Abstract
BACKGROUND:
Head and neck cancer (HNC) patients experience significant physical and psychological morbidity during radiotherapy (XRT) which contributes to treatment interruptions and poor quality of life. Although spouses/partners can help by encouraging patient self-management (e.g., self-care) during XRT, they often experience high psychological distress rates, lack basic healthcare knowledge/skills, and report increased marital conflict regarding patient self-management. This pilot study examined the feasibility and acceptability of a six-session telephone-based intervention called SHARE, which teaches self-management, communication, and coping skills to HNC patients and their spouses. Treatment effects of SHARE relative to usual medical care (UMC) in controlling patient physical symptoms and improving patient/spouse psychological and marital functioning were also examined.
METHODS:
Thirty patients initiating XRT and their spouses (N=60 participants; 40% racial/ethnic minorities) were randomized to SHARE or UMC, and pre- and post-intervention assessments were completed.
RESULTS:
Solid recruitment (70%) and low attrition rates (7%) demonstrated feasibility. Strong program evaluations and homework completion rates (72%) supported acceptability. Significant treatment effects (medium in magnitude) were observed for SHARE relative to UMC with regard to HNC-specific physical symptom burden (Cohen’s d=−.89) and symptom interference (d= −.86). Medium-to-large effects favoring SHARE were also found for patient and spouse depressive symptoms (d=−.84) and cancer-specific distress (d=−1.05).
CONCLUSION:
Findings support the feasibility, acceptability, and preliminary efficacy of SHARE. They also suggest that programs that empower HNC couples with the necessary skills to coordinate care and manage the challenges of XRT together hold great promise for controlling patient physical symptoms and improving both partners’ psychological functioning.
Keywords: head and neck cancer, couple-based intervention, MDASI-HN, psychological distress, supportive care
PRECIS:
Head and neck cancer patients participating in a couple-based intervention experienced significantly less physical symptom burden relative to those in usual medical care, with medium effect sizes. Medium-to-large effect sizes were also found for patient and spouse depressive symptoms and cancer-specific distress.
Introduction
Although head and neck cancers (HNC) account for only 3% of cancer cases in the United States,1 the disease and its treatment have disproportionate impact on quality of life (QOL). Patients often undergo intensive radiotherapy (XRT) either alone or combined with other treatments.2 XRT results in significant physical side-effects (e.g., xerostomia), functional challenges (e.g., difficulty swallowing/speaking), and psychosocial problems (i.e., depression/anxiety) that can persist long after treatment ends.3, 4 To control side-effects and reduce long-term functional problems, HNC patients must follow complicated and time-consuming self-management protocols,5, 6 which can reduce recovery time and improve QOL.5–9 However, non-adherence rates are high (up to 72% for oral care,10 68–87% for swallowing exercises),11 and almost half of patients experience significant weight loss and malnutrition during XRT.12, 13 Poorly managed side-effects lead to treatment interruptions and more complicated and costly rehabilitation.14 Possible contributing factors are the time-consuming nature of the self-care protocols, lack of knowledge and self-efficacy for implementing them, and patient depressed mood.15, 16 Adding to the challenge, the debilitating nature of XRT and rigorous treatment schedule make it difficult for patients to attend clinic-based supportive care programs.17, 18 Information/support to manage symptoms is a predictor of positive rehabilitation outcomes in HNC,19 thus, home-based programs that provide psychoeducation and support for self-management could enhance supportive care offerings in HNC and improve patient QOL.
Informal caregivers (e.g., spouses/partners) bear the responsibility for providing symptom management, emotional support, and rehabilitation assistance in the outpatient setting 20 – often with little or no training.16, 21 Therefore, problems with patient self-management could be related to unexamined factors in their informal caregivers. In HNC, spouse distress rates are comparable to or higher than those of patients,22 and patient symptom burden contributes to spousal distress.23 Despite the very best of intentions, spouses can also engage in maladaptive communication (e.g., nagging, criticizing) that can undermine patient self-management and exacerbate marital conflict.24, 25 Couple-based interventions hold great promise for this population because they can simultaneously address patient, partner, and relationship factors that hinder self-management and effective caregiving.
We developed a couple-based intervention called SHARE (Spouses coping with the Head And neck Radiation Experience) that is delivered by phone. SHARE provides psychoeducation, encourages self-management, and teaches strategies to improve teamwork and coping. The goal is to focus couples on self-management and coordination of care and support at the start of XRT to control/alleviate symptom burden (physical and psychological) and improve marital adjustment.
