, it could be argued that there are slightly more patients with mixed lesions (i.e., affecting cortical and subcortical structures) in the control group (n=9) as compared to the experimental group (n=6), and this bias could influence changes in motor outcomes after BMI-based rehabilitation. To investigate the influence of preserved cortex on motor recovery after BMI-based rehabilitation, we analyzed changes in cFMA scores between C+ and Sham groups after intervention in patients with either subcortical lesions only, i.e., when excluding patients with mixed lesions, or with mixed lesions only, i.e., when excluding patients with subcortical lesions.
A 2-way mixed model ANOVA with time (Pre, Post1 and Post2) as within-subjects factor (repeated measures) and group (C+ and Sham) as between-subjects factor indicated that in patients with subcortical lesion only a significant time × group interaction (F1,13=6.143, p=0.015), but no significant effect of time (F1,13=1.271, p=0.32) or group (F1,13=1.57, p=0.23). Post hoc t-tests indicated a significant increase in cFMA scores in the C+ group (n=10) only from Pre (10,4±2,33) to Post1 (13,7±2,74; p=0.002) sessions, but no significant changes between Pre and Post2 (12,1±2,82; p=0.17) or between Post1 and Post2 (p=0.24) sessions. No significant changes in cFMA scores were found in the Sham group (n=5).
Results in mixed lesion patients indicated no significant time × group interaction (F1,13=0.258, p=0.77), and no significant main effect of group (F1,13=1.312, p=0.28), but a significant main effect of time (F1,13=7.524, p=0.003). Post hoc t tests indicated that patients with mixed lesions (regardless on feedback modality) significantly increased cFMA scores from Pre (11±1.88) to Post1 (13.33±2.17; p=0.034) and from Pre to Post2 (13.23±1,7; p=0.005) sessions.
Furthermore, we separated the patients in 2 groups depending on lesion location (independent on feedback group) and performed a 2-way ANOVA (delta cFMA, Lesion) to analyze the difference in recovery based on lesion location. We found no significant difference in delta cFMA scores (delta Pre-Post1 F1,28=2.197 p=0.149; and delta Pre-Post2 F1,26=0.017 p=0.896) depending on lesion location. These results suggest that in mixed lesion patients the BMI effect might not be key in the overall patients’ minimal but significant motor improvement effect, as it is in the subcortical lesion only patients. However, lesion location was not the main factor regarding recovery, since when feedback contingency was ignored, no significant result was found. Nevertheless, due to the low number of patients these results should be carefully interpreted.
Secondary outcome measures: GAS, MAL and Ashworth scores
In the previous published analyses between Pre and Post1 data, we observed a significant improvement of MAL and GAS scores in both groups and no significant improvement of Ashworth scores (Ramos-Murguialday, et al., 2013). In the follow-up analyses, we found a significant increase in MAL and GAS scores from Pre to Post2 sessions and no significant change in MAL or GAS scores between Post1 and Post2 sessions in both C+ and Sham group (Table 2). We found a significant increase in Ashworth scores (i.e. increase in spasticity) from Pre to Post2 sessions in the Sham group (z=−2,63, p=0.009) but not in the C+ group (z=−1,269, p=0.2). Moreover, we found a significant increase in Ashworth scores from Post1 to Post2 sessions in both experimental (z=−2.764; p=0.006) and Sham groups (z=−2,271, p=0.023) (Table 2). In order to investigate the influence of the increase of spasticity in the cFMA, MAL and GAS scores during the follow-up measurement, we correlated delta (score difference between 2 sessions) of GAS and MAL with delta of Ashworth scores (Post2-Pre and Post2-Post1). We found no significant correlations between delta Ashworth and delta GAS, delta MAL scores or delta cFMA scores in any group (Supplementary Table 3). Furthermore, a Mann-Whitney U test on delta Ashworth scores (i.e., changes in Ashworth scores between Post1 and Post2 or between Pre and Post2 sessions) did not show significant differences between the experimental and control groups (Delta Ashworth scores from Post1 to Post2 session: C+=2.75±0.79; Sham=3.92±1.36; p=0.47; from Pre to Post2 session: C+=1.53±1.09; Sham=4.58±1.28; p=0.094).
Table 2.
