Abstract
Staphylococcus aureus (S. aureus) is a notorious pathogen and one of the most frequent causes of biofilm-related infections. The treatment of S. aureus biofilms is hampered by the ability of the biofilm structure to shield bacteria from antibiotics as well as the host’s immune system. Therefore, new preventive and/or therapeutic interventions, including the use of antibody-based approaches, are urgently required. In this review, we describe the mechanisms by which anti-S. aureus antibodies can help in combatting biofilms, including an up-to-date overview of monoclonal antibodies currently in clinical trials. Moreover, we highlight ongoing efforts in passive vaccination against S. aureus biofilm infections, with special emphasis on promising targets, and finally indicate the direction into which future research could be heading.
Keywords: Staphylococcus aureus, infection, biofilm, monoclonal antibodies, vaccine, passive immunization
1. Clinical significance of S. aureus biofilm-associated infections
The Gram-positive pathobiont Staphylococcus aureus (S. aureus) is one of the most frequent causes of nosocomial infections, and there is no vaccine available yet. S. aureus infections are highly diverse, ranging from acute diseases, such as bacteremia and skin abscesses to severe chronic infections that are often associated with biofilms [1]. Due to an arsenal of adhesins (see Glossary), S. aureus can attach to and persist on host tissues (e.g. heart valves and bones) as well as implanted materials (e.g. catheters, prosthetic joints and pace makers), and cause diseases such as endocarditis, and osteomyelitis [1–3]. On the other hand, about 20% of the human population is persistently colonized in the anterior nares and other body sites such as the intestine, while the remainder carry the bacteria intermittently [4]. In most cases, colonization is asymptomatic, but it can also lead to endogenous infections [5].
Over the past decades, the steady increase in the use of medical implants has been accompanied by a rise in infection risk. Indeed, implant or device-associated infections are important complications associated with the use of biomaterials [2,6], and account for one quarter of all healthcare-associated infections in the USA [7]. Among their deleterious consequences are failure of prosthetic devices, implant replacement with its associated risk of clinical complications, and chronic and/or relapsing diseases [2,8]. Staphylococci, including S. aureus, S. epidermidis (Box 1) and other coagulase-negative staphylococci (CoNS) are the main culprits of foreign body-associated infections, accounting together for an estimated 80% of all infections [2,9]. The diagnosis and targeted therapy of implant infections is often problematic, because they are frequently subclinical and culture-negative.
Box 1: Antibody-based therapies against S. epidermidis biofilm-associated infections.
S. epidermidis is the most frequent cause of device-related infections, with biofilm formation as the major virulence factor [2,9,121]. Comparable to S. aureus, targeting S. epidermidis biofilm-associated infections can be achieved either by preventing bacterial attachment to implants, or by blocking cell-to-cell adhesion during biofilm maturation. However, unlike S. aureus, S. epidermidis biofilm formation relies mainly on exopolysaccharides rather than proteins [20,122].
The two major biofilm matrix constituents, PNAG and Aap, have been targeted by monoclonal antibodies in order to prevent biofilm formation. Anti-PNAG antibodies inhibited biofilm formation in vitro and were protective in a rabbit endocarditis model [123]. However, the inhibitory effect on static biofilm formation seems to be strain-dependent [124]. Apparently, PNAG as a biofilm matrix constituent hinders antibody binding close to the bacterial cell surface, which is needed for efficient opsonic killing [45]. The surface-protein Aap promotes cell-to-cell adhesion within a biofilm. Monoclonal antibodies against Aap reduced S. epidermidis biofilm formation in vitro, but neither enhanced opsonophagocytosis nor protected mice in an experimental biomaterial-associated infection [110,125]. The lack of protection might result from shedded Aap, acting as a decoy for anti-Aap antibodies [125].
The anti-LTA monoclonal antibody Pagibaximab was designed primarily for the treatment of S. epidermidis biofilm-associated sepsis, which occurs particularly often in neonates [126,127]. After encouraging results in animals and a more limited phase II study in very low birth weight neonates, a larger phase III study in a similar patient cohort failed to show a reduction in staphylococcal sepsis (Table 2) [108].
As there are no toxins in S. epidermidis other than PSMs [128], anti-toxin monoclonal antibody development for the treatment of S. epidermidis catheter-related bacteremia has been limited to those peptides. This approach is however problematic due to the diversity and high production of PSMs. Although anti-PSMβ polyclonal antibodies showed some success in limiting the dissemination of S. epidermidis biofilm-associated infection in mice [33], an octavalent antigen mixture containing four α-type PSMs, despite their immunogenicity, did not protect against S. aureus bacteremia [129], dampening the enthusiasm for passive PSM-targeted vaccination approaches.
A more suitable candidate might be the surface protein SesC, which is expressed in biofilm-associated as well as planktonic cells. Polyclonal rabbit sera against SesC partially prevented in vitro biofilm formation by S. epidermidis and dissolved established biofilms [130]. A similar reduction in biofilm formation was observed with polyclonal anti-SesC antibodies in a mouse model of catheter-related infections [131].
Biofilm formation is an important virulence mechanism of many bacterial pathogens. A biofilm is defined as a sessile microbial community embedded within an amorphous slimy material [2]. Biofilm formation enables growth on natural and foreign surfaces, and shields bacteria from antibacterial therapies as well as the host immune system, often leading to persistent infections unresponsive to antibiotic therapy [2]. In addition to the matrix representing a penetration barrier for many antimicrobial agents, the efficacy of most antibiotics is reduced against biofilms, because cells in a biofilm are in a state of reduced metabolism [10,11], whereas most antibiotics target active cell processes, such as cell wall formation, translation or transcription [12]. Consequently, there is an immense medical need to develop innovative preventive and/or therapeutic interventions, including anti-infective biomaterials, biofilm-active antibiotics, and biofilm matrix-degrading enzymes [13]. Another appealing measure to prevent biofilm formation and/or treat established biofilms is the use of monoclonal antibodies targeting the invasive pathogen, which is the focus of this review. After describing aspects of S. aureus biofilm formation, the antibacterial antibody response in S. aureus biofilm infections as well as techniques to generate monoclonal antibodies, we provide an update on preclinical as well as clinical studies on monoclonal antibodies against S. aureus biofilm-associated infections and outline critical aspects for the development of a successful anti-biofilm vaccine.
2. Staphylococcal biofilm stages and composition
In order to develop protective antibody-based therapies, it is essential to gain an in-depth understanding of the process of biofilm formation as well as its composition, since gene and protein expression differ greatly between the planktonic and biofilm modes of bacterial growth [14,15]. In the past, proteomic analyses were usually based on examining intracellular proteomes of laboratory isolates in static biofilms. However, more recent studies used flow chamber systems or even analyzed biofilms from animal infection models, which may reflect the human clinical situation more closely and hence reveal novel biofilm-associated targets [14,16–19].
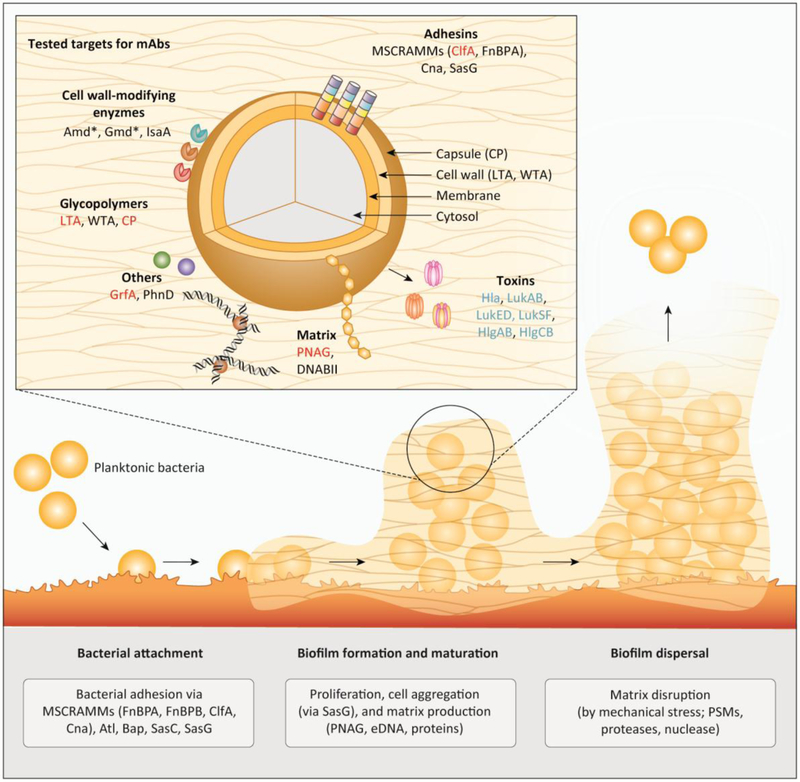
Biofilm formation in staphylococci has been described as a process comprising at least three main stages: (i) bacterial attachment to a surface, (ii) biofilm formation and maturation, and (iii) biofilm detachment / dispersal (Figure 1, Key Figure) [20]. Staphylococcal agglomerations that are not attached to a surface are also occasionally regarded as biofilms [21]; in those cases, intercellular aggregation substitutes for the initial adhesion step.
Figure 1: Overview on tested targets for an antibody-based preventive or therapeutic strategy against biofilm-associated S. aureus infections.
Main figure: Biofilm formation in staphylococci comprising three main stages: bacterial attachment to a surface, biofilm formation and maturation, and biofilm detachment / dispersal. For the attachment to (a)biotic surfaces, S. aureus relies on a broad spectrum of functionally redundant adhesins such as the MSCRAMMs (ClfA, Cna, FnbA, FnbB). After successful adhesion, bacteria start proliferation and production of the biofilm matrix consisting of eDNA, stabilized by DNABII, PNAG and proteins. Eventually, biofilm dispersal is mediated by mechanical shear stress (e.g. in a blood vessel) or by dispersion factors like PSMs, nuclease, and proteases. Insert: Molecular targets for antibody based therapies tested in preclinical in clinical studies include adhesins and cell-wall modifying enzymes and other cell wall-attached proteins, surface glycopolymers, biofilm matrix components, as well as toxins and immune evasion proteins. Targets from preclinical studies, ongoing clinical trials and failed clinical trials are shown in black, blue and red, respectively. The asterisk indicates that the S. aureus protein autolysin (Atl) is proteolytically processed into two enzymes, autolysin amidase (Amd) and autolysin glucosaminidase (Gmd), which stay non-covalently attached to the cell surface.
