Abstract
Background:
Low-grade glioma (LGG) and glioneuronal tumors (LGGNTs) diagnosed during the first year of life carry unique clinical characteristics and challenges in management. However, data on treatment burden, outcome, and morbidities are lacking.
Methods:
A retrospective study (1986–2015) of LGG/LGGNT diagnosed in patients younger than 12 months at St. Jude Children’s Research Hospital was conducted.
Results:
For the 51 patients (31 males), the mean age at diagnosis was 6.47 months (range, 0.17–11.76), and the mean follow-up period was 11.8 years (range, 0.21–29.19). Tumor locations were hypothalamic/optic pathway (61%), hemispheric (12%), brainstem (12%), cerebellar (8%), and spinal (8%). Of the 41 patients with histologic diagnoses, 28 had WHO grade I tumors; 6, grade II tumors; and 7, LGG/LGGNT not definitively graded. Forty-one patients required active intervention at diagnosis: 41 eventually underwent tumor-directed surgeries (median, 2 surgeries; range, 1–6); 39 received chemotherapy (median, 2 regimens; range, 1–13), and 21 received radiotherapy. Forty patients experienced disease progression (median, 2 progressions; range, 1–18). Ten patients died due to progression (n=5), malignant transformation (n=2), second cancer (n=2), or shunt infection (n=1). Ten-year overall survival, progression-free survival, and radiation-free survival rates were 85±5.3%, 16.9±5.3%, and 51.2±7.5%, respectively. Forty-nine patients experienced health deficits (e.g., endocrinopathies, obesity, seizure, visual/hearing impairment, neurocognitive impairment, and cerebrovascular disease). Predictors of progression and toxicities were defined.
Conclusion:
Infantile LGG/LGGNT is a chronic, progressive disease universally associated with long-term morbidities and requires multidisciplinary intervention.
Keywords: infant, low-grade glioma, treatment burden, morbidities, prognostic factors
Precis:
Low-grade gliomas and glioneuronal tumors diagnosed during the first year of life carry a high risk of progression, requiring repeated interventions. Chronic health deficits are universal in survivors, warranting preemptive counseling, systematic surveillance, and early intervention.
INTRODUCTION
Low-grade gliomas (LGGs) and glioneuronal tumors (LGGNTs) are the most common central nervous system (CNS) tumors in childhood.1 These histologically benign tumors are associated with excellent outcome, especially if completely removed.2–4 However, survivors are at risk of tumor- and treatment-related long-term health deficits.4–10 In infants, LGG/LGGNT carries a unique clinical profile and additional management challenges. Infant LGG/LGGNTs are more common in the hypothalamic or optic pathway (HT/OP) area, limiting resectability, and more likely to be metastatic and progressive than are LGG/LGGNTs in older children.11–20 Immature nervous systems of infants increase vulnerability to tumor- and treatment-induced toxicities.5, 16, 21 Radiation therapy (RT) is frequently deferred due to possible neurocognitive, neurodevelopmental, and endocrine complications.22–26 Tumor resection and systemic therapeutic agents also carry their own adverse effects.27–29
Studies on treatment burden, outcome, and chronic morbidities in LGG/LGGNT patients during the first year of life are lacking, despite their relevance to prognostication, family counseling, and design of multidisciplinary follow-up. We hypothesized that infants with LGG/LGGNTs experience significant health deficits and reviewed our institutional experience over 3 decades in managing LGG/LGGNTs diagnosed during the first year of life.
METHODS
Patient Characteristics and Treatment Outcome
This retrospective review included patients with LGG/LGGNT diagnosed during the first year of life at St. Jude Children’s Research Hospital (SJCRH) between January 1986 and July 2015. Eight patients were seen at SJCRH at diagnosis; 43 were referred from outside institutions (at median 3.1 months after diagnosis; range, 0–8.32 years). LGGs and glioneuronal tumors were diagnosed histologically (WHO grade I–II astrocytic, neuronal, and mixed neuronal-glial tumors; diagnosis extracted from histopathological reports issued by SJRCH) or radiographically (e.g., typical cases of optic pathway and brainstem glioma). Date of diagnosis was defined as date of first diagnostic neuroimaging. Medical records were reviewed for demographics, imaging, pathology, neurofibromatosis type 1 (NF1) status, treatment, progression, survival, and chronic morbidities. The study was approved by the Institutional Review Board; the need for informed consent was waived.
Treatment Burden
Tumor-directed surgery included attempted tumor resection or biopsy for diagnostic purposes and was classified as gross-total resection (GTR), subtotal resection (STR), or biopsy according to the operative record and post-operative radiographic findings. Data on use of chemotherapy and targeted agents, including duration and regimen, and on RT use, extent, and dosage were retrieved. Intervention at diagnosis was described; patients receiving no tumor-directed therapy or only biopsy were designated as being “observed only”. Treatment for transformed or secondary high-grade gliomas was also evaluated. Progression was defined as radiographic and/or clinical progression necessitating treatment initiation or modification.
Long-term Health Deficits
Endocrinopathy and obesity:
Diagnosed abnormal hormonal tests with/without corresponding treatment. The most recent body mass index (BMI) documented at age ≥2 years was used as marker for overweight and obese, defined as age- and sex-specific BMI percentiles ≥85% and ≥95%, respectively.
Neurological deficit and seizure:
Respectively defined as motor weakness at most recent clinical evaluation by an oncologist and/or neurologist and seizure and/or abnormal electroencephalogram requiring treatment.
Visual and hearing loss:
Most recent ophthalmological examination focusing on visual acuity (VA) and visual field (VF) assessment was evaluated, and most recent auditory evaluation by pure-tone audiometry or auditory brainstem responses was assigned an ototoxicity grade for each ear, according to the International Society of Pediatric Oncology (SIOP) ototoxicity scale.
Neurocognitive impairment:
Results from the most recent neurocognitive assessment were extracted when available. Intelligence quotient (IQ) was used as a performance measure of global cognitive functioning, and adaptive (real-world) functioning was assessed by parent ratings on the Vineland Adaptive Behavior Scales or Adaptive Behavior Assessment System.30–34 Normative scores were interpreted as continuous variables and divided into 2 groups, with 1 standard deviation (SD) from the population mean score used as threshold (IQ=85).
Cerebrovascular disease:
History of transient ischemic attack, ischemic stroke, cerebral hemorrhage, radiographic evidence of vascular stenosis, or moyamoya disease was included.
Scoliosis:
Clinically significant scoliosis requiring evaluation and follow-up by an orthopedic surgeon was included.
Statistical Analysis
Statistical analysis was performed using SAS v.14 and R v.3.5.0. Associations between demographic/diagnostic parameters and treatment burden were analyzed by the Wilcoxon test. Overall survival (OS) was calculated from date of diagnosis to date of death or last follow-up; progression-free survival (PFS) was calculated from date of diagnosis to date of disease progression (including malignant transformation of primary LGG/LGGNT), death from any cause, or last follow-up, whereas radiation-free survival (RFS) was calculated from date of diagnosis to start of RT, death from any cause, or last follow-up. The log-rank test was adopted to evaluate the relationship between survival and categorical predictor variables (age at diagnosis [<6 months/≥6 months], sex, period of diagnosis [1986–2000/2001–2015]), NF1 status, diencephalic syndrome, tumor location, metastasis at diagnosis, initial treatment modality, extent of upfront surgery, need for CSF diversion, RT [except in analysis of RFS]) and Cox regression to evaluate the impact of continuous predictor variables (number of tumor-directed surgeries, number of different chemotherapeutic regimens) on survival and in multivariate analysis. Associations between demographic (age at diagnosis [<6 months/≥6 months], sex, period of diagnosis [1986–2000/2001–2015]), diagnostic (NF1 status, tumor location, metastasis at diagnosis) /treatment (initial treatment modality, extent of upfront surgery, need for CSF diversion, RT, number of tumor-directed surgeries, number of different chemotherapeutic regimens) variables and long-term toxicities were studied by Chi-square or Fisher’s exact test for categorical variables and a non-parametric Wilcoxon test for continuous variables. Data on neuropsychological evaluation were compared with normative scores; the impact of potential demographic/diagnostic/treatment variables on neuropsychological outcomes was explored by the general univariate linear model. Multivariate regression was performed on predictor variables significant in univariate analysis.
