Abstract
Purpose:
Newborn genomic sequencing (nGS) has great potential to improve pediatric care. Parental interest and concerns about genomics are relatively unexplored. Understanding why parents decline research consent for nGS may reveal implementation barriers.
Methods:
We evaluated parental interest in a randomized trial of nGS in well-baby and ICU nursery settings. Interested families attended an informational enrollment session (ES) with a genetic counselor prior to consenting. Reason(s) for declining participation and sociodemographic associations were analyzed.
Results:
Of 3,860 eligible approached families, 10% attended ES, 67% of whom enrolled. Of 1,760 families queried for decline reasons, 58% were uninterested in research. Among 499 families considering research, principal reasons for decline prior to ES included burdensome study logistics (48%), feeling overwhelmed postpartum (17%), and lack of interest/discomfort with genetic testing (17%). Decliners after ES more often cited concerns about privacy/insurability (41%) and uncertain/unfavorable results (23%).
Conclusions:
Low interest in research and study logistics were major initial barriers to postpartum enrollment and are likely generic to many postpartum research efforts. Concerns over privacy and result implications were most commonly cited in decliners after ES. Understanding parental concerns around research nGS may inform future integration of nGS into newborn screening, predictive testing, and pediatric diagnostics.
Keywords: Newborn, Genomic Sequencing, Consent for Genomic Testing, Parental Concerns, Newborn Screening
INTRODUCTION
Since the first human genome was completely sequenced in 2003, technical advances have made clinical genomic information increasingly accessible in healthcare and direct-to-consumer settings. Common applications of genomic sequencing (GS) include diagnosis of rare disease and assessment of cancer risk and are rapidly expanding to include prenatal diagnostics and predictive and precision medicine in healthy adults.1–3 The Newborn Sequencing In Genomic medicine and public HealTh (NSIGHT) consortium, co-funded by the National Institutes of Child Health and Development (NICHD) and National Human Genome Research Institute (NHGRI), consists of four independent projects exploring the integration of genomic sequencing into the clinical care of newborns.4
Within NSIGHT, the BabySeq Project, is evaluating the medical, behavioral, and economic outcomes associated with integrating genomic information into the healthcare of healthy and sick newborns.4,5 GS in the newborn period may allow for more rapid diagnosis and treatment of newborns who are acutely ill,6 as well as early detection or prediction of potentially addressable childhood disorders in those who are clinically well. One major obstacle to the implementation of newborn GS (nGS) thus far has been the lack of data on the benefits and harms of performing genetic sequencing and returning test results to parents of newborns. Theoretical concerns pertaining to nGS include potential negative psychological impact on the patient, parents and family, limitations of the technology, and the potential use and misuse of genomic information.7,8 Further, there has been no formal exploration into whether parents of newborns are interested in or willing to consent to nGS, beyond assessing hypothetical interest.9
Here we describe our experiences offering parents enrollment in the first randomized trial of nGS to be offered in the immediate postpartum period. Fears and concerns about regarding negative potential consequences of GS, in the context of this research trial, may reflect actual parental willingness to consent to clinical genetic testing. Quantitating these parental perceptions may provide insight into barriers to be encountered in the broad application of nGS.
MATERIALS AND METHODS
Study Design and Participants
The BabySeq Project is a randomized controlled trial exploring the impact of clinical whole exome sequencing in the care of newborns. Details of the study design have been previously described.4,5 In addition to standard care, including state mandated newborn screening (NBS), enrolled newborns were randomized to receive either nGS in combination with a family history assessment, or a family history assessment only. nGS results were returned to parents in person by a study genetic counselor (GC) and physician, communicated to the infant’s primary care clinician and relevant specialists, and entered into the infant’s medical record as a formal Newborn Genomic Sequencing Report. Reportable results included moderately to highly penetrant pathogenic/likely pathogenic variants associated with monogenic disease risk or carrier status for genes associated with childhood onset disorders and variants with pharmacogenomic associations. Parents were required to consent to having 1cc of whole blood drawn from the newborn and to provide parental saliva samples. Study participation included a return to the hospital for results disclosure including randomization status (and any results of sequencing if applicable), physical exam, and family history assessment. Parents were asked to complete surveys at enrollment as well as three later time points during the infant’s first year of life. The Partners Human Research Committee, the Boston Children’s Hospital Office of Clinical Investigations, and the Baylor College of Medicine’s Institutional Review Board approved this study. All participating parents provided written informed consent.