This pilot trial tested the feasibility (i.e., recruitment, retention, and session completion rates), acceptability (i.e., program evaluations and homework completion rates), and preliminary efficacy of SHARE relative to usual medical care (UMC). We hypothesized that patients receiving SHARE would report less physical symptom burden at 1-month follow-up than those receiving UMC. We also hypothesized that patients and spouses receiving SHARE would report greater improvements in psychological functioning and marital adjustment at follow-up than those receiving UMC.
Methods
Procedures
An Institutional Review Board in the Southwestern U.S. approved this study. Patient eligibility criteria included: 1) initiating XRT-based HNC treatment, 2) having an Eastern Cooperative Oncology Group Performance Status of ≤ 2, and, 3) having a co-residing spouse/partner. Patients and spouses also had to be >18 years, speak/read English, and be able to provide informed consent. Couples were approached at a pre-treatment clinic visit. Those providing written informed consent completed baseline surveys and were randomly assigned to SHARE or UMC. Follow-up surveys were administered 1-month post-XRT.
Measures
Physical Symptom Burden.
The M D Anderson Symptom Inventory Head and Neck survey (MDASI-HN)26 comprises the 13-item MDASI-core (general cancer symptoms), 9-item MDASI-HNS (HNC-specific symptoms), and 6-item MDASI-Interference. Items are rated on a 0–10 scale, with higher scores indicating greater severity/interference. Internal reliability (Cronbach’s alpha) ranged from .85 to .89.
Psychological Functioning.
The 6-item Patient-Reported Outcomes Measurement Information System (PROMIS) depression short-form assesses negative mood/views of self.27 The 6-item PROMIS anxiety short-form assesses fear/worry.27 Items are rated from 1(never) to 5(always) and can be scaled into a t-score with a mean of 50 and standard deviation of 10. Internal reliability for depression was αpatients=.83, and αspouses=.90. For anxiety it was αpatients=.92, and αspouses=.90. The 22-item Impact of Events Scale-Revised (IES-R),28 measures intrusive/avoidant thoughts and hyperarousal related to cancer. Scores >33 (of 88) indicate high cancer-specific distress. Internal reliability was excellent (αpatients=.87 and αspouses=.93).
Marital Adjustment.
Scores on the 7-item short Dyadic Adjustment Scale (DAS-7) <21 (of 36) indicate marital distress.29 Internal reliability was good (α patients =.74 and αspouses = .85).
Sociodemographic/Medical Variables.
At baseline, patients/spouses reported their age, race/ethnicity, education, employment, marital status, length of relationship, and alcohol use (AUDIT-C 3-item screener; scores ≥3 for women and ≥4 for men indicate alcohol misuse30). Patients also reported on time since diagnosis, disease stage, and tobacco and psychiatric history. At 1-month follow-up, patients reported on any unplanned clinic visits, ED visits, or hospital stays.
Study Conditions
UMC. UMC comprised standard oncologic care visits (e.g., basic discussions about prognosis/treatment and routine symptom management). Spouses could attend these visits with patients but it was not a requirement.
SHARE. In addition to UMC, patients and spouses in SHARE each received a manual covering: 1) self-care, 2) symptom management, 3) stress management, 4) coping with cancer as a team, 5) managing post-treatment recovery, and 6) finding the new normal together after cancer. Units 1–3 focused on individual skills, with tailoring based on role. Patient-specific manual content included self-care, soliciting support, and balancing accepting help with autonomy. Spouse-specific content included caregiver self-care, caregiving skills (e.g., hygiene care, meal preparation, identifying red flag symptoms), and strategies for supporting patient self-management. Units 4–6 were dyadic, so manual content was the same for both partners.
In addition to the manual, participants received an educational CD and DVD that reinforced covered materials (e.g., relaxation and swallowing exercises), and six 60-minute telephone-sessions corresponding to the manual with interventionists who had Master’s level training in mental-health counseling (LPC, LCSW). During sessions, interventionists reviewed manual content, guided participants through skill-building activities, and assigned/reviewed homework. Weekly session goals, skills-building activities, and homework assignments are described in Supplemental Appendix A. Patients and spouses each received separate calls for units 1–3 to 1) build rapport; 2) allow in-depth coverage of tailored materials; and, 3) practice individual skills/receive feedback before moving to dyadic skills/content. Sessions covering units 4–6 were delivered to patients and spouses jointly via speakerphone. Fidelity checklists were developed and 50% of session recordings were reviewed. The average fidelity rating was 93%.
