Abstract
Rationale:
Red blood cells (RBC) undergo morphologic and biochemical changes during storage which may lead to adverse health risks upon transfusion. In prior studies, the effect of RBC age on health outcomes has been conflicting. We designed the study to assess the effects of RBC units’ storage duration on health outcomes specifically for hospitalized patients undergoing hip fracture surgery or coronary artery bypass grafting (CABG) surgery.
Methods:
Using International Classification of Diseases (ICD) 9 codes, hip fracture surgery and CABG surgery patients, who received RBC transfusions between 2008 and 2013, were retrospectively identified from the electronic medical records system. Hip fracture surgery and CABG cohorts were sub-divided into 3 blood age groups based upon RBC unit age at the time of transfusion: young blood (RBC units stored less than or equal to 14 days), old blood (RBC units were stored for greater than or equal to 28 days), or mixed blood for the remaining patients. Outcome variables were 30-day, 90-day, and inpatient mortality as well as hospital length of stay.
Results:
A total of 3,182 patients were identified: 1,121 with hip fractures and 2,061 with CABG. Transfusion of old blood was associated with higher inpatient mortality in the hip fracture surgery cohort (OR 166.8, 95% CI 1.067–26064.7, p=0.04) and a higher 30-day mortality in the CABG cohort (OR 4.55, 95% CI 1.01–20.49, p=0.03).
Conclusions:
Transfusing RBC units stored for greater than or equal to 28 days may be associated with a higher mortality for patients undergoing hip fracture or CABG.
Keywords: Surgery, red blood cell transfusions, hip surgery, cardiac surgery, outcomes
INTRODUCTION
Elderly patients account for a disproportionate use of blood transfusions. Although the life-saving benefits of red blood cell (RBC) transfusions for patients with acute hemorrhage are obvious, stricter thresholds for transfusions have resulted in equivalent or better outcomes for a variety of hospitalized patients, such as those with septic shock2, gastrointestinal bleeding3, and hip fracture repairs4. For unclear reasons, these disparate results suggest that the risks and outcomes associated with RBC transfusions are different in different patient groups, possibly due to the age of blood and its subsequent deterioration in storage.
This theory stems from scientific evidence unveiling deleterious biochemical and structural changes in red blood cells as they age while in storage, known as “storage lesions”5–7. The storage lesions comprise various metabolic, oxidative, and shape changes including but not limiting to decreased 2–3 diphosphoglycerate (DPG) with impaired oxygen delivery, decreased phosphate and glutathione levels, increased lactate levels, lipid peroxidation with generation of prostaglandins and isoprotanes, and increased RBC rigidity and adherence to vascular endothelium8–12.
Data from prior studies evaluating the effect of aged RBCs on patient-related outcomes have been mixed13 with a sizeable number of studies showing that transfusion with aged blood has been associated with increased morbidity and mortality in a variety of patient populations14–16. Conversely, a recent systematic review of 55 studies assessing the potential impact of RBC age on clinical outcomes among adult patients provided inconclusive results13. Two recently concluded clinical trials, the RECESS trial in cardiac surgery patients17 and the ABLE trial in critically ill adult patients18 compared blood stored for 1–2 weeks with blood stored for >3-weeks. Both the trials failed to show any impact of the age of transfused RBCs on patient related outcomes including mortality. Furthermore, a recent study of 23,247 transfused patients comparing clinical outcomes between older RBCs (aged ≥28 days and ≥35 days) and RBCs stored for ≤21 days showed adverse clinical outcomes in the older RBCs groups among selected subgroups of elderly and critically ill patients19.
Given the aforementioned conflicting results, with different patient groups exhibiting varied clinical outcomes to the transfused RBCs age thresholds, and prior studies comparing either younger blood (1–2 weeks) with standard transfusion practices or older blood (≥28 days) with standard transfusion, we sought to expand the literature by designing a study comparing very old blood (≥28 days storage) with fresh blood (≤14 days storage) among a priori defined patient subgroups based on the type of surgery (hip fracture, coronary artery bypass). Given the cardiac surgery and hip surgery population, our study also expands the literature on outcomes in elderly surgery patients, and those who receive a combination of old and fresh blood. We chose to study the cardiac surgery and hip surgery populations due to their high comorbidity profiles, and their likelihood of receiving multiple blood transfusions during the hospitalization, increasing the chances of also receiving blood products of various ages.