Eligible parents were approached for study enrollment either in a postpartum unit/well-baby nursery (WBN), or in one of five intensive care units (ICUs) that care for newborns at Boston Children’s Hospital, Brigham and Women’s Hospital, and Massachusetts General Hospital (three neonatal ICUs, a pediatric cardiac ICU, and a pediatric ICU). Exclusion criteria included: parental age under 18 years, baby age over 42 days, multiple gestations, non-English speaking parents, consent unavailable from both biological parents, or the clinical team deeming the family ineligible for maternal or newborn status reasons (for example, parental non-competence for consent, parent medically unable to consent, or inappropriateness of approach due to a dire clinical situation). The enrollment process consisted of: 1) an initial approach to assess interest in research and in this particular study, 2) agreement to attend an informational enrollment session (ES) with a GC for those who indicated interest, and 3) presentation of the study design, potential risks and benefits, a detailed description of the implications of the research and a copy of the consent form at the ES. These sessions generally lasted about 45 minutes. The parents were then given time to consider this information prior to signing the consent.
In a subset of families that elected not to enroll in the BabySeq Project, we requested basic demographic information and reasons for decline. This cohort (subsequently referred to as the “Reason for Decline Cohort” [RDC]) includes both families who declined participation at the initial approach and parents who attended an ES but declined participation during or after the session. Parents who declined prior to an ES were either not interested in participating in any newborn research or were willing to review a brief menu of research projects (including BabySeq) but were not interested in attending a BabySeq ES. Not all queried parents provided a reason for decline, and some provided multiple reasons. Additionally, not all parents who reported reasons provided complete demographic information. Participants provided reasons for decline in their own words which were later categorized by study staff into one of seven categories: Not Interested in Research, Study Design/Logistics (concerned about drawing additional blood from the newborn, time commitment of ES, time for travel to return to study site and time and effort necessary to complete questionnaires), Not Interested/Uncomfortable with GS (preferred standard testing and screening methods or found genome sequencing a new and unfamiliar technology), Privacy and Discrimination (concerned about placement of results into the medical record and insurance discrimination), Return of Results (concerned about the psychological impact of unfavorable or uncertain results), Overwhelmed (postpartum with either a well or an ill newborn), and Other (including respect for future autonomy of the infant and religious or spiritual reasons). All enrolled participants provided demographic information via survey.
Data Analysis
Descriptive statistics were calculated for the demographic characteristics of declining and enrolled parents, including frequencies and means. Reasons for decline were expressed as percentages of the total number of reasons collected. Demographic characteristics of parents who declined were compared to those who enrolled. Each family was characterized as to whether their decline occurred “-PRE” or “-POST” the ES with a GC (i.e., at the initial approach [Decliner-PRE] or during or after the ES [Decliner-POST]). Decliners were compared to enrolled participants using Chi Square and Fisher’s exact test or analysis of variance as appropriate. Logistic regression was used to examine variables associated with enrolling versus declining and time of decline. Variables included in the model were demographic characteristics: age (continuous), education level, annual household income, whether this was the parents’ first child, ethnicity, race, and study cohort (WBN vs. ICU). Statistical analyses were conducted using SPSS 24 (IBM Corp., Armonk, N.Y., USA). All p values were two-sided and with statistical significance set at p < 0.05.
Mothers’ demographic characteristics from both decliners and participants were used in the statistical analyses. Study staff often first approached mothers to describe the research project and were not always able to obtain demographic data from the other parent (typically the father) in the case of a declining family. Given the open-ended nature of the reason(s) for decline question, and that more than one reason could be provided, we present some descriptive data without statistical analyses.
RESULTS
Cohort Enrollment Outcomes
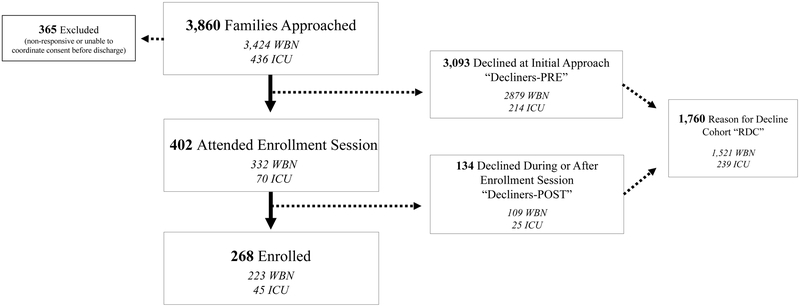
Between May 2015 and March 2017, the study team approached 3,860 eligible families for recruitment (Figure 1). Ten percent of families (n=365) were discharged or transferred from the nursery before a response regarding interest in this study could be obtained. Eighty percent of families (n=3,093) declined participation at the initial approach, often rejecting interest in any research and 10% (n=402) agreed to attend an ES with a GC. Of the families who expressed interest in this research and attended an ES, 33% (n=134) ultimately declined participation and 67% (n= 268) enrolled. The overall enrollment rate, based on all approached families, was 6.9% (6.5% (n=223) from the WBN cohort and 10.3% (n=45) from the ICU cohort).