Experimental and sham group MAL, GAS and Ashworth scores in assessment Pre, Post1 and Post2
| Session | p-values | |||||
|---|---|---|---|---|---|---|
| Group | Scale | Pre | Post1 | Post2 | Pre-Post2 | Post1-Post2 |
| C+ | MAL | 10.22±2.19 | 15.6±3.53 | 16.1±3.54 | 0.041 | 0.28 |
| GAS | 0.03±0.03 | 1.69±0.27 | 1.5±0.27 | 0.001 | 0.32 | |
| Ashworth | 10.34±1.85 | 9.13±1.83 | 11.88±1.8 | 0.2 | 0.006 | |
| Sham | MAL | 9.31±2.88 | 12.44±2.15 | 18.7±3.77 | 0.004 | 0.065 |
| GAS | 0 | 1.71±0.24 | 1.83±0.35 | 0.004 | 0.53 | |
| Ashworth | 6.46±1.35 | 6.36±1.48 | 10.33±1.5 | 0.009 | 0.023 | |
GAS = Goal attainment scale. MAL = Motor activity log. Values are given as mean ± standard error.
Electromyography (EMG) waveform length
Analyses of forearm EMG activity during continuous attempt to perform opening and closing of the hand (extend and flex the fingers) with Wilcoxon Signed Ranks test (data was not normally distributed) indicated no significant changes between Pre and Post2 sessions on the C+ (Pre=2.5±0.49, Post2=2.28±0.54, z=−0.943; p=0.35) or Sham groups (Pre=2.33±0.48, Post2=3.74±1.11, z=−0.628; p=0.53), confirming the results we observed previously when comparing Pre and Post1 data6. Similarly, we found no significant difference between Post1 and Post2 sessions in the C+ (Post1=3.66±0.74, z=−0.114, p=0.91) or Sham groups (Post1=3.58±0.97, z=−0.941, p=0.35). Mann Whitney U tests indicated no significant difference between experimental and Sham groups EMG waveform length Delta (after-before difference) from Post2 and Pre (C+=−0.57±0.66, Sham=1.41±1.07, U=60, p=0.33) or Post2 and Post1 (C+=−1.41±1.04, Sham=−0.29±1.25, U=75, p=0.89) sessions.
EMG waveform length was also assessed during performance of upper limb movements and posture holding. While in the previous analyses comparing Pre to Post1 data we observed significant improvement of EMG activity in the experimental group only during elbow and upper arm extension at the frontal and lateral parts of the deltoid and at the triceps, paired t tests on EMG waveform length of the different muscles evaluated during distinct upper limb movements revealed a statistically significant increase of muscle activity in both groups from Pre to Post2 and from Post1 to Post2 sessions (Supplementary Table 4). Independent t tests indicated no significant difference between experimental and Sham groups EMG waveform length changes from sessions Pre to Post2 or Post1 to Post2 in any of the upper limb muscles, indicating that both groups presented no significant difference on the amount of increase in upper limb EMG activity (Table 3).
Table 3.
EMG waveform length delta (after-before) values of arm and hand muscles during distinct upper limb movements
| Upper limb movements and and respective muscles |
|||||||
|---|---|---|---|---|---|---|---|
| Group | EMG waveform length delta | Shoulder flexion (deltoid) | Shoulder abduction (deltoid) | Elbow extension (triceps) | Arm supination (biceps) | Wrist extension (ext. dig.) | Fingers extension (ext. dig.) |
| C+ | Delta 1 (Post2-Post1) | 2,03±0,68 | 0,54±0,16 | 1,12±0,34 | 0,61±0,35 | 0,4±0,1 | 0,28±0,08 |
| Delta 2 (Post2-Pre) | 1,98±0,69 | 0,67±0,18 | 1,33±0,36 | 0,56±0,32 | 0,44±0,14 | 0,25±0,99 | |
| Sham | Delta 1 (Post2-Post1) | 2,51±0,78 | 0,67±0,3 | 1,34±0,3 | 0,09±0,08 | 0,81±0,33 | 0,45±0,19 |
| Delta 2 (Post2-Pre) | 2,09±0,79 | 0,86±0,21 | 1,13±0,21 | 0,24±0,1 | 0,79±0,3 | 0,4±0,18 | |
| independent t tests | |||||||
| p value (C+ Delta 1 vs. sham Delta1) | 0.66 | 0.69 | 0.65 | 0.27 | 0.36 | 0.35 | |
| p value (C+ Delta 2 vs. sham Delta2) | 0.92 | 0.5 | 0.64 | 0.4 | 0.31 | 0.42 | |
ext. dig. = extensor digitorum. Values are given as mean ± standard error.