RESULTS
Patient Demographics, Imaging, and Histological Characteristics
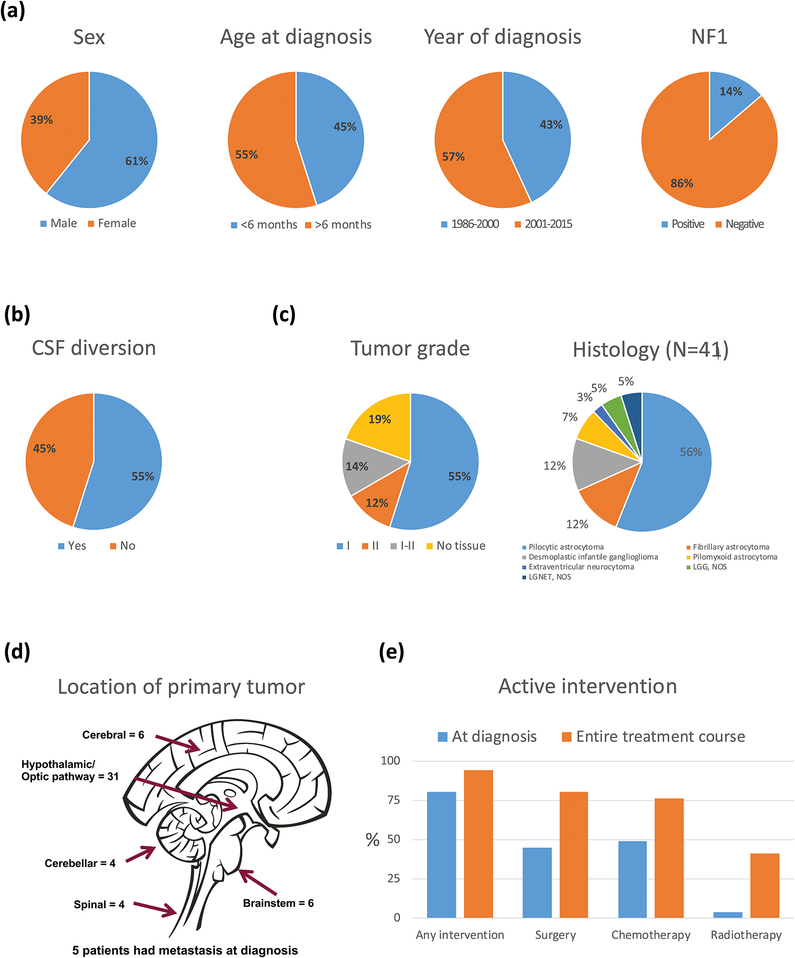
Of the 51 patients, 31 (61%) were male; mean age at diagnosis was 6.47 months (range, 0.17–11.76; Figure 1). Mean duration of follow-up was 11.8 years (range, 0.21–29.19), and mean age at last follow-up was 12.3 years (range, 0.57–29.80). Five patients were lost to follow-up. Seven patients (14%) had NF1 (Table 1); 12 had features of diencephalic syndrome at diagnosis. All patients underwent magnetic resonance imaging at diagnosis. Primary tumors were in the HT/OP (n=31, 61%), cerebral hemispheres (n=6, 12%), brainstem (n=6, 12%), cerebellum (n=4, 8%), or spinal cord (n=4, 8%). Five of 22 patients (23%) with complete radiographic staging at diagnosis had metastatic disease. Among the 41 patients who underwent tumor-directed surgery throughout the course of treatment, 28 (68%) had WHO grade I tumors (pilocytic astrocytoma=23; desmoplastic infantile ganglioglioma=5); 6 (15%), grade II tumors (fibrillary astrocytoma=5; extraventricular neurocytoma=1); and 7 (17%), LGG/LGGNT that could not be definitively graded (pilomyxoid astrocytoma=3; LGG/low-grade neuroepithelial tumor=4). BRAF duplication (a surrogate for BRAF-KIAA1549 fusion) was present in 6 of 9 patients in whom the test was performed, and BRAF V600E mutation, in 2 of 6. Patients with a diagnosis based on imaging alone included 8 with HT/OP tumors and 2 with brainstem tumors (involving pons/medulla and tectal plate); 5 had NF1.
Figure 1.
(a,b,d) Demographic, clinical, (c) histologic, and (e) treatment characteristics of infants with low-grade gliomas and glioneuronal tumors.
Table 1.
Features, outcome and long term morbidities of patients with neurofibromatosis type 1.
| Age at diagnosis of LGG (y) | Sex | Primary diagnosis and treatment details | LGG diagnosed by screening MRI | First mode of treatment | Treatment details and disease course | Number of progressions requiring Tx | Latest disease status | Duration of follow up from initial diagnosis (y) | Associated morbidities |
|---|---|---|---|---|---|---|---|---|---|
| 0.34 | F | Hypothalamic/optic pathway PA | NF1 diagnosed after LGG | Chemotherapy | Vincristine, at progression: cyclophosphamide → focal RT=49.5 Gy, biopsy at PD) – developed secondary GBM | 3 | Died of secondary GBM | 3.52 | VF defect, endocrinopathy, stroke, hemiparesis, seizure (after diagnosis) |
| 0.34 | F | Hypothalamic/optic pathway PA | NF1 diagnosed after LGG | Observation | At progression: carboplatin, topotecan (prolonged myelosuppression) → vincristine → focal RT | 2 | SD off therapy | 22.23 | VF defect, impaired VA, endocrinopathy, scoliosis, moyamoya, TIA, history of seizure, obesity, IQ = 74 |
| 0.68 | F | Optic pathway glioma (no biopsy) | Yes | Observation | At progression: carboplatin, vincristine → focal RT | 2 | SD off therapy | 14.21 | Endocrinopathy, scoliosis, moyamoya, stroke, hemiparesis, history of seizure, IQ = 77 |
| 0.98 | F | Optic pathway glioma (no biopsy) | Yes | Observation | At progression: carboplatin, vincristine, temozolomide → vinblastine | 2 | SD off therapy | 11.54 | Endocrinopathy, scoliosis, overweight, history of seizure, IQ = 76 |
| 0.01 | F | Tectal glioma (no biopsy) | NF1 diagnosed after LGG | Observation | Observation only | 0 | SD without therapy | 9.74 | Impaired VA, strabismus, seizure, IQ = 89 |
| 0.61 | F | Optic Pathway Glioma (no biopsy) | NF1 diagnosed after LGG | Observation | At progression: carboplatin, vincristine, temozolomide → vinblastine → selumetinib | 3 | SD on therapy | 5.33 | Impaired VA, scoliosis, IQ = 65 |
| 0.21 | F | Optic Pathway Glioma (no biopsy) | NF1 diagnosed after LGG | Chemotherapy | Carboplatin/vincristine → at progression: vinblastine → bevacizumab/irinotecan | 2 | SD off therapy | 6.78 | Impaired VA, endocrinopathy, obesity, scoliosis, hemiparesis, history of seizure |
Abbreviations: GBM, glioblastoma multiforme; IQ, intelligent quotient; LGG, low-grade glioma; PA, pilocytic astrocytoma; PD, progressive disease; RT, radiotherapy; SD, stable disease; TIA, transient ischemic attack; Tx, treatment; VA, visual acuity; VF, visual field; y, years
Intervention and Treatment Burden
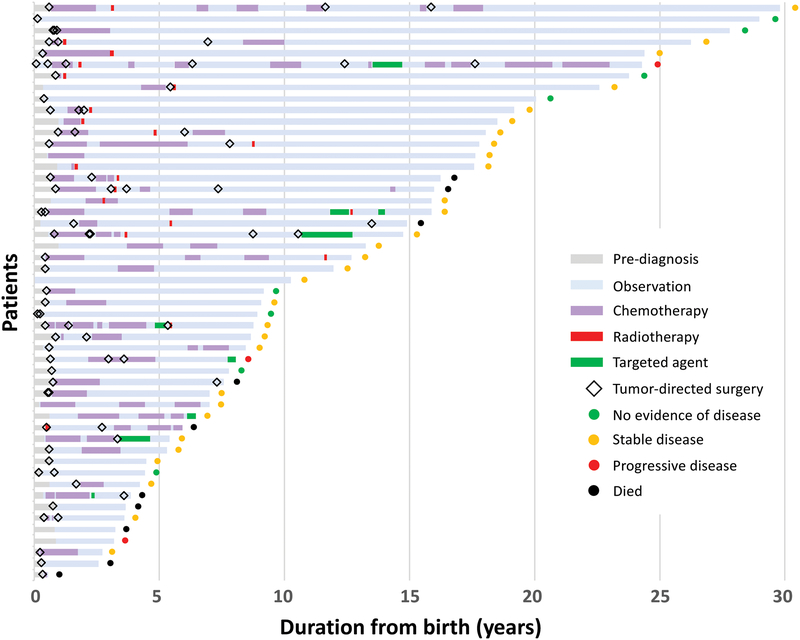
Forty-one patients required active intervention at diagnosis, and 10 were observed (4 with biopsy only; Figure 2). Intervention at diagnosis included tumor resection in 23 (8 also received adjuvant chemotherapy), chemotherapy alone in 16, RT alone in 1, and combined chemotherapy and radiation in 1. Of the 10 patients observed upfront, 7 received treatment for progression (at median 2.61 years from diagnosis; range, 1.09–5.33). Treatment was recommended in 2 patients but refused by family: one patient died of disease and the other remained alive with disease. One patient with tectal glioma remained stable without progression with observation alone.
