Abstract
In the 1960s and 70s, biochemical and pharmacological evidence was pointing toward glutamate as a synaptic transmitter at a number of distinct receptor classes, known as NMDA and non-NMDA receptors. The field, however, lacked a potent and highly selective antagonist to block these putative postsynaptic receptors. So, the discoveries in the early 1980s of d-AP5 as a selective NMDA receptor antagonist and of its ability to block synaptic events and plasticity were a major breakthrough leading to an explosion of knowledge about this receptor subtype. During the next 10 years, the role of NMDA receptors was established in synaptic transmission, long-term potentiation, learning and memory, epilepsy, pain, among others. Hints at pharmacological heterogeneity among NMDA receptors were followed by the cloning of separate subunits. The purpose of this review is to recognize the important contributions made in the 1980s by Graham L. Collingridge and other key scientists to the advances in our understanding of the functions of NMDA receptors throughout the central nervous system.
Keywords: Long-term potentiation (LTP), NMDA, NMDA receptors, APV, d-AP5, Synaptic plasticity
Introduction
The 1980s proved to be a decade where N-methyl-d-aspartate (NMDA) receptor-mediated neurotransmission became firmly established. Perhaps this is epitomized by the 1983 paper published by Graham Collingridge and colleagues in the Journal of Physiology that changed our understanding of neuronal plasticity and, more widely, of the functional role of NMDA receptors in the central nervous system (CNS) [1]. With Steven Kehl and Hugh McLennan, they showed that a new selective NMDA receptor antagonist, 2-amino-5-phosphonovalerate (APV), inhibited the induction of long-term potentiation (LTP) of the synaptic input to CA1 neurones in hippocampal slices (Fig. 1a). Although cautious at the time, stating that ‘NMA receptors…may play a role in synaptic plasticity’, this observation, and the use of this new pharmacological tool are at the core of the now established role of NMDA receptors in excitatory neurotransmission, in many forms of synaptic plasticity and hence in learning and memory. In this brief review, we will consider what led up to this important discovery, what other related events surrounded it, and what directly followed from these studies with APV in the 1980s.
Fig. 1.
d-AP5, NMDA and NMDA receptor-dependent synaptic plasticity in 1983. a Iontophoretic application of d-AP5 blocks induction of LTP, which can be readily induced after washout of the antagonist [1]. b Brief iontophoretic application of NMDA leads to a transient enhancement of field potential amplitude, which declines to baseline over time [1]. c Longer, bath application, of NMDA leads to a permanent depression of synaptic transmission [49]
APV is now more commonly known as 2-amino-5-phosphonopentanoate (AP5). Although some studies state that either the racemic mixture, d,l-AP5, or the single active isomer, d-AP5, was used, it is unclear from some reports, however, which chemical entity was used. Thus, for simplicity and because d-AP5 is the active moiety within the racemate, d-AP5 has been used throughout the main body of this review.
Background to 1980 Discoveries
In 1949, Hebb had proposed that changes in synaptic strengthening underlying learning required coincident pre- and post-synaptic activity [2] and, by the end of the 1960s, short lasting forms of synaptic plasticity were described in invertebrates and in the spinal cord. In their seminal review of 1968, Kandel and Spencer stated that ‘In contrast to the extensive data on spinal synapses, data on cortical synaptic plasticity are meager and, specifically, post-tetanic potentiation (PTP) has not yet been studied in detail comparable to that in the cord. This is unfortunate, since the complex morphology of cortical synapses may indicate a capability for unusual plastic alteration.’ Indeed, although long lasting depression [3] and facilitation [4] were already observed in hippocampal synapses there were very few other accounts of synaptic plasticity in the mammalian brain [5]. The phenomenon of long-term potentiation (LTP) was first detailed by Bliss and Lomo [6] in the dentate gyrus in vivo. Soon, however, hippocampal slices [7] became the preferred preparation for studying LTP [8–10]. LTP was shown to require cooperativity between strong afferent input from many fibres and a resulting strong depolarization of the postsynaptic neurone [11, 12]. Such potentiation was input specific so that other afferent inputs were unaffected [9] or reduced, i.e. heterosynaptic depression [13]. By contrast, a low rate of stimulation could lead to a long-term depression of all inputs [12]. The nature of the chemical transmitters involved in such processes was largely conjectural.