As spasticity can be seen as an agonist/antagonist EMG ratio (ratEMG) conflict, we calculated and analyzed ratEMG changes and their relationship with recovery and spasticity and we found no significant interactions or main effects (See Supplementary Information).
fMRI analyses
While in the previous analysis between Pre1 and Post1 we found significant differences in LI in the experimental group only towards normalized contralateral activation during paretic hand movements, no significant changes between Pre1 and Post2 sessions in the C+ (Pre1=−0.044±0.097; Post2=−0.157±0.124; p=0.17) or Sham (Pre1=−0.119±0.149; Post2=−0.066±0.245; p=0.77) groups were observed. We also found no significant changes between Post1 and Post2 sessions in the C+ (Post1=−0.271±0.13; p=0.41) or Sham (Post1=0.271±0.159; p=0.33) groups and this time no correlation was found between changes in cFMA and LI (Supplementary Table 5).
Discussion
In a previous study we demonstrated that BMI training associated with behavioral physiotherapy is an efficient strategy to promote arm and hand motor recovery in severely paretic chronic stroke patients (Ramos-Murguialday, et al., 2013). Our current findings complement this previous study and indicate that significant improvements in upper limb motor function (FMA, GAS, MAL) are partly preserved six months after BMI-based rehabilitation. Specifically, the experimental group only showed a sustained significant improvement in cFMA motor scores six months after intervention as compared to baseline assessments. When analysing hand and arm FMA scores separately we found that long-term effects are preserved for the arm FMA scores only.
The exact instructions for the home training were design to maintain the residual movement the patients presented at post1. Not having a day-by-day control on the training might be a confounding factor, as some patients may train more than others and the ones with more residual movement capacities could train more and might be more motivated to do so. However, if one looks at the mean cFMA scores the sham group patients presented higher scores than the control group and therefore could have presented a larger gain at Post2, which was not the case. Furthermore, no significant difference related to frequency of home training after the intervention between experimental and Sham groups was found, and in the same line no correlation between frequency of home training and any of the outcome measures changes was found. These findings indicate that significantly higher cFMA scores six months after intervention as compared to baseline measurements found only in the experimental group cannot be attributed to differences in home training between groups and were not due to behavioural physiotherapy only. These results strengthen the importance of the contingent visual and proprioceptive feedback attempting to “associatively bridge” the lesion via BMI and may overcome the learned non-use effect produced by paralysis (Krakauer, 2006; Pomeroy, et al., 2012).
The significant increase in spasticity in the Post2 session on both did not correlate with any of the motor assessment (cFMA, EMG, GAS, MAL) scores. This strengthens the functional motor improvement result from our interventional study as independent from changes in spasticity indicating no long-term effect of the BMI intervention in the reduction of spasticity seen after intervention in the experimental group only.
Moreover, besides increased spasticity, both groups also presented significant increase in voluntary EMG activity during upper arm and hand movements, suggesting an overall increase in patients’ capacity for muscular contraction – either voluntarily (as indicated by EMG) or associated with spasticity. As both groups significantly increased spasticity and upper limb EMG activity between Post1 and Post2, and both groups increased EMG activity from Post1 to Post2 by a similar degree (no statistical difference was found between increase in voluntary EMG activity in both groups), these results may reflect an effect of home training and increase of muscle use instead of BMI training. However, there was no correlation between reported frequency of home training and increase of EMG activity from Post1 to Post2. Alternatively, increased spasticity and EMG activity in the Post2 session in both groups could be an effect of the physiotherapy, provided in equal extent to both groups during training. Physiotherapy may affect muscle strength in the long run more than BMI, because BMI is focused on the association between intention and movement while physiotherapy is focused on visible muscle activation. Therefore, the importance of the BMI to guide correct, or at least well timed muscle activation seems to be confirmed. Furthermore, as it was found no significant correlation between frequency of home training and increased Ashworth scores or EMG activity during upper limb movements we hypothesize that this effect is associated with the quality (i.e., type) of exercises instead of frequency of exercises during home training. For example, without proper therapeutic guidance patients may have performed more strenuous movements during home training plausibly inducing increased spasticity and increasing non-specific EMG activation during attempt to perform the requested task as a consequence. This would likely be associated with less efficient movements (i.e., more “noisy” movements). This assessment of movement quality may not be identifiable by EMG, but could explain why increased EMG activity between Pre and Post1 in the experimental group [Ramos-Murguialday, et al., 2013] was not preserved in the follow-up assessment (Post2). Alternatively, participation in this highly motivating treatment (for most patients may be the last resource of medical hope since no other treatment was proposed for these patients by their medical consultants) increased EMG activity and Ashworth as an unspecific side effect.
