Abstract
Background:
Alterations in reward processing are a central feature of depression and may be influenced by inflammation. Indeed, inflammation is associated with deficits in reward-related processes in animal models and with dysregulation in reward-related neural circuitry in humans. However, the downstream behavioral manifestations of such impairments are rarely examined in humans.
Methods:
The influenza vaccination was used to elicit a mild inflammatory response in 41 healthy young adults (age range: 18–22, 30 female). Participants provided blood samples and completed behavioral measures of three key aspects of reward—reward motivation, reward learning, and reward sensitivity—before and 1 day after receiving the influenza vaccine.
Results:
The influenza vaccine led to mild but significant increases in circulating levels of the pro-inflammatory cytokine interleukin-6 (IL-6) (p < .001). Consistent with hypotheses, increases in IL-6 predicted lower reward motivation (p = .029). However, contrary to hypotheses, increases in IL-6 predicted increased performance on a reward learning task (p = .043) and were not associated with changes in reward sensitivity (p’s > .288).
Conclusions:
These findings contribute to an emerging literature on the nuanced associations between inflammation and reward and demonstrate that even mild alterations in inflammation are associated with multiple facets of reward processing.
Keywords: Inflammation, depression, anhedonia, reward motivation, reward learning, sickness behavior
1. Introduction
Depression is a debilitating, chronic, and widespread condition characterized by a constellation of affective, cognitive, and behavioral symptoms (Hasler et al., 2004; Kessler et al., 2012). Compelling evidence links dysregulated inflammatory biology to depression broadly (Dantzer et al., 2008; Valkanova et al., 2013), but less is known about specific dimensions of depression that are sensitive to alterations in inflammation. One critical dimension is reward processing, with reward dysfunction linked to anhedonia (Craske et al., 2016). Commonly defined as reduced ability to experience pleasure, anhedonia actually reflects a broad array of potential deficits in reward-related processes, including reward motivation, reward learning, and reward sensitivity (Treadway and Zald, 2013). Inflammation has been shown to disrupt neural reward processing (Capuron et al., 2012; Eisenberger et al., 2010b; Harrison et al., 2016), although the facets of reward tested vary. By contrast, effects of inflammation on behavioral measures of reward processing have rarely been studied, and no study, to date, has tested the associations between inflammation and multiple reward domains. Thus, the overarching goal of this study was to examine the extent to which changes in inflammation were related to changes in three domains of reward processing: motivation, learning, and sensitivity.
Reward motivation refers to the willingness to exert effort to achieve a reward. In animal models, inducing inflammation reliably reduces reward motivation (La Garza, 2005), which is frequently measured by manipulating the amount of effort required to obtain palatable food (Der-Avakian and Markou, 2012). To the best of our knowledge, only two previous studies have examined inflammation and reward motivation in humans, with conflicting results. Using endotoxin to elicit an acute inflammatory response in a sample of men, Draper et al. (2017) found lower reward motivation as assessed by a novel “effort-stake” task in which participants could select to reject or work for offers of reward at varying levels of monetary value and physical effort expenditure. By contrast, in a mixed sex sample, Lasselin and colleagues (2016) found higher reward motivation following endotoxin versus saline, but only when the probability of receiving the reward was high. This study assessed reward motivation with the Effort Expenditure for Rewards task (EEfRT) (Treadway et al., 2009), which is based on animal models of depression and reward motivation, and requires participants to choose between working at lower versus higher levels of physical effort for monetary reward. Patients with major depressive disorder have been shown to work less hard than healthy controls on the EEfRT (Treadway et al., 2012). Further, EEfRT performance has also been linked to trait anhedonia (Geaney et al., 2015) and is sensitive to psychological (Anand et al., 2015) and pharmacological (e.g., Wardle et al., 2011) manipulation.
Reward learning, including the ability to respond to positive reinforcement, is a core component of motivated behavior and consists of both explicit and implicit processes (Thomsen, 2015). There is some evidence that inflammation is associated with decreased explicit reward learning in humans. Specifically, one study found decreased ventral striatal encoding of reward prediction error during an instrumental learning task following typhoid vaccine versus placebo control (Harrison et al., 2016). A similar pattern, though correlational, was observed in association with stress-induced inflammatory responses (Treadway et al., 2017). Much work on reward learning in the context of depression has relied on the Probabilistic Reward Task (PRT), which assesses implicit reinforcement learning (Goldstein and Klein, 2014; Pizzagalli et al., 2005). Higher depressive symptoms (particularly anhedonic symptoms) are associated with blunted response to reward on the PRT (Fletcher et al., 2015; Vrieze et al., 2013). Moreover, there is evidence for reduced reward responsiveness on the PRT in both rats and humans following acute stress and pharmacological challenges hypothesized to decrease dopamine (e.g., Der-Avakian et al., 2013, 2017; Bogdan and Pizzagalli, 2006; Pizzagalli et al., 2008a). However, the relationship between inflammation and implicit reward learning has not yet been tested.
Reward sensitivity refers to the hedonic impact of reward, and is comparable to the consummatory reward response, or “liking” (Dantzer et al., 2014; Huys et al., 2013). Inducing inflammation decreases reward sensitivity in animal models, as measured by preference for palatable substances or intracranial self-stimulation (Koo et al., 2008; La Garza, 2005; Van Heesch et al., 2013; Yirmiya et al., 2000; but cf. Vichaya et al., 2014). Indices of reward sensitivity can be derived from learning tasks like the PRT, particularly when used in conjunction with computational modeling that parses participants’ performance into learning rate (i.e., the ability to learn from and accumulate rewards over time) and reward sensitivity (i.e., the immediate behavioral impact of rewards) (Huys et al., 2013). To date, no studies have examined the relationship between inflammation and these components of the PRT. By contrast, both Draper et al. (2017) and Lasselin et al. (2016) assessed reward sensitivity in the context of reward motivation. Interestingly, neither of those studies found an effect of inflammation on reward sensitivity; in the placebo and control conditions, increases in hedonic value (e.g., more money) predicted similar increases in effort.