In the late 1970s, the concept emerged of different subtypes of glutamate receptor that mediate synaptic excitation in the central nervous system [14, 15]. Initial observations with several natural and recently synthesized acidic amino acids indicated that that N-methyl-d-aspartate (NMDA) was a considerably more potent excitant of central neurones than l-glutamate and l-aspartate [16, 17]. An early indication that there might be subtypes of receptors for these acidic acids was the finding that the ratios of potency between d,l-homocysteate or l-aspartate and l-glutamate, and later between NMDA and kainate, varied between different neuronal populations [18–20]. These findings were part of the developing concept of subtypes of glutamate receptors. Studies with other structurally constrained glutamate analogues from natural resources, such as kainic, domoic and quisqualic acids, suggested potential diversity of receptors mediating synaptic excitation. This diversity was supported by the observation that Mg2+ reduced the effectiveness of NMDA to a greater extent than most other glutamate analogues [21]. Further development of this concept required discovery of suitable antagonists. Longer chain analogues of glutamate, namely α-amino-adipic and -suberic and diaminopimelic acids, were weak, selective antagonists of NMDA-induced excitation rather than that induced by quisqualate, kainate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and reduced some synaptic events [22–27]. Thus the concept of NMDA and non-NMDA (later to be known as AMPA and kainate) receptors became accepted [28].
By the mid-1970 s, there were a number of papers reporting the presence, uptake and calcium-dependent release of l-glutamate and/or l-aspartate, which provided powerful evidence toward a transmitter role for these acidic amino acids [29] but the lack of selective, potent and established receptor antagonists slowed further progress in this field.
d-AP5, NMDA and LTP
Hence, the description of 2-amino-phosphonovaleric acid [30] as a potent and selective NMDA receptor antagonist at synapses on spinal neurones was the breakthrough needed to allow a thorough investigation of the physiological role of NMDA receptors.
Collingridge, being a Bristol graduate with Jeff Watkins, a PhD student with John Davies and a postdoc with Hugh McLennan, was in a good position to examine the effects of this new pharmacological tool on hippocampal slices in vitro, a preparation being used for detailed electrophysiology. Thus, Collingridge and collaborators first demonstrated that d-AP5, a gift from Jeff Watkins, was a more potent and selective NMDA receptor antagonist than previously used compounds, the activity lying mainly in the d-isomer [31]. With his co-authors, he then went on to show that d-AP5 reduced the synaptic potentiation in the CA1 region that followed high frequency stimulation of the Schaffer collateral input (Fig. 1a) with minimal effect on synaptic potentials at low stimulation frequencies [1]. Thus, the role of NMDA receptors in the initiation of LTP following high frequency stimulation was established in this highly quoted paper (1830 citations; Web of Science; September 2018).
This basic observation, in hippocampal slices, of the role of NMDA receptors in synaptic plasticity was rapidly seized upon and replicated by other major researchers in LTP using different paradigms but with a common d-AP5-sensitive theme: Schaffer collateral/commissural pathways to CA1 [32–35], perforant pathway to dentate gyrus in vivo [36, 37]. However, LTP at some hippocampal synapses appeared not to be mediated by NMDA receptors. For example, only the commissural, and not the mossy fibre, input to CA3 was sensitive to d-AP5 [38].
The use of d-AP5 allowed the role of NMDA receptors in LTP to be extended to rat visual cortical slices, although in this tissue GABAergic inhibition appears to play a more important modulating role than in the hippocampus [39]. In parallel, Wolf Singer’s group showed that, at a critical period of development in the kitten visual cortex, d-AP5 also prevented the normal developmental process of activity-dependent modifications, which results in orientation selectivity of neurones in the visual cortex [40, 41]. NMDA receptor antagonists prevent both the loss of inappropriate synaptic connections and the strengthening of correct connections. Another form of learning during development mediated by NMDA receptors is imprinting in day-old chicks, a phenomenon in which both the learning itself and the subsequent increase in glutamate binding are sensitive to d-AP5 [42, 43].