Abbreviations: Atl (autolysin); Amd (autolysin amidase); Bap (biofilm-associated protein ); ClfA (clumping factor A); Cna (collagen-binding protein); CP (capsular polysaccharides); DNABII (DNABII family proteins); eDNA (extracellular DNA); FnBPA/FnBPB (fibronectin-binding protein A and B); Gmd (autolysin glucosaminidase); GrfA (ABC transporter); Hla (α-toxin); Hlg (γ-haemolysin); IsaA (Immunodominant staphylococcal antigen A); LTA (lipoteichoic acid); Luk (Leukotoxins); mAb (monoclonal antibody); MSCRAMM (microbial surface components recognizing adhesive matrix molecule); PhnD (subunit of alkylphosphonate ABC transporter); PNAG (poly-N-acetyl-ß-(1,6)-glucosamine); PSMs (phenol soluble modulins); Sasc/G (S. aureus surface protein C and G); WTA (wall teichoic acid).
Attachment of bacteria to abiotic plastic surfaces of indwelling medical devices may happen via hydrophobic attraction. However, soon after insertion, human matrix proteins cover the device surfaces, and thus, initial attachment in vivo proceeds mainly via the interaction of staphylococcal surface binding proteins with human extracellular matrix [20]. Many of the former belong to the “microbial surface components recognizing adhesive matrix molecule” (MSCRAMM) family [22]. MSCRAMMs (discussed in detail in Chapter 5) are anchored to the cell wall via the enzyme sortase and contain cell wall-spanning domains that end with an exposed domain binding to human matrix proteins (Figure 1). Overall, there is pronounced redundancy among the MSCRAMMs, reflecting their key role in bacterial colonization and survival in the host [22].
The biofilm formation / maturation phase, in addition to bacterial growth, is characterized by the secretion of biofilm matrix components and the creation of a three-dimensional biofilm structure. The composition of biofilm matrix is heterogeneous, comprising proteins, extracellular DNA (eDNA) and polysaccharides (discussed in detail in Chapter 5). Several secreted proteins have been implicated in biofilm formation, many of them are surface binding proteins, whose contribution to the initial adhesion versus subsequent phases of biofilm development is often hard to discern. In contrast, the S. epidermidis accumulation-associated protein Aap and its S. aureus homologue SasG, appear to have a very specific biofilm matrix function, forming polymeric fibrils that link together cells in a biofilm [23–25].
Another biofilm-characteristic component is the cell surface associated exopolysaccharide PNAG (ß-1,6-poly-N-acetylglucosamine, also called polysaccharide intercellular adhesin, PIA) [26]. PNAG is not omnipresent in staphylococcal biofilm-forming isolates [27], but its production supports cell to cell adhesion, leading to more robust biofilms [28,29]. PNAG’s cationic nature facilitates bacterial attachment to host cell surfaces [30], which is possibly mediated by negatively charged molecules such as teichoic acids and eDNA that is released by dying cells.
Biofilms do not grow as undifferentiated “bricks”, but contain channels that are deemed important for nutrient delivery to all layers of a biofilm. Enzymatic digestion of biofilm matrix molecules, such as eDNA and proteins by nucleases and proteases, respectively, has been implicated in channel formation [1]. However, no enzyme capable of degrading PNAG has so far been identified in staphylococci. Moreover, phenol-soluble modulins (PSMs) are amphipathic and surfactant-like peptides that structure biofilms independently of biofilm matrix composition, most likely by disrupting hydrophobic as well as hydrophilic interactions between biofilm matrix molecules [31,32].
Detachment of cells or cell clusters from a biofilm can be triggered solely by mechanical shear forces as encountered in the blood stream. However, this process, which is crucial for the systemic dissemination of a biofilm-associated infection, can also be facilitated by pronounced activity of biofilm-structuring factors. For instance, PSMs disrupt interactions of biofilm matrix molecules, such as PNAG, with each other in vivo, contributing to biofilm dispersal [31,33].
3. Antibacterial antibody response in S. aureus biofilm infections
How the innate and adaptive immune systems react to biofilms and what type of immune response is protective is still not well understood. Biofilm infections trigger an inflammatory response, as reflected by the infiltration and activation of phagocytes at the site of infection, and the release pro-inflammatory cytokines, promoting a Th1/Th17 response and the production of antibodies, predominantly of the human IgG1 subclass [34–36]. While neutrophils are capable of infiltrating the biofilm and efficiently phagocytose enclosed cells, this defense mechanism is less effective in mature biofilms [37]. This exemplifies the inefficiency of the induced host response in clearing a persistent biofilm infection [37,38].
Similarly, the protective potential of antibodies in biofilm infections is not well defined. S. aureus infection stimulates the production of specific antibodies against a broad range of surface and secreted staphylococcal proteins, but these generally do not prevent a re-infection with this notorious pathogen [39]. However, antibody profiling in sepsis patients at the time of diagnosis showed that high antibody titers might confer protection from an adverse outcome [40]. This implies that the immunological “starting position” is important for disease outcome, a fact that is encouraging for efforts in vaccine development. For biofilm infections, clinical data are scarce, but suggest that biofilms also trigger or boost an antibody response against a broad range of S. aureus antigens: adhesins and cell wall-modifying enzymes, biofilm matrix components, toxins and immune evasion factors (discussed in detail in Chapter 5) [41–43].
In line with these patient data, animal experiments indicate that boosting the antibody response by active or passive vaccination prevents or at least reduces the severity of biofilm-associated S. aureus infections [18,35,44]. For example, in a murine model of mesh-associated biofilm infection, a vaccination approach using biofilm matrix exoproteins significantly reduced the number of bacterial cells inside a biofilm and on the surrounding tissue [18]. Another multivalent S. aureus vaccine comprising four cell wall-associated proteins prevented the formation of biofilm-mediated osteomyelitis in the majority of the treated animals when combined with an antimicrobial therapy [44]. Hence, animal data suggest that antibodies can contribute to biofilm prevention and clearance.
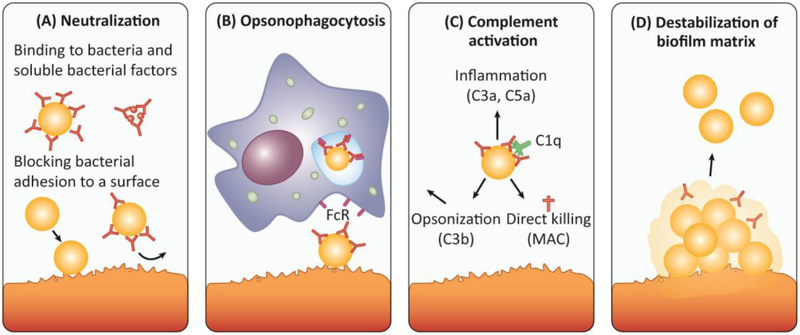
Anti- S. aureus antibodies can penetrate the biofilm matrix [45,46], and interfere with all three stages of biofilm formation. Initial attachment can be prevented by targeting surface-bound or soluble adhesins (Figure 2). Biofilm maturation is disturbed by blocking surface proteins involved in cell-to-cell adhesion, and biofilm dispersal is enhanced by targeting matrix-stabilizing proteins. Moreover, high affinity IgA and IgG antibodies can neutralize secreted bacterial factors (e.g. toxins, enzymes, immune evasion molecules). Finally, surface-bound antibodies can enhance biofilm elimination by neutrophils and macrophages, either via antibody-binding Fc receptors or by inducing complement activation and C3b deposition on the bacterial surface (Figure 2) [18,47,48]. In conclusion, antibodies can potentially interfere with biofilm formation and/or promote dispersal of established biofilms by several mechanisms. However, since the natural antibody response in many cases seems to be insufficient to eliminate established biofilms, boosting the antibody response by active or passive vaccination seems a promising approach to reduce the severity of biofilm-associated S. aureus infections.
Figure 2: Antibodies can interfere with biofilm formation and promote dispersal of established biofilms by several mechanisms.
A) Secreted staphlyococcoal proteins (e.g. immune evasion molecules, toxins, exoenzymes) as well as surface proteins are involved in biofilm development and are hence potential targets for therapeutic purposes. High affinity IgA and IgG antibodies can neutralize the action of bacterial toxins and surface proteins. Moreover, antibodies can bind to bacterial adhesins (e.g. ClfA, FnBPA) and cell wall components (e.g. PNAG), thereby blocking initial attachment to host matrices and subsequent initiation of biofilm formation. B) Surface-bound antibodies (most prominently IgG) can trigger the uptake and destruction by neutrophils and macrophages expressing Fc receptors (FcR) on their surface (opsonophagocytosis). Activation of neutrophils can also trigger granule release, oxidative burst and NETosis. C) Surface-bound antibodies (IgM and IgG) trigger complement activation via the classical pathway. Following binding of C1q to the surface-bound antibody, the complement cascade is initiated resulting in the formation of the C3 convertase, which cleaves the central component of all complement pathways, C3, into C3a and C3b. C3b acts as an opsonin, enabling phagocytes that express the C3b receptor to ingest C3b-coated bacteria more easily. The soluble C3a (as well as C5a) act as chemo-attractants that recruit immune cells to the site of infection causing inflammation. C3 activation also triggers the formation of the membrane attack complex (MAC) that generates lytic pores in certain pathogens. Gram-positive bacteria, including S. aureus, are protected from MAC-dependent lysis by their thick peptidoglycan layer [132]. D) Antibodies targeting different components of the biofilm matrix, e.g. DNABII, can destabilize a biofilm matrix and thereby promote bacterial dispersal and clearance by immune cells or antibiotics.