We want to emphasize that the patients in the control group received contingent correct feedback by chance sometimes during the intervention. The random movements of the orthoses coincided sometimes with desynchronization of the SMR rhythm. This difference was computed to be on average around 20% of the training time implying that even random feedback can sometimes result in correct feedback and may induce some limited neuroplastic changes. These results emphasize the importance of the link between brain motor activity and the relevance of improving decoding of brain activity to allow instrumental learning to stimulate functional neuroplasticity. The consequences of neuroplastic mechanisms during the chronic phase have been demonstrated to be very limited compared to the time windows during the acute phase (Carmichael & Krakauer, 2013). BMI might allow access to some of the neuroplastic mechanisms to allow significant motor functional recovery even in the chronic phase. How movement intention decoding accuracy, intensity of training (intervention schedule: how many days, hours and how often), movement controlled by BMI and physiotherapy would affect the functioning and stability of neuroplastic mechanisms needs to be further investigated.
Our fMRI results suggest a trend towards significance when comparing Pre and Post2 in the experimental group only. This might represent that if the associative link between brain and behavior is no longer there, the brain activity change towards normalized ipsilesional activity, which might be considered as functional plasticity, is slowly being reversed, as residual movement based training might not suffice to regain impaired movement specific ipsilesional activity. Furthermore, our results demonstrating that LI changes did not correlate with cFMA changes between Post1 and Post2 nor between Pre and Post2, suggest that BOLD activity changes observed during the intervention might represent motor learning (BMI motor control learning) and not motor recovery, as we hypothesized in our previous work (Ramos-Murguialday et al., 2013). On the other hand, the recovery might have consolidated in spinal neural networks and therefore, their appearance might last longer than the brain effect and are not captured by the LI analysis.
We demonstrated that altering a brain signal (increase in SMR desynchronization) time-contingent with visual and proprioceptive sensory feedback associated with orthosis and thus limb movement leads to significant motor improvement and functional neural reorganization, promoting motor function improvement and partial maintenance of the found effect. However, most other findings (secondary outcomes, hand cFMA, imaging, EMG) are not pointing to the same direction. The sustained significant improvement in cFMA scores six months after intervention might reflect recovery from motor impairment. For example, a clinically meaningful change from no activity to some activity in lifting and stretching the arm, turning the forearm, extending the wrist and/or fingers was preserved six months after intervention. It is conceivable that BMI training engaging a crucial network of brain regions related to intent (visuomotor task) could stimulate activation of neuroplastic mechanisms that allow physiotherapy to promote functional motor recovery in chronic severe stroke (evidenced in cFMA scores and EMG activity immediately after intervention6 and in the sustained cFMA scores six months after intervention). However, the experimental design employed here does not allow firm conclusions conceiving the relevance of behavioral physiotherapy to the improvements in motor function.
Despite the small samples we used in the analyses regarding lesion location relevance in motor recovery when using BMI coupled with physiotherapy as intervention, our results suggest that lesion location might not be a limiting factor. However, these results should be considered with caution. Further studies, including more patients or including only patients with specific lesion locations (e.g., mixed or subcortical lesions) in the inclusion criteria, are necessary to better evaluate BMI training effects in motor recovery according to patient’s lesion location. Moreover, it may be considered that motor recovery after BMI-training and physiotherapy has not reached a level of motor improvement that has a significant clinical impact on chronic stroke patients. Still, we believe that as a proof-of-concept this study demonstrates the potential of BMI to promote long-term motor recovery in chronic stroke patients with severe paresis, a population that is currently unable to undergo any rehabilitation strategy. In any case, to promote stronger clinical impacts, further development of BMI trainings may include implementation of (1) functional electrical stimulation (FES) to induce paretic limb movements, as it also activates muscle contractions [Biasiucci, et al., 2018]; (2) invasive SMR recordings (invasive BMI), which record SMR with superior signal quality (e.g., less noise and better resolution), (3) broader range of practiced movements, and (4) longer rehabilitation interventions.