METHODS
Settings
After obtaining approval from the local institutional review board, data were retrieved from the electronic medical records (EMR). Patients were included in the study if they were 18 years or older and had received an RBC transfusion between November 1, 2008 and November 30, 2013. The date of transfusion was used to identify the specific inpatient encounter in the EMR for which the blood transfusion occurred, and from which the outcomes of interest were extracted.
Patient Cohorts
The cohorts were identified based on the International Classification of Diseases (ICD) 9 diagnosis codes. The hip fracture surgery cohort included patients with a diagnosis of hip fracture who underwent surgical repair. Patients were excluded if they had either hip fracture without undergoing surgery, or conversely, hip surgery without having a hip fracture. Patients who underwent coronary artery bypass grafting (CABG) were placed in the CABG cohort.
Age of Blood
All transfused blood units from a participant’s hospital admission, defined as occurring between admission through discharge, were attributed to the inpatient encounter. The overall age of transfused blood was classified into one of three “blood age groups” based on the age of the transfused RBC units; Young blood: when all RBC units received during an encounter were less than or equal to 14 days old; old blood: when all blood units received during an encounter were greater than or equal to 28 days old; and, mixed blood: if the units of blood were a combination of young blood and old blood, thereby not being exclusively 14 days or newer, or 28 days or older. This process helped in isolating the patients with a large number of units of blood transfused. The greater number of units a patient received, the greater the probability that they would have variation in the age of the RBC units. Thus, most patients with a larger number of transfused units ended up in the mixed group.
Outcome Variables
The primary outcome variables were inpatient, 30-day and 90-day mortality. Thirty and 90-day mortality were defined as death occurring within 30 and 90 days post admission date. The secondary outcome variables were length of hospital stay (LOS), and 30-day and 90-day readmission rates. Thirty and 90-day readmissions were defined as any cause readmissions occurring within 30 and 90-days post discharge date.
Other Data
Age, gender, race, chronic co-morbidities using the Charlson comorbidity index21 (which assigns points for diabetes, chronic liver disease, heart failure, cancer, HIV), number of units transfused, and type of blood groups were also collected from the electronic medical records. The Acute Physiology Score (APS) was recoded to the Acute Physiology and Chronic Health Evaluation II (APACHE II)20 using an algorithm incorporating the following variables: pulse, respiratory rate, serum sodium, serum potassium, serum pH, serum creatinine, oxygenation, hematocrit, white blood cell count, and mean arterial pressure.
Statistical Analysis
Descriptive statistics for continuous variables were calculated, and non-parametric tests were used where appropriate. Multivariable logistic regression analyses were performed to determine if RBC age was associated with 30-day, 90-day, and inpatient mortality. Multivariable linear regression was performed to determine if RBC age was associated with hospital LOS. As hospital LOS appeared to be right skewed, it was log-transformed. Patients’ age at admission, gender, race, pre-transfusion APACHE II score (recoded from APS as described above) and pre-transfusion Charlson comorbidity index were always included as predictors in the model; the rest of the covariates were selected through stepwise procedures with entry and exit criteria (alpha) of 0.05. Covariates placed into the model for selection were: number of units of blood, standard deviation of the number of days between units of blood per patient, the interaction between the age of the blood and the number of units of blood, the interaction between Charlson score and the age of the blood, the interaction between the age of the blood and the severity of illness. Outliers were eliminated from analysis by only including patient encounters where the number of transfusions received was within two standard deviations of the mean for that sub-cohort. This led to an upper limit of seven units of transfused blood for the hip fracture cohort and 17 units of transfused blood for the CABG cohort. Additional constraints applied to the data were that of a length of stay between zero and 180 days, receiving a blood transfusion of only red blood cells, having a pre-transfusion hemoglobin of 17 or less, and having a pre-transfusion creatinine of 32 or less. Use of constraints was used to ensure more robust comparison of differences between groups receiving old versus young blood. All analyses and numbers listed in the paper are based on all of the previous assumptions unless otherwise noted.
RESULTS
Patient characteristics
We identified 45,099 unique medical record numbers (MRNs) as having received a blood transfusion between November 1, 2008 and November 30, 2013 from the EMR. Of these records, 39,330 MRNs were patients 18 years of age or older (defined from the patient’s most recent transfusion date). Accounting for multiple MRNs for the same patients resulted in 39,003 distinct patients. Limiting the analyses to hip fracture surgery and CABG cohorts, a total of 3,182 patients met the inclusion criteria: 1,121 with hip fracture surgeries, and 2,061 with CABG.