Session delivery was based on the known timeline for symptom onset and recovery for HNC XRT. Sessions 1–4 were delivered weekly upon treatment commencement with the goal of teaching individual and basic dyadic skills before acute symptom onset. A 4-week break followed to allow time to apply skills learned and to allow for patients to recuperate. Sessions 5–6 were survivorship-focused and delivered after the break.
Data Analysis
Recruitment rates and descriptive statistics for feasibility/acceptability measures were computed. t-tests examined study outcomes by treatment group (SHARE and UMC). Pearson and partial correlations among outcome variables for patients and spouses were examined. Analyses of covariance (ANCOVAs) were performed with baseline (T0) scores as covariates and 1-month follow-up (T1) scores as outcomes. Sociodemographic/medical variables significantly correlated with the outcomes were also included as covariates. For each outcome, treatment group (SHARE or UMC) and role (patient or spouse) main effects were examined as well as their interaction. An intent-to-treat framework was implemented, using the last observation carried forward method to address missing data. Effect sizes (Cohen’s d) were calculated for significant effects at T1.31
Results
Sample Descriptives
Demographic/Medical Characteristics. As Table 1 shows, patients were predominantly male, middle-aged, and married. Spouses were mostly female and middle-aged. Most patients had pharynx cancers (N=19-oropharynx, 2-nasopharynx, 2-hypopharynx); 63% of oropharynx patients had HPV-positive (p16) tumors. At baseline, 3 patients were current smokers, 4 were recent quitters (<6 months), and 12 were former smokers. Nine patients and 11 spouses met AUDIT-C criteria for alcohol misuse. Five patients had psychiatric histories significant for depression. Average length of relationship in years was = 28.85 (SD = 12.65; Range = 3 to 54).
Table 1.
Sample Descriptives (N=30 patients and 30 spouses)
| Patients(%) | Spouses(%) | |
|---|---|---|
| Gender | ||
| Male | 24(80) | 7(23) |
| Female | 6(20) | 23(77) |
| Age in years | =58.43, SD=10.49 (Range=21 to 78) | =58.07, SD=10.11 (Range=27 to 79) |
| Ethnicity | ||
| Hispanic | 6(20) | 3(10) |
| Race* (%) | ||
| White | 24(80) | 22(73) |
| Black | 4(13) | 3(10) |
| Asian | -- | 2( 7) |
| > 1 race | 2( 7) | 3(10) |
| Employment Status | ||
| Full-time | 18(60) | 17(57) |
| Part-time | 3(10) | 3(10) |
| Unemployed | 9(30) | 9(30) |
| Did not answer | -- | 1( 3) |
| Education | ||
| High school diploma or less | 6(20) | 8(27) |
| At least some college | 7(23) | 3(10) |
| College degree | 17(57) | 19(63) |
| Type of HNC | ||
| Oral cavity | 1( 3) | |
| Pharynx | 23(77) | |
| Larynx | 3(10) | |
| Paranasal sinus/Nasal cavity | 2( 7) | |
| Salivary gland | 1( 3) | |
| Cancer Stage | ||
| 1 | 3(10) | |
| 2 | 3(10) | |
| 3 | 1( 3) | |
| 4A | 23(77) | |
| Treatment | ||
| XRT | 5(17) | |
| Surgery + XRT | 7(23) | |
| CRT | 6(20) | |
| Surgery + CRT | 12(40) |
Note: HNC=head and neck cancer, XRT=radiotherapy, CRT=chemoradiation
Physical Symptom Burden.
At T0, the most commonly reported symptoms were pain, disturbed sleep, fatigue, dry mouth, and difficulty swallowing. Over 30% of patients reported moderate to severe (≥5 of 10) pain, fatigue, and disturbed sleep; 20–30% reported moderate to severe dry mouth and swallowing problems. At T1, the most common symptoms were problems with dry mouth, mucus, fatigue, and taste, swallowing, and speech. Over 30% of patients rated these symptoms as moderate to severe.
Psychological Functioning and Marital Adjustment.
At T0, several participants had high anxiety (patients-27%, spouses-37%), depression (spouses only-30%), and/or cancer-specific distress (patients-27%, spouses-47%); 3% of patients and 10% of spouses reported marital distress. Partial correlations for patients and spouses for anxiety were significant. Spouses had significantly higher depression and cancer-specific distress than patients (Table 2).
Table 2.