Conclusions
In summary, our proof of concept study demonstrated that BMI-based rehabilitation in chronic stroke patients with severe paresis successfully promotes motor recovery and cortical reorganization associated with recovery of function; and induces long-lasting improvements in motor function.
Supplementary Material
Acknowledgements
We would like to thank Dr. Massimiliano Rea, Dr. Leonhard Laeer, and Dr. Manuel Agostini for their assistance during data acquisition.
Funding
This work was supported by the German Federal Ministry of Education and Research (BMBF, FKZ 13GW0053 and FZ 16SV7754) as well as the Deutsche Forschungsgemeinschaft (DFG, Koselleck); Baden-Württemberg Stiftung (GRUENS ROB1); Diputacion Foral de Gipuzkoa (INKRATEK), the Intramural Research Program (IRP) of the National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, Maryland, USA; the Center for Neuroscience and Regenerative Medicine (CNRM); Uniformed Services University of Health Sciences, Bethesda, Maryland, US. Several authors were supported by the DAAD (Deutscher Akademischer Austauschdienst) to EGC and WC; CNPq (Brazilian National Counsel of Technological and Scientific Development) to FB; CAPES (Coordination for the Improvement of Higher Level -or Education-Personnel, Brazil) to MRC and Humbolt Award to LC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Potential conflicts of interest
Nothing to report.
References
- Ang Kai Keng, Chua Karen Sui Geok, Phua Kok Soon, Wang Chuanchu, Chin Zheng Yang, Kuah CWK, Low Wilson, Guan C, 2014. A Randomized Controlled Trial of EEG-Based Motor Imagery Brain-Computer Interface Robotic Rehabilitation for Stroke. Clin. EEG Neurosci. doi: 10.1177/1550059414522229 [DOI] [PubMed] [Google Scholar]
- Biasiucci A, Leeb R, Iturrate I, Perdikis S, Al-Khodairy A, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nature Communications. 2018. vol: 9 (1) pp: 2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N and Cohen L. 2007. Brain–computer interfaces: communication and restoration of movement in paralysis. J Physiol. 579(3):621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broetz D and Augustinski P. 2010. Aus heiterem Himmel Mein bewegtes Leben vor und nach dem Schlaganfall. Wieder selbstständig werden mit dem Brötz-Training. 1th ed. Hrsg. Stuttgart (Germany): Trias. [Google Scholar]
- Broetz D 2010. Combination of brain-computer interface training and goal-directed physical therapy in chronic stroke: a case report. Neurorehabil Neural Rep. 24(7):674–679. [DOI] [PubMed] [Google Scholar]
- Broetz D et al. 2014. A new hand assessment instrument for severely affected stroke patients. NeuroRehabil. 34(3):409–427. [DOI] [PubMed] [Google Scholar]
- Caria A et al. , 2011. Chronic stroke recovery after combined BCI training and physiotherapy: a case report. Psychophysiology. 48(4):578–582. [DOI] [PubMed] [Google Scholar]
- Carmichael S and Krakauer J. 2013. The promise of neuro-recovery after stroke. Stroke. 44:S103. [DOI] [PubMed] [Google Scholar]
- Crow J and Harmeling-van der Wel BC. 2008. Hierarchical properties of the motor function sections of the fugl-meyer assessment scale for people after stroke: a retrospective study. Phys Ther. 88(12):1554–1567. [DOI] [PubMed] [Google Scholar]
- Dahl A et al. 2008. Short- and long-term outcome of constraint-induced movement therapy after stroke: a randomized controlled feasibility trial. Clin Rehabil. 22(5): 436–447. [DOI] [PubMed] [Google Scholar]
- Daly J and Wolpaw J. 2008. Brain–computer interfaces in neurological rehabilitation. Lancet Neurology. 7(11):1032–1043. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer A et al. 1975. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 7(1):13–31. [PubMed] [Google Scholar]
- Hurn J, Kneebone I and Cropley M. 2006. Goal setting as an outcome measure: a systematic review. Clin Rehabil. 20(9):756–772. [DOI] [PubMed] [Google Scholar]
- Krakauer J 2006. Motor Learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 19(1):84–90. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Bernhardt J and Kwakkel G. 2011. Stroke rehabilitation. Lancet. 377(9778):1693–1702. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Coupar F and Pollock A. 2009. Motor recovery after stroke: a systematic review. Lancet Neurol. 8(8):741–754. [DOI] [PubMed] [Google Scholar]