Figure 1:
Flow diagram of study enrollment and decline (May 2015 – March 2017). WBN = Well baby nursery cohort. ICU = Intensive care unit cohort.
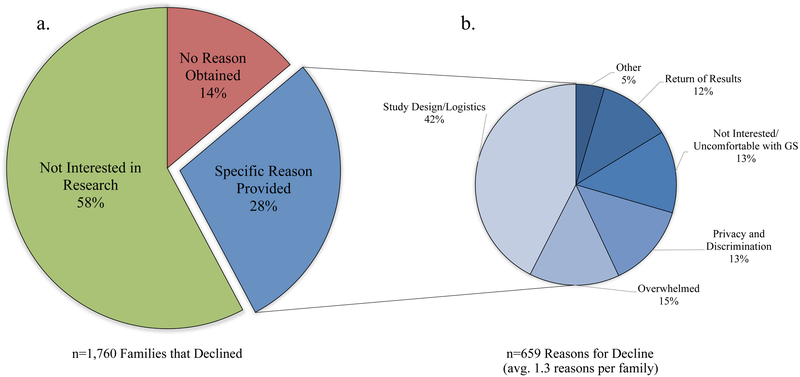
Reasons for decline are shown in Figure 2a (n=1760). Of these families, 58% (n=1,017) stated that they were not interested in participating in any research and 28% (n=499) provided specific reasons for why they declined this nGS randomized trial. A specific reason for decline was not available for 14% (n=244), including those who preferred not to answer, those who were discharged prior to returning a response, or those who were not queried as it was deemed inappropriate under the immediate clinical circumstances.
Figure 2:
Reasons for declining study enrollment reported by the Reason for Decline Cohort (RDC): a. Percent of subjects identified as “Not interested in Research”, “Specific Reason Provided” (see Figure 2b) or “No Reason Obtained” category of reason for decline. b. Specific reasons for decline provided by families who were potentially willing to participate in research. Families could provide multiple reasons for decline. GS = Genomic sequencing
Demographic Characteristics
Characteristics of Declining vs. Enrolled Families
Demographic characteristics for enrolled participants (n=258 of 268 available at the time of this analysis) and for RDC families that volunteered this information (n=245) are presented in Table 1. Of the RDC families providing demographics, most (n=231) were from the 499 that provided specific reasons for not participating in this study. The majority of mothers from both groups were non-Hispanic white and had completed college. After controlling for demographic characteristics and study cohort in a multivariate logistic regression model we found no independent predictors of enrolling versus declining participation (Table 1).
Table 1.
Characteristics of study participants and decliners+
| N (%), unless noted* | Total (n=503) |
Decliners+ (n=245) |
Participants (n=258) |
p-value (95% CI)*** |
|---|---|---|---|---|
| Age, Mean in years (SD) | 34.1 (4.4) | 33.6 (4.5) | 34.5 (4.3) | 0.890 (0.944–1.069) |
| Ethnicity | 1.110 (0.678–1.820) | |||
| Hispanic or Latino | 42 (10.7) | 26 (14.7) | 16 (7.4) | |
| Not Hispanic or Latino | 350 (89.3) | 151 (85.3) | 199 (92.6) | |
| Race** | 0.230 (0.331–1.305) | |||
| White | 333 (80.2) | 150 (77.7) | 183 (82.4) | |
| All Others | 82 (19.8) | 43 (22.3) | 39 (17.6) | |
| Education level | 0.055 (0.981–5.155) | |||
| Less than Bachelor’s | 64 (14.3) | 45 (20.5) | 19 (8.3) | |
| Bachelor’s or higher | 384 (85.7) | 175 (79.5) | 209 (91.7) | |
| Annual household income | 0.391 (0.748–2.104) | |||
| Less than $149,999 | 184 (50.7) | 98 (59.0) | 86 (43.7) | |
| More than $150,000 | 179 (49.3) | 68 (41.0) | 111 (56.3) | |
| First child | 0.675 (0.678–1.820) | |||
| No | 207 (46.5) | 111 (48.7) | 96 (44.2) | |
| Yes | 238 (53.5) | 117 (51.3) | 121 (55.8) | |
| Study cohort | 0.374 (0.420–1.385) | |||
| Intensive Care Unit | 106 (21.1) | 68 (27.8) | 38 (14.7) | |
| Well Baby Nursery | 397 (78.9) | 177 (72.2) | 220 (85.3) |
Reasons for Decline Cohort (RDC) who volunteered demographic information
Categories do not sum to column totals because of individual non-response.