Figure 2.
Treatment burden for infants with low-grade gliomas and glioneuronal tumors.
Throughout follow-up, 41 patients underwent tumor resection (median, 2 surgeries; range, 1–6). Maximal extent of surgery was biopsy in 11, STR in 19, and GTR in 11 patients. Patients with NF1 had fewer tumor-directed surgeries than those without (P=0.001). Thirty-nine patients received chemotherapy (median, 2 regimens; range, 1–13), and 21 received RT (focal radiation, 19; craniospinal irradiation [CSI], 2). Mean age of cohort at RT was 4.54 years (median, 3.07; range, 0.45–18.71); mean duration from diagnosis to RT was 4.01 years (median, 2.46; range, 0–18.19). Absence of GTR at diagnosis (P=0.001) and presence of tumors in the HT/OP (P<0.001) predicted a higher number of chemotherapy regimens than did absence of tumors. CSF diversion was required in 28 patients (55%).
Progression, Survival, and Predictors of Outcome
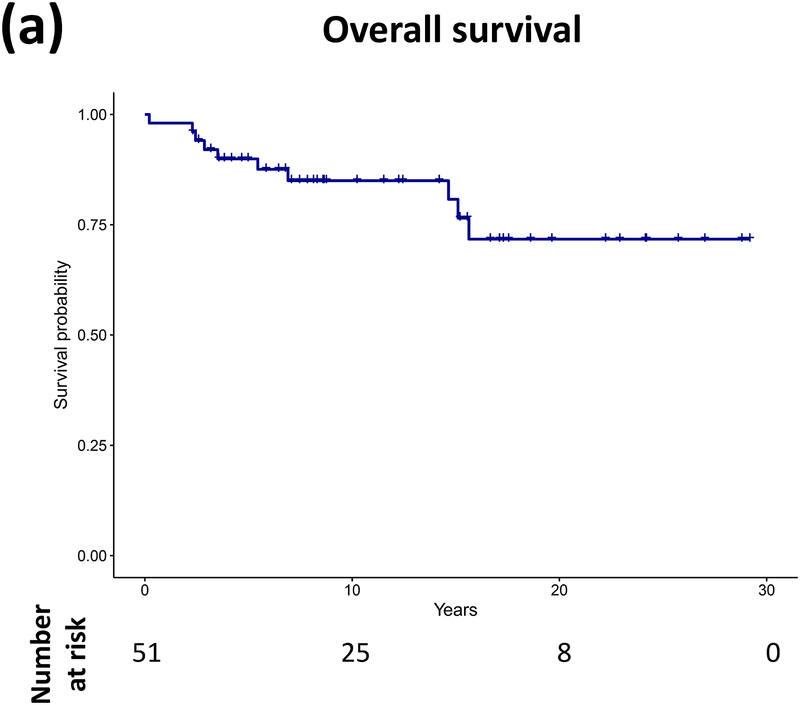
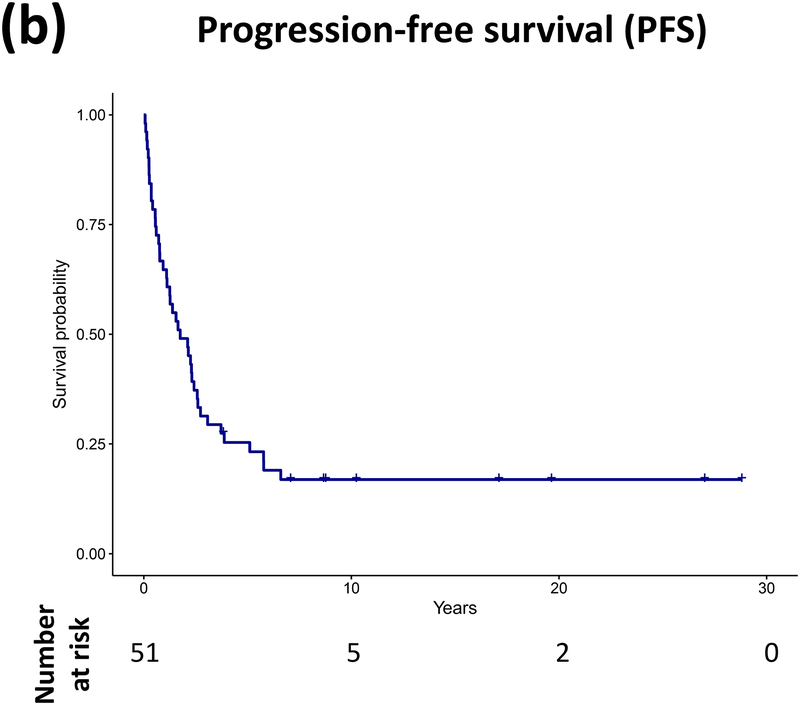
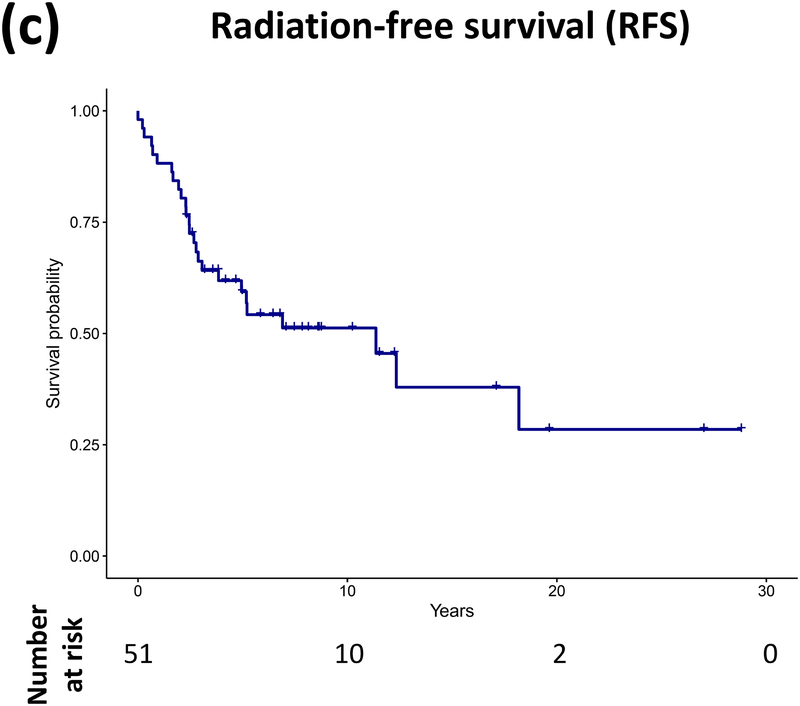
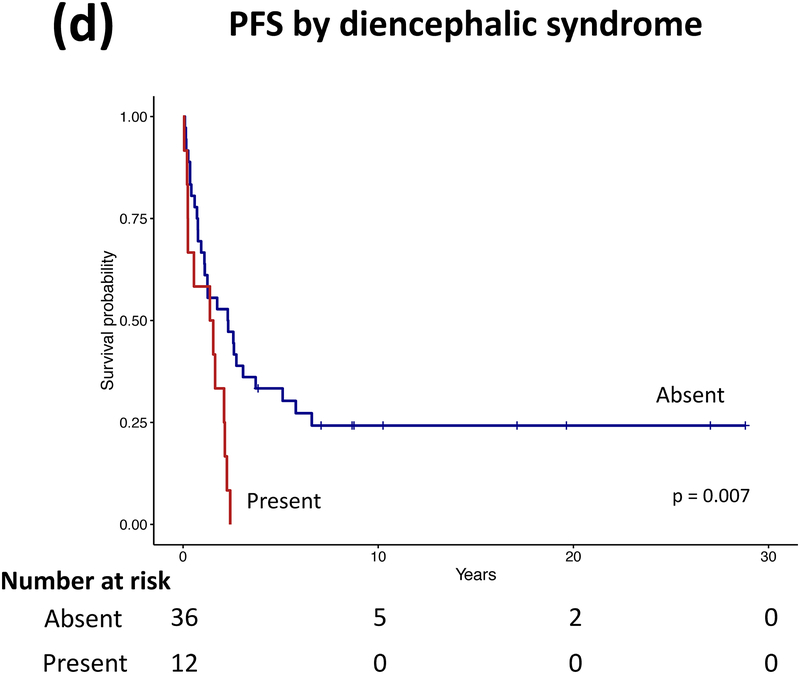
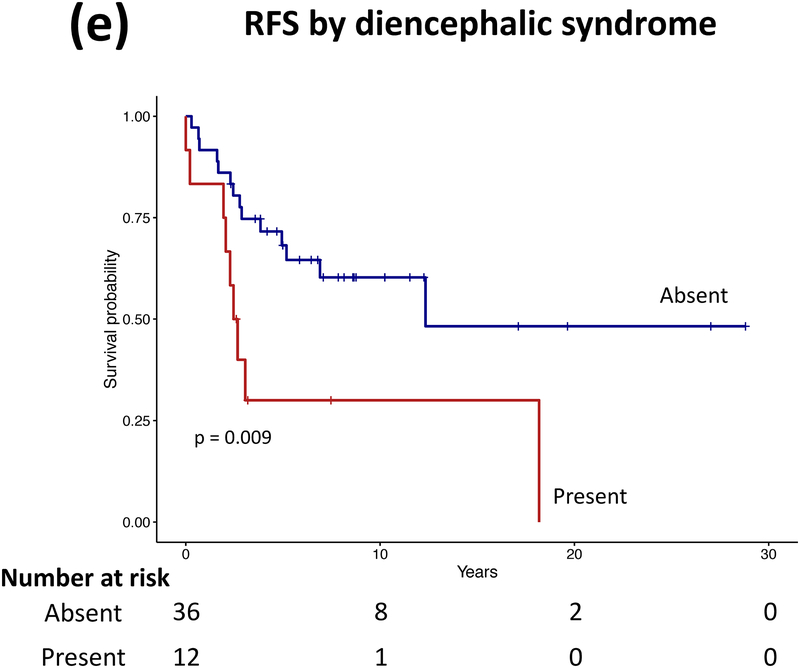
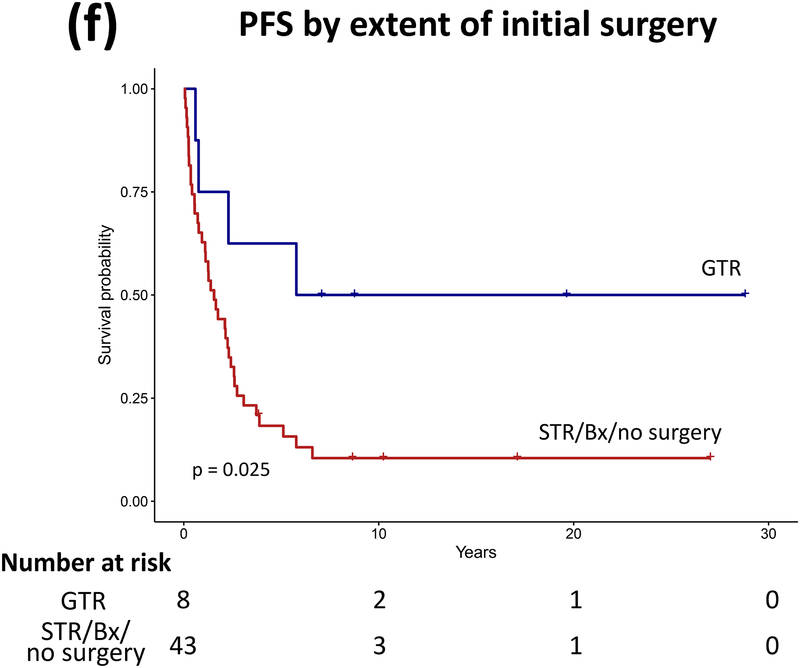
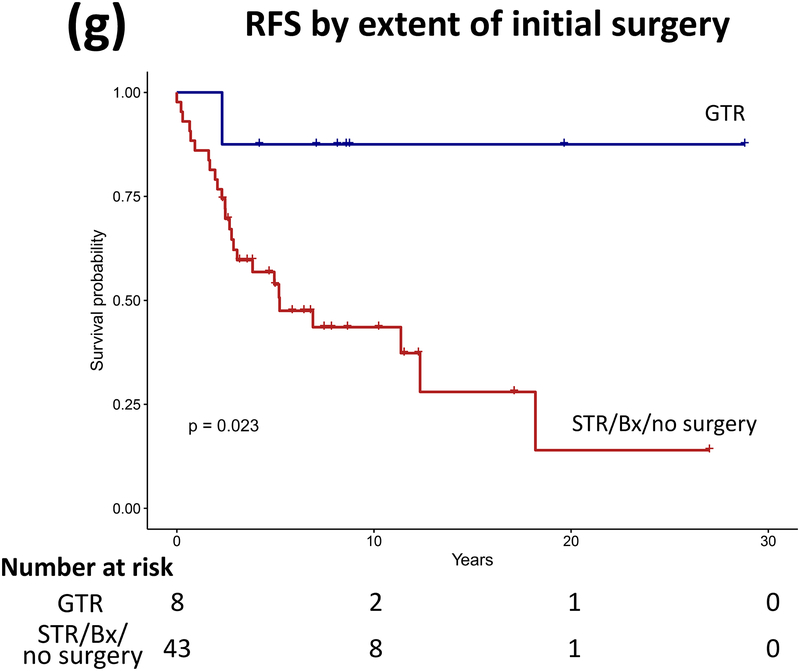
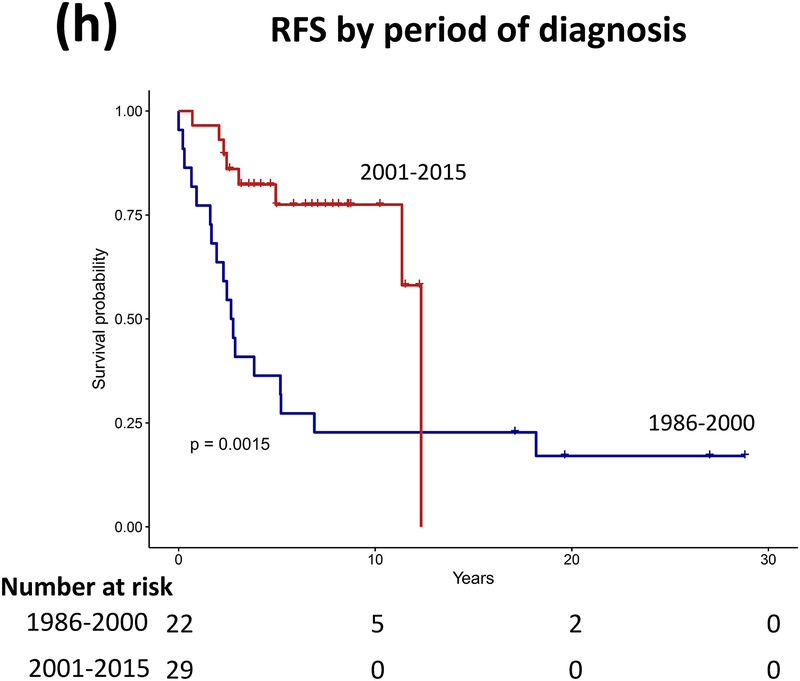
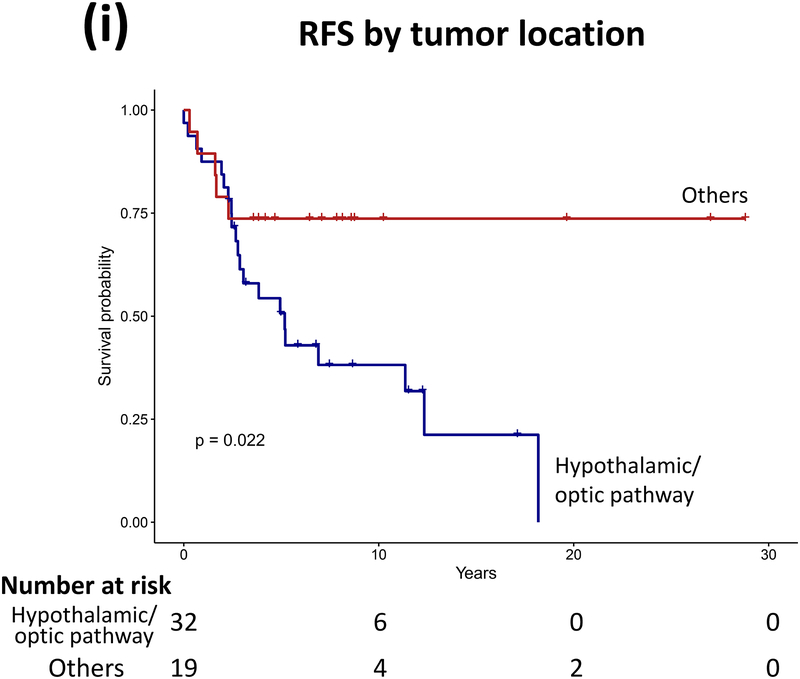
Forty patients (78%) experienced progression during follow-up: single progression in 13, and multiple progressions in 27. Median number of progressions requiring initiation or change of intervention was 2 (range, 1–18). Five patients not undergoing complete staging at diagnosis had metastasis at progression. At latest assessment, 8 patients had no evidence of disease, 30 had stable disease, and 3 had progressive disease (PD). Ten patients (20%) died due to progression (n=5), malignant transformation (n=2), second cancer (n=2), or shunt infection (n=1). Respective 5-year/10-year/20-year OS were 89.9±4.3%/85±5.3%/71.7±8.4%, PFS 25.3±6.1%/16.9±5.3%/16.9±5.3%, and RFS rates 59.4±7.1%/51.2±7.5%/28.5±11.1% (Figure 3). Among the 21 patients who received RT, 11 experienced progression afterwards, with 5-year/10-year PFS after RT being 60.8±11.6%/42.6±12% respectively.