Abbreviations: FcR (Fc receptor); MAC (membrane attack complex).
4. Generation of monoclonal antibodies
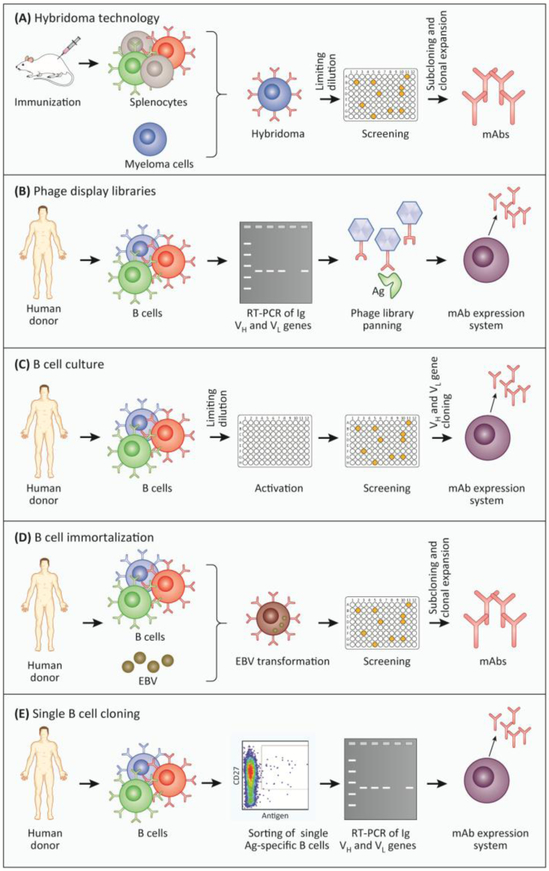
Monoclonal antibodies are superior to polyclonal sera in studying anti-biofilm activities, since they allow for molecular interaction studies and can potentially be applied in human patients. Over the past decade, technical advances have been made in the production and modification of monoclonal antibodies. Traditionally, monoclonal antibodies were generated using the hybridoma technology (Figure 3A) resulting in murine antibodies, which can, however, have severe side effects if introduced into the human host [49,50].
Figure 3: Methods commonly used for the generation of monoclonal antibodies (mAbs).
A) Hybridoma technology. Following immunization with an antigen, mice start producing large amounts of antigen-specific B cells. These cells are harvested from the spleen and fused with myeloma cells. The resulting hybridoma cells are screened for the secretion of antigen-specific antibodies. Antigen-specific hybridoma cells are selected by limiting dilution (subcloning) [3]. B) Phage display library. Initially, mRNA is isolated from B cells or plasma cells and then reverse-transcribed into cDNA. The variable light and heavy chains are amplified via PCR and ligated into a phage display vector. The resulting phage library consists of 108-1010 different phages, each encoding a single surface-expressed mAb, generated by random combination of heavy and light chains. The antigen is subsequently “displayed” to the phage library in successive rounds, to enrich antigen-specific phages (panning). The genes encoding the desired antigen-specific mAbs can then be cloned into an appropriate expression system for the generation of the mAbs of interest [1,2]. C) B cell culture. After isolation and limiting dilution, B cells are cultivated and activated in vitro leading to the secretion of antibodies. B cell culture supernatants are screened for antigen-specific antibodies, and positive cultures are used for the amplification of heavy and light Ig genes via PCR. The antibody sequence is finally cloned into an expression system to produce the mAbs [6]. D) EBV immortalization. Human B cells or plasma cells are isolated and immortalized using Epstein-Barr virus followed by subsequent single cell distribution. Supernatants of B cell cultures are screened for specific antigen binding and subsequently subcloned to produce mAbs [4]. E) Single B cell cloning. Human B cells are isolated and single antigen-specific B cells are sorted by fluorescence-activated cell sorting (FACS). The mRNA of those single cells is reverse-transcribed into cDNA followed by amplification of Ig heavy and light chains via PCR. The extracted antibody sequences can be cloned into a vector and ultimately introduced into an expression system. Finally, the resulting monoclonal antibodies are validated for their antigen-specificity [5].
Abbreviations: Ag (antigen); EBV (Epstein-Barr virus ); Ig (Immunoglobulin); mAb (monoclonal antibody).
Within less than a decade after the first monoclonal antibody was described, two approaches were developed to reduce antigenicity and enhance antibody-mediated effector functions. The recombinant attachment of a murine antigen-specific variable region to a human constant region resulted in the rise of chimeric antibodies [51]. Almost concurrently, the first humanized antibody was generated by transfering the murine complementarity-determining regions (CDRs) into a human antibody sequence. Especially the latter approach reduced the murine proportion and hence the immunogenicity of the antibody [52]. Both methods enabled the selection of the desired human constant region, which defines the antibody class and hence antibody-mediated effector functions. Nowadays it is possible to generate chimeric and humanized antibodies by combining the use of transgenic mice expressing chimeric or CDR-drafted antibodies with the hybridoma technology.
The four most common techniques for the generation of numerous fully human monoclonal antibodies are phage display libraries, B cell cultures, B cell immortalization using Epstein-Barr virus (EBV) as well as cloning of antibodies from single antigen-specific B cells (Figure 3B-E). While phage display libraries are generated by random combination of immunoglobulin heavy and light chains, the other three methods are based on antigen-specific B cells from exposed donors and thus reflect the physiological antibody response [53,54]. These methods can also be elegantly combined. For example, instable hybridomas and EBV-transformed cell lines can be rescued by cloning the antibody-coding sequences into an expression system.
Using antibody engineering, it is also possible to tailor the affinity and effector functions of antibodies to their application. For example, monoclonal antibodies can be genetically modified to increase antigen affinity and antibody half-life or to increase/decrease affinity towards Fc receptors [55,56]. Moreover, two antibodies can be combined to create bispecific antibodies by linking Fab fragments of two different specificities [57,58], or used as a shuttle to target antimicrobial drugs directly to the S. aureus cells [59–61]. This tool box for antibody engineering promises to be very helpful in designing protective anti-S. aureus monoclonal antibodies in the future.
5. Preclinical studies on antibodies targeting S. aureus biofilms
Despite the huge clinical impact of biofilm infections, research on antibodies targeting S. aureus infections often ignores biofilms during antigen selection and preclinical antibody testing. Nevertheless, there are several interesting S. aureus vaccine candidates with promising results in pre-clinical studies that we would like to highlight in this chapter, including adhesins, cell-wall modifying enzymes, surface glycopolymers, biofilm matrix components, and toxins (Figure 1).
Adhesins
Staphylococcal adhesins, including MSCRAMMs, are one of the most studied targets for antibody-based therapies [22]. Antibodies against adhesins exert their action via two mechanisms (Figure 2): (i) preventing the initial microbial adherence to abiotic as well as biotic surfaces [62–64], and (ii) coating the bacterial surface, thereby facilitating the clearance of an organism through opsonophagocytic bacterial killing [65,66]. In the context of biofilm vaccine development, interesting candidates are clumping factor (Clf) A and B, and the fibronectin-binding proteins (FnBPA and B) as these cell-wall associated proteins are involved in biofilm formation and are widely distributed among the S. aureus clinical isolates, while the collagen-binding protein (Cna), biofilm-associated protein (Bap), as well as the S. aureus surface protein C (SasC) and SasG are present only in a subset of isolates [67].
The MSCRAMM ClfA promotes bacterial binding to fibrinogen. ClfA plays an important role in the colonization of implanted biomaterials or damaged endothelial surfaces at the site of endovascular infections [22,68]. Over the past 15 years, several monoclonal antibodies against ClfA were shown to block biofilm formation in vitro (Table 1) [62,66,69,70]. In animal infection models anti-ClfA monoclonal antibodies protected from biofilm (e.g. IE) and non-biofilm-associated (e.g. sepsis) infections [69,70]. In contrast, an anti-ClfA monoclonal antibody alone had only a moderate effect in a murine hematogenous implant infection, but in combination with antibodies against alpha toxin (Hla) it effectively inhibited biofilm formation both in vitro and in vivo [62]. Moreover, a humanized anti-ClfA monoclonal antibody (tefibazumab) conferred full protection against infective endocarditis (IE) in rabbits when applied prophylactically [69].
Table 1:
Molecular targets for antibody-based therapies against S. aureus biofilms in pre-clinical studies.