The current study used a mild inflammatory stimulus to interrogate within-subject associations between inflammation and behavioral measures of reward motivation, learning and sensitivity. To do so, we recruited healthy undergraduate students to complete behavioral reward tasks before and after receiving the annual influenza vaccine, which elicits mild increases in peripheral levels of IL-6 (e.g., Bucasas et al., 2011; Christian et al., 2013; Kuhlman et al., 2018; Tsai et al., 2005). This within-subjects design was appropriate given past research demonstrating significant within-person associations between the magnitude of the induced inflammatory response and the degree of change in mood and behavior following administration of endotoxin (Eisenberger et al., 2010a; 2009; Grigoleit et al., 2011) and typhoid vaccine (Harrison et al., 2009). Notably, most past studies assessing reward have utilized more potent inflammatory challenges, such as typhoid vaccine, endotoxin and interferon-alpha therapy, which can provoke increases in inflammation ranging from 250% (e.g., typhoid vaccine, Harrison et al., 2015) to 49,900% (e.g., endotoxin, Lasselin et al., 2016) (Dooley et al., 2018). Here, we were interested in examining smaller increases in inflammation, more comparable to those induced by psychological stress (Gruenewald et al., 2009; Muscatell et al., 2015; Rohleder, 2014), given the strong association between stress and depression (Hammen, 2005; Slavich et al., 2009). In addition, we used behavioral tasks commonly used in the depression literature to focus on three domains of reward. Based primarily on preclinical evidence, we predicted that increases in IL-6 following the vaccine would be associated with decreased reward motivation (using the EEfRT), implicit reward learning (using the PRT), and reward sensitivity (using indices derived from both the EEfRT and the PRT) from pre- to post-vaccine.
2. Methods and Materials
2.1. Participants and Procedure
Forty-one undergraduate students at the University of California, Los Angeles (UCLA) participated in a study investigating the affective, cognitive, and behavioral effects of inflammatory activation following influenza vaccination (Kuhlman et al., 2018). Participants were recruited during the Fall of 2015 and 2016 through flyers posted on the university campus. Participants were eligible if they were age 18 to 22 and had not yet received the annual influenza vaccine. Exclusion criteria were current illness, presence of a major medical condition, use of tobacco products, or use of mood or immune-altering medications. Out of 46 eligible and enrolled individuals, three withdrew due to illness and two were unable to provide blood samples.
After providing informed consent, eligible participants completed questionnaires and behavioral reward tasks during an in-person baseline visit. Participants then completed 1 week of daily diaries before a second in-person visit, when they provided a morning blood sample and received the influenza vaccine (between 7am and 12pm). Results for daily diary analysis of changes in mood, social disconnection, sleep and physical symptoms are reported in Kuhlman et al. (2018). The next day, at the expected peak of the inflammatory response (Carty et al., 2006; Christian et al., 2013; Tsai et al., 2005), participants returned to the lab and completed a morning blood draw and behavioral reward tasks. The post-vaccine blood draw and behavioral tasks occurred between 21 and 29 hours after the vaccination (M = 24:35, SD = 2:10); of note, sampling time was not correlated with levels of IL-6 at pre- or post-vaccine (all p ’s > .453).
Data were collected over a 2-year period with two cohorts (October-November 2015 and October-November 2016). The two cohorts did not differ in terms of age (p = .589), sex (p = .303), body mass index (BMI; p = .257), baseline levels of IL-6 (p = .764) or change in IL-6 (p = .062). There were more IL-6 non-responders (i.e., showing no change or a decrease in IL-6 from pre to post-vaccine) in cohort 1 (n = 6) compared to cohort 2 (n = 2), χ2 = 4.61, p = .032. The influenza vaccine was trivalent and, for cohort 1, included A/Califomia/7/2009 (H1N1) pdm09-like virus, A/Switzerland/9715293/2013 (H3N2)-like virus, and B/Phuket/3073/2013. The vaccine for cohort 2 included A/California/7/2009 (H1N1) pdm09-like virus, A/Hong Kong/4801/2014 (H3N2)-like virus, and B/Brisbane/60/2008-like virus (B/Victoria lineage). Participants were compensated up to $200.00. Performance on the EEfRT and the PRT was incentivized with raffle tickets for $50 gift cards rather than immediate compensation. All participants, regardless of performance, received the same compensation. All study procedures were approved by the UCLA Institutional Review Board.
2.2. Measures
2.2.1. Inflammation.
IL-6 was selected as a marker of inflammation based on previous studies demonstrating increases in IL-6 following the influenza vaccine (Christian et al., 2013; Tsai et al., 2005) and correlations of within-person changes in IL-6 with changes in mood following typhoid vaccine (Harrison et al., 2009) and endotoxin administration (Grigoleit et al., 2011). Further, meta-analyses demonstrate that individuals with depression have elevated levels of IL-6 (e.g., Valkanova et al., Haapakoski et al., 2015). In the current study, blood samples were collected between 8:21 am and 12:45pm (M = 9:59am, SD = 1:04) by venipuncture into EDTA tubes, placed on ice, centrifuged for acquisition of plasma, and stored at −80 C for subsequent batch testing at study completion for each cohort. Samples were assayed in duplicate using a high sensitivity ELISA (R&D Systems, Minneapolis, Minnesota) at the UCLA Inflammatory Biology Core (inter- and intra-assay CVs <9%). The lower limit of detection was 0.20pg/mL and there were no undetectable values.
2.2.2. Effort Expenditure for Rewards Task.
The computerized EEfRT was used to examine reward motivation and reward sensitivity at post-vaccine (Treadway et al., 2009). Lower motivation for reward is operationalized as less willingness to exert greater effort for higher monetary reward. Lower sensitivity to reward is operationalized as an attenuated association between the extent to which variations in potential monetary reward predict the decision to exert effort for reward (Lasselin et al., 2016; Treadway et al., 2012). During the task, participants were presented with a series of trials in which they chose between an easy task (worth $1.00) and a hard task (worth between $1.24-$4.30). For the current study, easy trials required 30 button presses using the non-dominant index finger in 7 seconds, while hard trials required 100 button presses with the pinky finger of the dominant hand in 21 seconds. Of note, the EEfRT typically uses the non-dominant hand for the hard trials1. Participants were told that only some of the successfully completed trials would be rewarded, and that the monetary reward would be converted to raffle tickets. Each trial presented the probability that a successful response would be rewarded (12%, 50%, 88% probability). No one reward value was paired with a given probability more than once. Participants had 5 seconds to choose to work for a hard or easy trial; if they did not make a choice they were randomly assigned to a hard or easy trial. In the current study, the EEfRT duration was shortened from 20 minutes to 10 minutes due to the number of tasks administered.