Figure 3.
(a–c) Overall, progression-free, and radiation-free survival of the cohort and (d–i) significant predictors of survival.
Patients receiving GTR upfront (vs all other patients, log rank P=0.025, Cox regression P=0.085) and those not experiencing diencephalic syndrome had better PFS on univariate analysis, with the latter also being significant in multivariate analysis (log rank P=0.007, Cox regression P=0.036). Receiving GTR upfront (vs all other patients, P=0.023), having a more recent diagnosis (2001–2015) (P=0.005), or having non-HT/OP tumors (P=0.021) predicted better RFS in univariate analysis, with diagnosis period remaining significant in multivariate analysis (P=0.008; Table A1). No predictor variable was significantly associated with OS. Cerebellar or HT/OP lesions (P=0.009) and initial resection other than GTR (P=0.005) were associated with a higher number of progressions. Table 2 lists malignant transformation and second cancers in patients (n=7).
Table 2.
Features, risk factors and outcome of patients with second cancer and/or malignant transformation.
| Age at diagnosis of LGG (y) | Sex | Primary diagnosis and treatment details | Age at diagnosis of second cancer/malignant transformation (y) | Second cancer/malignant transformation and treatment details | Possible risk factor(s) for second cancer/malignant transformation | Latest disease status | Duration of follow up from initial diagnosis (y) |
|---|---|---|---|---|---|---|---|
| 0.77 | F | FA of spinal cord, STR × 2, adjuvant (vincristine, cyclophosphamide, cisplatin, etoposide × 2 years) | 21.9 | SCC of tongue Resection + radiation | N/A | NED | 27.01 |
| 0.34 | F | NF1, PA of hypothalamus/optic pathway. Diagnosed by imaging, chemotherapy (vincristine × 1 month, cyclophosphamide × 3 months, carboplatin × 15 months, focal RT=49.5 Gy, biopsy at PD) | 3.74 | Cerebellar GBM (diagnosis on autopsy) Palliation | NF1, cranial RT | Died of secondary cancer | 3.52 |