| Targeta | In vitro anti-biofilm activitiesb | Protection in ex vivo / in vivo biofilm modelsc | Antibodyd | Reference(s) |
|---|---|---|---|---|
| Adhesins, including MSCRAMMs | ||||
| ClfA | block FBG binding / agglutination of human plasma; displace FBG-bound bacteria; promote OPK |
P: full - rabbit IE model T: partial (+vancomycin) - rabbit IE model |
Mu/mAb (MAb 12-9. 11H10), Huz/mAb (tefibazumab) | [69,70] |
| FnBPA | block FBN binding; promote OPK and nGr activation; reduce biofilm formation | Not tested | Mu/mAb | [63,64] |
| Cna | block CN binding; displace CN from bacterial surface; promote OPK; block laminin and C1q binding | P: strong - sepsis model (less animals developed arthritis) | Murine pAb, Mu/mAb | [133–135] |
| SasG | reduce biofilm formation | Not tested | Rabbit pAb | [136] |
| Cell wall-modifying enzymes | ||||
| Atl1 | inhibit biofilm formation; promote OPK | Not tested | Murine pAb | [71,72,137] https://www.ncbi.nlm.nih.gov/pubmed/27044299 |
| Atl-Amd | promote OPK | Not tested | Murine pAb | [72] |
| Atl-Gmd | promote OPK; block cell division (binary fission); induce agglutination | P: strong - murine model of implant-associated osteomyelitis | IgG1 Mu/mAb (1C11) | [73] |
| IsaA | promote nGr activation (ox. burst) and OPK by nGrs (UK-66); promote OPK In whole blood (hUK-66); promote nGr activation, but not phagocytosis (1D9) |
P: partial, strain-dependent (1D9) - murine bacteremia model T: no (1D9) - murine bacteremia model; partial (UK-66) - catheter-related infection model |
Mu/mAb (UK-66), Huz/mAb (hUK-66) Hu/mAb (1D9) | [138–140] |
| Glycopolymers | ||||
| WTA | promote C3 deposition and OPK by nGr (hu pAb) | P: no - murine bacteremia model (Hu/mAb) | Hu/mAb, IgG Hu/mAb (THIOMAB) | [61,75] |
| CP | promote OPK (mu/mAb) | P: partial – rat endocarditis model (Ra/pAb); no protection – rat endocarditis model (Mu/pAb) | Mu/mAb, Ra/pAb, Mu/pAb | [77–79] |
| LTA | promote OPK | Not published | murine/human chimeric mAb (Pagibaximab®) | [141] |
| Matrix components | ||||
| PNAG / dPNAG | promote OPK | P: partial - murine bacteremia model | IgG1 Hu/mAb (F598) | [29,65] |
| DNABII | disrupt established biofilms |
T: partial (+daptomycin) - murine tissue cage infection model T: partial (+vancomycin) - rat IE model |
native Hu/mAb (TRL1068) | [84,85] |
| Immune evasion proteins | ||||
| Spa | neutralize Fcγ and VH3+ Fab binding activities of Spa; promote OPK in mouse and human blood | P: strong - murine sepsis model with renal abscess formation | Mu/mAb against Spa toxoid (3F6) | [142] |
| Toxins | ||||
| Hla | neutralize toxin activity; modestly inhibit biofilm formation |
P: complete - ex vivo porcine vaginal mucosa explants P: partial - hematogenous orthopedic implant infection in mice (increased protection in combination with anti-ClfA 11H10 mAb) |
Hu/mAb (MEDI4893) | [62,91,92] |
| LukAB | neutralize LukAB-mediated cytotoxicity; inhibit LukAB binding to I domain of CD11b | P: partial - murine sepsis model (1:1 mixture of SA-15 and SA-17) | Hu/mAb (SA-13, -15 and -17) | [96] |
| Other proteins | ||||
| PhnD | inhibit biofilm formation under shear flow (S. aureus and S. epidermidis), promote OPK by nGr | Not tested | Ra/pAb | [143] |
Protein names: ClfA (clumping factor A); FnBP (fibronectin-binding protein); Cna (collagen-binding protein); SasG (S. aureus surface protein G); Atl (autolysin: bifunctional enzyme that undergoes proteolytic cleavage to yield two catalytically active proteins, an amidase (Amd) and a glucosaminidase (Gmd), which both are involved in peptidoglycan cleavage); IsaA (Immunodominant staphylococcal antigen A); WTA (wall teichoic acid); CP (capsular polysaccharides); LTA (lipo teichoic acid); PNAG/ dPNAG, PNAG (poly-N-acetyl-ß-(1,6)-glucosamine); dPNAG (deacetylated PNAG); DNABII (DNABII family proteins); Spa (staphylococcal protein A); Hla (α-toxin); LukAB (Leukotoxins A and B); PhnD (subunit of alkylphosphonate ABC transporter).
Footnotes:
FBG: fibrinogen; FBN: fibronectin; CN: collagen
P (prophylaxis); T (therapy); IE (infective endocarditis)
Abbreviations: mAb (monoclonal antibody); Hu/mAb (human mAb); Huz/mAb (humanized mAb); Mu/mAb (murine mAb); Ra/mAb (rabbit mAb); pAb (polyclonal antibodies); Hu/pAb (human pAb); Mu/pAb (murine pAb); Ra/pAb (rabbit pAb); IHF (integration host factor )
FnPBs recognize fibronectin, fibrinogen and elastin, and promote intercellular accumulation and biofilm development (Table 1) [22]. Antibodies against FnBP inhibit S. aureus biofilm formation in vitro and partially protected mice against endocarditis following sepsis [63,64].
Cell wall-modifying enzymes
The surface-associated murein hydrolase autolysin, Atl is a bifunctional enzyme that undergoes proteolytic cleavage to yield two cell wall-active enzymes, an amidase (Amd) and a glucosaminidase (Gmd). Both enyzmes are involved in bacterial cell separation after cell division, host extracellular matrix adhesion and biofilm formation (Table 1) [71]. Polyclonal antibodies to Amd and Gmd inhibit biofilm formation and enhance opsonophagocytosis [71,72]. In addition, a monoclonal antibody against Gmd (1C11) reduced infection severity in a murine model of implant-associated osteomyelitis [73].
Glycopolymers
Staphylococcal cells are decorated with glycopolymers, including wall teichoic acids (WTA), peptidoglycan, lipoteichoic acids (LTA), and capsular polysaccharides (CP). These surface glycopolymers are recognized by serum antibodies and a variety of pattern recognition molecules, including mannose-binding lectin. Anti-WTA antibodies facilitate complement C3 deposition via the classical pathway as well as opsonophagocytosis of laboratory and clinical S. aureus isolates by neutrophils (Table 1) [74,75]. Although a human monoclonal anti-WTA antibody was ineffective in preventing S. aureus infection in an intravenous mouse infection model, it showed promising in vivo results when conjugated to an antibiotic [61]. Further human monoclonal antibodies targeting WTA are currently characterized [76]. However, to the best of our knowledge, anti-WTA antibodies have never been tested in biofilm-related infection models. Antibodies against the capsular polysaccharides promote opsonophagocytosis but yielded contradictory results when tested in a rat endocarditis models. While rabbit polyclonal antibodies conferred partial protection, murine antibodies were not protective [77–79].
Biofilm matrix
The biofilm matrix has been recently brought into the focus of anti-biofilm vaccine research. This is in part due to the widely conserved nature of some of its components, making those components suitable conserved vaccine candidates for protection against various human pathogens.
PNAG has been extensively evaluated as a potential vaccine candidate in relation to biofilm-associated infections (Table 1). In contrast to many S. aureus-specific biofilm factors, it is expressed among a variety of bacteria, fungi and protozoa [80,81]. For instance, the immunological cross-reactivity of an opsonic antibody against S. aureus PNAG and Escherichia coli polyglucosamine has led scientists to investigate the possibility of developing a vaccine against both pathogens [80]. Several studies have highlighted the superiority of deacetylated PNAG (dPNAG) to PNAG in terms of immunogenicity and protection in animal models [80,82]. Anti-dPNAG immune sera provided efficient protection in a murine intraperitoneal [80,82], as well as a bacteremia model [82]. More interestingly, the human IgG1 monoclonal antibody F598 (which binds both PNAG and dPNAG) has opsonic and protective activities against multiple microbial pathogens in vivo [65,81] and is currently undergoing preclinical and clinical assessments as a broad-spectrum antimicrobial therapeutic [83].
Bacterial DNA-binding proteins (DNABII family) have conserved homologs in a wide variety of bacterial species and are involved in a number of biofilm-associated infections [84,85]. They serve as adapter proteins for eDNA strands and hence stabilize the biofilm matrix (Figure 1; Table 1) [86]. Loss of these scaffolding proteins, for instance by neutralization with specific antibodies, causes dispersal of the biofilm. The released bacteria regain their susceptibility to killing by antibiotics and are more easily cleared by phagocytes [86,87].
Recently, Estellés et al. generated a native human monoclonal antibody (TRL1068) recognizing a DNABII epitope conserved across a range of Gram-positive and Gram-negative bacterial species [84]. TRL1068 showed anti-biofilm efficacy in an in vitro biofilm assay as well as in a murine infectious implant model, and a catheter-related biofilm infection model in rats [84,85]. However, as this antibody promotes biofilm dispersal, it is essential to eliminate the released bacteria to prevent subsequent dissemination to distant organs. Therefore, TRL1068 was proposed as a clinical candidate for the treatment of implant-associated infections in combination with standard-of-care antibiotics (Table 1) [84,85].
Toxins
Proteomic studies demonstrated that several pore-forming toxins (e.g. Hla, LukAB, and γ hemolysin (HlgAB)), and immune evasion molecules (e.g. SCIN, and CHIPS) are produced within a biofilm in vitro and in vivo, some even in higher amounts than in planktonic cultures, whereas others, including the immune evasion protein A, are down-regulated [14,17,88]. The pore-forming toxins Hla, LukAB and HlgAB lyse a range of host immune cells, including T cells, monocytes and neutrophils [89], thereby torpedoing the anti-biofilm immune response. Neutralizing these toxins by monoclonal antibodies may enhance host defenses and facilitate clearance of planktonic and biofilm cells (Figure 1).
Apart from destroying immune cells, Hla promotes biofilm formation in vitro, as well as in vivo by disrupting the host epithelium, providing nutrients for bacterial survival through promoting host cell lysis, and facilitating bacterial cell-to-cell interactions [90,91]. The human monoclonal anti-AT neutralizing antibody (MEDI4893) sterically inhibits binding of Hla to its cellular receptor ADAM10, effectively blocking pore formation [92]. It successfully abrogated ex vivo biofilm formation on porcine vaginal mucosa explants [91]. Considering that prophylactic treatment with MEDI4893 in a mouse model of S. aureus wound infection also promotes wound healing [93], this suggests that neutralization of Hla may be useful in biofilm-related S. aureus wound infections. MEDI4893 has been extensively tested in various biofilm and non-biofilm infection models (Table 1) [62,91,93].
The pore-forming toxin leukocidin A/B (LukAB) kills professional phagocytic cells, and together with AT facilitates the persistence of staphylococcal biofilms [94]. Badarau et al. first reported on the discovery of a highly potent neutralizing human IgG1 monoclonal antibody against LukAB (ASN-2), with a high affinity antibody binding site on the LukAB dimer [95]. In 2017, Thomsen et al. reported on three potently neutralizing naturally occurring LukAB-specific human monoclonal antibodies, which reduced the bacterial load in murine sepsis model (Table 1) [96].