Concurrent with these observations in mammals, the development of a retinotopic map in the tectum of frogs and goldfish was also reported to be impaired by d-AP5 [44–46]. Part of this re-wiring may require the growth of neurites and dendrites as well as cell survival processes that are also NMDA receptor-dependent [47, 48]. Interestingly both the ability to induce cortical LTP and the density of NMDA receptors appeared to peak during this critical period for development of cortical connections, stressing the importance of NMDA receptors in this form of plasticity [49].
Is NMDA Receptor Activation Sufficient for Inducing LTP?
The discovery that d-AP5 blocked induction of LTP suggested that application of NMDA alone should be sufficient to induce plasticity. As shown in the original paper, brief exposure to NMDA results only in a transient enhancement of field potentials (Fig. 1b, [1]). In contrast, a longer application of NMDA (Fig. 1c, [50]) or glutamate [50] resulted in a depression of synaptic transmission, later recognized as NMDA receptor-dependent chemical LTD [51]. Similarly, low frequency afferent stimulation, besides limiting the induction of LTP [52], can also induce a long-term depression of synaptic transmission [12], shown in the 1990s to be d-AP5-sensitive [53, 54].
The transient enhancement of the amplitude of the field potentials, seen following NMDA application (Fig. 1b), seemed similar to the initial decremental phase of LTP (Fig. 1a), termed short-term potentiation (STP); STP, just like LTP, was d-AP5-sensitive raising the question whether STP was essential to the establishment of LTP or whether it was a mechanistically distinct parallel event [1, 55, 56]. Eventually it was shown that NMDA receptors of different subunit composition mediate induction of STP versus LTP [57] and that NMDA-induced enhancement of the field potential amplitude is distinct from STP, which is associated with a change in slope of field responses [58]. Gary Lynch’s group, did, however, show that successful induction of chemical LTP could be achieved when application of NMDA was followed by a brief application of d-AP5 [59], the antagonist possibly preventing the longer activation of NMDA receptors required for the induction of LTD, thus revealing the chemical LTP.
Why NMDA Receptors for LTP?
Understanding why NMDA receptors play a unique role in synaptic potentiation depended on two key observations.
The first relates to the explanation of (i) the strange current–voltage curve of the NMDA receptor [60] and (ii) why Mg2+ ions inhibit responses to bath application of NMDA [21]. The discovery was that Mg2+ ions produce a voltage-dependent brake on channel conductance particularly at hyperpolarised membrane potentials [61, 62]. The Schaffer collaterals release glutamate onto both NMDA and AMPA receptors, the latter dominating the synaptic potential because of the Mg2+ block of the NMDA receptor. Removal of Mg2+ ions uncovered a slow NMDA component of the EPSP [63]. The NMDA receptor component also rises more slowly than the AMPA receptor component, which decays quickly not giving sufficient time for the Mg2+ block to be fully removed. The depolarisation resulting from AMPA receptor activation is not an absolute requirement: with AMPA receptors blocked, a slow synaptic depolarisation mediated by NMDA receptors is uncovered [64–67]. Thus the depolarization that follows temporal (or spatial) summation during high frequency stimulation of excitatory synaptic inputs is required to relieve the Mg2+ ion block, which immediately increases the conductance of the NMDA receptor-coupled channel [68, 69]. This slow NMDA receptor component can be observed during high frequency stimulation beneath the AMPA receptor-mediated synaptic potentials [70].
The second key observation is that NMDA receptors are readily permeable to calcium when the voltage-dependent Mg2+ ion block is relieved [71]. The resultant increase in intracellular calcium, which can be visualised in dendritic spines receiving NMDA receptor activation [72] is the main driving force for plasticity in LTP induction protocols [73, 74]. Calcium activates a complex array of secondary intracellular events, including up-regulation of AMPA receptors at the potentiated synapse [75–78] and activation of protein kinases [79–82], that act as molecular switches [83, 84] and that also regulate protein synthesis dependence of the late phases of LTP [85, 86]. Much of this, including recruitment of glutamate receptors to dendritic spines, was debated early [87, 88] and detailed in subsequent decades [89–91]. Nevertheless, although the field was in general agreement about the central role of NMDA receptors in initiating LTP, there was little consensus about the mechanisms of LTP expression, which could be mediated by pre-synaptic and post-synaptic mechanisms alike [92]. The differences in the outcome of various NMDA receptor activation protocols depends among others on the extent to which different intracellular messaging systems are engaged and the type of synaptic plasticity that is induced or maintained [93, 94].