Baseline Correlations and Descriptive Results (N=30 patients and 30 spouses)
| 1 | 2 | 3 | 4 | 5 | 6 | Patients Mean(SD) | Spouses Mean (SD) | t | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. MDASI Core | -- | .77** | .67** | .21 | .50** | .43* | .18 | 1.61(1.30) | -- | -- |
| 2. MDASI-HNS | -- | -- | .56** | .09 | .24 | .30 | .24 | 1.57(1.61) | -- | -- |
| 3. MDASI-Interference | -- | -- | -- | .37* | .30 | .42* | .15 | 2.04(1.86) | -- | -- |
| 4. PROMIS Depression1 | -- | -- | -- | −.13 | .41* | .59** | −.35 | 9.27(3.31) | 12.40(5.87) | −2.40* |
| 5. PROMIS Anxiety1 | -- | -- | -- | .53** | .49** | .51** | .11 | 11.53(4.17) | 13.00(4.97) | −1.66 |
| 6. IES-R Total | -- | -- | -- | .81** | .48** | .31 | −.24 | 20.50(14.70) | 34.13(21.74) | −3.42** |
| 7. DAS-7 | -- | -- | -- | −.10 | −.08 | −.08 | .38* | 28.80(4.01) | 27.57(5.28) | 1.39 |
Note: Partial correlations between patients and spouses are in bold, on the diagonal. Correlations for patients are above the diagonal. Correlations for spouses are below the diagonal. t=paired samples t-test *p<.05, **p<.01. 1PROMIS raw scores are provided.
Feasibility
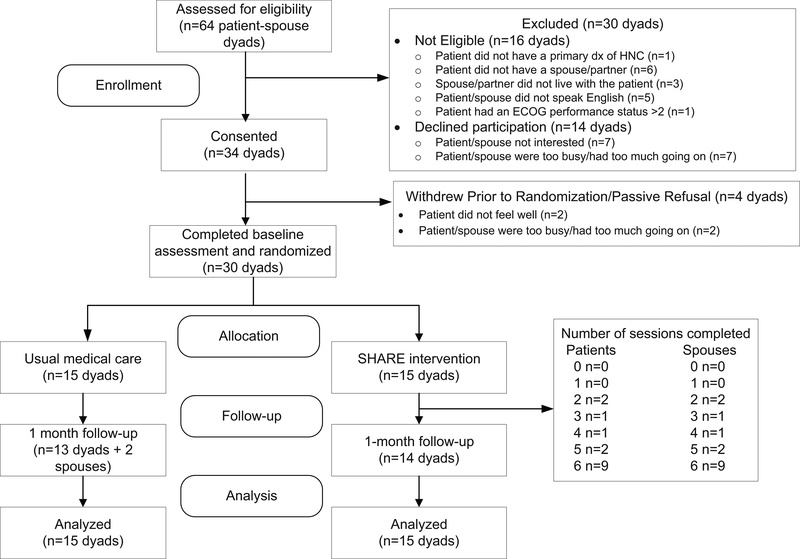
As Figure 1 shows, 64 couples were screened over an 18-month period; 16 were excluded due to one partner being ineligible. Thirty-four of the forty-four eligible couples (70%) consented, but 4 withdrew before returning the baseline survey. The remaining 30 couples were randomized to SHARE (15) and UMC (15).
Figure 1.
Consort Diagram
Overall, the telephone session completion rate was good (84%); 60% of patients and spouses completed all sessions. However, completion rates varied for individual (95%) and dyadic sessions (71%). Patients missed sessions because they were not feeling well and spouses missed sessions because they had too much going on/other demands on their time. In 13% of dyadic sessions, even though the patient was not feeling well, spouses requested to continue and completed the session alone. Retention at follow-up was excellent (93%) and retention rates did not differ by treatment group.
Acceptability
Patients and spouses completed 72% of homework assignments. They rated SHARE favorably in terms of format, helpfulness, and usefulness (Table 3). Although homework and in-session activities were rated as enjoyable/helpful, approximately 1/3 of patients and spouses felt the time to review study materials, complete homework, and participate in the 60-minute telephone sessions was too long.
Table 3.
Program Evaluations
| Patients M(SD) | Spouses M(SD) | |
|---|---|---|
| How helpful were the sessions? | 7.9(2.5) | 8.7(1.7) |
| How useful was the manual? | 8.8(2.8) | 9.5(.9) |
| How relevant was the material covered to what you are experiencing? | 7.5(1.8) | 7.8(2.0) |
| How satisfied were you with the telephone delivery format? | 8.5(2.1) | 9.0(2.1) |
| How enjoyable were the homework assignments? | 7.2(2.6) | 8.0(3.0) |
| How helpful were the in-session activities? | 8.4(2.3) | 8.6(2.6) |
| How much did you enjoy participating with your partner? | 8.0(3.1) | 8.2(2.8) |
| How much did you like having some individual and some joint sessions? | 8.6(2.0) | 7.5(3.8) |
| How attentive was the interventionist? | 10.0 (0) | 9.6(.8) |
| To what extent did you feel heard, understood, and respected by the interventionist? | 9.9 (.1) | 10.0(0) |
| Overall, how satisfied are you with the program? | 8.2(2.6) | 9.2(.8) |
M=mean, SD=standard deviation; Ratings are on a 0–10 scale; higher scores indicate greater endorsement.