| 0.30 | F | PA of hypothalamus/optic pathway, initial STR then cyst resection, chemotherapy (carboplatin/vincristine × 15 months, vinblastine × 11 months, temozolomide × 11 months, selumetinib × 9 months, focal RT=54 Gy, vemurafenib × 3 months) | 14 | SCC of skin Resection × 2 | Vemurafenib | Alive with SD of PA | 15.56 |
| 0.29 | M | Cerebral desmoplastic infantile ganglioglioma with GTR done | 1.37 | T cell ALL Chemotherapy, HSCT | N/A | Died of secondary cancer | 2.29 |
| 0.09 | M | Extraventricular neurocytoma of posterior fossa, 3 tumor-directed surgeries, 2 chemotherapy regimens (vincristine, cyclophosphamide, cisplatin, etoposide, carboplatin, topotecan), CSI (30 Gy/49.5 Gy); followed by 8 more chemotherapy regimens with agents including irinotecan, etoposide, temozolomide, oral cyclophosphamide, erlotinib, oral topotecan, and vinblastine) | 14.4 | Follicular carcinoma of thyroid Thyroidectomy | CSI | PD on therapy | 24.20 |
| 17.45 | HGG (Grade III) STR, chemotherapy (bevacizumab +/− irinotecan) | CSI | |||||
| 0.69 | F | FA of hypothalamus/optic pathway diagnosed by biopsy, chemotherapy (carboplatin/vincristine × 10 months, vincristine/actinomycin D × 11 months) | 7.3 | GBM STR | N/A | DOD | 6.91 |
| 0.23 | M | FA of hypothalamus/optic pathway diagnosed by biopsy, chemotherapy (carboplatin/vincristine × 9 months) | 13.5 | GBM STR, chemotherapy (temozolomide/bevacizumab) | N/A | DOD | 14.65 |
Abbreviations: ALL, acute lymphoblastic leukemia; CSI, craniospinal irradiation; DOD, died of disease; FA, fibrillary astrocytoma; GBM, glioblastoma; GTR, gross-total resection; HSCT, hematopoietic stem cell transplantation; N/A, not applicable; NED, no evidence of disease; NF1, neurofibromatosis type 1; PA, pilocytic astrocytoma; PD, progressive disease; RT, radiation therapy; SD, stable disease; SCC, squamous cell carcinoma; STR, subtotal resection
Burden of and Risk Factors for Chronic Health Deficits
Survivors experienced several tumor- and treatment-related sequelae requiring medical attention, which affected their daily lives. Almost all patients (49/51, 96%) experienced ≥1 health deficits given below. Table A2 summarizes significant predictors of health deficits in univariate and multivariate analyses.