2.2.3. Probabilistic Reward Task.
The PRT is a 15-min computerized task derived from signal detection theory (Pizzagalli et al., 2005; adapted from Tripp and Alsop, 1999). Performance on the PRT encompasses both implicit learning rate and reward sensitivity components (e.g., Huys et al., 2013), which together have been termed reward responsiveness (e.g., Bogdan and Pizzagalli, 2006). In the current study, participants completed a total of 240 trials, with a 30-sec break every 80 trials. In each trial, participants were asked to identify which of two difficult-to-differentiate stimuli were presented. The stimuli were cartoon faces with one of two straight mouths (10mm short mouth versus 11mm long mouth). Each trial presented a fixation cross [750ms], followed by a mouthless cartoon face [500ms], and then a face with a mouth [100ms]. Participants made their choice of mouths by pressing the ‘c’ or ‘m’ key and were then presented with either feedback or a blank screen [1750ms]. The feedback was “Congratulations! You just won 1 ticket!” While both the long and short stimuli were presented equally often, an asymmetric (3:1) pseudo-randomized reinforcement schedule was used to induce a response bias toward the more frequently rewarded stimuli across the 240 trials. Participants were not presented with more than three instances of the same stimulus consecutively, and if they did not make a correct response on a trial scheduled for reward, reward feedback was delayed until the next correct identification of that stimulus. Under this differential reinforcement schedule, healthy controls reliably develop a response bias favoring the more frequently rewarded stimulus (e.g., Pizzagalli et al., 2005, Bogdan and Pizzagalli, 2006); the degree to which the magnitude of this response bias changed from pre- to post-vaccine was used to operationalize reward responsiveness. Computational analyses of trial-level responses using the Bayesian model developed by Huys and colleagues (2013) were then used to derive for each participant two parameters: learning rate and reward sensitivity. Participants completed 10 practice trials to familiarize themselves with the task. Post-vaccine, all participants completed a different version of the PRT in which they had to choose between a long (5.31mm) and short (5.00mm) nose.
2.3. Analytic Approach
Analyses were carried out using Stata version 13.1. All analyses controlled for sex, cohort, and BMI. IL-6 values were positively skewed and log transformed prior to analyses, and one post-vaccine IL-6 value that was more than 4 standard deviations above the mean was winsorized. Change in IL-6 was operationalized as a single change score (post-vaccine minus pre-vaccine), with higher values indicating a greater increase in IL-6.
2.3.1. Data Reduction for the EEfRT and PRT.
For the EEfRT analyses, trials in which the participant did not choose between an easy or hard task were excluded (0.39% of all trials). In the current study, participants successfully completed 96% of all trials on average, which is consistent with previous studies (Treadway et al., 2009). For the PRT, in line with prior work and current recommendations, inclusion criteria were: accuracy greater than 50%, ratio of rewards received greater than 2.4, more than 80% trials within valid range (between 150ms and 1500ms), and fewer than 16 outliers at each administration. This procedure excluded 8 participants (19.5%) which is consistent with some studies (e.g., Fletcher et al., 2015) but higher than others (e.g., Kaiser et al., 2017).
2.3.2. Assessment of Reward Motivation and Sensitivity with the EEfRT.
The association between reward motivation (likelihood of choosing high-effort trials during the EEfRT) and change in IL-6 was tested with generalized estimating equations (GEEs) with a binary logistic model and exchangeable working correlation structure. GEEs are a typical approach for the EEfRT (Treadway et al., 2009), account for correlated data, and are appropriate for a binary dependent variable. In the current study, predictors included experimental session (0 = pre-vaccine, 1 = post-vaccine), sex (0 = male; 1 = female), cohort (0 = cohort 1; 1 = cohort 2), BMI, and change in IL-6. Consistent with previous studies (Treadway et al., 2009), the following task-specific variables were included as continuous time-varying covariates: reward magnitude, probability, expected value (reward magnitude X probability), and trial number. The dependent variable was hard trial choice (0 = no, 1 = yes). Reward sensitivity on the EEfRT was tested as the interaction between change in IL-6 and reward magnitude. A significant interaction would indicate that reward magnitude predicted hard trial choice differently depending on levels of IL-6; reduced reward sensitivity was operationalized as an attenuated association between reward magnitude and hard trial choice. Exploratory analyses tested for two-way interactions between change in IL-6 and other task specific variables (probability, expected value), consistent with previous studies (Treadway et al., 2009). All models converged successfully.
2.3.3. Assessment of Reward Responsiveness (Learning Rate and Sensitivity) with the PRT.
The association between change in IL-6 and change in reward responsiveness (total response bias) was examined using regression analysis with robust standard errors. First, a total response bias score across the 240 trials at each administration was calculated with the following formula, with “Rich” referring to
the more frequently rewarded stimulus, and “Lean” referring to the less frequently rewarded stimulus:
Change in reward responsiveness was calculated as change in total response bias (subtracting the total response bias at pre-vaccine from the total response bias at post-vaccine).
To further probe reward responsiveness and parse the contribution of learning rate (which operationalizes participants’ ability to learn from reward feedback) and reward sensitivity (which operationalizes reduction in consummatory pleasure) on PRT performance, a computational model of trial-level performance was implemented. A series of reinforcement-learning models were fitted to the PRT choice data (Huys et al., 2013) using an empirical Bayesian random-effects approach that ultimately yielded a parameter assessing learning rate, or participants’ ability to accumulate rewards over time and learn from the rewards, and a parameter assessing reward sensitivity , or the immediate behavioral impact of rewards. These parameters were analyzed in the transformed space to prevent issues with non-Gaussianity (detailed information on the computational modeling approach is provided in the supplementary material). Once coefficients representing learning rate and reward sensitivity at each administration were derived, change scores were created by subtracting pre-vaccine scores from postvaccine scores. These difference scores were then examined in regression analysis with robust standard errors. Thus, we conducted three separate analyses to assess change in 1) reward responsiveness; 2) learning rate; 3) reward sensitivity.
3. Results
3.1. Participant Characteristics
Participants were 41 undergraduate students, predominantly female (n = 30), and ranged in age from 18 to 22 (M = 18.5, SD = .75). Participants self-identified as Asian (n = 25), non-Hispanic White (n = 7), and Hispanic (n = 9). BMI ranged from 19.05 to 41.34, with average BMI in the normal weight category (M = 23.96, SD = 3.87). The influenza vaccine led to mild but significant increases in circulating levels of IL-6 (Mpre = 1.14, SD = 0.95; Mpost = 1.46, SD = 1.22; t(40) = −4.79,p < .001; Cohen’s d = 0.75)2. Thirty-three of 41 participants (80%) had an increase in IL-6, and average ΔIL-6 was 0.28 pg/mL (SD = 0.57; range - 1.44 to 2.70 pg/mL; see Kuhlman et al., 2018 for additional details).