The hip fracture surgery cohort had a higher number of females (70%) compared with the CABG cohort (38%). Both cohorts were predominately Caucasians (92% hip, 88% CABG). The mean age of patients in the hip fracture surgery cohort was 77.7 (15.5) years whereas the mean age in the CABG cohort was 67.1 (10.7) years (p<0.01). The mean severity of illness scores were lower in the hip fracture surgery cohort 5.3 (SD: 3.5) as compared to the CABG cohort 7.1 (SD: 3.8) (p<0.01). The Charlson comorbidity index was 1.1 (2.0) in the hip fracture surgery cohort and 1 (1.8) in the CABG cohort (p=0.40). Patient characteristics within each cohort stratified on the age of the blood transfused are shown in Table 1.
Table 1:
Patient Characteristics for Each Cohort
| HIP FRACTURE SURGERY | |||||
|---|---|---|---|---|---|
| Young Blood (n=127) |
Mixed Blood (n=718) |
Old Blood (n=276) |
Total (n=1121) |
P-Value | |
| Age in years | 79 (16) | 78 (15) | 77 (17) | 78 (16) | 0.54* |
| Males, n (%) | 32 (25.2) | 227 (31.6) | 80 (28.9) | 339 (30.2) | 0.30** |
| Caucasians, n (%) | 118 (92.9) | 667 (92.9) | 253 (91.7) | 1038 (92.6) | 0.58** |
| APACHE IIa | 5.1 (3.3) | 5.4 (3.5) | 5.1 (3.5) | 5.3 (3.5) | 0.32* |
| CCIb | 1.2 (2.1) | 1.1 (2.1) | 1.1 (2.0) | 1.1 (2.0) | 0.82* |
| CORONARY ARTERY BYPASS GRAFTING (CABG) | |||||
| Young Blood (n=203) |
Mixed Blood (n=1562) |
Old Blood (n=296) |
Total (n=2061) |
P-Value | |
| Age in years | 67 (11) | 67 (11) | 66 (11) | 67 (11) | 0.02* |
| Males, n (%) | 114 (56.2) | 970 (62.1) | 190 (64.2) | 1274 (61.8) | 0.17** |
| Caucasians, n (%) | 179 (88.2) | 1383 (88.5) | 249 (84.1) | 1811 (87.9) | 0.17** |
| APACHE IIa | 6.4 (3.6) | 7.0 (3.9) | 7.1 (3.8) | 7.0 (3.9) | 0.05* |
| CCIb | 0.9 (1.6) | 1.0 (1.9) | 1.2 (1.9) | 1.0 (1.8) | 0.46* |
Data presented as mean (SD) unless otherwise specified
APACHE II: Acute Physiology and Chronic Health Evaluation Score
CCI: Charlson Co-morbidity Index
Wilcoxon-Rank Sum test
Chi-Squared test
The average age of transfused RBCs was 23.3 (8.6) days in the hip fracture surgery cohort and 21.8 (7.9) days (p<0.01) in the CABG cohort. Table 2 shows the age of transfused RBCs, units of RBCs transfused, and the donor blood types in the young, mixed and old blood age groups in both hip fracture surgery and CABG cohorts. Data presented in table 2 represents data prior to constraints being applied, resulting in large variance values (standard deviation) in pre-surgery hemoglobin.
Table 2.