Quorum-sensing blocking monoclonal antibodies
Targeting quorum sensing, i.e. bacterial cell density-dependent gene regulation, is a frequently promoted antivirulence strategy [97]. In S. aureus and other staphylococci, the quorum-sensing system Agr controls virtually all known virulence factors, such as toxins and secreted degradative enzymes [98]. In an exceptionally strict fashion, Agr controls thePSMs [99], which – as previously mentioned – trigger biofilm structuring and detachment [100]. Owing to this control, interfering with Agr quorum sensing results in the formation of thick undifferentiated biofilms [101]. Another less well characterized potential quorum-sensing system, LuxS, controls exopolysaccharide synthesis in a negative fashion [102,103]. Thus, interfering with quorum-sensing in staphylococci by the use of monoclonal antibodies or any other means does not represent a promising/efficient anti-biofilm strategy.
In summary, several S. aureus vaccine candidates, including adhesins, cell-wall modifying enzymes, biofilm matrix components and toxins, showed promising results in pre-clinical studies. To combat biofilm-related infections, future vaccination studies should aim at identifying and testing bacterial target structures expressed by both planktonic and biofilm cells, for instance using proteomic approaches.
6. Clinical trials on antibodies targeting S. aureus
Several of the above described targets, including ClfA, CP5 and 8, PNAG, Hla and HlgAB (Table 2), have been tested as passive vaccines in clinical phase II and/or III trials. However, none of them improved the clinical outcome in the treated patient cohorts. For instance, the anti-ClfA monoclonal antibody tefibazumab failed to achieve statistically significant improvement of clinical outcome in bacteremia and cystic fibrosis patients [104]. Similarly, polyclonal antiserum against CP5 and CP8 (AltaStaph), as well as a monoclonal antibody against LTA (Pagibaximab) failed in phase II and III trials, respectively (Table 2, Box 1) [105–108]. Moreover, a phase IIa study on using an anti-PNAG monoclonal antibody in ventilated intensive care unit (ICU) patients was terminated.
Table 2:
Clinical trials involving therapeutic antibodies/antisera against S. aureus infections*
| Targeta | Name [Company; NCT numberb] |
Study Design | Status (study result) | Interventionc | Ref |
|---|---|---|---|---|---|
| Single component | |||||
| ClfA (adhesin) | Tefibazumab (Aurexis®) [Inhibitex] |
randomized, double-blind, placebo-controlled trial of bacteremia patients receiving standard antibiotic treatment plus Tefibazumab (N = 63) | Phase II (failed) | Huz/mAb (IgG1) | [104] |
| Tefibazumab (Aurexis®) [Inhibitex; NCT00198289] |
dose escalation study of Aurexis® in cystic fibrosis patients chronically colonized with S. aureus in their lung (N = 30) | Phase IIa (failed) | Huz/mAb (IgG1) | i | |
| CP 5 and CP8 (capsular polysaccharides) | AltaStaph™ [Nabi Biopharmaceuticals; NCT00063089] |
randomized, double-blind, placebo-controlled trial involving adult S. aureus bacteremia patients receiving standard treatment plus Altastaph (N = 40) | Phase II (halted) | polyclonal human IgG with high antibody titers against CP5 and CP8, purified from the plasma of healthy donors that have been vaccinated with StaphVAXd | [105] |
| AltaStaph™ [Nabi Biopharmaceuticals; NCT00066989] |
randomized, double-blind, placebo-controlled trial for prevention of nosocomial S. aureus infections in very low birth weight (VLBW) neonates (N = 206) | Phase II (failed) | same as above | [106] | |
| LTA (cell wall component) | Pagibaximab® [Biosynexus; NCT00631800] |
randomized, double-blind, placebo-controlled dose-ranging study on prevention of CoNS and S. aureus sepsis in VLBW neonates (N = 88) | Phase II (finished) | murine/human chimeric mAb | [127] |
| Pagibaximab® [Biosynexus; NCT00646399] |
randomized, double-blind, placebo-controlled study on prevention of staphylococcal sepsis in VLBW neonates (N = 1579) | Phase III (failed) | murine/human chimeric mAb | [108] | |
| WTA (wall teichoic acid) | DSTA4637S [Roche/Genentech; NCT03162250] |
randomized double-blind, placebo-controlled multiple-ascending dose study on safety, tolerability, and pharmacokinetics in S. aureus bacteremia (N = 24) | Phase lb (ongoing) | THIOMAB™ antibody (Hu/mAb; IgG1)-antibiotic conjugate | [114,115] |
| PNAG (cell wall component) | SAR279356 [Sanofi-Aventis; NCT01389700] |
randomized, double-blind, placebo-controlled study to assess a single dose of SAR279356 in ICU patients on mechanical ventilation (N = 7) | Phase IIa (terminated due to difficulty in patient recruitment) | Hu/mAb | ii |
| Hla (toxin) | MEDI4893 (Suvratoxumab) [MedImmune LLC; NCT02296320] |
randomised, double-blind, placebo-controlled, single-dose, dose-ranging study in mechanically ventilated adult subjects (N = 213) | Phase II (ongoing) | Hu/mAb (IgG1) | [111] |
| AR-301 (Salvecin®) [Aridis Pharmaceuticals; NCT01589185] |
randomized, double-blind, placebo-controlled, single dose study of AR-301 as an adjunctive therapy against severe S. aureus-related pneumonia (N = 48) | Phase IIa (successful) | Hu/mAb (IgG1) | iii | |
| GrfA (ABC transporter) | Aurograb® [NeuTec Pharma Ltd./Novartis Pharma AG; NCT00217841] |
randomised, double-blind, placebo-controlled trial of patients with severe, deep-seated staphylococcal infections receiving vancomycin plus Aurograb (N = 180) | Phase II (failed), development stopped | single chain antibody fragment (Fab) | [134,144] |
| Multi-component | |||||
| ClfA (S. aureus), SdrG (S. epidermidis) (adhesins) | INH-A21 (Veronate®) [Inhibitex / Bristol Myers-Squibb; NCT00113191] |
randomized, double-blind, placebo-controlled study of INH-A21 for prevention of staphylococcal late-onset sepsis in VLBW infants (N = 1983) | Phase III (failed) | Pooled human Ig purified from the serum of donors with high titers against ClfA and SdrG | [113] |
| Hla, HlgAB, HlgCB, LukED, LukSF, LukAB (toxins) | ASN100 [Arsanis Biosciences GmbH; NCT02940626] |
randomized, double-blind, single-dose, placebo-controlled study of ASN100 for the prevention of S. aureus pneumonia in heavily-colonized, mechanically ventilated subjects (N = 354) | Phase II (halted) | Hu/mAb combination of ASN-1 (IgG1, crossreactive mAb with affinity for Hla, HlgAB, HlgCB, LukED and LukSF) and ASN-2 (IgG1, mAb against LukAB) | [95,112] |
data based on publications, review of sponsor website information as well as clinical trial data accessible via www.clinicaltrials.gov on 20.08.2018.
Abbreviations: ClfA, clumping factor A; LTA, lipoteichoic acid; PNAG, ß-1,6-poly-N-acetylglucosamine; Hla, α-toxin; SdrG, Serine- aspartate repeat- containing protein G; HlgAB and HlgCB, γ-haemolysin AB and CB; LukED and LukSF, leukotoxin ED and SF; NA, data not available; VLBW, very low birth weight.
ClinicalTrials.gov Identifieriv
Antibodies applied in clinical studies were either murine, chimeric, humanized or human (see Glossary)
StaphVAX® is a bivalent S. aureus vaccine which contains the purified capsular polysaccharides (CPS) types 5 and 8v. Its development was halted by Nabi due to failure in preventing S. aureus infections in kidney disease patients in a confirmatory phase III clinical trialvi.. The company also halted the development of Altastaph™, as it is based on the same capsular polysaccharide technology as StaphVAX®.
Resources
This failure of trials using surface-directed monoclonal antibodies against adhesins and surface glyocopolymers forced S. aureus researchers to revisit S. aureus pathogenesis and potential correlates of protection. One lesson learned is that targeting a single adhesin is prone to failure due to the high functional redundancy of these proteins. For instance, there are at least five fibrinogen-binding proteins in S. aureus [22]. Moreover, it has been suggested that adverse effects could be caused by antibody-induced agglutination, since large aggregates of bacteria in the blood may not be cleared by the host and could become trapped in various tissues, particularly in the lungs [109,110]. Finally, in contrast to other pathogens, opsonophagocytosis may not be the most important mechanism of protection, since this species produces a whole arsenal of toxins and immune evasion proteins that are decisive for pathogenesis. In consequence, current research and clinical trials are focusing on poreforming toxins as targets for an antibody-based therapy (Table 2) [95,111,112]. MedImmune as well as Aridis Pharmaceuticals are testing human anti-Hla antibodies for prevention of S. aureus pneumonia [111].
Apart from a shift towards toxins, there is now a trend towards multivalent vaccines in order to combat the multifactorial nature of S. aureus pathogenesis [112,113]. For instance, Arsanis Biosciences has tested a combination of two human monoclonal antibodies (ASN-1 targeting Hla and four other bi-component leukocidins; ASN-2 targeting LukAB) in the ASN100 phase II clinical trial for the prevention of S. aureus pneumonia (Table 2) [95,112]. The trial was however recently halted due to insufficient efficacy. Another approach involves the use of monoclonal antibodies as means of targeted delivery of antimicrobials. For instance, an antibody-antibiotic conjugate (AAC) specifically binding wall teichoic acid is currently used in a phase Ib clinical trial targeting S. aureus bacteremia patients [114,115] (Table 2).
7. Concluding Remarks and Future Perspectives
Although biofilm infections have been recognized as an important mediator of chronic infection associated with high morbidity and mortality, vaccine research has seemingly overlooked biofilms with regard to discovery and efficacy studies. A better understanding of the immune response against biofilms, and of how biofilms manipulate this response, is therefore essential for the development of protective staphylococcal vaccines (see Outstanding Questions). Nevertheless, in the reasonably near future, the identification and testing of new combinations of monoclonal antibodies, which are effective against planktonic as well as biofilm cells in a broad range of disease settings, will hopefully achieve more success than past attempts.