3.2. Effort Expenditure for Rewards Task - Reward Motivation and Sensitivity
On average, participants completed between 24 and 44 trials on the EEfRT (M = 33.3, SD = 4.92), and the overall proportion of hard trials chosen was .56. Higher reward magnitude and expected value each predicted higher likelihood of choosing a hard trial in the GEE model, indicating that participants worked harder for trials that offered greater potential reward, which is consistent with prior studies (e.g., Treadway et al., 2009) (see Table 1). In support of our hypothesis, AIL-6 significantly predicted lower likelihood of choosing a hard trial, b = −0.65, p = .029, OR = 0.52. Thus, greater increases in IL-6 were associated with lower reward motivation, over and above the effects of administration session (pre- vs. post-vaccine), task variables, sex, cohort, and BMI (see Figure 1, Table 1). To examine the influence of contextual factors on trial choice, exploratory analyses tested for 2-way interactions between ΔIL-6 and task-specific variables, including probability and expected value. However, these were not significant (see Supplemental Tables 1 and 2).
Table 1.
Results of GEE model predicting hard trial choices on the EEfRT from change in IL-6
| Variable | b | SE | z | p-value | CI (lower) | CI (upper) |
|---|---|---|---|---|---|---|
| Session (pre/post-vaccine) | −0.11 | 0.09 | −1.21 | .228 | −0.28 | 0.07 |
| △IL-6 | −0.65 | 0.30 | −2.18 | .029 | −1.24 | −0.07 |
| Cohort | −0.87 | 0.21 | −4.16 | < .001 | −1.28 | −0.46 |
| Expected Value | 2.03 | 0.24 | 8.44 | < .001 | 1.56 | 2.50 |
| Probability | −0.44 | 0.19 | −2.34 | .020 | −0.81 | −0.07 |
| Reward Magnitude | 0.36 | 0.08 | 4.28 | < .001 | 0.19 | 0.52 |
| Trial number | −0.02 | 0.004 | −3.48 | .001 | −0.02 | −0.01 |
| Sex | .030 | 0.24 | 1.26 | .209 | −0.17 | 0.78 |
| BMI | −0.04 | 0.03 | −1.31 | .191 | −0.09 | 0.02 |
| Constant | −0.45 | 0.93 | −0.48 | .630 | −2.27 | 1.37 |
Note.△ IL-6 = change in IL-6 (post-vaccine minus pre-vaccine); BMI= Body Mass Index
Figure 1.
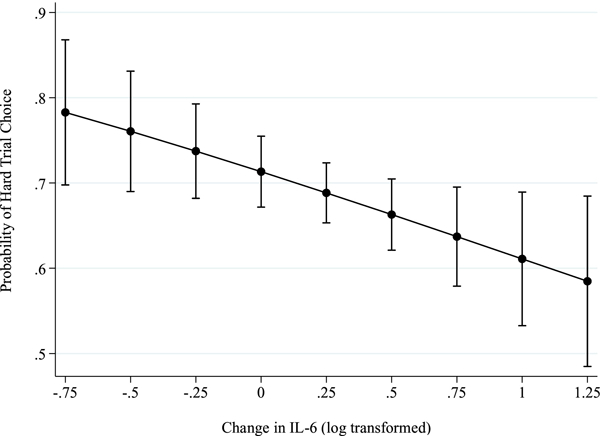
Results from generalized estimating equations for the Effort Expenditure for Rewards Task with 95% confidence interval error bars. Greater increases in IL-6 from pre- to post-influenza vaccine were significantly associated with fewer hard trial choice over and above the effects of time (pre-vs. post-vaccine), task specific variables (i.e., probability, expected value, reward magnitude, trial number), sex, BMI and cohort (b = −0.65, p = .029).
Next, we examined reward sensitivity with the EEfRT task by testing the significance of the interaction between changes in reward magnitude and ΔIL-6. There was no interaction between reward magnitude and ΔIL-6 in the prediction of hard trial choice (b = .14, p = .344), suggesting that ΔIL-6 was not associated with alterations in reward sensitivity on the EEfRT (see Supplemental Table 3)3.
3.3. Probabilistic Reward Task - Reward Responsiveness (Learning Rate and Reward Sensitivity)
On average, there were no significant differences in total response bias, sensitivity, or learning from pre- to post-vaccine on the PRT for the 33 participants who provided evaluable PRT data (all p’s > .085; see Supplementary Table 4), though there was substantial individual variability in these changes. Our primary analysis examined whether individual differences in ΔIL-6 were associated with the magnitude of change in total response bias (reward responsiveness) from pre- to post-vaccine. Contrary to hypotheses, ΔIL-6 was associated with increases in reward responsiveness (see Table 2, Figure 2a). Specifically, an increase in IL-6 was associated with an increase in total response bias from pre-to post-vaccine, b = 0.32, SE = 0.15, p = .043, ß = .44, over and above the effects of sex, cohort, and BMI. Of note, when we repeated this analysis with all 41 participants included we found similar effects, b = 0.29, SE = 0.15, p = .054, ß = .38.
Table 2.
Results from regression model predicting change in reward responsiveness on the PRT from change in IL-6
| Variable | B | β | SE | t | p-value | CI(lower) | CI(upper) |
|---|---|---|---|---|---|---|---|
| △IL-6 | 0.32 | .44 | 0.15 | 2.12 | .043 | 0.01 | 0.64 |
| BMI | −0.02 | −.26 | 0.01 | −1.36 | .185 | −0.05 | 0.009 |
| Sex | 0.02 | .03 | 0.12 | 0.16 | .875 | −0.23 | 0.26 |
| Cohort | −0.20 | −.34 | 0.12 | −1.58 | .124 | −0.45 | 0.06 |
| Intercept | 0.43 | 0.44 | 0.96 | .345 | −0.48 | 1.33 |
Note. △ IL-6 = change in IL-6 (post-vaccine minus pre-vaccine); BMI= Body Mass Index. Reward responsiveness refers to total response bias scores.
Figure 2.