Preliminary Efficacy
Table 4 reports descriptive statistics for study outcomes at T0 and T1. No significant (p≤.05) baseline differences by treatment group were found other than that spouses receiving SHARE reported significantly higher anxiety at T0 than spouses receiving UMC (t=3.07, p=.005). Table 5 reports results of ANCOVAs for each outcome, controlling for age and length of relationship. None of the role main effects or interactions was statistically significant, so we present only treatment effects for simplicity.
Table 4.
Treatment and Control Group Means For Patients and Spouses
| Patients |
Spouses |
|||||||
|---|---|---|---|---|---|---|---|---|
| SHARE | UMC | SHARE | UMC | |||||
| Baseline M (SD) | Follow-up M (SD) | Baseline M (SD) | Follow-up M (SD) | Baseline M (SD) | Follow-up M (SD) | Baseline M (SD) | Follow-up M (SD) | |
| Physical Symptom Burden1 | ||||||||
| MDASI Core | 1.99 (1.54) | 2.30 (1.65) | 1.23 (.90) | 2.04 (1.57) | -- | -- | -- | -- |
| MDASI HN Severity | 2.19 (1.94) | 2.93 (2.29) | 1.17 (.93) | 2.87 (2.24) | -- | -- | -- | -- |
| MDASI Interference | 2.21 (1.77) | 1.65 (1.77) | 1.87 (1.99) | 2.40 (2.14) | -- | -- | -- | -- |
| Psychological Functioning | ||||||||
| PROMIS Depression2 | 45.77 (8.49) | 45.21 (8.34) | 49.17 (7.52) | 48.82 (6.80) | 56.02 (8.63) | 50.95 (9.16) | 50.07 (9.66) | 52.91 (10.28) |
| PROMIS Anxiety2 | 55.99 (8.75) | 49.83 (7.28) | 50.94 (6.82) | 49.01 (6.95) | 59.71 (8.16) | 55.05 (8.61) | 50.97 (7.39) | 50.28 (8.49) |
| IES3 | 23.47 (16.98) | 19.07 (17.76) | 17.53 (11.86) | 19.47 (14.51) | 41.07 (20.62) | 30.80 (26.93) | 27.20 (21.23) | 29.40 (20.18) |
| Marital Adjustment | ||||||||
| DAS-74 | 29.33 (5.31) | 29.87 (5.45) | 28.27 (2.12) | 27.93 (2.89) | 26.07 (6.86) | 25.27 (7.71) | 29.07 (2.43) | 25.73 (3.53) |
Note:
Scores can range from 0–10; higher scores indicate greater severity/interference
Standardized scores >60 suggest need for further psychological evaluation.
Scores > 33 indicate significant cancer-specific distress.
Higher scores indicate better marital adjustment.
Table 5.
ANCOVA results for primary outcomes
| Treatment Group | |||
|---|---|---|---|
| Measure at T1 | F-value | p-value | Least square means |
| Physical Symptom Burden | |||
| MDASI-Core | n.s. | n.s. | -- |
| MDASI-HNS | 5.69 | .03 | UMC=3.91, SHARE=2.06 |
| MDASI-Interference | 4.55 | .05 | UMC=2.79, SHARE=1.76 |
| Psychological Functioning | |||
| PROMIS Depression | 4.21 | .04 | UMC=11.22, SHARE=8.90 |
| PROMIS Anxiety | n.s. | n.s. | -- |
| IES-R Total | 6.52 | .01 | UMC=30.09; SHARE=18.90 |
| Marital Adjustment | |||
| DAS-7 | n.s. | n.s. | -- |
Note: n.s.= not significant
Physical Symptom Burden.
A significant difference by treatment group was observed for MDASI-HNS (p=.04), with patients in SHARE reporting less severe HNC-specific symptoms at T1 than those in UMC. The effect size was medium32 (d=−.89, 95% CI=−1.64 to −.14). MDASI-interference also showed significant difference by treatment group (p=.01) with a medium effect size in favor of SHARE (d=−.86, 95% CI=−1.61 to −.11).