Outstanding questions box:
What is the proteome (surface proteins, secreted factors) of staphylococcal biofilms in ex vivo or in vivo-like conditions?
How does the adaptive immune system (antibodies, T cells) respond to biofilm as compared to non-biofilm infections?
How do biofilm-embedded bacteria modulate and subvert innate and adaptive defense mechanisms?
What are correlates of protection in biofilm infections – type1/3, type 2 or regulatory responses?
Are specific epitopes of antigens more effective in destabilising biofilms and/or preventing biofilm formation?
Can the efficacy of monoclonal antibodies be enhanced by using a multivalent vaccine or by combing antibodies with antibiotics or specific enzymes (such as nucleases, proteases)?
How well can ‘reverse vaccinology’, a genome-based unbiased discovery process for the prediction of candidate vaccine antigens, supplement traditional vaccine approaches?
Ideally, the following factors should be considered while selecting potential biofilm-related antigens: (i) prevalence in clinical isolates [67], (ii) antigenic variability of the target protein [66,116,117], (iii) expression profiling of proteins within the biofilm in vivo [14,16], (iv) its relevance in many different staphylococcal diseases, (v) immunological relevance, i.e. accessibility to antibodies within the biofilm matrix, and (vi) ability to induce not only a strong but the correct (i.e. protective) type of immune response (governed by the right choice of adjuvant / route of antigen application) [118].
In order to meet all or most of these criteria, multivalent vaccines are the only strategy of choice for active as well as passive immunization [18,44,44,66]. The most effective therapeutic approach for the biofilm lifestyle will likely require a combinatorial approach of bactericidal and immunostimulatory treatments. It may be an unrealistic goal to achieve a complete clearance of S. aureus from our body, bearing in mind that the microorganism is part of the human normal microbiota and an expert in evading host immune defense, but rather clinical protection to reduce the severity of staphylococcal infections and prevent chronification.
Recapitulating the unsuccessful clinical trials for a passive S. aureus vaccine, several hurdles can be named: (i) the multiplicity and redundancy of S. aureus virulence factors which challenges the selection of protective antigens, (ii) the production of numerous immune evasion factors, including protein A (iii), a lack of knowledge about the nature of protective immunity against S. aureus infection, and (iv) a lack of transition from animal models to human studies [109,119,120]. One explanation for a lack of transition could be the use naive animals, whereas humans are immunologically primed against S. aureus. This may explain why huge effects of different passive or active vaccination strategies in animals cannot be reproduced in humans. If these points are considered in antigen selection processes and subsequent preclinical tests, we will hopefully be more successful in the near future in developing a protective passive vaccine against this notorious pathogen.
Highlights.
S. aureus and other staphylococci are the most common cause of persistent biofilm-associated infections, which are inherently resistant to antibiotics as well as the host’s immune system.
Antibody-based approaches can be used to combat biofilms. Antibodies can prevent bacterial attachment and/or biofilm maturation, or even disperse mature biofilms as shown in vitro and in pre-clinical studies.
Several sophisticated techniques can be used for the generation of human monoclonal antibodies, to be ultimately employed in research or clinical settings.
Since antibodies against surface structures proved unsuccessful in clinical trials so far, current research is focussed on S. aureus toxins, and biofilm matrix components.
Multivalent vaccines, with a special emphasis on biofilm-related targets, are the strategy of choice for active as well as passive immunization.
Acknowledgements
The authors would like to acknowledge the valuable comments and suggestions of Barbara M. Broker and Murthy N. Darisipudi. S.H., K.R., and J.I. were supported by the European Union (European Social Funds, Card-ii-Omics, ESF/14-BM-A55-0037/16, http://ec.europa.eu/esf/home.jsp). MO was supported by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health, 1 ZIA AI001080.
Glossary
- Active vaccination:
immunization with an antigen to provoke adaptive immunity. It usually induces long-lasting and robust protective immune memory, but requires several weeks to become fully effective.
- Adhesins:
bacterial cell surface proteins that enable them to bind to the surfaces of host cells or the extracellular matrix.
- Antibody:
also known as immunoglobulin (Ig), is secreted by B cells upon antigen contact. It is highly specific and binds its target structure, the antigen, with very high affinity. While each B cell produces identical antibodies, an individual can produce a total number of at least 107 antibody specificities, enabling the immune system to respond to wide range of antigens. Structurally, antibodies are heterodimeric proteins composed of two heavy and two light chains which are linked by disulfide bonds.
- Chimeric antibody:
antibody whose constituent parts are derived from different species, mostly human and murine. The replacement of the murine with a human Fc part allows chimeric antibodies to efficiently interact with the human immune system and reduces the risk of an adverse immune response to the applied monoclonal antibodies.
- Human antibody:
antibody that is composed of fully human antibody heavy and light chains.
- Humanized antibody:
antibody, where the mouse antigen binding regions (= hypervariable loops) are genetically engineered into otherwise human antibodies.
- Hybridomas:
hybrid cell lines formed by fusing a myeloma cell (no antibody production, but immortal) with a specific antibody-producing B cell (antibody production, but mortal). The resulting immortal hybridoma cells are grown in tissue culture and produce antibodies of a single specificity (i.e. monoclonal).
- mAb (monoclonal antibody):
antibodies produced by a single clone of B cells, which are hence all identical. They are generated either by immortalizing the antibody-producing B cell or by cloning the respective genes into an expression system.
- MAC (membrane-attack complex):
a protein complex composed of the terminal complement proteins, which generates lytic pores in certain pathogens.
- MSCRAMMs (microbial surface components recognizing adhesive matrix molecules):
cell wall-attached adhesin proteins, which share a similar protein structure and a common mechanism of ligand binding, including ClfA, ClfB, SdrC, SdrD, SdrE, bone sialoprotein-binding protein, FnBPA, FnBPB and Cna. They mediate the initial attachment of bacteria to abiotic/biotic surfaces, providing a critical step in the establishment and persistence of infections.
- Murine antibody:
antibody that has been generated in mice. Murine antibodies are recognized by the human immune system as foreign antigens, and can thus - upon repeated application - lead to allergic reactions, reduced therapeutic effectiveness and shorter circulating antibody half-life.
- OPK (opsonophagocytic killing):
the deposition of antibody and/or complement onto the surface of a pathogen makes it more easily ingested by phagocytes.
- Passive immunization:
transfer of antibodies / immune sera / immune cells to provide immediate and specific – albeit short-lived – immunological protection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lister JL and Horswill AR (2014) Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Frontiers in cellular and infection microbiology 4, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arciola CR et al. (2018) Implant infections: adhesion, biofilm formation and immune evasion. Nature reviews. Microbiology 16, 397–409 [DOI] [PubMed] [Google Scholar]
- 3.Hoerr V et al. (2018) S. aureus endocarditis: Clinical aspects and experimental approaches. International journal of medical microbiology : IJMM 308, 640–652 [DOI] [PubMed] [Google Scholar]
- 4.Mulcahy ME and McLoughlin RM (2016) Host-bacterial crosstalk determines Staphylococcus aureus nasal colonization. Trends in microbiology 24, 872–886 [DOI] [PubMed] [Google Scholar]
- 5.von Eiff C et al. (2001) Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. The New England journal of medicine 344, 11–16 [DOI] [PubMed] [Google Scholar]
- 6.Arciola CR et al. (2015) Biofilm-based implant infections in orthopaedics. Advances in experimental medicine and biology 830, 29–46 [DOI] [PubMed] [Google Scholar]
- 7.Magill SS et al. (2014) Multistate point-prevalence survey of health care-associated infections. The New England journal of medicine 370, 1198–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montanaro L et al. (2011) Scenery of Staphylococcus implant infections in orthopedics. Future microbiology 6, 1329–1349 [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal VK et al. (2014) Organism profile in periprosthetic joint infection: pathogens differ at two arthroplasty infection referral centers in Europe and in the United States. The journal of knee surgery 27, 399–406 [DOI] [PubMed] [Google Scholar]
- 10.Resch A et al. (2005) Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Applied and environmental microbiology 71, 2663–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Y et al. (2005) Genomewide analysis of gene expression in Staphylococcus epidermidis biofilms: insights into the pathophysiology of S. epidermidis biofilms and the role of phenol-soluble modulins in formation of biofilms. The Journal of infectious diseases 191, 289–298 [DOI] [PubMed] [Google Scholar]
- 12.Mah TF and O'Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends in microbiology 9, 34–39 [DOI] [PubMed] [Google Scholar]
- 13.Kiedrowski MR and Horswill AR (2011) New approaches for treating staphylococcal biofilm infections. Annals of the New York Academy of Sciences 1241, 104–121 [DOI] [PubMed] [Google Scholar]
- 14.Brady RA et al. (2006) Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infection and immunity 74, 3415–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resch A et al. (2006) Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics 6, 1867–1877 [DOI] [PubMed] [Google Scholar]
- 16.den Reijer PM et al. (2017) Combining in vitro protein detection and in vivo antibody detection identifies potential vaccine targets against Staphylococcus aureus during osteomyelitis. Medical microbiology and immunology 206, 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei MG et al. (2017) Proteomics of Staphylococcus aureus biofilm matrix in a rat model of orthopedic implant-associated infection. PloS one 12, e0187981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gil C et al. (2014) Biofilm matrix exoproteins induce a protective immune response against Staphylococcus aureus biofilm infection. Infection and immunity 82, 1017–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassat JE et al. (2013) A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell host & microbe 13, 759–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otto M (2008) Staphylococcal Biofilms. Current topics in microbiology and immunology 322, 207–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dastgheyb S et al. (2015) Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. The Journal of infectious diseases 211, 641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster TJ et al. (2014) Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nature reviews. Microbiology 12, 49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banner MA et al. (2007) Localized tufts of fibrils on Staphylococcus epidermidis NCTC 11047 are comprised of the accumulation-associated protein. Journal of bacteriology 189, 2793–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corrigan RM et al. (2007) The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology (Reading, England) 153, 2435–2446 [DOI] [PubMed] [Google Scholar]