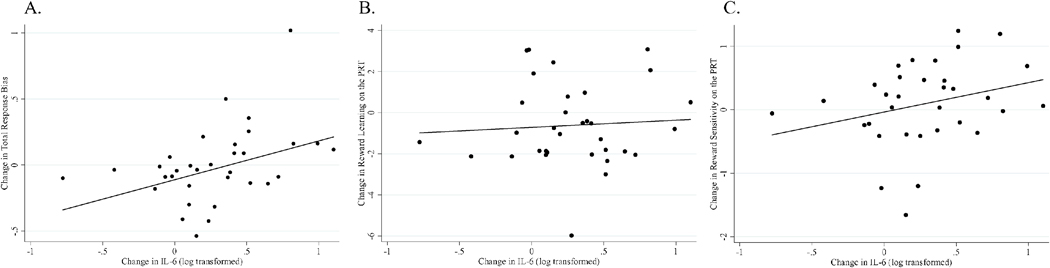
Greater increases in IL-6 were significantly associated with greater increases in total response bias (i.e., reward responsiveness) from pre- to post-vaccine on the PRT (r = .40, p = .019) (Panel A). Analyses remain significant when removing the outlier on changes in total response bias (i.e., the participant with the highest values on the y axis in Panel A). The relationship between change in IL-6 and change in reward learning did not reach significance (r = .07, p = .710) (Panel B) nor did the association between change in IL-6 and change in reward sensitivity on the PRT (r = .28, p = .121) (Panel C). Note that reward sensitivity (logβ) and learning rate () parameters in the transformed space were used to prevent issues with non-Gaussianity.
We next conducted regression analysis with the learning rate parameter derived from the computational analyses. Greater increases in IL-6 were not associated with significantly greater increases in learning rate, b = 1.06, SE = 0.95, p = .274, ß = .21 (see Table 3, Figure 2b). Similarly, greater increases in IL-6 were not significantly associated with greater increases in sensitivity, b = 0.31, SE = 0.28, p = .288, ß = .18 (see Table 3, Figure 2c). Thus, greater increases in IL-6 from pre-to post-vaccine were associated with increases in reward responsiveness on the PRT, but we were unable to determine if this was primarily due to changes in learning rate or reward sensitivity. See Table 4 for correlations between ΔIL-6, IL-6 at pre-and post-vaccine, and performance on the PRT, and supplementary material (Tables 5, 7–9) for analyses controlling for baseline levels of IL-6 and reward task performance.
Table 3.
Results from regression models predicting change in learning rate and change in reward sensitivity on the PRT from change in IL-6
| Outcome | Predictor | b | β | SE | t | p-value | CI(lower) | CI (upper) |
|---|---|---|---|---|---|---|---|---|
| △Learning Rate | ||||||||
| △IL-6 | 1.06 | .21 | 0.95 | 1.12 | .274 | −0.88 | 3.00 | |
| BMI | 0.003 | .01 | 0.11 | 0.03 | .977 | −0.22 | 0.22 | |
| Sex | −0.48 | −.10 | 1.18 | −0.41 | .688 | −2.89 | 1.94 | |
| Cohort | −1.37 | −.34 | 0.83 | −1.66 | .109 | −3.06 | 0.32 | |
| Intercept | 0.69 | 4.14 | 0.17 | .868 | −7.79 | 9.17 | ||
| △Reward Sensitivity | ||||||||
| △IL-6 | 0.31 | .18 | 0.28 | 1.08 | .288 | −0.27 | 0.89 | |
| BMI | −0.03 | −.20 | 0.04 | −0.76 | .451 | −0.12 | 0.05 | |
| Sex | 0.10 | .07 | 0.22 | 0.46 | .651 | −0.36 | 0.56 | |
| Cohort | 0.09 | .07 | 0.28 | 0.32 | .751 | −0.49 | 0.67 | |
| Intercept | 0.54 | 1.08 | 0.50 | .620 | −1.68 | 2.76 | ||
Note. IL-6= interleukin-6 (log transformed); BMI= Body Mass Index; △ = change (calculated as post-vaccine minus pre-vaccine). Learning rate() and reward sensitivity (logβ) coefficients were derived from computational modeling of trial-level responses (Huys et al., 2013).
Table 4.
Correlations among IL-6, △IL-6 and PRT performance at pre- and post-vaccine
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IL-6_T1 | |||||||||||
| 2 | IL-6_T2 | .826* | ||||||||||
| 3 | △IL-6 | −.408* | .179 | |||||||||
| 4 | Reward Responsiveness _T1 | .080 | −.018 | −.169 | ||||||||
| 5 | Reward Responsiveness _T2 | −.236 | −.011 | .393* | −.101 | |||||||
| 6 | △Reward Responsiveness | −.232 | .000 | .405* | −.588* | .864* | ||||||
| 7 | Reward Sensitivity_T1 | .096 | .095 | −.014 | .558* | .144 | −.165 | |||||
| 8 | Reward Sensitivity_T2 | −.187 | −.001 | .325 | −.163 | .728* | .674* | .033 | ||||
| 9 | △Reward Sensitivity | −.212 | −.058 | .276 | −.467* | .513* | .653* | −.569* | .803* | |||
| 10 | Learning Rate_T1 | −.040 | −.167 | −.200 | .285 | −.208 | −.313 | −.425* | −.208 | .083 | ||
| 11 | Learning Rate_T2 | .094 | .034 | −.109 | .220 | .127 | −.008 | .069 | −.422* | −.388* | .233 | |
| 12 | △Learning Rate | .109 | .159 | .067 | −.042 | .269 | .240 | .391* | −.185 | −.386* | −.594* | .644* |
Note. T1 = pre-vaccine; T2 = post-vaccine; IL-6 = interleukin-6 (log transformed); △ = change (calculated as post-vaccine minus pre-vaccine). Reward responsiveness refers to total response bias scores. Learning rate () and reward sensitivity (logβ) coefficients were derived from computational modeling of trial-level responses (Huys et al., 2013).
4. Discussion
This study examined the association between inflammation and reward processing using influenza vaccination as a mild inflammatory stimulus. We focused on three key dimensions of reward processing: reward motivation, reward learning, and reward sensitivity. Consistent with hypotheses, we found that larger increases in circulating concentrations of the pro-inflammatory cytokine IL-6 following vaccination were associated with lower reward motivation. Contrary to hypotheses, we found that greater increases in IL-6 were associated with increased reward responsiveness on the reward learning task; however, computational analyses were not able to clarify whether this was attributable to effects of learning rate or reward sensitivity, possibly due to the sample size.