Psychological Functioning.
A significant difference by treatment group was observed for depression (p<.05), with patients and spouses in SHARE reporting less depressive symptoms than those in UMC. The effect size was medium (d=−.84, 95% CI=−1.59 to −.10). Cancer-specific distress also showed significant difference by treatment group (p=.01), with a large effect size32 in favor of SHARE (d=−1.05, 95% CI=−1.81 to −.28).
Marital Adjustment.
No significant effects were found.
Clinical Significance
Physical Symptom Burden.
We examined patients in SHARE and UMC who either did not change from T0 to T1 or got worse. At T0, 4 patients had scores >1SD above the MDASI-HNS mean; at T1 there were 11 patients. At T1, Only 7% of patients in SHARE reported more severe physical symptoms compared to 47% of patients in UMC (χ2=6.14, p=.01). Moreover, 27% of patients in SHARE reported no change and 53% reported less symptom interference (MDASI) at T1. In UMC, 40% of patients reported no change and 47% reported greater symptom interference (χ2=5.60, p=.06).
Psychological Functioning.
Patients and spouses with high baseline distress levels (e.g., above PROMIS depression/anxiety and IES-R cut-offs) who improved at T1 were identified and differences by treatment group were examined. Chi-square analyses revealed that for spouses with high depression levels, 83% of those receiving SHARE improved and none got worse whereas 66% of those receiving UMC stayed the same and 33% got worse at T1 (χ2=6.75, p=.03).
Healthcare Use.
No significant group differences were found. At T1, 3 patients in SHARE and 3 in UMC reported an unplanned clinic visit. Reasons were skin rash (3), dehydration (1), and problems with taste/swallowing (2). One patient in UMC also reported an ED visit.
Discussion
This study examined the feasibility, acceptability, and preliminary efficacy of the SHARE intervention. The recruitment rate was 70%, which is higher than rates for most couple-based interventions in cancer33 and supports the feasibility of recruiting HNC patients undergoing XRT. Participants rated the intervention as useful, enjoyable, and helpful. Retention (93%) and homework completion (72%) rates were strong. Medium effect sizes were found for the impact of SHARE relative to UMC on HNC-specific symptom severity (d=−.89) and symptom interference (d= −.86). Medium to large effect sizes favoring SHARE were also found for both partners’ depressive symptoms and cancer-specific distress. The number of patients whose HN-specific symptoms remained at/returned to baseline levels at follow-up and the number of spouses with high depression levels who improved were also significantly greater in SHARE than UMC.
To our knowledge, SHARE is the first couple-based intervention focusing on self-management for HNC patients and their spousal caregivers. Of note, 20% of the US HNC population comprises racial/ethnic minorities,34 and minority enrollment in couple-based interventions is notoriously poor, averaging around 16%.33 In this study, 40% of patients and 36% of spouses were minorities, which bolsters generalizability. However, given the manualized format, more work is needed to determine the effectiveness of SHARE in a more diverse sample with regard to health literacy/education.
Although SHARE evidenced solid effect sizes, there is room for improvement. For example, some interesting findings emerged related to innovative design features we incorporated to maximize enrollment and retention. Spouses evaluated telephone-delivery more favorably than patients; and some patients found it difficult to talk on the telephone as XRT progressed. Thus, we may need to consider other delivery formats (e.g., web-based) that are still interactive but less burdensome. Additionally, unlike most couple-based interventions that treat patients and spouses together (all dyadic sessions),33 we used a hybrid approach comprising individual and dyadic sessions. Couples rated this feature favorably, but it required delivering nine 60-minute sessions (3 patients, 3 spouses, 3 joint/dyadic), which is resource intensive. Dyadic session completion rates were also lower than individual session completion rates. The most likely reason is timing, as dyadic sessions were delivered on/after week 4 of XRT, when patients typically begin experiencing more severe physical symptoms. Moving forward, we will consider a shorter timeline for delivery and restructuring the order of individual and dyadic sessions.
Even though SHARE resulted in statistically significant and clinically meaningful control of HNC-specific symptoms and improvements in depression, no significant treatment effects were found for marital adjustment. Since 97% of patients and 90% of spouses reported satisfying marriages at baseline, there was little room for improvement. This could reflect a selection bias, whereby satisfied couples were more likely to participate. The fact that so many couples had satisfying relationships and varying degrees of physical/psychological symptom burden could have also contributed to the lower ratings for relevance of the program materials (Mean=7.5 for patients and 7.8 for spouses) relative to other aspects of the program. Given this, interventions with a pre-specified number of sessions on predetermined topics may not be realistic for this population. Adaptive intervention designs that change in intensity, duration, and focus based on participant needs may help maximize effectiveness and improve uptake of the SHARE intervention in HNC clinical settings.