Race All Others includes African American, Other, and More than one race.
CI=Confidence interval; P-value and CI based on multivariable logistic regression
Characteristics of Declining Families by Time of Decline
The ES represents a critical time point in the enrollment process, creating two separate and unique groups of study decliners: those who declined prior to ES (Decliners-PRE, n=203), and those who declined during or after an ES (Decliners-POST, n=42) (Table 2). Although a majority (80%) of decliners were college graduates or higher, this proportion was slightly higher in the Decliners-POST compared to Decliners-PRE group (93% vs. 77%). However, after adjusting models to control confounding factors, we found no participant characteristics to be independent predictors of whether parents were more likely to decline participation at initial approach (Decliner-PRE) versus declining during or after attending ES (Decliner-POST).
Table 2.
Characteristics of families who declined participation before (PRE) vs. during or after (POST) an enrollment session
| N (%), unless noted* | Total (n=245) |
Before Consent (n=203) |
Time of Consent (n=42) |
p-value (95% CI)*** |
|---|---|---|---|---|
| Age, Mean in years (SD) | 33.6 (4.5) | 33.3 (4.6) | 34.7 (3.5) | 0.448 (0.846–1.077) |
| Ethnicity | 0.428 (0.398–8.78) | |||
| Hispanic or Latino | 26 (14.7) | 23 (15.8) | 3 (9.7) | |
| Not Hispanic or Latino | 151 (85.3) | 123 (84.2) | 28 (90.3) | |
| Race** | 0.768 (0.232–2.547) | |||
| White | 150 (77.7) | 125 (78.1) | 25 (75.8) | |
| All Others | 43 (22.3) | 35 (21.9) | 8 (24.2) | |
| Education level | 0.131 (0.048–1.482) | |||
| Less than Bachelor’s | 45 (20.5) | 42 (23.3) | 3 (7.5) | |
| Bachelor’s or higher | 175 (79.5) | 138 (76.7) | 37 (92.5) | |
| Annual household income | 0.363 (0.254–1.651) | |||
| Less than $149,999 | 98 (59.0) | 85 (62.0) | 13 (44.8) | |
| More than $150,000 | 68 (41.0) | 52 (38.0) | 16 (55.2) | |
| First Child | 0.135 (0.202–1.239) | |||
| No | 111 (48.7) | 95 (50.8) | 16 (39.0) | |
| Yes | 117 (51.3) | 92 (49.2) | 25 (61.0) | |
| Study cohort | 0.452 (0.237–1.898) | |||
| Intensive Care Unit | 68 (27.8) | 58 (28.6) | 10 (23.8) | |
| Well Baby Nursery | 177 (72.2) | 145 (71.4) | 32 (76.2) |
Categories do not sum to column totals because of individual non-response.
Race All Others includes African American, Other, and More than one race.
CI=Confidence interval; P-value and CI based on multivariable logistic regression.
Reasons for Decline
Overall Reasons for Decline
Irrespective of time of decline, among the 499 RDC families who provided a reason other than a general lack of interest in research, an average of 1.3 reasons were cited per family (Figure 2b). The most commonly cited category of reason for decline, 42% (n=280), was Study Design/Logistics, i.e., not specific to concerns about GS. Fifteen percent (n=95) of reasons related to feeling Overwhelmed due to the delivery of a new baby or due to having a critically ill child. Fewer reasons for decline were specific to nGS, such as Not Interested/Uncomfortable with GS (13%, n=88), concerns about Privacy and Discrimination (13%, n=89), and Return of Results (12%, n=77).