Potential Confounders for Each Cohort
| HIP FRACTURE SURGERY | |||||
|---|---|---|---|---|---|
| Young Blood (n=127) |
Mixed Blood (n=731) |
Old Blood (n=286) |
Total (n=1144) |
P-Value | |
| Age of RBCs in days, mean (SD) | 11 (3) | 21 (6) | 34 (4) | 23 (9) | <0.01* |
| Pre-Surgery Creatinine** (SD) | 1.2 (0.9) | 1.2 (1.0) | 1.1 (0.8) | 1.2 (0.9) | 0.50* |
| Pre-Surgery Hemoglobin*** (SD) |
10.5 (1.8) | 10.8 (11.5) | 12.3 (21.2) | 11.1 (13.6) | 0.02* |
| Number of transfused units, mean (SD) | 1.8 (0.7) | 2.6 (1.4) | 2.0 (1.0) | 2.3 (1.3) | <0.01* |
| CORONARY ARTERY BYPASS GRAFTING (CABG) | |||||
| Young Blood (n=203) |
Mixed Blood (n=1563) |
Old Blood (n=298) |
Total (n=2064) |
P-Value | |
| Age of RBCs in days, mean (SD) | 11 (3) | 21 (7) | 32 (4) | 22 (8) | <0.01* |
| Pre-Surgery Creatinine** (SD) | 1.3 (1.2) | 1.4 (1.2) | 1.3 (1.2) | 1.4 (1.2) | 0.30* |
| Pre-Surgery Hemoglobin*** (SD) |
12.0 (4.6) | 11.9 (11.8) | 11.4 (1.9) | 11.8 (11.0) | <0.01* |
| Number of transfused units, mean (SD) | 2.6 (1.8) | 4.5 (3.2) | 2.8 (2.2) | 4.1 (3.1) | <0.01* |
Wilcoxon Rank Sum test
Creatinine (mg/dL)
Hemoglobin (gm/dL)
Outcomes
Hip fracture surgery cohort
Mortality, length of stay, and hospital readmission data for young-blood and old-blood recipients are shown in Table 3. Data for mixed-blood recipients was not constrained and therefore is not shown in Table 3 (comparisons between groups were performed between old blood and young blood). There were four hospital deaths (4/127, 3%) in the young blood group compared to 11 in the old blood group (11/286, 3.8%) (p=0.33). Eight patients (8/127, 6%) in the young blood group died within 30 days compared to 25 in the old blood group (25/286, 8%) (p=0.09). Nine patients (9/127, 7%) in the young blood group died within 90 days compared to 27 in the old blood group (27/286, 9%) (p=0.11).
Table 3:
Mortality, Length of Stay, and Readmission Outcomes
| Hip Surgery Cohort | |||
|---|---|---|---|
| Mortality | |||
| Hospital, n (%) | 4 (3) | 11 (3.8) | 0.33 |
| 30-Day, n (%) | 8 (6) | 25 (8) | 0.09 |
| 90-Day, n (%) | 9 (7) | 27 (9) | 0.11 |
| Length of Stay | 10.8 (22.3) | 11.5 (21.9) | 0.36 |
| Readmissions | |||
| 30-Day, n (%) | 30 (26.3) | 52 (21.1) | 0.48 |
| 90-Day, n (%) | 38 (34.6) | 79 (32.9) | 0.950.95 |
| CABG Surgery Cohort | |||
| Mortality | |||
| Hospital, n (%) | 2 (1) | 8 (0.3) | 0.18 |
| 30-Day, n (%) | 2 (1) | 14 (4.7) | 0.02 |
| 90-Day, n (%) | 4 (1.9) | 14 (4.7) | 0.11 |
| Length of Stay | 9.82 (6.44) | 11.89 (13.67) | 0.24 |
| Readmissions | |||
| 30-Day, n (%) | 37 (18.7) | 58 (21.2) | 0.51 |
| 90-Day, n (%) | 56 (28.7) | 88 (32.3) | 0.40 |
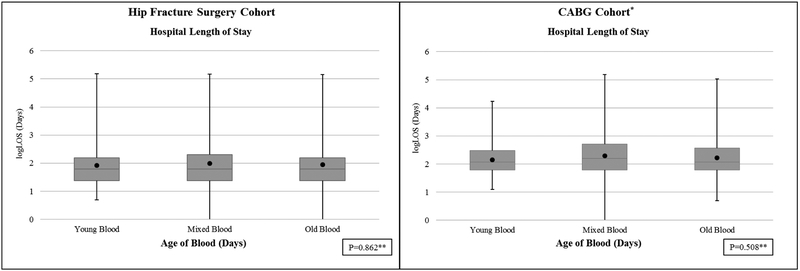
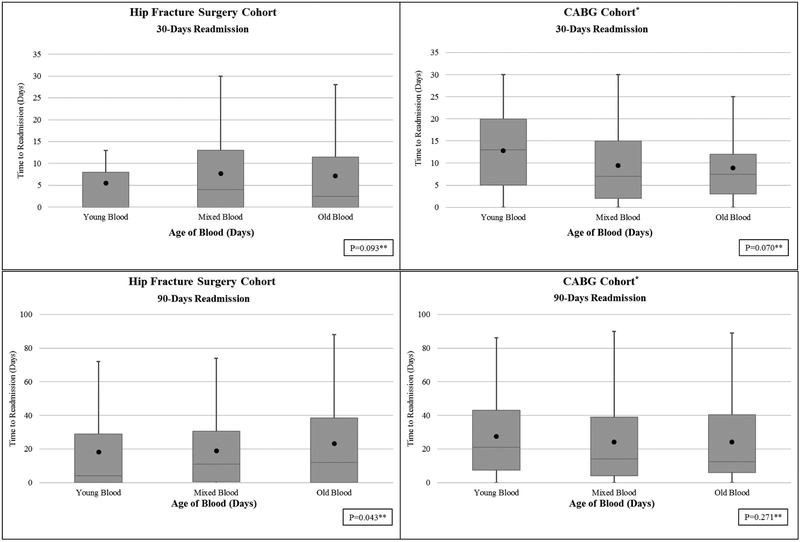
There were no differences in the hospital length of stay between young and old blood groups, 10.8 (22.3) days in young versus 11.5 (21.9) days in old blood group (p=0.86) (Figure 1). Similarly, the 30-day readmission rates (Figure 2) was not different among young and old blood age groups [young blood 30-day readmission rate (30/114, 26.3%), old blood (52/247, 21.1%) (p=0.09). In the hip surgery cohort, there were no differences in the 90-day readmission rate for young blood (38/110, 34.6%) compared to the old blood cohort (79/240, 32.9%), though the median time to readmission was shorter in the young blood group (p=0.04) (see Figure 2).