A further factor to consider is the role of inhibitory synapses, which are recruited when afferent pathways are stimulated with a tetanic pattern, including Schaffer collateral-commissural fibres into CA1. GABAergic hyperpolarisation helps maintain the Mg2+ brake on the NMDA receptor conductance. Blocking GABA-A receptor-mediated inhibition reveals the NMDA receptor component at low and high frequencies of stimulation [68, 95] and facilitates LTP [96, 97]. During high frequency bursts, postsynaptic GABAergic inhibition declines and hence allows calcium flux through NMDA receptor channels [98]. The more natural theta stimulation allows very short trains of stimuli to induce LTP [99, 100] in which postsynaptic GABAergic inhibition is less prominent, itself being regulated by presynaptic GABA-B receptor-mediated inhibition [93, 94].
Temporal summation or frequency dependence of the recruitment of NMDA receptors, the resultant dendritic depolarization and calcium entry are the driving forces of LTP [70, 101]. Thus, NMDA receptors function as coincidence detectors that sense synchronised pre- and post-synaptic activity and uniquely allow for the Hebbian principle of cooperativity, between strong afferent input and marked postsynaptic depolarization, which is required for synaptic strengthening [68, 69, 102, 103]. This aspect of cooperativity can be side-stepped, as described above, by a small postsynaptic depolarization, reducing extracellular Mg2+ ion concentration or reducing post- or pre-synaptic GABAergic inhibition, when low frequency stimulation can induce LTP [68, 103–105].
Ubiquity of NMDA Receptors
As is apparent from the above sections, NMDA receptors are not unique to the Schaffer collateral synapse on the CA1 hippocampal pyramidal neurones. The development of d-AP5, as a potent and selective NMDA receptor antagonist, allowed the role of NMDA receptors to be more widely investigated.
Indeed reports of a transmitter role for NMDA receptors onto spinal neurones in vivo using weaker NMDA receptor antagonists (see above) preceded the hippocampal papers. Interestingly using d-AP5, a single stimulus of peripheral afferents, unlike the initial reports in the hippocampus [1], could evoke NMDA receptor-mediated synaptic potentials in spinal neurones [30, 106]. The causal features of this difference are likely to be the more depolarized state in vivo and the temporal and spatial summation that occurs following stimulation of a mixed population of primary afferents and internuncial neurones in the spinal cord experiments. Frequency-dependent depolarization and potentiation, sensitive to NMDA receptor antagonists such as d-AP5, are also seen in these spinal pathways [107, 108].
Throughout the 1980s, d-AP5 was used to demonstrate a transmitter role for NMDA receptors throughout the brain; substantia nigra [109], dentate gyrus [110], interpeduncular nucleus [111], cerebellar Purkinje cells [112], neocortical neurones [113], red nucleus [114] and ventro-basal thalamus [115] as well as in sympathetic ganglia [116].
Most of the above studies were performed on rats or other mammals, although NMDA receptor-mediated d-AP5-sensitive synaptic excitation was earlier demonstrated in the spinal cord of amphibians [117–119] and of fish [120, 121] and in the retina of fish [122, 123]. Interestingly, superfusion of the exposed spinal cord with NMDA is able to initiate fictive locomotion in both frogs and lampreys, a pattern blocked by d-AP5 [118, 120].
Thus it became apparent in the 1980s that NMDA receptors were important mediators of synaptic transmission throughout the central nervous system of vertebrates, although the question of the natural transmitter was still unanswered. l-Aspartate and l-glutamate, although mimicking the effects of NMDA, were considerably less potent than NMDA itself in most assays, despite biochemical evidence supporting a transmitter role for these two amino acids [29]. Two key observations in the 1980s supported l-glutamate as the candidate. Firstly, in the absence of amino acid transport processes, l-glutamate became tenfold more potent than NMDA on dissociated neurones [124]. Secondly, in binding studies, l-glutamate was tenfold more potent than l-aspartate as an inhibitor of radioactive d-AP5 binding to NMDA receptors in rat brain membranes [125] and had an indistinguishable autoradiographic distribution to d-AP5 in rat brain [126].