Reasons for Decline by Time of Decline
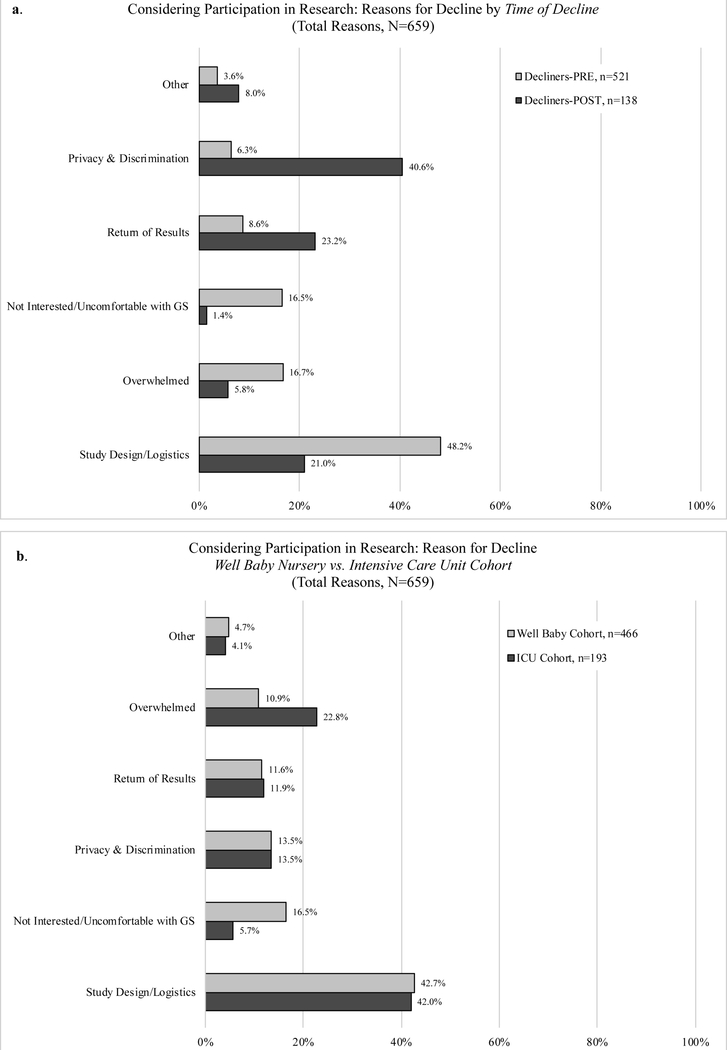
Figure 3a compares reasons from families declining before (Decliners-PRE) versus during or after an ES (Decliners-POST). While Decliners-PRE most often cited reasons unrelated to nGS (Study Design/Logistics [48.2% vs 21.0%] and Overwhelmed [16.7% vs 5.8%]) they also more commonly cited Not Interested/Uncomfortable with GS (16.5% vs 1.4%) than Decliners-POST. Decliner-POST parents more often reported concerns specific to the potential ramifications of nGS, including Privacy and Discrimination (40.6% vs 6.3%) and Return of Results (23.2% vs. 8.6%).
Figure 3:
Reasons for declining study enrollment reported by the Reason for Decline Cohort (RDC) a. comparison of reasons from families declining PRE vs. POST an enrollment session. b. comparison of reasons from families with babies in the well baby nursery (WBN) vs. intensive care unit (ICU). GS = Genomic sequencing
Reasons for Decline by Cohort Location
Reasons for decline were also compared between the WBN and ICU cohorts (Figure 3b). Parents of ICU babies more often cited Overwhelmed (22.8% vs 10.9%) as a reason for decline, while parents of WBN babies more often cited Not Interested or Uncomfortable with GS (16.5% vs 5.7%) as their reason. All other reasons for decline were similarly distributed among both cohorts.
DISCUSSION
While clinical use of GS in the newborn period is increasing, parental interest and concerns about this technology have not been fully explored.4,6,7 Of the families approached for participation in the BabySeq Project, many were uninterested in any type of research, 10% were willing to meet with a GC for an ES, and of those, 67% enrolled in the study. Among those providing reasons for decline, the majority of families (58%) were not interested in participating in any research. This highlights an intrinsic difficulty in recruiting parents during the immediate postpartum period. Our findings are consistent with National Institutes of Child Health and Development (NICHD) neonatal network experience with interventional randomized clinical trials, which estimated that 57–70% of parents of eligible newborns decline to participate in research.10–12 We found that parents who provided reasons for decline beyond a general lack of interest in any research most commonly cited study logistics and inconvenience as reasons for non-participation. Parental concerns regarding a blood draw and follow-up logistics have been observed in other studies with similar participation requirements of healthy newborns in the neonatal period, and are therefore not unique to genomic research.13–15 An additional barrier specific to our study was the requirement that both parents (if known) provide consent, necessitating that they both are available and agree to participate. Of note, 11 families cited discordance over the decision to participate and therefore were not enrolled. It is reasonable to consider that many of the barriers created by the research setting and study design may not be impediments to the future clinical implementation of newborn GS, and therefore represent a context-specific reduction in parental interest in nGS.
We examined the reasons for declining participation between Decliners-PRE and Decliners-POST and found Decliners-POST more often expressed concerns about privacy and discrimination, including concerns about future insurability of their children, as well as discomfort with the potential return of unfavorable and uncertain results. Electing to attend an ES allowed Decliners-POST a one-on-one session with a GC who communicated in detail the risks, benefits, and potential for discrimination if sequencing was performed. Those who declined study participation at initial approach were not exposed to the additional information provided in the ES. This demonstrates the impact of a robust consent process that clearly communicates study risks and benefits on parental perception of the study. A study of informed consent for genetic research in a NICU setting found that 79% of non-consenting families identified institutionally required risk language in the consent process as the primary reason for refusal.16 Informed consent for complex genomic research appears to impact how parents perceive nGS. In addition to content and setting of consent, our data suggest that the timing of such communication (i.e. being overwhelmed due to high postpartum stress) has additional consequences on parental willingness to engage. Parental response to informed consent for this complex genomic research may be relevant to the current and future implementation of newborn clinical genetic testing and nGS.