Figure 1.
Distribution of Length of Hospital Stay by Blood Group for Each Cohort
Black dot represents mean
LOS: Length of Stay
P value by Wilcoxon Rank Sum test used to compare Young Blood to Old Blood.
Figure 2.
Thirty and Ninety-Day Readmission Rates for Hip Surgery and Coronary Artery Bypass Graft Surgery Cohorts
Black dot represents mean
P value by Wilcoxon Rank Sum test used to compare Young Blood to Old Blood.
Table 4 shows the odds ratios (OR) for inpatient, 30-day, and 90-day mortality in the hip fracture surgery cohort. Patients in the old blood group had significantly higher odds for inpatient mortality compared with those in the young blood group (OR 166.8, 95% CI 1.067- >100) after adjusting for other relevant variables. Additionally, the age of the patient (OR 1.04, 95% CI 1.003–1.070) and the severity of illness (OR 1.95, 95% CI 1.28–2.95) were also found to be statistically significant. In contrast, receipt of mixed blood was not associated with higher odds of inpatient mortality (OR 20.9, 95% CI 0.13->100). After adjusting for relevant variables, receipt of old blood did not concur higher odds of 30-day readmission (OR 0.74, 95% CI 0.43–1.23), or of 90-day readmission (OR 0.91, 95% CI 0.55–1.47).
Table 4:
Multivariable Logistic Regression Odds Ratios (OR) for Inpatient, 30-day, and 90-day Mortality for Hip Fracture Surgery Cohort
| Inpatient mortality# OR (95% CI) |
30-day mortality OR (95% CI) |
90-day mortality OR (95% CI) |
|
|---|---|---|---|
| Old Blood vs. Young Blood | 166.80 (1.07, >100) |
1.51 (0.64,3.59) |
1.45 (0.63,3.33) |
| Mixed Blood vs. Young Blood | 20.90 (0.13,>100) |
0.73 (0.32,1.67) |
0.72 (0.33,1.60) |
| Age of Patient (per year) | 1.04 (1.00,1.07) |
1.04 (1.02,1.06) |
1.04 (1.02,1.06) |
| Male | 1.10 (0.49,2.48) |
1.31 (0.77,2.23) |
1.08 (0.64,1.83) |
| Caucasian race | 145665.78 (0.00, 9.42) |
2.13 (0.49,9.20) |
2.48 (0.57,10.73) |
| APACHE IIa | 1.95 (1.28,2.95) |
1.24 (1.16,1.32) |
1.28 (1.20,1.36) |
| CCIb | 1.05 (0.90,1.23) |
1.08 (0.97,1.20) |
1.10 (0.99,1.21) |
Interaction between Old Blood and APACHE II: p=0.02
APACHE II: Acute Physiology and Chronic Health Evaluation Score
CCI: Charlson Co-morbidity Index (per additional point)
CABG cohort
There were two hospital deaths (2/203, 1%) in the young blood group compared to eight in the old blood group (8/298, 0.3%) (p=0.18) (see Table 3). Data for mixed-blood recipients was not constrained and therefore is not shown in Table 3 (comparisons between groups were performed between old blood and young blood). Two patients (2/203, 1.0%) in the young blood group died within 30 days compared to 14 in the old blood group (14/298, 4.7%) (p=0.02). Four patients (4/203, 1.9%) in the young blood group died within 90 days compared to 14 in the old blood group (14/298, 4.7%) (p=0.11).