Past literature has linked inflammation to reward motivation, particularly in animal models. Further, inflammation alters dopaminergic function and is associated with activity in neural areas associated with reward (Capuron et al., 2012; Eisenberger et al., 2012; Felger and Treadway, 2017). Elevated inflammation may signal an organism to prioritize resources toward the facilitation of healing (Dantzer et al., 2008) and, in turn, shift priorities away from physical mobilization to attain reward. Consistent with this perspective, the current study found an association between increased inflammation and lower reward motivation; participants with a larger IL-6 response from pre- to 1 day post influenza vaccination selected fewer hard trial choices on the EEfRT. A similar pattern was evident in Draper et al. (2017), who, using a far more potent inflammatory challenge, found lower acceptance of high effort trials following endotoxin administration. We found no evidence that the context of the task moderated the association between increased IL-6 and reward motivation, in contrast to results of Lasselin et al. (2016). Specifically, their results suggested that higher levels of inflammation shifted priorities toward reward that was more likely to be attained (i.e., higher probability reward) rather than causing a global reduction in reward motivation. This shift was attributable to symptoms of sleepiness following endotoxin. It is possible that the levels of inflammation induced in the current study, which were not associated with notable sickness symptoms (Kuhlman et al., 2018), were not sufficient to elicit such a shift. Furthermore, our modifications to the EEfRT (specifically, instructing participants to use their dominant hand for hard trials) may have rendered hard trials less physically effortful. Thus, while these three studies converge in terms of linking inflammation to reward motivation, additional work is needed to clarify the relationship.
Consistent with both Draper et al., (2017) and Lasselin et al., (2016), there was little evidence that reward sensitivity on the EEfRT was altered in tandem with changes in inflammation. Specifically, the relationship between reward magnitude and reward motivation was not attenuated with greater increases in inflammation. However, it should be noted that this index of reward sensitivity entails a cost-benefit analysis (i.e., the tradeoff between anticipated effort and potential reward) and captures cognitive valuation of potential monetary reward. This may be distinct from actual receipt of monetary reward, rewards that elicit an automatic response (e.g., primary rewards like food or water) or rewards that are not in the context of a motivational task (e.g., viewing positive images). Indeed, there is some work suggesting that the neural response to monetary reward versus positive images may be differentially altered in the context of depression (Smoski, Rittenberg, & Dichter, 2011), and the effects of inflammation on social reward may be similarly nuanced (Irwin & Eisenberger, 2017).
Contrary to hypotheses, we found that greater increases in IL-6 were associated with increased reward responsiveness (encompassing both learning rate and sensitivity) on the PRT. This was surprising, as both stress and depressive symptoms are associated with blunted performance on this task (Bogdan and Pizzagalli, 2006; Pizzagalli et al., 2005; Pizzagalli et al., 2008b), and we expected that increases in inflammation would act similarly, perhaps indicating withdrawal from environmental cues. For example, Harrison and colleagues (2015) found that inflammation reduced prediction-error signaling in the ventral striatum during an instrumental learning task. However, unlike the current study, their task assessed explicit learning and included punishment learning cues. While in need of greater study, these methodological differences may reflect meaningful differences in behavioral sensitivity to inflammation. For example, explicit learning requires greater cognitive resources than implicit learning, and the presence of punishment may obfuscate or moderate effects of inflammation on reward learning.
It is also important to note that our finding for a facilitative effect of inflammation on reward responsiveness is not without precedent. Several studies have found improvements in some measures of cognition following an inflammatory stimulus (e.g., Grigoleti et al., 2011), and it is conceivable that greater awareness of environmental cues is adaptive in the context of threat or illness. Furthermore, there is increasing evidence that inflammation can increase reward sensitivity (Vichaya et al., 2014) although this may vary by context (e.g., Lasselin et al., 2016; Inagaki et al., 2015; Muscatell et al. 2016). For example, an inflammatory stimulus has been shown to elicit greater neural sensitivity to rewarding social stimuli, including images of loved ones (Inagaki et al., 2015). More studies testing the dimensions and contextual factors that shape how inflammation may alter reward are clearly warranted.
The results of this study need replication and should be interpreted in light of several limitations. Most notably, the lack of a placebo or wait-list control group precludes establishing a causal relationship between the vaccine and change in IL-6, and between change in IL-6 and performance on the reward tasks. It is important to note, however, that our hypotheses centered on within-person change (Kuhlman et al., 2018) based on prior work demonstrating within-person associations between changes in IL-6 following typhoid vaccination and changes in negative mood (Wright et al., 2005), fatigue and mental confusion (Brydon et al., 2008) and reinforcement learning (Harrison et al., 2015; Treadway et al., 2017). While relatively small, our sample size was comparable to previous studies investigating affective, cognitive, or behavioral changes following the typhoid vaccine (Harrison et al., 2015) or interferon alpha therapy (Dowell et al., 2016). Our sample consisted of healthy young adults, and the extent to which these results generalize to other populations remains to be established. There was variability in time between blood draws, and while we based our assessment schedule on previous research showing increases in IL-6 at 1-day post-influenza vaccine (Carty et al., 2006; Tsai et al., 2005), it is possible that the peak response for some individuals may have occurred later (e.g., Christian et al., 2011). Although we made modifications to the reward tasks, past studies have shown both tasks to be robust to these types of modifications (Damiano et al., 2012; Pechtel et al., 2013). Finally, it would have been ideal to use immediate monetary reward, rather than raffle tickets, and to counterbalance task order to address potential habituation effects.
Reward processing is a critical organizer of behavior involving the willingness to work for reward, the hedonic/consummatory response to reward, and reward learning (Schultz, 2015), all of which may be dysregulated in the context of depression (Keedwell et al., 2005; Pechtel et al., 2013; Treadway et al., 2012). Increasing evidence, particularly in the neuroimaging literature, suggests that inflammation plays an important role in the dysregulation of reward-related processing (Capuron et al., 2012; Eisenberger et al., 2010b; Felger et al., 2015), and the current study extends this literature by assessing multiple domains of reward behaviorally. Our results indicate that even very mild increases in inflammation are associated with alterations in dimensions of reward processing. This line of research is an important step towards the development of more targeted and effective treatments for depression and may also be relevant for other clinical conditions characterized by impaired reward processing, such as Alzheimer’s disease, schizophrenia, and post-traumatic stress disorder.