Epilepsy
Epilepsy results from changes in brain circuitry excitability that lead to bursts of cortical activity arising spontaneously or from otherwise subthreshold events. A prime example of such epileptogenesis is the phenomenon of kindling, a form of plasticity following repetitive brain stimulation that leads to epilepsy-like convulsions. Kindling has features in common with LTP [127, 128]. In particular, d-AP5 prevents the induction of the epileptiform activity, but also reduces the resultant seizure-like discharges, following kindling protocols [129, 130].
However, the first real evidence of the role of NMDA receptors in epilepsy came from in vivo studies in Harry Bradford’s and Brian Meldrum’s laboratories. They showed that local administration of d-AP5 reduced seizures resulting from a cobalt-induced lesion [131] and both sound-induced seizures in DBA-2 mice and pentylenetetrazol-induced seizures in Swiss mice [132] as well as photic stimulated epilepsy in primates [133]. The striking correlation in potency between NMDA receptor antagonism in vitro and that against seizures of three competitive NMDA receptor antagonists substantiated the importance of NMDA receptors as anticonvulsants [132].
This was followed by many publications showing that both induction and maintenance of many forms of epileptiform activity in hippocampal slices [95, 134–137] and in cortical slices [138, 139] could be prevented by d-AP5. This included the blocking of ex vivo bursting epileptogenic foci in kainate-lesioned rat hippocampi [140] and in surgically removed human neocortex [141]. The bursting pattern of layer 4/5 neurones during slow wave sleep was also blocked by local ejection of d-AP5 [142].
The role of NMDA receptors in, and the use of NMDA receptor antagonists for, various forms of epilepsy is still a subject of therapeutic interest.
Pain
Another therapeutically important aspect of plasticity is the development of neuropathic and other chronic pain conditions, for example phantom limb pain. Such maladapted plasticity may lead to hyperalgesia and allodynia, two symptoms that indicate nociceptive pathways have been abnormally strengthened or new ones formed.
Because, as mentioned earlier, the polysynaptic excitation of spinal neurones following afferent stimulation of hind limb nerves is mediated by NMDA receptors [22–24, 30, 106], it was not surprising that NMDA and d-AP5, respectively, induced and reduced nociceptive responses following local application to the spinal cord [143–145].
Concerning plasticity, the phenomenon of ‘wind-up’ whereby repetitive nociceptor fibre stimulation leads to a potentiated response of spinal neurones [146] is thought to underlie central sensitization leading to hyperalgesia. This form of plasticity is prevented by d-AP5 following local spinal application in vivo [107] and following bath application in vitro [147]. Unilateral foot paw tissue damage may result in secondary hyperalgesia in the contralateral limb, which can be prevented by spinal administration of d-AP5 [148]. Nevertheless weak bioavailability of d-AP5 has limited its use in vivo for researching the role of NMDA receptors in various pain states.
d-AP5 and Memory
Because LTP is thought to be one of the mechanisms underlying learning and memory, it was not surprising that the effects of d-AP5 were assessed in learning paradigms rapidly following the description of its block of LTP [1]. Parallels had already been drawn between the rate of decline of LTP and of loss of memory in older rats (reviewed in [149]).
Because of the low bioavailability of competitive NMDA receptor antagonists in general, d-AP5 was injected into the cerebral ventricles (i.c.v.) in the early experiments of Richard Morris and collaborators. Treated and untreated rats, placed in a large pool of opaque water, were compared for their ability to learn the position of a hidden escape platform over a 5 day period, a task now known as the Morris Water Maze. The results were highly significant in that the d-AP5 treated animals took much longer to learn the location of the platform, spending much less time than the controls in the correct quadrant of the pool [36]. d-AP5-treated animals did not show deficits in a visual discrimination test suggesting a role for NMDA receptors specifically in spatial learning, which is thought to be a hippocampus-based phenomenon. By changing the time of administration of d-AP5, they were able to show that, in parallel with LTP, NMDA receptors were required for the acquisition or encoding of memory but not for its storage or retrieval [150], see also [151].