- 25.Conrady DG et al. (2013) Structural basis for Zn2+-dependent intercellular adhesion in staphylococcal biofilms. Proceedings of the National Academy of Sciences of the United States of America 110, E202–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack D et al. (1996) The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol 178, 175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohde H et al. (2007) Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 28, 1711–1720 [DOI] [PubMed] [Google Scholar]
- 28.Schommer NN et al. (2011) Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infection and immunity 79, 2267–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerca N et al. (2007) Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infection and immunity 75, 3406–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuong C et al. (2004) A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. The Journal of biological chemistry 279, 54881–54886 [DOI] [PubMed] [Google Scholar]
- 31.Otto M (2013) Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annual review of medicine 64, 175–188 [DOI] [PubMed] [Google Scholar]
- 32.Le KY et al. (2014) Molecular determinants of staphylococcal biofilm dispersal and structuring. Frontiers in cellular and infection microbiology 4, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R et al. (2011) Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. The Journal of clinical investigation 121, 238–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherr TD et al. (2014) Hiding in plain sight: Interplay between staphylococcal biofilms and host immunity. Frontiers in immunology 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gogoi-Tiwari J et al. (2015) Comparative studies of the immunogenicity and protective potential of biofilm vs planktonic Staphylococcus aureus vaccine against bovine mastitis using non-invasive mouse mastitis as a model system. Biofouling 31, 543–554 [DOI] [PubMed] [Google Scholar]
- 36.Prabhakara R et al. (2011) Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infection and immunity 79, 1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Günther F et al. (2009) Host defence against Staphylococcus aureus biofilms infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN). Molecular immunology 46, 1805–1813 [DOI] [PubMed] [Google Scholar]
- 38.Thurlow LR et al. (2011) Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. Journal of immunology (Baltimore, Md. : 1950) 186, 6585–6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bröker BM et al. (2014) Immune control of Staphylococcus aureus - regulation and counterregulation of the adaptive immune response. International journal of medical microbiology : IJMM 304, 204–214 [DOI] [PubMed] [Google Scholar]
- 40.Stentzel S et al. (2015) Specific serum IgG at diagnosis of Staphylococcus aureus bloodstream invasion is correlated with disease progression. Journal of proteomics 128, 1–7 [DOI] [PubMed] [Google Scholar]
- 41.Grumann D et al. (2011) Characterization of infecting strains and superantigen-neutralizing antibodies in Staphylococcus aureus bacteremia. Clinical and vaccine immunology : CVI 18, 487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishitani K et al. (2015) A diagnostic serum antibody test for patients with Staphylococcus aureus osteomyelitis. Clinical orthopaedics and related research 473, 2735–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.den Reijer PM et al. (2013) Characterization of the humoral immune response during Staphylococcus aureus bacteremia and global gene expression by Staphylococcus aureus in human blood. PloS one 8, e53391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brady RA et al. (2011) Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment. Infection and immunity 79, 1797–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerca N et al. (2006) Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infection and immunity 74, 4849–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brady RA et al. (2007) Immunoglobulins to surface-associated biofilm immunogens provide a novel means of visualization of methicillin-resistant Staphylococcus aureus biofilms. Applied and environmental microbiology 73, 6612–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stroh P et al. (2011) Host defence against Staphylococcus aureus biofilms by polymorphonuclear neutrophils: oxygen radical production but not phagocytosis depends on opsonisation with immunoglobulin G. Immunobiology 216, 351–357 [DOI] [PubMed] [Google Scholar]
- 48.Skurnik D et al. (2010) Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. The Journal of clinical investigation 120, 3220–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiner GJ (2015) Building better monoclonal antibody-based therapeutics. Nature reviews. Cancer 15, 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stern M and Herrmann R (2005) Overview of monoclonal antibodies in cancer therapy: present and promise. Critical reviews in oncology/hematology 54, 11–29 [DOI] [PubMed] [Google Scholar]
- 51.Morrison SL et al. (1984) Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proceedings of the National Academy of Sciences 81, 6851–6855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones PT et al. (1986) Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 [DOI] [PubMed] [Google Scholar]
- 53.Frenzel A et al. (2016) Phage display-derived human antibodies in clinical development and therapy. mAbs 8, 1177–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammers CM and Stanley JR (2014) Antibody phage display: Technique and applications. The Journal of investigative dermatology 134, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saeed AFUH et al. (2017) Antibody engineering for pursuing a healthier future. Frontiers in microbiology 8, 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li B et al. (2014) In vitro affinity maturation of a natural human antibody overcomes a barrier to in vivo affinity maturation. mAbs 6, 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kontermann RE and Brinkmann U (2015) Bispecific antibodies. Drug discovery today 20, 838–847 [DOI] [PubMed] [Google Scholar]
- 58.Tkaczyk C et al. (2017) Multimechanistic monoclonal antibodies (MAbs) targeting Staphylococcus aureus alpha-toxin and Clumping factor A: Activity and efficacy comparisons of a MAb combination and an engineered bispecific antibody approach. Antimicrobial agents and chemotherapy 61, e00629–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meeker DG et al. (2018) Versatility of targeted antibiotic-loaded gold nanoconstructs for the treatment of biofilm-associated bacterial infections. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 34, 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim M-H et al. (2013) Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Annals of biomedical engineering 41, 598–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lehar SM et al. (2015) Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 527, 323–328 [DOI] [PubMed] [Google Scholar]
- 62.Wang Y et al. (2017) Mouse model of hematogenous implant-related Staphylococcus aureus biofilm infection reveals therapeutic targets. Proceedings of the National Academy of Sciences of the United States of America 114, E5094–E5102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Neill E et al. (2008) A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. Journal of bacteriology 190, 3835–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rennermalm A et al. (2001) Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine 19, 3376–3383 [DOI] [PubMed] [Google Scholar]
- 65.Kelly-Quintos C et al. (2006) Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infection and immunity 74, 2742–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tkaczyk C et al. (2016) Targeting alpha toxin and ClfA with a multimechanistic monoclonal-antibody-based approach for prophylaxis of serious Staphylococcus aureus disease. mBio 7, e00528–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindsay JA et al. (2006) Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. Journal of bacteriology 188, 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siboo IR et al. (2001) Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infection and immunity 69, 3120–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Domanski PJ et al. (2005) Characterization of a humanized monoclonal antibody recognizing clumping factor A expressed by Staphylococcus aureus. Infection and immunity 73, 5229–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall AE et al. (2003) Characterization of a Protective Monoclonal Antibody Recognizing Staphylococcus aureus MSCRAMM Protein Clumping Factor A. Infection and immunity 71, 6864–6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCarthy H et al. (2016) The major autolysin is redundant for Staphylococcus aureus USA300 LAC JE2 virulence in a murine device-related infection model. FEMS microbiology letters 363, fnw087. [DOI] [PubMed] [Google Scholar]
- 72.Nair N et al. (2015) Amidase, a cell wall hydrolase, elicits protective immunity against Staphylococcus aureus and S. epidermidis. International journal of biological macromolecules 77, 314–321 [DOI] [PubMed] [Google Scholar]
- 73.Varrone JJ et al. (2014) Passive immunization with anti-glucosaminidase monoclonal antibodies protects mice from implant-associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus megaclusters. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 32, 1389–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J-H et al. (2015) Surface glycopolymers are crucial for in vitro anti-wall teichoic acid IgG-mediated complement activation and opsonophagocytosis of Staphylococcus aureus. Infection and immunity 83, 4247–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jung D-J et al. (2012) Specific serum Ig recognizing staphylococcal wall teichoic acid induces complement-mediated opsonophagocytosis against Staphylococcus aureus. Journal of immunology (Baltimore, Md. : 1950) 189, 4951–4959 [DOI] [PubMed] [Google Scholar]
- 76.Fong R et al. (2018) Structural investigation of human S. aureus-targeting antibodies that bind wall teichoic acid. mAbs, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nemeth J and C Lee J (1995) Antibodies to capsular polysaccharides are not protective against experimental Staphylococcus aureus endocarditis. 63, 375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JC et al. (1997) Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infection and immunity 65, 4146–4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu B et al. (2017) Antibodies to Staphylococcus aureus capsular polysaccharides 5 and 8 perform similarly in vitro but are functionally distinct in vivo. Virulence 8, 859–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cerca N et al. (2007) Protection against Escherichia coli infection by antibody to the Staphylococcus aureus poly-N-acetylglucosamine surface polysaccharide. Proceedings of the National Academy of Sciences of the United States of America 104, 7528–7533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cywes-Bentley C et al. (2013) Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens. Proceedings of the National Academy of Sciences of the United States of America 110, E2209–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maira-Litrán T et al. (2005) Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated Staphylococcal Poly-N-acetyl-beta-(1-6)-glucosamine. Infection and immunity 73, 6752–6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soliman C et al. (2018) Structural basis for antibody targeting of the broadly expressed microbial polysaccharide poly-N-acetylglucosamine. The Journal of biological chemistry 293, 5079–5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Estellés A et al. (2016) A high-affinity native human antibody disrupts biofilm from Staphylococcus aureus bacteria and potentiates antibiotic efficacy in a mouse implant infection model. Antimicrobial agents and chemotherapy 60, 2292–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong YQ et al. (2017) A human biofilm-disrupting monoclonal antibody potentiates antibiotic efficacy in rodent models of both Staphylococcus aureus and Acinetobacter baumannii infections. Antimicrobial agents and chemotherapy 61, e00904–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goodman SD et al. (2011) Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal immunology 4, 625–637 [DOI] [PubMed] [Google Scholar]