Table 5 shows the odds ratios (OR) for inpatient, 30-day, and 90-day mortality in the CABG cohort. Patients in the old blood group had significantly higher odds of 30-day mortality compared with those in the young blood group (OR 4.55, 95% CI 1.01–20.49, p=0.03). In addition, severity of illness (OR 1.10, 95% CI 1.04–1.16, p<0.01) and number of transfused units (OR 1.25, 95% CI 1.19–1.33) were also found to be statistically significant. In contrast, receipt of mixed blood was not associated with a higher 30-day mortality (OR 2.8, 95% CI 0.69–12.02).
Table 5:
Multivariable Logistic Regression Odds Ratios (OR) for Inpatient, 30-day, and 90-day Mortality for CABG Cohort
| Inpatient mortality OR (95% CI) |
30-day mortality OR (95% CI) |
90-day mortality OR (95% CI) |
|
|---|---|---|---|
| Old Blood vs. Young Blood | 2.48 (0.51,12.11) |
4.55 (1.01,20.49) |
2.19 (0.69,6.94) |
| Mixed Blood vs. Young Blood | 1.92 (0.45,8.21) |
2.88 (0.69,12.02) |
1.40 (0.49,4.01) |
| Age of Patient (Per year) | 1.00 (0.98,1.02) |
1.00 (0.99,1.03) |
1.01 (0.99,1.03) |
| Male | 0.79 (0.47,1.31) |
0.70 (0.46,1.07) |
0.84 (0.54,1.30) |
| Caucasian race | 0.79 (0.37,1.71) |
0.89 (0.44,1.77) |
0.92 (0.46,1.84) |
| APSa | 1.42 (1.24,1.60) |
1.10 (1.04,1.16) |
1.38 (1.24,1.53) |
| CCIb | 0.96 (0.85,1.08) |
0.98 (0.88,1.08) |
0.98 (0.88,1.08) |
| Number of transfused Units | 1.73 (1.48,2.01) |
1.25 (1.19,1.33) |
1.54 (1.34,1.77) |
| Pre-surgery Hemoglobin | 1.24 (1.09,1.42) |
1.18 (1.05,1.32) |
APACHE II: Acute Physiology and Chronic Health Evaluation Score
CCI: Charlson Co-morbidity Index (per additional point)
There were no differences in the hospital length of stay between young and old blood groups (see Figure 2), 9.82 (6.44) days in young versus 11.89 (13.67) days in old (p=0.51) (Figure 1). Similarly, the 30 and 90-day readmission rates (Figure 2) were not different among young and old blood age groups [young blood 30-day readmission rate (37/198, 18.7%), old blood (58/274, 21.2%) (p=0.07); young blood 90-day readmission rate (56/195, 28.7%), old blood (88/272, 32.3%) (p=0.27)]. After adjusting for relevant variables, receipt of old blood did not concur higher odds of 30-day readmission (OR 1.07, 95% CI 0.66–1.71), or of 90-day readmission (OR 1.09, 95% CI 0.72–1.65).
DISCUSSION
Our study results indicate that transfusion with red blood cells stored for ≥ 28 days could be associated with increased mortality as compared to transfusions with red blood cells stored for ≤14 days in both hip fracture surgery and CABG surgery patients. This suggests that the possible harmful effects that arise from the transfusion of older RBCs may occur in blood stored for ≥ 28 days. As our study focused on selected patient groups instead of a heterogeneous medical or surgical population, taking into account relevant clinical variables, we raise the possibility that transfusing old RBCs at the end of their storage limit could potentially be associated with adverse patient-related outcomes. Specifically, our multivariable logistic regression model accounted for differences in age, race, severity of illness, as well as comorbidities. Our study findings question the current transfusion practices and are suggestive of reevaluating our transfusion practices in hip fracture and CABG surgery patients.
Our findings are at odds with the recently concluded ABLE and RECESS clinical trials. Both ABLE and RECESS trials failed to show a negative impact with transfusion of relatively older blood. However, there are some caveats that need to be accounted for when interpreting their results17, 18. The ABLE trial was focused on a heterogeneous group of critically ill patients with majority of the patients (71%) admitted to the medical ICU. The average duration of storage was 6.1±4.9 days in the fresh-blood group versus 22.0±8.4 days in the standard-blood group. So along with being a diverse group, the patients were not exposed to RBCs nearing the end of their storage limit. This is in contrast to our study of specific patient groups undergoing hip fracture and CABG surgeries and receiving older blood for transfusion.