Similarly, i.c.v. d-AP5 disrupted acquisition of short term memory (radial maze) and attenuated retention of long term memory (passive avoidance) provided the drug was injected before the learning phase [152] and prior administration of d-AP5 could result in memory decline in an operant learning paradigm [153]. Acquisition of odour discrimination was also prevented by d-AP5 but previously learned memories were not disrupted [154]. These data extended the concept of an NMDA receptor-mediated LTP-like plasticity requirement from the hippocampal-based spatial domain to other forms of learning and memory.
Neurotoxicity and Neuroprotection
In contrast to its positive role in neuroplasticity, excess NMDA receptor activation can lead to d-AP5-sensitive neurodegeneration [155, 156]. This sensitivity to NMDA-induced neurotoxicity varies between populations of neurones, a finding likely related to the differences in NMDA receptor expression and/or calcium buffering [157]. Release of glutamate following excessive and/or prolonged stimulation of neuronal pathways can also result in d-AP5-sensitive degeneration of targetted neurones [158] similar to that following epileptiform activity in hippocampal slices [159, 160].
Brain ischaemia and hypoglycaemia lead to high extracellular levels of glutamate [161–163]. Although competitive NMDA receptor antagonists have been shown to be effective in reducing neuronal cell loss following temporary carotid artery occlusion [164] and hypoglycaemia [165], the hope for them as clinical agents [166] has not yet been realized.
Beyond d-AP5: Medicinal Chemistry Around the NMDA Receptor
Although the synthesis of NMDA was first reported in 1962 [167], it wasn’t until much later that chemists developed more potent agonists by conformational restriction of either aspartate or glutamate (reviewed in [174]). Such agonists include α-tetrazolylglycine [168], the cyclobutane trans-ACBD [169, 170] and the cyclopropanes d-CCG-II and l-CCG-IV [171–173].
Following the success of d-AP5 in forwarding our understanding of the role of NMDA receptors, medicinal chemists in academia and industry continued to develop new compounds in order to increase potency and/or bioavailability (reviewed in [174]). Increasing the affinity of d-AP5 was achieved by conformational restriction for example by incorporating a double bond into the side chain (e.g. CGP 37849 and its α-carboxyethyl ester CGP 39551, [175]), or incorporating the α-amino group and some of the side chain into a piperidine ring (e.g. CGS 19755, [176]). Like d-AP5, D-AP7, a longer chain analogue, was also found to be a competitive NMDA receptor antagonist and blocked LTP whereas d-AP4, D-AP6 and D-AP8 were essentially inactive [32, 177, 178]. Conformational restriction of D-AP7 led to the development of high affinity antagonists such as the piperazine derivatives d-CPP [179, 180] and d-CPPene [181], the decahydroisoquinoline LY274614 [182] and the phenylalanine SDZ EAB515 [183].
Several of these high affinity NMDA receptor antagonists were radiolabelled (e.g. [3H]AP5 [125], [3H]CPP [184], [3H]CGS19755 [185] and [3H]CGP 39653 [186]). They were used in binding assays and alongside [3H]glutamate [187, 188] and [3H]MK-801 [189] (a high affinity channel blocker) in autoradiography, to study the distribution of native NMDA receptors throughout different brain regions.
High affinity NMDA receptor antagonists were used in animal models of CNS disorders and were found to be anticonvulsant in models of epilepsy, neuroprotective in models of cerebral ischaemia and to be effective in models of chronic pain. Some, such as d-CPPene, also were taken into clinical trials for prevention of brain damage following stroke or head injury and for treatment resistant forms of epilepsy. Positive outcomes from such clinical trials, e.g. with d-CPPene and CGS19755, have been prevented by the occurrence of side effects, particularly of a psychogenic nature [190, 191] .
Coincidental but Related Pharmacological Discoveries of the 1980s
Interestingly, Collingridge and collaborators were not the only group studying LTP pharmacologically in 1983. Patrice Guyenet’s laboratory was independently showing that the effects of phencyclidine, ketamine and sigma opiates blocked the long term potentiation of the population spike in CA1 region of the hippocampal slices [192, 193]. Equally independent was the observation that phencyclidine, ketamine and sigma opiates were selective NMDA receptor antagonists on spinal neurones in vivo [194–196]. Thus, these two independent groups coincidentally provided extra support for the role of NMDA receptors in LTP [1].