Endocrinopathy and obesity:
Twenty-six patients (51%) had endocrinopathies: precocious puberty (n=19, 37%), hypothyroidism (n=15, 29%; including 1 patient undergoing thyroidectomy for thyroid cancer), growth hormone deficiency (n=15, 29%), hypogonadism (n=6, 12%), and adrenocortical deficiency (n=3, 6%). Twenty of 43 patients (47%) with BMI data were overweight, of whom 12 (28%) were obese. RT use was significantly associated with development of any endocrine problem in multivariate analysis (p=0.032), and tumor location (HT/OP) was the only predictor of obesity (p=0.037).
Neurological deficit and seizure:
Residual weakness occurred in 22 patients (43%): hemiparesis (n=21, 41%) and paraplegia (n=1, 2%). Thirty-five patients (69%) had seizure disorder or abnormal electroencephalogram requiring anti-epileptic medications; among them, 7 had seizures at or before presentation, and 28 had seizures after presentation. Twenty-one (41%) required anti-epileptic agent(s) at latest follow-up; the median time between diagnosis and seizure development in those who presented after diagnosis among these 21 patients (n=15) was 3.98 years (range, 0.03–17.5). Higher number of chemotherapy regimens was significantly associated with occurrence of seizure (p=0.014).
Visual and hearing loss:
VA was decreased in 27 of 48 patients (56%), of whom 13 (27%) were legally blind. VF defect occurred in 14 of 31 patients (45%), including 4 having normal VA. Of 39 patients with audiological test results, 17 (44%) had some degree of hearing loss (unilateral=6, bilateral=11). Considering hearing impairment in the better ear, 5 patients had SIOP grade 1, 1 had grade 2, 3 had grade 3, and 2 had grade 4 impairment. Hearing aids were recommended for 7 patients (18%). Tumor location (HT/OP) was significantly associated with decreased VA (p=0.002) and more episodes of tumor-directed surgeries was the only predictor for hearing loss (p=0.007).
Neurocognitive impairment:
Twenty-five patients (49%) had data from neurocognitive evaluations. Mean age at testing was 10.5 years (range, 1.9–17.9), and mean duration from diagnosis to assessment was 9.9 years (range, 1–17.6). Patients with neurocognitive data were significantly older at first treatment than those without neurocognitive data (P=0.003). However, no other demographic and treatment parameters differed significantly between both groups. Patients’ mean IQ score was significantly lower than the normative score (n=21; mean=75.5, median=77; SD=20.6; range, 40–115; P<0.001), with 16/21 patients (76.2%) having IQ scores <85. Ten of 21 patients with IQ scores available received RT (median age, 3.3 years; range, 1.6–18.7 years): 9 of them received focal RT (HT/OP=7, posterior fossa=1, brainstem=1); 1 received CSI (HT/OP primary). Median IQ scores were 75.5 (range, 40–115) for those who received RT and 79 (range, 45–109) for those who did not. Five of 21 patients with IQ scores available had NF1: the median IQ scores were 76 (range, 65–89) for those with NF1 and 77 (range, 40–115) for those without. Receiving more chemotherapy regimens was significantly associated with lower IQ (p=0.0116) in multivariate analysis. Adaptive functioning (n=17; mean=71.3, median=72; SD=23.6; range=26–110; P<0.001) was significantly lower in 12/17 patients (70.6%) having scores <85. Per parental report, IEP was required in 32 (62.7%) patients.
Cerebrovascular disease and scoliosis:
Fourteen patients (27%) had cerebrovascular disease: stroke (n=9; 3 with moyamoya disease), vascular stenosis (n=3), arteriovenous malformation (n=1), and cerebral venous thrombosis (n=1). Seven of 14 patients presented after RT (all had HT/OP primaries; median 1.21 years after RT; range, 0.26–26.29 years); the remaining patients had cerebrovascular events attributed to operative complication (n=5), congenital lesion (n=1), or uncertain cause (n=1). Scoliosis occurred in 18 patients (35%). RT use was significantly associated with cerebrovascular disease (only significant factor, P=0.011) and scoliosis (multivariate analysis, P=0.015). Cerebrovascular complications (stroke=2, moyamoya disease=1) developed in 3 of 7 patients with NF1, all of whom had received RT.
DISCUSSION
We report long-term outcomes of LGG/LGGNT patients diagnosed during the first year of life. Poor PFS, multiple interventions, and universal occurrence of chronic health deficits define disease in these infants. Compared with outcomes of 361 children with LGGs (<21 years at diagnosis),4 PFS of our cohort was significantly worse, with 10-year PFS rates of 16.9% (vs 58%) despite similar 10-year OS rates (85% vs 87%). The drastically lower PFS observed is in keeping with previous reports by SIOP and GPOH on LGG diagnosed during the first year of life.19, 20 Inability to achieve GTR of tumor upfront, diencephalic syndrome and, possibly HT/OP tumor location were associated with inferior PFS in our cohort, similar to observations in pediatric LGG over the entire age spectrum.1, 4, 35 Consistent with previous studies, the proportion of infants with metastasis in our cohort was higher than in pediatric LGG, but unlike in older patients, metastasis in infant LGG did not affect survival.36, 37
Three-quarters of patients experienced disease progression after LGG/LGGNT diagnosis during infancy, which increased treatment burden and treatment-related toxicities in patients. Half our patients diagnosed during infancy experienced multiple progressions, with 1 patient experiencing 18 progressions requiring interventions; in comparison with multiple progressions in 20% of children with LGG in previous studies.4 In our cohort, up to 6 tumor-directed surgeries per patient and up to 13 systemic therapy regimens per patient were required. NF-1 status predicted fewer surgeries, consistent with the more benign course of glioma in these patients, but tumors not completely resectable at diagnosis and those in the HT/OP—commonly found in infants—resulted in increased chemotherapy/systemic therapy.38 Common LGG regimens (e.g., carboplatin/vincristine and vinblastine) involve weekly hospital attendance over months, disrupting daily lives and family routines. Radiation therapy, which can often achieve tumor control in lesions that are not completely resectable, was required in almost half our patients, with a mean deferral of 4 years from diagnosis. The better RFS in patients receiving diagnoses more recently (2001–2005) likely reflects a change in practice towards RT avoidance in patients with LGG diagnosed at a very young age.39 Although this change in practice means that efficacy of RT in our infant cohort cannot be compared directly with that in older children due to differences in the timing of RT administration, tumors progressed further in half of our patients who were irradiated (5-year PFS after RT ~60%), whom also had an increased risk of long-term morbidities, including endocrinopathy, cerebrovascular disease, and scoliosis.9
Chronic health conditions are prevalent in childhood cancer survivors and affect their well-being.40 In a Childhood Cancer Survivor Study (CCSS), 62% of survivors at a mean age of 26.6 years had at least 1 chronic condition, with survivors of CNS tumors at second-highest risk of severe or life-threatening events. A recent CCSS study revealed a 66% prevalence of chronic health conditions in survivors as young as 5 years, with prevalence increasing to 88% in survivors aged 40–49 years.41 Survivors of CNS tumors had endocrinopathy, neurological deficits, seizure, sensory conditions, cognitive impairment, and vascular events; these findings formed the basis for collecting data on health deficits in our infant cohort.42–44
Endocrine disorders occurred in half our patients, with RT use being the only significant predictor in multivariate analysis. Hormonal deficiencies and osteoporosis characterize the spectrum of endocrine disorders in survivors of CNS tumors.44 However, precocious puberty was the most common endocrinopathy in our cohort, likely due to the common hypothalamic tumor location and pre-pubertal age at diagnosis and treatment, including RT.3, 45, 46 A previous study from our institution revealed precocious puberty in 15.2% of patients with CNS tumors and 29.2% with hypothalamic/pituitary tumors.47 Documenting pubertal stage, growth velocity, and if necessary, radiographic bone age, is essential in addition to hormonal profiles during follow-up for timely intervention. As for older patients,4, 45, 47 excessive weight gain remained problematic in infants with hypothalamic LGG.