Supplementary Material
Highlights.
Mild increases in IL-6 following influenza vaccination were associated with alterations in behavioral measures of reward processing.
Increases in IL-6 were associated with lower reward motivation, but not lower reward sensitivity for monetary reward.
Increases in IL-6 were associated with increased performance on an implicit reward learning task.
Acknowledgments
This study was supported by the George F. Solomon Endowed Term Chair in Psychobiology, Cousins Center for Psychoneuroimmunology, UCLA Semel Institute. Supervision of this study was supported by a postdoctoral fellowship to Kate R. Kuhlman (T32MH015750). Chloe C. Boyle was supported by the NIH/NIMH Predoctoral Fellowship 5T32MH015750–35 (2014–2016) and the Cousins Center for Psychoneuroimmunology. Dr. Pizzagalli was partially supported by R37 MH068376. Dr. Bower was partially supported by R01 CA160427. Data from this study were previously presented at the Psychoneuroimmunology Research Society meeting (2017, June) and the Society of Affective Sciences (2017, April).
Footnotes
Declarations of interest: Over the past 3 years, Dr. Pizzagalli has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Posit Science and Takeda Pharmaceuticals, for activities unrelated to the current research. Dr. Pizzagalli has a financial interest in BlackThorn Therapeutics, which has licensed the copyright to the Probabilistic Reward Task through Harvard University. Dr. Pizzagalli’s interests were reviewed and are managed by McLean Hospital and Partners HealthCare in accordance with their conflict of interest policies. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. All other authors report no biomedical financial interests.
This modification was made to facilitate comparison with an unrelated study conducted by our group in which participants were expected to have an indwelling catheter in their dominant arm, which may have rendered “easy trials” less attractive.
Raw IL-6 values are presented for descriptive statistics; log-transformed IL-6 was used for inferential statistics. Note that one post-vaccine IL-6 value was winsorized from 6.31 to 4.82 pg/mL.
Results for the EEfRT were similar using other analytic approaches (e.g., controlling for baseline levels of IL-6, modeling IL-6 as a time-varying covariate) and are available in Supplemental Tables 6a-c and Table 10/figure 1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bogdan R, Pizzagalli DA, 2006. Acute stress reduces reward responsiveness: Implications for depression. Biological Psychiatry 60, 1147–1154. doi: 10.1016/j.biopsych.2006.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA, Pizzagalli DA, 2008. The heritability of hedonic capacity and perceived stress: A twin study evaluation of candidate depressive phenotypes. Psychological Medicine 39, 211–218. doi: 10.1017/S0033291708003619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Niño D, … & Belmont JW 2011. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. The Journal of Infectious Diseases, 203, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD, 2008. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological Psychiatry 63, 1022–1029. doi: 10.1016/j.biopsych.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH, 2012. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 69, 1044. doi: 10.1001/archgenpsychiatry.2011.2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty CL, Heagerty P, Nakayama K, McClung EC, Lewis J, Lum D, Boespflug E, McCloud-Gehring C, Soleimani BR, Ranchalis J, J Bacus T, E Furlong C, Jarvik GP, 2006. Inflammatory response after influenza vaccination in men with and without carotid artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology 26, 2738–2744. doi: 10.1161/01.ATV.0000248534.30057.b5 [DOI] [PubMed] [Google Scholar]
- Christian LM, Porter K, Karlsson E, Schultz-Cherry S, Iams JD, 2013. Serum proinflammatory cytokine responses to influenza virus vaccine among women during pregnancy versus non-pregnancy. Am J Reprod Immunol 70, 45–53. doi: 10.1111/aji.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour HJ, 2016. Treatment for anhedonia: A neuroscience driven approach. Depression and Anxiety 33, 927–938. doi: 10.1002/da.22490 [DOI] [PubMed] [Google Scholar]
- Damiano CR, Aloi J, Treadway M, Bodfish JW, Dichter GS, 2012. Adults with autism spectrum disorders exhibit decreased sensitivity to reward parameters when making effort-based decisions. J Neurodev Disord 4, 13. doi: 10.1186/1866-1955-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci 9, 46–56. doi : 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A, 2012. The neurobiology of anhedonia and other reward-related deficits. Trends in Neurosciences 35, 68–77. doi: 10.1016/j.tins.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, D’souza MS, Pizzagalli DA, & Markou A, 2013. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: Implications for cross-species translational research. Translational Psychiatry, 3(8), e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley LN, Kuhlman KR, Robles TF, Eisenberger NI, Craske MG, & Bower JE 2018. The role of inflammation in core features of depression: Insights from paradigms using exogenously-induced inflammation. Neuroscience and Biobehavioral Reviews, 94, 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell NG, Cooper EA, Tibble J, Voon V, Critchley HD, Cercignani M, Harrison NA, 2016. Acute changes in striatal microstructure predict the development of interferon-alpha induced fatigue. Biological Psychiatry 79, 320–328. doi: 10.1016/j.biopsych.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper A, Koch RM, van der Meer JW, Apps M, Pickkers P, Husain M, van der Schaaf ME, 2017. Effort but not reward sensitivity is altered by acute sickness induced by experimental endotoxemia in humans. Neuropsychopharmacology 26, 1–39. doi: 10.1038/npp.2017.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR, 2010a. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior, and Immunity 24, 558–563. doi: 10.1016/j.bbi.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Irwin MR, Inagaki TK, Berkman ET, Rameson LT, Mashal NM, 2010b. Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to rew. Biological Psychiatry 68, 748–754. doi: 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Rameson LT, Inagaki TK, Mashal NM, Irwin MR, 2009. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. NeuroImage 47, 881–890. doi : 10.1016/j.neuroimage.2009.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2015. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry 21, 1358–1365. doi: 10.1038/mp.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Treadway MT, 2017. Inflammation effects on motivation and motor activity: Role of dopamine. Neuropsychopharmacology 42, 216–241. doi: 10.1038/npp.2016.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher K, Parker G, Paterson A, Fava M, Iosifescu D, & Pizzagalli DA, 2015. Anhedonia in melancholic and non-melancholic depressive disorders. Journal of Affective Disorders 184, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geaney JT, Treadway MT, Smillie LD, 2015. Trait anticipatory pleasure predicts effort expenditure for reward. PLoS ONE 10, e0131357. doi: 10.1371/journal.pone.0131357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Klein DN, 2014. A review of selected candidate endophenotypes for depression. Clinical Psychology Review 34, 417–427. doi: 10.1016/j.cpr.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoleit J-S, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M, 2011. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS ONE 6, e28330–10. doi: 10.1371/journal.pone.0028330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE, 2009. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Social Science & Medicine 69, 451–459. doi: 10.1016/j.socscimed.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, 2005. Stress and depression. Annu. Rev. Clin. Psychol. 1, 293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938 [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD, 2009. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry 66, 407–414. doi: 10.1016/j.biopsych.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD, 2016. A Neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biological Psychiatry 80, 73–81. doi: 10.1016/j.biopsych.2015.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS, 2004. Discovering endophenotypes for major depression. Neuropsychopharmacology 29, 1765–1781. doi: 10.1038/sj.npp.1300506 [DOI] [PubMed] [Google Scholar]