Unlike competitive NMDA receptor antagonists, ketamine blocks within the receptor-coupled channel [197], pharmacologically mimicking the voltage-dependent block of Mg2+ ions but with slower kinetics. Although there are concerns related to the specificity of ketamine and phencyclidine as NMDA receptor antagonists, particularly at higher concentrations [198], its rapid CNS bioavailability and reversibility following systemic administration makes low doses of ketamine particularly useful for studying the effects of NMDA receptors in vivo.
By the 1980s, many of the pharmacological and clinical properties of ketamine were already established in the absence of knowledge of it as an NMDA receptor antagonist. Developed as an anaesthetic, it was known for its good analgesia and its safety but with recognized emergence phenomena including hallucinations [199]. In the 1980s striking similarities between the actions of ketamine and d-AP5 emerged. For example, ketamine’s effect on polysynaptic responses of spinal neurones [194, 195], on hippocampal LTP [192], on spinal ‘wind-up’ [108], on cortical synaptic transmission [200], on cortical epileptiform activity [201], on sound-induced seizures [202] and on ocular dominance in the visual cortex [203] echoed the effects of d-AP5 cited above.
Another major aspect of the NMDA receptor’s pharmacology was discovered in the 1980s. Philippe Ascher’s group showed that glycine, or a glycine-like substance such as d-serine, was a required co-agonist for NMDA receptor activation [204]. This glycine-site was not sensitive to the traditional inhibitory antagonist, strychnine. Instead, compounds such as HA-966 and 7-chlorokynurenate were shown to be NMDA receptor antagonists acting via this glycine site (for example [205–208]).
It is beyond the scope of this review to describe all the contributions that using ketamine and other non-competitive NMDA antagonists including glycine-site antagonists have made to our understanding of the importance of NMDA receptors. Some of this literature, concerning the effects of ketamine in synaptic plasticity, neuroprotection, epilepsy, pain and behaviour, is cited in previous reviews [198, 209].
Heterogeneity Within NMDA Receptors
Also in the 1980s, the possibility of subtypes of the NMDA receptor was first raised. Differential sensitivity of brain regions to quinolinic acid, a weak naturally occurring NMDA receptor agonist [210–212] suggested NMDA1 and NMDA2 receptor subtypes. Similarly, regional differences in the sensitivity to glycine and to a variety of NMDA receptor antagonists [213] and to differential stimulation of [3H]MK-801 binding by l-glutamate in different brain regions [214, 215] and relative affinity of various competitive antagonists in autoradiography studies [216, 217] reinforced the idea of heterogeneity in NMDA receptor subtypes. Specific profiles were noted between the rat medial thalamus, the forebrain and the cerebellum [218].
Such suggestions pre-dated the cloning of NMDA receptor subunits in the early 1990s, which confirmed this heterogeneity. The first cloned subunit [219] is now called GluN1 and is the glycine-sensitive subunit. Cloning of the four glutamate-sensitive subunits, GluN2A-D followed soon [220] and of two more glycine-sensitive GluN3 subunits followed later (reviewed in [221]). Defining the roles of the NMDA receptor subunits in aspects of plasticity has become a major interest of Collingridge and many others [57, 222–230].