Visual impairment is common in infant LGG/LGGNT, given its predilection for occurring in the HT/OP and the inability to deliver RT upfront.4,6, 48 More than half our cohort had reduced VA, of whom approximately one-quarter were legally blind. VF defect also occurred in half of those who could be reliably assessed. Six patients had dual sensory impairment (reduced VA and hearing), increasing their vulnerability to neurodevelopmental deficiencies. Overall, the prevalence of significant (SIOP grade 3/4) ototoxicity in our cohort was lower than in patients with embryonal tumors, because of using carboplatin rather than cisplatin in LGG/LGGNT and only few patients receiving significant RT doses to the posterior fossa, which are both predictors of hearing impairment in pediatric brain tumor survivors.8, 43, 49 However, infant LGG/LGGNT survivors still require regular, age-appropriate auditory evaluations, as both surgery itself and carboplatin use are associated with hearing loss, especially if initiated before 6 months of age for the latter.50, 51
Neurocognitive outcome is central to studies of childhood cancer survivors and affects their quality of life, functionality, educational attainment, job opportunities, and psychosocial well-being.4, 42, 52–56 Deficits in survivors vary by CNS tumor type, with poorer outcomes in medulloblastoma than in LGG.57 Medulloblastoma survivors display a universal, steady decline in IQ, with younger age at RT and higher CSI doses being risk factors for faster deterioration.56–58 Other factors include mass effect from tumor, tumor-directed surgery, chemotherapy, hydrocephalus, and posterior fossa syndrome. In one study, one-third of pediatric LGG survivors had an IQ <85, with a mean IQ of 92.5 at an average of 6 years post diagnosis.4 Epilepsy, need for CSF shunting, and age <3 years are significantly associated with lower IQ.4, 10 In our cohort, mean IQ was 75.5, with three-quarters of tested patients having an IQ lower than 85. Predictors of lower IQ included supratentorial location of primary tumor and more chemotherapy regimens but not RT use. RT use in infants with LGG/LGGNT, often focal in extent and delivered after surgery and chemotherapy, could thus had less impact on neurocognitive outcomes of survivors.6, 10 Other domains of functionality and psychosocial well-being are also important in the follow-up of LGG/LGGNT survivors.59–62 In our study, adaptive functioning or the child’s ability to complete age-appropriate tasks of daily living was as affected as was IQ.
Our study has several limitations. First, due to its retrospective design, morbidity data were not collected in a standardized manner, and some assessments were performed at other facilities, restricting accurate determination of morbidity incidence and possibly underestimating health deficits. Regardless, the documented high prevalence of morbidities confirmed our hypothesis that survivors of infant LGG/LGGNT commonly experience chronic health deficits. Second, the treatment approach for patients was heterogeneous, precluding evaluation of the efficacy of individual therapeutic regimens and confirmation of causality between treatment factors and observed morbidities. Third, histopathologic diagnoses in our cohort should be interpreted considering the evolution of tumor biology, diagnostic tests, and classification terminology over the past decades. Molecular alterations that have recently been shown to be of prognostic significance, such as BRAF V600E mutation and CDKN2A deletion, were not tested for in most patients and could not be factored into the current analysis.63 Notwithstanding these shortcomings, the natural history and clinical profile of LGG/LGGNT diagnosed during the first year of life has been described at a granular level in a cohort with long-term follow-up, illustrating the uniqueness of LGG/LGGNT and highlighting the need to develop specific consensus guidelines in management.64
CONCLUSION
Low-grade glioma and glioneuronal tumors diagnosed during the first year of life are clinically unique and associated with multiple progressions and repeated interventions. Treatment recommendations should be made with anticipation of possible toxicities. Chronic health deficits are highly prevalent and multi-systemic in survivors, warranting pre-emptive counseling and dedicated multidisciplinary management.
Supplementary Material
Table A1. Predictors of overall, progression-free, and radiation-free survival.
Table A2. Clinical and treatment variables significantly associated with long-term toxicities.
ACKNOWLEDGEMENTS
We thank Cherise Guess, PhD, ELS, for editing the manuscript.
This work was sponsored in part by ALSAC, the St. Jude Children’s Research Hospital Pediatric Oncology Education Program, National Cancer Institute Grant R25CA23944, and Cancer Center Support Grant CA21765.
Footnotes
Authors declare no conflict of interest.
Data from the study were presented in part at the International Symposium on Pediatric Neuro-Oncology 2018, Denver, USA.
REFERENCES
- 1.Qaddoumi I, Sultan I, Gajjar A. Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer. 2009;115: 5761–5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol. 1997;15: 2792–2799. [DOI] [PubMed] [Google Scholar]
- 3.Benesch M, Lackner H, Sovinz P, et al. Late sequela after treatment of childhood low-grade gliomas: a retrospective analysis of 69 long-term survivors treated between 1983 and 2003. J Neurooncol. 2006;78: 199–205. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong GT, Conklin HM, Huang S, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnautovic A, Billups C, Broniscer A, Gajjar A, Boop F, Qaddoumi I. Delayed diagnosis of childhood low-grade glioma: causes, consequences, and potential solutions. Childs Nerv Syst. 2015;31: 1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberger BA, Pulsifer MB, Ebb DH, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2014;89: 1060–1068. [DOI] [PubMed] [Google Scholar]
- 7.Jalali R, Mallick I, Dutta D, et al. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77: 974–979. [DOI] [PubMed] [Google Scholar]
- 8.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late Effects of Conformal Radiation Therapy for Pediatric Patients With Low-Grade Glioma: Prospective Evaluation of Cognitive, Endocrine, and Hearing Deficits. Journal of Clinical Oncology. 2009;27: 3691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA. Phase II Trial of Conformal Radiation Therapy for Pediatric Low-Grade Glioma. Journal of Clinical Oncology. 2009;27: 3598–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto MD, Conklin HM, Li C, Merchant TE. Learning and Memory Following Conformal Radiation Therapy for Pediatric Craniopharyngioma and Low-Grade Glioma. Int J Radiat Oncol Biol Phys. 2012;84: e363–e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opocher E, Kremer LC, Da Dalt L, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer. 2006;42: 1807–1816. [DOI] [PubMed] [Google Scholar]
- 12.Lasky JL, Choi EJ, Johnston S, Yong WH, Lazareff J, Moore T. Congenital brain tumors: case series and review of the literature. J Pediatr Hematol Oncol. 2008;30: 326–331. [DOI] [PubMed] [Google Scholar]
- 13.Qaddoumi I, Carey SS, Conklin H, et al. Characterization, treatment, and outcome of intracranial neoplasms in the first 120 days of life. J Child Neurol. 2011;26: 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosino MM, Hernanz-Schulman M, Genieser NB, Wisoff J, Epstein F. Brain tumors in infants less than a year of age. Pediatr Radiol. 1988;19: 6–8. [DOI] [PubMed] [Google Scholar]
- 15.Rivera-Luna R, Medina-Sanson A, Leal-Leal C, et al. Brain tumors in children under 1 year of age: emphasis on the relationship of prognostic factors. Childs Nerv Syst. 2003;19: 311–314. [DOI] [PubMed] [Google Scholar]
- 16.Schmandt SM, Packer RJ. Treatment of low-grade pediatric gliomas. Curr Opin Oncol. 2000;12: 194–198. [DOI] [PubMed] [Google Scholar]
- 17.Alfaar AS, Hassan WM, Bakry MS, Qaddoumi I. Neonates with cancer and causes of death; lessons from 615 cases in the SEER databases. Cancer Med. 2017;6: 1817–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larouche V, Huang A, Bartels U, Bouffet E. Tumors of the central nervous system in the first year of life. Pediatr Blood Cancer. 2007;49: 1074–1082. [DOI] [PubMed] [Google Scholar]