Privacy concerns, such as the safe storage of genomic data and the potential for genetic discrimination, are common concerns related to GS in general.17–20 The BabySeq Project ES provided an explanation of the federal protections provided by the Genetic Information Nondiscrimination Act (GINA)21 as well as Massachusetts state-specific protections. GINA prevents health insurance companies from utilizing genetic information or requesting genetic testing to make decisions about an individual’s eligibility, coverage, or premium. It also prevents employers with 15 or more employees from using family health history and genetic information to make decisions about employment, pay, and workplace treatment.21 However, GINA does not provide federal protections against genetic discrimination by life, long-term care, and disability insurers. Further, GINA does not apply to the U.S. military health system, the Indian Health Service, the Veterans Health Administration, the Federal Employees Health Benefits Program, or to private companies with less than 15 employees, however additional protections outside of GINA do exist for these individuals19. Additional state protections against genetic discrimination in life, long-term care, and disability insurance may exist, but vary from state to state22. In similar sequencing studies, 40–79% of families still cited privacy and/or discrimination as a reason for decline following such discussion.16,18–20 In our study, families who declined for this reason often expressed a general interest in obtaining nGS, but would only participate if the results remained private and were not integrated into the infant’s medical record.
Discomfort with potential results which could be returned was another common theme among Decliners-POST. These parents often voiced concerns about their own psychological reactions if they should learn about health risks that are not currently treatable or curable. Consideration of this study asked parents to weigh the potential benefits of early diagnosis, such as early intervention and improved outcome, versus the potential psychological burdens of learning unfavorable results about their infant’s future health. Unique to the ICU setting, some parents were not willing to risk learning about additional health risks unrelated to their infant’s presenting phenotype, or simply felt too overwhelmed to consider an additional test or study. The BabySeq Project differs from other nGS studies in NICU populations since our reports cast a broad net for all childhood-relevant results, as opposed to results solely focused on the diagnosis of the presenting phenotype.6,23 In addition, we included ICU infants not suspected of having a genetic condition (e.g., ICU admissions for prematurity or birth injuries), differing from other genomic studies focused on newborn ICU patients with likely genetic etiologies underlying the cause for admission.6 The NICU is a notably difficult environment for research enrollment, with some studies reporting increased enrollment rates compared to healthy infant units due to the potential influence of doctor-family relationships,24 while others report lower enrollment rates due to parents feeling overwhelmed.25 Both the inclusion of findings unrelated to the indication for ICU admission and the inclusion of ICU infants with a low likelihood of a genetic etiology may have played a role in the lower than anticipated study uptake in this critically ill population.
Aside from fears surrounding the type of results returned, some families who declined during or after an ES were uncomfortable with any potential for uncertainty in risk information about future illness that they might receive. Uncertainties related to conditions with less than complete penetrance elicited fears of unnecessary worry and the possibility for “false positive” results. In this study, return of results criteria focused on highly penetrant conditions and/or actionable moderately penetrant conditions,5 yet the lingering possibility for a positive molecular diagnosis in an infant who might never go on to develop related symptoms was a repeated concern. Such hesitations highlight the need for a comprehensive explanation of concepts such as allelic frequency and reduced penetrance for both parents and clinicians. Further, our experiences illuminate a lack of comprehensive and reliable penetrance data, that will only come with additional sequencing and longitudinal follow-up of clinically healthy populations. Despite well-defined study criteria guiding the types of findings reported to parents,5 the vast amount of information made available by nGS, and its potential future implications, was a factor in dissuading some parents from participating.
We acknowledge limitations of this study. Because we could not collect reasons for decline from all families approached or queried, it is possible that the reasons for decline analyzed may not be fully representative. The study population was largely non-Hispanic white with college degrees or higher, so results might not be generalizable to more diverse populations. Further, the relatively high mean age at delivery in the both decliners and study participants is likely to reflect a combination of Massachusetts’ elevated mean age at first delivery compared to the national average (29 vs 26 years) as well as the high level of education and SES in our cohort, which are known to delay childbearing age26,27.This lack of diversity might be particularly important to explore in future studies. Study participants are likely to be enriched with the inclusion of “early adopters”, although no significant differences in demographics were found between decliners and participants in adjusted models.