- Huys QJ, Pizzagalli DA, Bogdan R, Dayan P, 2013. Mapping anhedonia onto reinforcement learning: A behavioural meta-analysis. Biology of Mood & Anxiety Disorders. doi: 10.1186/2045-5380-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Treadway MT, Wooten DW, Kumar P, Goer F, Murray L, … & Alpert NM, 2017. Frontostriatal and dopamine markers of individual differences in reinforcement learning: A multi-modal investigation. Cerebral Cortex, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Williams SCR, Brammer MJ, Brammer MJ, Phillips ML, Phillips ML, 2005. The neural correlates of anhedonia in Major Depressive Disorder. Biological Psychiatry 58, 843–853. doi: 10.1016/j.biopsych.2005.05.019 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U, 2012. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 21, 169–184. doi: 10.1002/mpr.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Robles TF, Dooley LN, Boyle CC, Haydon MD, Bower JE, 2018. Within-subject associations between inflammation and features of depression: Using the flu vaccine as a mild inflammatory stimulus, Brain, Behavior, and Immunity, doi: 10.1016/j.bbi.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Koo JW, Duman RS, Duman RS, 2008. IL-1 is an essential mediator of the antineurogenic and anhedonic effects of stress. Proceedings of the National Academy of Sciences 105, 751–756. doi: 10.1073/pnas.0708092105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza La, De, R., II, 2005 Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neuroscience & Biobehavioral Reviews 29, 761–770. doi : 10.1016/j.neubiorev.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Lasselin J, Treadway MT, Lacourt TE, Soop A, Olsson MJ, Karshikoff B, Paues-Göranson S, Axelsson J, Dantzer R, Lekander M, 2016. Lipopolysaccharide Alters motivated behavior in a monetary reward task: A randomized trial. Neuropsychopharmacology, 1–10. doi: 10.1038/npp.2016.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Roiser JP, Wang LZ, Zhu YH, Huang J, Neumann DL, Shum DHK, Cheung EFC, Chan RCK, 2016. Anhedonia is associated with blunted reward sensitivity in first-degree relatives of patients with major depression. Journal of Affective Disorders 190, 640–648. doi: 10.1016/j.jad.2015.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, Irwin MR, Eisenberger NI, 2015. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain, Behavior, and Immunity 43, 46–53. doi: 10.1016/j.bbi.2014.06.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA, 2013. Blunted reward responsiveness in remitted depression. Journal of Psychiatric Research 47, 1864–1869. doi: 10.1016/j.jpsychires.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, 2014. Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 10, 393–423. doi: 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, & Culhane M, 2008a. Single dose of a dopamine agonist impairs reinforcement learning in humans: Behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology. 196(2), 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, & Fava M, 2008b. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. Journal of Psychiatric Research, 43(1), 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP, 2005. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological Psychiatry 57, 319–327. doi: 10.1016/j.biopsych.2004.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, 2014. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosomatic medicine 76, 181–189. doi: 10.1097/PSY.0000000000000049 [DOI] [PubMed] [Google Scholar]
- Schultz W, 2015. Neuronal reward and decision signals: From theories to data. Physiol Rev 95, 853–951. doi: 10.1152/physrev.00023.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Muscatell KA, Monroe SM, Gotlib IH, 2009. Stressful life events, chronic difficulties, and the symptoms of clinical depression. The Journal of Nervous and Mental Disease 197, 154–160. doi: 10.1097/NMD.0b013e318199f77b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen KR, 2015. Measuring anhedonia: Impaired ability to pursue, experience, and learn about reward. Frontiers in Psychology 6, 1639. doi: 10.3389/fpsyg.2015.01409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH, 2012. Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. Journal of Abnormal Psychology 121, 553–558. doi: 10.1037/a0028813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH, 2009. Worth the “EEfRT?” The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS ONE 4, e6598. doi: 10.1371/journal.pone.0006598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH, 2013. Parsing anhedonia: Translational models of reward-processing deficits in psychopathology. Current Directions in Psychological Science 22, 244–249. doi: 10.1177/0963721412474460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Hanson NQ, Straka RJ, Hoke TR, Ordovas JM, Peacock JM, Arends VL, Arnett DK, 2005. Effect of influenza vaccine on markers of inflammation and lipid profile. Journal of Laboratory and Clinical Medicine 145, 323–327. doi: 10.1016/j.lab.2005.03.009 [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B 1999. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. Journal of Clinical Child Psychology 28, 366–75 [DOI] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL, 2013. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. Journal of Affective Disorders 150, 736–744. doi: 10.1016/j.jad.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Van Heesch F, Prins J, Konsman JP, Westphal KGC, Olivier B, Kraneveld AD, Korte SM, 2013. Lipopolysaccharide-induced anhedonia is abolished in male serotonin transporter knockout rats: An intracranial self-stimulation study. Brain, Behavior, and Immunity 29, 98–103. doi: 10.1016/j.bbi.2012.12.013 [DOI] [PubMed] [Google Scholar]
- Vichaya EG, Hunt SC, & Dantzer R, 2014. Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology. 39(12), 2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, … & Claes, S., 2013. Reduced reward learning predicts outcome in major depressive disorder. Biological Psychiatry. 73(7), 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A, 2005. Acute inflammation and negative mood: Mediation by cytokine activation. Brain, Behavior, and Immunity 19, 345–350. doi: 10.1016/j.bbi.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmächer T, 2000. Illness, cytokines, and depression. Annals of the New York Academy of Sciences 917, 478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