The RECESS trial on the other hand focused exclusively on cardiac surgery patients and compared outcomes between patients transfused with red cells stored for ≤ 10 days and patients transfused with red cells ≥ 21 days. Although the trial did not show differences in Multiple Organ Dysfunction Score or mortality, the authors acknowledged the limitation that their trial was not designed to assess the effects of RBCs transfused closer to the end of the storage time period. We exclusively studied patients receiving older RBCs stored for ≥ 28 days and comparing them with transfusions with RBCs stored for ≤ 14 days, hence leading to no overlap in age of RBCs transfused between study groups. These findings are also consistent with a recent similar analysis that showed adverse outcomes when patients were transfused blood stored for ≥ 28 and ≥ 35 days19. In contrast to mortality, our study did not show differences in LOS or readmission rates between patients transfused RBCs stored for ≥ 28 days and patients transfused RBCs stored for ≤ 14 days. This finding was similar to other large studies22, 23 and the aforementioned trials17, 18.
The potential harm in transfusing older red blood cells is attributed to storage lesions that occur over time5–7. While the exact mechanism of these changes is not completely delineated, RBCs undergo both structural and biochemical changes that impair their function and remain stagnant during storage in a low-temperature environment24. Reduction in adenosine tri-phosphate, glutathione, and pH can lead to morphological changes of the RBC membranes, decreasing RBC flexibility and stability25, 26. Additionally, pro-inflammatory proteins and lipids accumulate while decreased 2,3 DPG results in reduced RBC function and oxygen delivery27–29. Thus, transfusions of aged red blood cells have been postulated to result in a state of microvascular and metabolic derangement. There is also increasing hemolysis of RBCs as storage time increases, which could result in release of free hemoglobin. Excess free hemoglobin may affect the availability of nitric oxide, hence creating a disruption of vascular homeostasis30, 31. These changes could potentially explain the association seen between mortality and transfusion of old RBCs in our CABG and hip fracture surgery cohorts.
Our study has significant limitations, particularly due to the retrospective nature of the study, and our use of an administrative database. We can only imply an association between old blood transfusions and mortality but cannot ascertain cause and effect between transfusing RBC units older than 28 days and higher mortality in CABG and hip fracture repair patients. Additionally, the majority of the patients in each cohort were classified to the mixed blood group, which dilutes the strength of our execution gathering a large cohort and strictly defining old blood and young blood. However, this provided further contrast as receiving mixed blood was not associated with increased mortality. We focused only on hip fracture surgery and CABG patients and hence our findings are not applicable to other medical or surgical cohorts. Our analysis has several strengths. We adjusted for all the relevant variables including severity of illness and chronic comorbidities. We also included the number of units transfused in the CABG analysis to reduce confounding by indication.
In conclusion, we found that transfusing RBC units stored for greater than or equal to 28 days is associated with higher mortality for patients with hip fracture surgery and CABG procedure. Despite conflicting results in the literature, this suggests these specific patient populations may benefit from receiving transfusions with younger RBCs. However, this needs to be further explored in future studies.
Source of Funding and Conflict of Interest:
SK is supported by NHBLI 5T32HL091816–07. BK is supported by NIA K23-AG043476. BK received grant funding from the sponsor for conducting research. ML is employed by the sponsor, with stock ownership. AG is employed by a division of the sponsor company, with stock ownership.
The study was supported by an unrestricted grant awarded to the Regenstrief Institute from Zimmer Biomet, Inc. Data extraction and analyses are conducted independently at Regenstrief Institute and are not influenced by any financial interest that could affect or be affected by this research.
Footnotes
Sponsor’s Role:
The study sponsor’s role was limited to funding and technical consultation.