- 19.Gnekow AK, Walker DA, Kandels D, et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (</=16 years) low grade glioma - A final report. Eur J Cancer. 2017;81: 206–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirow C, Pietsch T, Berkefeld S, et al. Children< 1 year show an inferior outcome when treated according to the traditional LGG treatment strategy: A report from the german multicenter trial HIT-LGG 1996 for children with low grade glioma (LGG). Pediatr Blood Cancer. 2014;61: 457–463. [DOI] [PubMed] [Google Scholar]
- 21.Young HK, Johnston H. Intracranial tumors in infants. J Child Neurol. 2004;19: 424–430. [DOI] [PubMed] [Google Scholar]
- 22.Duffner PK, Horowitz ME, Krischer JP, et al. The treatment of malignant brain tumors in infants and very young children: an update of the Pediatric Oncology Group experience. Neuro Oncol. 1999;1: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato O, Tamura A, Sano K. Brain tumors of early infants. Childs Brain. 1975;1: 121–125. [DOI] [PubMed] [Google Scholar]
- 24.Askin DF. Neonatal cancer: a clinical perspective. J Obstet Gynecol Neonatal Nurs. 2000;29: 423–431. [DOI] [PubMed] [Google Scholar]
- 25.Huynh-Le MP, Walker AJ, Burger PC, et al. Management of pediatric intracranial low-grade gliomas: long-term follow-up after radiation therapy. Childs Nerv Syst. 2016;32: 1425–1430. [DOI] [PubMed] [Google Scholar]
- 26.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328: 1725–1731. [DOI] [PubMed] [Google Scholar]
- 27.Gajjar A, Heideman RL, Kovnar EH, et al. Response of pediatric low grade gliomas to chemotherapy. Pediatr Neurosurg. 1993;19: 113–118; discussion 119–120. [DOI] [PubMed] [Google Scholar]
- 28.Lafay-Cousin L, Sung L, Carret AS, et al. Carboplatin hypersensitivity reaction in pediatric patients with low-grade glioma: a Canadian Pediatric Brain Tumor Consortium experience. Cancer. 2008;112: 892–899. [DOI] [PubMed] [Google Scholar]
- 29.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86: 747–754. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, Texas: Psychological Corporation; 2014. [Google Scholar]
- 31.Wechsler D Wechsler intelligence scale for children–Fifth Edition (WISC–V). San Antonio, Texas: Psychological Corporation; 2014. [Google Scholar]
- 32.Wechsler D, Scales PI, Index VC. Wechsler Preschool and Primary Scale of Intelligence—Fourth Edition 2012. [Google Scholar]
- 33.Harrison P, Oakland T. Adaptive behavior assessment system (ABAS-II). San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 34.Sparrow SS, Cicchetti DV, Balla DA, Doll EA. Vineland adaptive behavior scales: Survey forms manual. American Guidance Service, 2005. [Google Scholar]
- 35.Bandopadhayay P, Bergthold G, London WB, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: An analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61: 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamdine O, Broniscer A, Wu S, Gajjar A, Qaddoumi I. Metastatic Low-Grade Gliomas in Children: 20 Years’ Experience at St. Jude Children’s Research Hospital. Pediatr Blood Cancer. 2016;63: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Hornstein S, Kortmann RD, Pietsch T, et al. Impact of chemotherapy on disseminated low-grade glioma in children and adolescents: report from the HIT-LGG 1996 trial. Pediatr Blood Cancer. 2011;56: 1046–1054. [DOI] [PubMed] [Google Scholar]
- 38.Ater JL, Xia C, Mazewski CM, et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children’s Oncology Group. Cancer. 2016;122: 1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bishop AJ, McDonald MW, Chang AL, Esiashvili N. Infant brain tumors: incidence, survival, and the role of radiation based on Surveillance, Epidemiology, and End Results (SEER) Data. International Journal of Radiation Oncology* Biology* Physics. 2012;82: 341–347. [DOI] [PubMed] [Google Scholar]
- 40.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine. 2006;355: 1572–1582. [DOI] [PubMed] [Google Scholar]
- 41.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiology and Prevention Biomarkers. 2015;24: 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. JNCI: Journal of the National Cancer Institute. 2009;101: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21: 3255–3261. [DOI] [PubMed] [Google Scholar]
- 44.Gurney JG, Kadan-Lottick NS, Packer RJ, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors. Cancer. 2003;97: 663–673. [DOI] [PubMed] [Google Scholar]
- 45.Fouladi M, Wallace D, Langston JW, et al. Survival and functional outcome of children with hypothalamic/chiasmatic tumors. Cancer. 2003;97: 1084–1092. [DOI] [PubMed] [Google Scholar]
- 46.Gan HW, Phipps K, Aquilina K, Gaze MN, Hayward R, Spoudeas HA. Neuroendocrine Morbidity After Pediatric Optic Gliomas: A Longitudinal Analysis of 166 Children Over 30 Years. J Clin Endocrinol Metab. 2015;100: 3787–3799. [DOI] [PubMed] [Google Scholar]
- 47.Chemaitilly W, Merchant TE, Li Z, et al. Central precocious puberty following the diagnosis and treatment of paediatric cancer and central nervous system tumours: presentation and long-term outcomes. Clin Endocrinol (Oxf). 2016;84: 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awdeh RM, Kiehna EN, Drewry RD, et al. Visual Outcomes in Pediatric Optic Pathway Glioma After Conformal Radiation Therapy. Int J Radiat Oncol Biol Phys. 2012;84: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armstrong GT, Liu Q, Yasui Y, et al. Long-Term Outcomes Among Adult Survivors of Childhood Central Nervous System Malignancies in the Childhood Cancer Survivor Study. JNCI: Journal of the National Cancer Institute. 2009;101: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qaddoumi I, Bass JK, Wu J, et al. Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol. 2012;30: 1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghazwani Y, Qaddoumi I, Bass JK, et al. Profound hearing loss following surgery in pediatric patients with posterior fossa low-grade glioma. Neuro-oncology practice. 2017;5: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirchhoff AC, Krull KR, Ness KK, et al. Physical, mental, and neurocognitive status and employment outcomes in the childhood cancer survivor study cohort. Cancer Epidemiology and Prevention Biomarkers. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological Status in Childhood Cancer Survivors: A Report From the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2009;27: 2396–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive Status in Long-Term Survivors of Childhood CNS Malignancies: A Report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23: 705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brinkman TM, Krasin MJ, Liu W, et al. Long-Term Neurocognitive Functioning and Social Attainment in Adult Survivors of Pediatric CNS Tumors: Results From the St Jude Lifetime Cohort Study. Journal of Clinical Oncology. 2016;34: 1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. The lancet oncology. 2004;5: 399–408. [DOI] [PubMed] [Google Scholar]
- 57.Mulhern RK, Reddick WE, Palmer SL, et al. Neurocognitive deficits in medulloblastoma survivors and white matter loss. Annals of neurology. 1999;46: 834–841. [DOI] [PubMed] [Google Scholar]
- 58.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. Journal of Clinical Oncology. 2005;23: 5511–5519. [DOI] [PubMed] [Google Scholar]
- 59.Brinkman TM, Liptak CC, Delaney BL, Chordas CA, Muriel AC, Manley PE. Suicide ideation in pediatric and adult survivors of childhood brain tumors. J Neurooncol. 2013;113: 425–432. [DOI] [PubMed] [Google Scholar]
- 60.Bhat SR, Goodwin TL, Burwinkle TM, et al. Profile of daily life in children with brain tumors: an assessment of health-related quality of life. J Clin Oncol. 2005;23: 5493–5500. [DOI] [PubMed] [Google Scholar]
- 61.Aarsen FK, Paquier PF, Reddingius RE, et al. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer. 2006;106: 396–402. [DOI] [PubMed] [Google Scholar]
- 62.Beebe DW, Ris MD, Armstrong FD, et al. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130). Journal of Clinical Oncology. 2005;23: 5198–5204. [DOI] [PubMed] [Google Scholar]
- 63.Lassaletta A, Zapotocky M, Mistry M, et al. Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. Journal of Clinical Oncology. 2017;35: 2934–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. Version 4.0 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A1. Predictors of overall, progression-free, and radiation-free survival.
Table A2. Clinical and treatment variables significantly associated with long-term toxicities.