The overall enrollment rate of 6.9% in this study is low in comparison to previously reported studies on parental interest in nGS.28,29 In 2015, our group surveyed parents within 48 hours of birth regarding their hypothetical interest in nGS and found that the majority (83%) of parents willing to answer the survey expressed at least some interest (37% some interest, 28% very interested, 18% extremely interested). However, those who were so uninterested in research that they declined to take the survey on hypothetical interest were not included in the denominator used to calculate levels of interest, making refusal to consider research participation a primary factor underlying discordance between the two studies. Among the Decliners-PRE, identifying 17% of reasons related to disinterest or discomfort in genetic testing is consistent with the proportion of the prior study cohort that would have declined testing had it been actually offered. It should be noted that while we made every effort to approach any accessible family, clinical trials do not typically approach a truly neutral population such as this. Rather, typical clinical trials are advertised to specific populations where interested persons first self-identify, and consent/enrollment rates are estimated from there.
The recruitment and enrollment experiences in the BabySeq Project provide important insights into potential barriers to the integration of nGS into screening, predictive testing, and diagnostics in newborn and pediatric care. The BabySeq Project enrollment rate of 67% after attending a session with a GC, may be a more accurate reflection of parental interest in nGS than the overall decliner rate, if unfettered by the initial barriers of the timing of approach within a day of the baby’s birth, lack of interest in research participation in general, and factors related to our clinical trial design. Thus, the unique environment of the postpartum period needs to be taken into account when considering the timing of consent, and logistical barriers to participation related to research and study design should be minimized in order to optimize rate of uptake and diversity of subjects.
The outcomes of this study reveal the influence of risk communication during the education process and informed consent and identify concerns about privacy, discrimination, and return of results in some parents. Some parents struggled with weighing the benefits of having genetic risk information integrated into their infant’s medical records, with the perceived risks of having this information permanently linked to their child. Such concerns highlight the disconnect between advancements in genomic medicine and current legislation protecting against discrimination, as well as the importance of presenting a balanced depiction of the benefits and risks in both written and verbal consent. In addition to the data presented here, prospective survey data being collected from enrolled families will continue to quantify the actual impact of nGS and provide important guidance for its future integration into clinical care of newborns.
Acknowledgements:
The authors would like to thank the families and clinicians for their participation in The BabySeq Project. Special thank you to the current and former research assistants, genetic counselors and research nurses involved in The BabySeq Project recruitment at BWH, BCH, and MGH. This work was supported by grants U19 HD077671 and R01 HD075802 from the National Institute of Child Health and Human Development and National Human Genome Research Institute of the National Institutes of Health, as well as by The Manton Center for Orphan Disease Research of Boston Children’s Hospital.
Footnotes
Pankaj B. Agrawal, Alan H. Beggs, Wendi N. Betting, Ozge Ceyhan-Birsoy, Kurt D. Christensen, Dmitry Dukhovny, Shawn Fayer, Leslie A. Frankel, Casie A. Genetti, Chet Graham, Robert C. Green, Amanda M. Guiterrez, Maegan Harden, Ingrid A. Holm, Joel B. Krier, Matthew S. Lebo, Harvey L. Levy, Xingquan Lu, Kalotina Machini, Amy L. McGuire, Jaclyn B. Murry, Medha Naik, Tiffany T. Nguyen, Richard B. Parad, Hayley A. Peoples, Stacey Pereira, Devan Petersen, Uma Ramamurthy, Vivek Ramanathan, Heidi L. Rehm, Amy Roberts, Jill O. Robinson, Serguei Roumiantsev, Talia S. Schwartz, Tina K. Truong, Grace E. VanNoy, Susan E. Waisbren, Timothy W. Yu.
Conflicts of Interest:
Dr. Green receives compensation for speaking or consultation from AIA, Helix, and Veritas; and is co-founder, advisor and equity holder in Genome Medical, Inc.
All other authors disclose no conflicts of interest.