The small sample limited statistical power for detecting effects and may have contributed to the group imbalance on spousal anxiety at baseline, despite randomization. A larger trial would allow replication of findings, analysis of sub-groups, exploration of the impact of SHARE on health service use, and examination of whether sociodemographic or medical variables moderate treatment effects. It would also allow possible expansion of the eligibility criteria to include other informal caregivers (e.g., adult children) and an examination of whether type of relationship (i.e., marital, familial, other) between caregiver and patient matters.
A more thorough examination of clinical impact is needed. Since timely completion of XRT directly affects outcome in HNC, our future work will seek to examine whether participation in SHARE can facilitate patient compliance and tolerance with treatment, and ultimately contribute to outcome. Finally, we compared SHARE to UMC so it is possible that the improvements found were due to nonspecific effects. At the same time, the goal of this pilot was to achieve better outcomes for patients and spouses over and above the existing standard of care, so UMC was a reasonable and appropriate comparison condition.35 In the future, after establishing efficacy in a larger trial, we hope to explore what aspects of SHARE are of benefit and whether the “active ingredients” are the same for patients and spouses.
In conclusion, the SHARE intervention appears beneficial in HNC, helping to control patient physical symptom burden and minimizing the adverse of effects of XRT on both partners’ psychological functioning. Given this and the fact that attending to the needs/concerns of patients and caregivers are important components of quality care, more research on ways to integrate couple-based interventions in HNC supportive care is warranted.
Supplementary Material
Acknowledgments
This work was supported by R21CA178478 (PI: Badr) and P30CA125123, and the facilities and resources of the Center for Innovations in Quality, Effectiveness, and Safety (CIN13–413).
Footnotes
Conflicts of Interest: None.
Clinical Trial Registry: NCT02409485
References
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Head and neck cancers version 2.2013. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf [accessed October 20 2015].
- 3.Bjordal K, Ahlner‐Elmqvist M, Hammerlid E, et al. A prospective study of quality of life in head and neck cancer patients. Part II: Longitudinal data. The Laryngoscope. 2001;111: 1440–1452. [DOI] [PubMed] [Google Scholar]
- 4.Gritz ER, Carmack CL, de Moor CA, et al. The first year after head and neck cancer: Quality of life. Journal of Clinical Oncology. 1999;17: 352–360. [DOI] [PubMed] [Google Scholar]
- 5.Jansma J, Vissink A, Spijkervet F, et al. Protocol for the prevention and treatment of oral sequelae resulting from head and neck radiation therapy. Cancer. 1992;70: 2171–2180. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen N, Smith H, Sallah S. Evaluation and management of swallowing dysfunction following chemoradiation for head and neck cancer. Current Opinion in Otolaryngology & Head and Neck Surgery. 2007;15: 130–133. [DOI] [PubMed] [Google Scholar]
- 7.Jensen S, Pedersen AML, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Supportive care in cancer. 2010: 1–19. [DOI] [PubMed] [Google Scholar]
- 8.Vissink A, Burlage F, Spijkervet F, Jansma J, Coppes R. Prevention and treatment of the consequences of head and neck radiotherapy. Critical Reviews in Oral Biology & Medicine. 2003;14: 213. [DOI] [PubMed] [Google Scholar]
- 9.Chambers MS, Garden AS, Kies MS, Martin JW. Radiation-induced Xerostomia in patients with head and neck cancer: Pathogenesis, impact on quality of life, and management. Head & neck. 2004;26: 796–807. [DOI] [PubMed] [Google Scholar]
- 10.Epstein J, Meij E, Emerton S, Le N, Stevenson-Moore P. Compliance with fluoride gel use in irradiated patients. Special Care in Dentistry. 1995;15: 218–222. [DOI] [PubMed] [Google Scholar]
- 11.Shinn EH, Basen-Engquist K, Baum G, et al. Adherence to preventive exercises and self-reported swallowing outcomes in post-radiation head and neck cancer patients. Head & Neck. 2013;35: 1707–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravasco P, Monteiro-Grillo I, Camilo ME. Does nutrition influence quality of life in cancer patients undergoing radiotherapy? Radiotherapy and Oncology. 2003;67: 213–220. [DOI] [PubMed] [Google Scholar]