- Liang C et al. 2012. Effectiveness of thermal stimulation for the moderately to severely paretic leg after stroke: serial changes at one-year follow-up. Arch Phys Med Rehabil. 93(11):1903–1910. [DOI] [PubMed] [Google Scholar]
- Luft A et al. 2004. Repetitive bilateral arm training and motor cortex activation in chronic stroke. 292(15):1853–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastos M, Miller K, Eliasson A, Imms C. 2007. Goal-directed training: linking theories of treatment to clinical practice for improved functional activities in daily life. Clin Rehabil. 21(1):47–55. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G and Aranibar A 1979. Review of stroke rehabilitation. ElectroencephClin Neurophysiol. 46(2):138–146. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G and Lopes da Silva F. 1999. Event-related eeg/meg synchronization and desynchronization: basic principles. Clin Neurophysiol. 110(11):1842–1857. [DOI] [PubMed] [Google Scholar]
- Pichiorri F, Morone G, Petti M, Toppi J, Pisotta I, Molinari M, Paolucci S, Inghilleri M, Astolfi L, Cincotti F, Mattia D, 2015. Brain-computer interface boosts motor imagery practice during stroke recovery. Ann. Neurol 77, 851–865. doi: 10.1002/ana.24390 [DOI] [PubMed] [Google Scholar]
- Pomeroy V et al. 2012. The SWIFT Cast trial protocol: a randomized controlled evaluation of the efficacy of an ankle-foot cast on walking recovery early after stroke and the neural-biomechanical correlates of response. Int J Stroke. 7(1):86–93. [DOI] [PubMed] [Google Scholar]
- Ono T, Shindo K, Kawashima K, Ota N, Ito M, Ota T, Mukaino M, Fujiwara T, Kimura A, Liu M, Ushiba J, 2014. Brain-computer interface with somatosensory feedback improves functional recovery from severe hemiplegia due to chronic stroke. Front. Neuroeng 7, 19. doi: 10.3389/fneng.2014.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Murguialday A et al. 2013. Brain-machine interface in chronic stroke rehabilitation: A controlled study. Ann Neurol. 74(1):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Murguialday A et al. 2015. Residual EMG in chronic paralyzed stroke patients revealed decodable muscle contraction control during impaired movements. Ann TranslClin Neurol. 2(1):1–11. [Google Scholar]
- Ramos-Murguialday A et al. 2012. Proprioceptive feedback and brain computer interface (bci) based neuroprostheses. PloS One, 7(10):e47048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehm B et al. 2010. Functional neuroanatomy of mirroring during a unimanual force generation task. Cerebral Cortex. 20(1):34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu A et al. 1995. Congruent unilateral impairments for real and imagined hand movements. Neuroreport. 6(7):997–1001. [DOI] [PubMed] [Google Scholar]
- Stinear C 2010. Prediction of recovery of motor function after stroke. Lancet Neurol. 9(12):1228–1232. [DOI] [PubMed] [Google Scholar]
- Taub E et al. 1993. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 74(4):347–354. [PubMed] [Google Scholar]
- Timmermans A et al. 2013. Effect of mental practice on the improvement of function and daily activity performance of the upper extremity in patients with subacute stroke: a randomized clinical trial. J Am Med Dir Assoc. 14(3):204–212. [DOI] [PubMed] [Google Scholar]
- Uswatte G et al. 2005. Reliability and validity of the upper-extremity Motor Activity Log-14 for measuring real-world arm use. Stroke. 36(11):2493–2496. [DOI] [PubMed] [Google Scholar]
- Whitall J et al. , 2011. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabil Neural Repair. 25(2):118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M and Lidzba K. 2007. LI-tool: a new toolbox to assess lateralization in functional MR-data. J NeurosciMeth. 163:128–136. [DOI] [PubMed] [Google Scholar]
- Wolf S et al. 2006. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke. JAMA. 296(17):2095–2104. [DOI] [PubMed] [Google Scholar]
- Young J and Forster A. 2007. Review of stroke rehabilitation. BMJ. 334(7584): 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