REFERENCES
- 1.Krier JB, Kalia SS, Green RC. Genomic sequencing in clinical practice: applications, challenges, and opportunities. Dialogues Clin Neurosci 2016;18:299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biesecker LG, Green RC. Diagnostic clinical genome and exome sequencing. N Engl J Med 2014;371:1170. [DOI] [PubMed] [Google Scholar]
- 3.Vassy JL, Christensen KD, Schonman EF, et al. The impact of whole-genome sequencing on the primary care and outcomes of health adult patients: A pilot randomized trial. Ann Intern Medicine 2017;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg J, Agrawal P, Bailey D, et al. Newborn sequencing in genomic medicine and public health. Pediatrics 2017;132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birsoy O, Machini K, Lebo M, et al. A curated gene list for reporting results in newborn genomic sequencing. Gen Med 2016;19:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrikin JE, Cakici JA, Clark MM, et al. The NSIGHT1-randomized controlled trial: rapid whole-genome sequencing for accelerated etiologic diagnosis in critically ill infants. NPJ Genom Med 2018;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frankel LA, Pereira S, McGuire AL. Potential psychosocial risks of sequencing newborns. Pediatrics 2016;137 Suppl 1:S24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botkin JR, Belmont JW, Berg JS, et al. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet 2015;97:6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waisbren SE, Bäck DK, Liu C, et al. Parents are interested in newborn genomic testing during the early postpartum period. Genet Med 2015;17:501–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodian DL, Klein E, Iyer RK, et al. Utility of whole-genome sequencing for detection of newborn screening disorders in a population cohort of 1,696 neonates. Genet Med 2016;18:221–30. [DOI] [PubMed] [Google Scholar]
- 11.Foglia EE, Nolen TL, DeMauro SB, et al. Short-term outcomes of infants enrolled in randomized clinical trials vs those eligible but not enrolled. JAMA 2015;313:2377–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rich W, Finer NN, Gantz MG, et al. Enrollment of extremely low birth weight infants in a clinical research study may not be representative. Pediatrics 2012;129:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maayan-Metzger A, Kedem-Friedrich P, Kuint J. Motivations of mothers to enroll their newborn infants in general clinical research on well-infant care and development. Pediatrics 2008;121:e590–6. [DOI] [PubMed] [Google Scholar]
- 14.Skinner D, Choudhury S, Sideris J, et al. Parents’ decisions to screen newborns for FMR1 gene expansions in a pilot research project. Pediatrics 2011;127:e1455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lernmark B, Johnson SB, Vehik K, et al. Enrollment experiences in a pediatric longitudinal observational study: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Contemp Clin Trials 2011;32:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamvas A, Madden KK, Nogee LM, et al. Informed consent for genetic research. Arch Pediatr Adolesc Med 2004;158:551–5. [DOI] [PubMed] [Google Scholar]
- 17.Tabor HK, Stock J, Brazg T, et al. Informed consent for whole genome sequencing: a qualitative analysis of participant expectations and perceptions of risks, benefits, and harms. Am J Med Genet A 2012;158A:1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson JO, Carroll TM, Feuerman LZ, et al. Participants and study decliners’ perspectives about the risks of participating in a clinical trial of whole genome sequencing. J Empir Res Hum Res Ethics 2016;11:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green RC, Lautenbach D, McGuire AL. GINA, genetic discrimination, and genomic medicine. N Engl J Med 2015;372:397–9. [DOI] [PubMed] [Google Scholar]
- 20.Amendola LM, Robinson JO, Hart R, et al. Why patients decline genomic sequencing studies: experiences from the CSER consortium. J Genet Couns 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genetic Information Nondiscrimination Act (GINA) of 2008. National Institutes of Health, 2008. (Accessed August 18, 2009, at http://www.genome.gov/24519851.)
- 22.Genetics and health insurance state anti-discrimination laws. 2008. (Accessed March 14, 2018, at http://www.ncsl.org/research/health/genetic-nondiscrimination-in-health-insurance-laws.aspx.)
- 23.Stark Z, Tan TY, Chong B, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med 2016;18:1090–6. [DOI] [PubMed] [Google Scholar]
- 24.Singhal N, Oberle K, Burgess E, Huber-Okrainec J. Parents’ perceptions of research with newborns. J Perinatol 2002;22:57–63. [DOI] [PubMed] [Google Scholar]
- 25.Golec L, Gibbins S, Dunn MS, Hebert P. Informed consent in the NICU setting: an ethically optimal model for research solicitation. J Perinatol 2004;24:783–91. [DOI] [PubMed] [Google Scholar]
- 26.For most highly educated women, motherhood doesn’t start until the 30s. 2015. (Accessed May, 2018, at http://www.pewresearch.org/fact-tank/2015/01/15/for-most-highly-educated-women-motherhood-doesnt-start-until-the-30s/.)
- 27.User guide to the 2016 Natality Public Use File. Centers for Disease Control and Prevention, 2016. (Accessed May, 2018, at https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm.)
- 28.Waisbren SE, Weipert CM, Walsh RC, Petty CR, Green RC. Psychosocial factors influencing parental interest in genomic sequencing of newborns. Pediatrics 2016;137 Suppl 1:S30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldenberg AJ, Dodson DS, Davis MS, Tarini BA. Parents’ interest in whole-genome sequencing of newborns. Genet Med 2013;16:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]