- 13.Lees. Incidence of weight loss in head and neck cancer patients on commencing radiotherapy treatment at a regional oncology centre. European Journal of Cancer Care. 1999;8: 133–136. [DOI] [PubMed] [Google Scholar]
- 14.Nitenberg G, Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Critical Reviews in Oncology/Hematology. 2000;34: 137–168. [DOI] [PubMed] [Google Scholar]
- 15.DiMatteo M, Lepper H, Croghan T. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160: 2101–2107. [DOI] [PubMed] [Google Scholar]
- 16.Barriers McGuire D. and strategies in implementation of oral care standards for cancer patients. Supportive Care in Cancer. 2003;11: 435–441. [DOI] [PubMed] [Google Scholar]
- 17.Edwards D. Head and neck cancer services: views of patients, their families and professionals. British Journal of Oral and Maxillofacial Surgery. 1998;36: 99–102. [DOI] [PubMed] [Google Scholar]
- 18.Ostroff J, Ross S, Steinglass P, Ronis-Tobin V, Singh B. Interest in and barriers to participation in multiple family groups among head and neck cancer survivors and their primary family caregivers. Family Process. 2004;43: 195–208. [DOI] [PubMed] [Google Scholar]
- 19.de Boer M, Pruyn J, van den Borne B, Knegt P, Ryckman R, Verwoerd C. Rehabilitation outcomes of long-term survivors treated for head and neck cancer. Head & Neck. 1995;17: 503–515. [DOI] [PubMed] [Google Scholar]
- 20.Schulz R, Tomkins C. Informal Caregivers in the United States: Prevalence, Caregiver Characteristics, and Ability to Provide Care National Research Council (US) Committee on the Role of Human Factors in Home Health Care: Workshop Summary. Washington DC: National Academies Press, 2010:117–144. [PubMed] [Google Scholar]
- 21.Silverman S Oral Cancer, Volume 1 5th ed. Hamilton, Ontario: B.C. Decker, 2003. [Google Scholar]
- 22.Verdonck-de Leeuw IM, Eerenstein SE, Van der Linden MH, Kuik DJ, de Bree R, Leemans CR. Distress in spouses and patients after treatment for head and neck cancer. Laryngoscope. 2007;117: 238–241. [DOI] [PubMed] [Google Scholar]
- 23.Harding R, Higginson IJ, Donaldson N. The relationship between patient characteristics and carer psychological status in home palliative cancer care. Supportive Care in Cancer. 2003;11: 638–643. [DOI] [PubMed] [Google Scholar]
- 24.Badr H, Herbert K, Reckson B, Rainey H, Sallam A, Gupta V. Unmet needs and relationship challenges of head and neck cancer patients and their spouses. Journal of Psychosocial Oncology. 2016;34: 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badr H, Yeung C, Lewis MA, Milbury K, Redd WH. An observational study of social control, mood, and self-efficacy in couples during treatment for head and neck cancer. Psychology & Health. 2015;30: 783–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenthal DI, Mendoza TR, Chambers MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the MD Anderson symptom inventory, head and neck module. Head & Neck. 2007;29: 923–931. [DOI] [PubMed] [Google Scholar]
- 27.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18: 263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss DS. The impact of event scale: revised Cross-cultural assessment of psychological trauma and PTSD: Springer, 2007:219–238. [Google Scholar]
- 29.Hunsley J, Best M, Lefebvre M, Vito D. The seven item short form of the dyadic adjustment scale: Further evidence for the construct validity. The American Journal of Family Therapy. 2001;29: 325–335. [Google Scholar]
- 30.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Archives of Internal Medicine. 1998;158: 1789–1795. [DOI] [PubMed] [Google Scholar]
- 31.Rosnow RL, Rosenthal R. Computing contrasts, effect sizes, and counternulls on other people’s published data: general procedures for research consumers. Psychological Methods. 1996;1: 331. [Google Scholar]
- 32.Cohen J Statistical power analysis for the behavioral sciences. New Jersey: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 33.Badr H, Krebs P. A systematic review and meta-analysis of psychosocial interventions for couples coping with cancer. Psycho-Oncology. 2013;22: 1688–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.PDQ Online. Head and Neck Cancer. Available from URL: http://www.cancer.gov/cancerinfo/types/head-and-neck 2005].
- 35.Freedland KE, Mohr DC, Davidson KW, Schwartz JE. Usual and unusual care: existing practice control groups in randomized controlled trials of behavioral interventions. Psychosomatic Medicine. 2011;73: 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.