REFERENCES
- 1.Anderson SA, Menis M, Burwen DR. Blood use by inpatient elderly population in the United States. Transfusion. 2007;47:582–592 [DOI] [PubMed] [Google Scholar]
- 2.Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014; 371(15):1381–91. [DOI] [PubMed] [Google Scholar]
- 3.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368(1):11–21. [DOI] [PubMed] [Google Scholar]
- 4.Brunskill SJ, Millette SL, Shokoohi A, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev 2015(4):CD009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood 2006; 108(7):2455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kucukakin B, Kocak V, Lykkesfeldt J, et al. Storage-induced increase in biomarkers of oxidative stress and inflammation in red blood cell components. Scand J Clin Lab Invest 2011; 71(4):299–303. [DOI] [PubMed] [Google Scholar]
- 7.Ogunro PS, Ogungbamigbe TO, Muhibi MA. The influence of storage period on the antioxidants level of red blood cells and the plasma before transfusion. Afr J Med Med Sci 2010; 39(2):99–104. [PubMed] [Google Scholar]
- 8.Hamasaki N, Yamamoto M. Red blood cell function and blood storage. Vox Sang 2000; 79(4):191–7. [DOI] [PubMed] [Google Scholar]
- 9.Korgun DK, Bilmen S, Yesilkaya A. Alterations in the erythrocyte antioxidant system of blood stored in blood bags. Res Commun Mol Pathol Pharmacol 2001; 109(5–6):357–63. [PubMed] [Google Scholar]
- 10.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet 2007; 370(9585):415–26. [DOI] [PubMed] [Google Scholar]
- 11.Kay MM, Flowers N, Goodman J, et al. Alteration in membrane protein band 3 associated with accelerated erythrocyte aging. Proc Natl Acad Sci U S A 1989; 86(15):5834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimshaw K, Sahler J, Spinelli SL, et al. New frontiers in transfusion biology: identification and significance of mediators of morbidity and mortality in stored red blood cells. Transfusion 2011; 51(4):874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lelubre C, Vincent JL. Relationship between red cell storage duration and outcomes in adults receiving red cell transfusions: a systematic review. Crit Care 2013; 17(2):R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janz DR, Zhao Z, Koyama T, et al. Longer storage duration of red blood cells is associated with an increased risk of acute lung injury in patients with sepsis. Ann Intensive Care 2013; 3(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Likosky DS, Paone G, Zhang M, et al. Red Blood Cell Transfusions Impact Pneumonia Rates After Coronary Artery Bypass Grafting. Ann Thorac Surg 2015; 100(3):794–800; discussion 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008; 358(12):1229–39. [DOI] [PubMed] [Google Scholar]
- 17.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med 2015; 372(15):1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med 2015; 372(15):1410–8. [DOI] [PubMed] [Google Scholar]
- 19.Goel R, Johnson DJ, Scott AV, et al. Red blood cells stored 35 days or more are associated with adverse outcomes in high-risk patients. Transfusion 2016; 56(7):1690–8. [DOI] [PubMed] [Google Scholar]
- 20.Wagner DP, Draper EA. Acute physiology and chronic health evaluation (APACHE II) and Medicare reimbursement. Health Care Financing Review. 1984;1984(Suppl):91–105. [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME, Sax FL, MacKenzie CR, et al. Assessing illness severity: does clinical judgment work? J Chronic Dis 1986; 39(6):439–52. [DOI] [PubMed] [Google Scholar]
- 22.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg 1999; 178(6):570–2. [DOI] [PubMed] [Google Scholar]
- 23.van de Watering L, Lorinser J, Versteegh M, et al. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion 2006; 46(10):1712–8. [DOI] [PubMed] [Google Scholar]
- 24.Antonelou MH, Seghatchian J. Insights into red blood cell storage lesion: Toward a new appreciation. Transfus Apher Sci 2016. [DOI] [PubMed] [Google Scholar]
- 25.Nakao K, Wada T, Kamiyama T, et al. A direct relationship between adenosine triphosphate-level and in vivo viability of erythrocytes. Nature 1962; 194:877–8. [DOI] [PubMed] [Google Scholar]
- 26.Lang E, Pozdeev VI, Xu HC, et al. Storage of Erythrocytes Induces Suicidal Erythrocyte Death. Cell Physiol Biochem 2016; 39(2):668–76. [DOI] [PubMed] [Google Scholar]
- 27.Valeri CR, Collins FB. The physiologic effect of transfusing preserved red cells with low 2,3-diphosphoglycerate and high affinity for oxygen. Vox Sang 1971; 20(5):397–403. [DOI] [PubMed] [Google Scholar]
- 28.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2015; 55(1):205–19. [DOI] [PubMed] [Google Scholar]
- 29.Hess JR. Red cell changes during storage. Transfus Apher Sci 2010; 43(1):51–9. [DOI] [PubMed] [Google Scholar]
- 30.Roback JD, Neuman RB, Quyyumi A, et al. Insufficient nitric oxide bioavailability: a hypothesis to explain adverse effects of red blood cell transfusion. Transfusion 2011; 51(4):859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol 2009; 16(6):515–23. [DOI] [PubMed] [Google Scholar]