- 87.Brockson ME et al. (2014) Evaluation of the kinetics and mechanism of action of antiintegration host factor-mediated disruption of bacterial biofilms. Mol Microbiol 93, 1246–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu Y et al. (2016) In vivo gene expression in a Staphylococcus aureus prosthetic joint infection characterized by RNA sequencing and metabolomics: a pilot study. BMC microbiology 16, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seilie ES and Bubeck Wardenburg J (2017) Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Seminars in cell & developmental biology 72, 101–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caiazza NC and O'Toole GA (2003) Alpha-toxin is required for biofilm formation by Staphylococcus aureus. Journal of bacteriology 185, 3214–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson MJ et al. (2018) Alpha-toxin contributes to biofilm formation among Staphylococcus aureus wound isolates. Toxins 10, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oganesyan V et al. (2014) Mechanisms of neutralization of a human anti-α-toxin antibody. The Journal of biological chemistry 289, 29874–29880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ortines RV et al. (2018) Neutralizing alpha-toxin accelerates healing of Staphylococcus aureus-infected wounds in nondiabetic and diabetic mice. Antimicrobial agents and chemotherapy 62, e02288–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scherr TD et al. (2015) Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. mBio 6, e01021–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Badarau A et al. (2016) Context matters: The importance of dimerization-induced conformation of the LukGH leukocidin of Staphylococcus aureus for the generation of neutralizing antibodies. mAbs 8, 1347–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomsen IP et al. (2017) Monoclonal antibodies against the Staphylococcus aureus bicomponent leukotoxin AB isolated following invasive human infection reveal diverse binding and modes of action. The Journal of infectious diseases 215, 1124–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dickey SW et al. (2017) Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nature reviews. Drug discovery 16, 457–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Novick RP and Geisinger E (2008) Quorum sensing in staphylococci. Annual review of genetics 42, 541–564 [DOI] [PubMed] [Google Scholar]
- 99.Queck SY et al. (2008) RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Molecular cell 32, 150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Periasamy S et al. (2012) How Staphylococcus aureus biofilms develop their characteristic structure. Proceedings of the National Academy of Sciences of the United States of America 109, 1281–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vuong C et al. (2003) Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. The Journal of infectious diseases 188, 706–718 [DOI] [PubMed] [Google Scholar]
- 102.Ma R et al. (2017) AI-2 quorum sensing negatively regulates rbf expression and biofilm formation in Staphylococcus aureus. International journal of medical microbiology : IJMM 307, 257–267 [DOI] [PubMed] [Google Scholar]
- 103.Xu L et al. (2006) Role of the luxS quorum-sensing system in biofilm formation and virulence of Staphylococcus epidermidis. Infection and immunity 74, 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weems JJ et al. (2006) Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrobial agents and chemotherapy 50, 2751–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rupp ME et al. (2007) Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrobial agents and chemotherapy 51, 4249–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Benjamin DK et al. (2006) A blinded, randomized, multicenter study of an intravenous Staphylococcus aureus immune globulin. Journal of perinatology : official journal of the California Perinatal Association 26, 290–295 [DOI] [PubMed] [Google Scholar]
- 107.Fattom A et al. (2015) Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Human vaccines & immunotherapeutics 11, 632–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Patel M and Kaufman DA (2015) Anti-lipoteichoic acid monoclonal antibody (pagibaximab) studies for the prevention of staphylococcal bloodstream infections in preterm infants. Expert opinion on biological therapy 15, 595–600 [DOI] [PubMed] [Google Scholar]
- 109.Salgado-Pabón W and Schlievert PM (2014) Models matter: the search for an effective Staphylococcus aureus vaccine. Nature reviews. Microbiology 12, 585–591 [DOI] [PubMed] [Google Scholar]
- 110.Hu J et al. (2011) Monoclonal antibodies against accumulation-associated protein affect EPS biosynthesis and enhance bacterial accumulation of Staphylococcus epidermidis. PloS one 6, e20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu X-Q et al. (2017) Safety, tolerability, and pharmacokinetics of MEDI4893, an investigational, extended-half-life, anti-Staphylococcus aureus alpha-toxin human monoclonal sntibody, in healthy adults. Antimicrobial agents and chemotherapy 61, e01020–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rouha H et al. (2015) Five birds, one stone: neutralization of α-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. mAbs 7, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DeJonge M et al. (2007) Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. The Journal of pediatrics 151, 260–5, 265.e1 [DOI] [PubMed] [Google Scholar]
- 114.Zhou C et al. (2016) Pharmacokinetics and pharmacodynamics of DSTA4637A: A novel THIOMAB™ antibody antibiotic conjugate against Staphylococcus aureus in mice. mAbs 8, 1612–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang-Lin SX et al. (2018) Minimal physiologically-based pharmacokinetic modeling of DSTA4637A, A novel THIOMAB™ antibody antibiotic conjugate against Staphylococcus aureus, in a mouse model. mAbs 10, 1131–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burke FM et al. (2010) Fibronectin-binding protein B variation in Staphylococcus aureus. BMC microbiology 10, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loughman A et al. (2008) Sequence diversity in the A domain of Staphylococcus aureus fibronectin-binding protein A. BMC microbiology 8, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bröker BM et al. (2016) The T Cell Response to Staphylococcus aureus. Pathogens (Basel, Switzerland) 5, 10.3390/pathogens5010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Otto M (2010) Novel targeted immunotherapy approaches for staphylococcal infection. Expert opinion on biological therapy 10, 1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fowler VG and Proctor RA (2014) Where does a Staphylococcus aureus vaccine stand? Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 20 Suppl 5, 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Becker K et al. (2014) Coagulase-negative staphylococci. Clinical microbiology reviews 27, 870–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Büttner H et al. (2015) Structural basis of Staphylococcus epidermidis biofilm formation: mechanisms and molecular interactions. Frontiers in cellular and infection microbiology 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takeda S et al. (1991) Protection against endocarditis due to Staphylococcus epidermidis by immunization with capsular polysaccharide/adhesin. Circulation 84, 2539–2546 [DOI] [PubMed] [Google Scholar]
- 124.França A et al. (2013) Monoclonal antibody raised against PNAG has variable effects on static S. epidermidis biofilm accumulation in vitro. International journal of biological sciences 9, 518–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Broekhuizen CAN et al. (2009) The influence of antibodies on Staphylococcus epidermidis adherence to polyvinylpyrrolidone-coated silicone elastomer in experimental biomaterial-associated infection in mice. Biomaterials 30, 6444–6450 [DOI] [PubMed] [Google Scholar]
- 126.Cheung GYC and Otto M (2010) Understanding the significance of Staphylococcus epidermidis bacteremia in babies and children. Current opinion in infectious diseases 23, 208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weisman LE et al. (2011) A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics 128, 271–279 [DOI] [PubMed] [Google Scholar]
- 128.Otto M (2009) Staphylococcus epidermidisQ--the 'accidental' pathogen. Nature reviews. Microbiology 7, 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van den Berg S et al. (2015) Active immunization with an octa-valent Staphylococcus aureus antigen mixture in models of S. aureus bacteremia and skin infection in mice. PloS one 10, e0116847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shahrooei M et al. (2009) Inhibition of Staphylococcus epidermidis biofilm formation by rabbit polyclonal antibodies against the SesC protein. Infection and immunity 77, 3670–3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shahrooei M et al. (2012) Vaccination with SesC decreases Staphylococcus epidermidis biofilm formation. Infection and immunity 80, 3660–3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berends ETM et al. (2013) Distinct localization of the complement C5b-9 complex on Grampositive bacteria. Cellular microbiology 15, 1955–1968 [DOI] [PubMed] [Google Scholar]
- 133.Visai L et al. (2000) Monoclonal antibodies to CNA, a collagen-binding microbial surface component recognizing adhesive matrix molecules, detach Staphylococcus aureus from a collagen substrate. The Journal of biological chemistry 275, 39837–39845 [DOI] [PubMed] [Google Scholar]
- 134.Valotteau C et al. (2017) Single-Cell and Single-Molecule Analysis Unravels the Multifunctionality of the Staphylococcus aureus Collagen-Binding Protein Cna. ACS nano 11, 2160–2170 [DOI] [PubMed] [Google Scholar]
- 135.Nilsson IM et al. (1998) Vaccination with a recombinant fragment of collagen adhesin provides protection against Staphylococcus aureus-mediated septic death. The Journal of clinical investigation 101, 2640–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Belyi Y et al. (2018) Staphylococcus aureus surface protein G is an immunodominant protein and a possible target in an anti-biofilm drug development. The open microbiology journal 12, 94–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haghighat S et al. (2017) Cloning, expression and purification of autolysin from methicillin-resistant Staphylococcus aureus: potency and challenge study in Balb/c mice. Molecular immunology 82, 10–18 [DOI] [PubMed] [Google Scholar]
- 138.van den Berg S et al. (2015) A human monoclonal antibody targeting the conserved staphylococcal antigen IsaA protects mice against Staphylococcus aureus bacteremia. International journal of medical microbiology : IJMM 305, 55–64 [DOI] [PubMed] [Google Scholar]
- 139.Oesterreich B et al. (2014) Characterization of the biological anti-staphylococcal functionality of hUK-66 IgG1, a humanized monoclonal antibody as substantial component for an immunotherapeutic approach. Human vaccines & immunotherapeutics 10, 926–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ohlsen K and Lorenz U (2010) Immunotherapeutic strategies to combat staphylococcal infections. International journal of medical microbiology : IJMM 300, 402–410 [DOI] [PubMed] [Google Scholar]
- 141.Weisman LE (2007) Antibody for the prevention of neonatal noscocomial staphylococcal infection: a review of the literature. Archives de Pédiatrie 14, S31–S34 [DOI] [PubMed] [Google Scholar]
- 142.Kim HK et al. (2012) Protein A-specific monoclonal antibodies and prevention of Staphylococcus aureus disease in mice. Infection and immunity 80, 3460–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lam H et al. (2014) Antibodies to PhnD inhibit staphylococcal biofilms. Infection and immunity 82, 3764–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burnie JP et al. (2000) Identification of an immunodominant ABC transporter in methicillin-resistant Staphylococcus aureus infections. Infection and immunity 68, 3200–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]