Conclusions
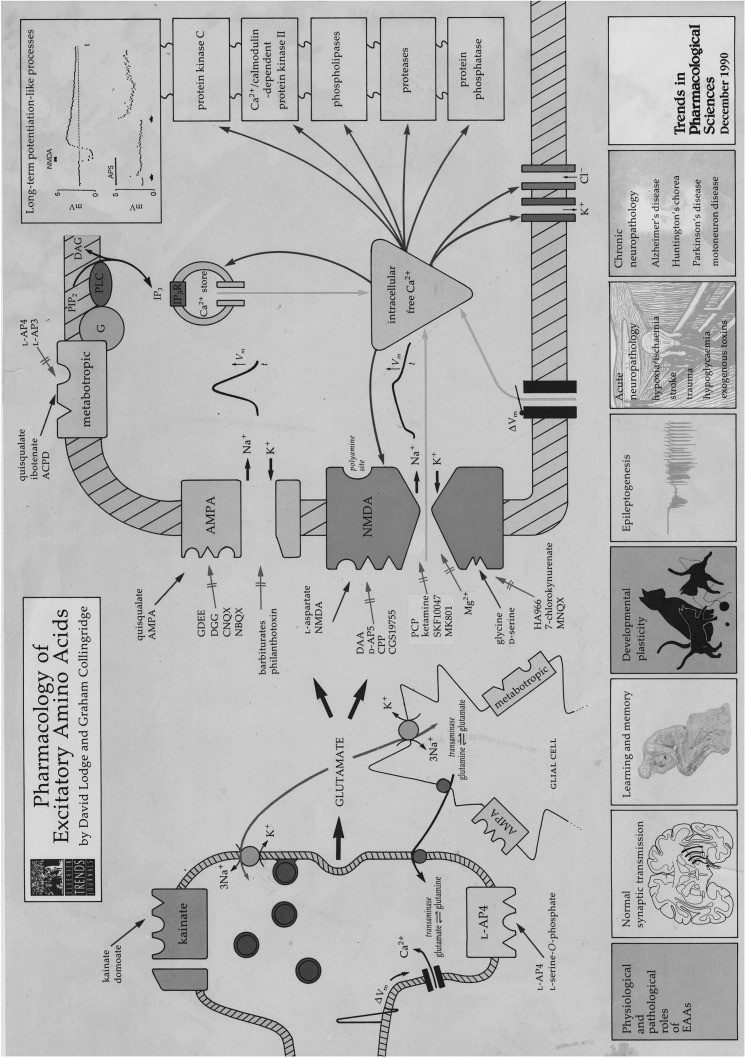
The growing evidence of the role of glutamate and of NMDA receptors in particular, in synaptic transmission received a considerable boost in the 1980s. This was largely driven by the discovery of the highly selective NMDA receptor antagonist, d-AP5, which enabled its use to establish a role for NMDA receptors in synaptic transmission and plasticity [1, 30]. This review has focussed on some examples of the resulting explosion in knowledge, which were more thoroughly described in a 1991 Supplement of Trends in Pharmacological Sciences, which also included a poster depicting pharmacological tools that were available for targeting of glutamate receptors, synaptic transmission and plasticity Figs. 2, 3.
Fig. 2.
Trends in pharmacological sciences: Special Report 1991. Cover page: This supplement was a compilation of the articles published each month during 1990 on the theme: “The Pharmacology of Excitatory Amino Acids” edited by David Lodge, and Graham L. Collingridge with Alison Abbott of Elsevier. The supplement was sponsored by Leslie L. Iversen of Merck & Sharp and Dohme Research Laboratories. The Glutamate Tree of Life is represented in the “Cover design by Nigel Hynes, based on an original idea of David Lodge”
Fig. 3.
Pharmacology of excitatory amino acids: 1991 Poster. This accompanying Poster summarised what had been published in the Special Report. Much of what was simplistically sketched in 1991 still holds true today in 2018 with major advances that have been made in many areas. In particular our knowledge about glutamate receptor involvement in disease has shown huge advances together with advances in the molecular biology, crystal structure, genetics and intracellular signalling of glutamate receptors. Absence of metabotropic glutamate receptors on the illustrated glutamatergic terminal and of pharmacological tools for these G-protein coupled receptors are obvious omissions. Two areas of neuropsychiatry, namely schizophrenia and depression, are now widely linked with NMDA receptor function
Acknowledgements
Supported by The Royal Society (RSG\R1\180384) and the MRC (MR/K023098/1).
Compliance with Ethical Standards
Conflict of interest
The authors have no competing interests to declare.
Footnotes
Special issue: In honor of Graham Collingridge
References
- 1.Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebb DO. The organization of behavior: a neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- 3.Andersen P. Interhippocampal impulses. III. Basal dendritic activation of CA3 neurons. Acta Physiol Scand. 1960;48:209–230. doi: 10.1111/j.1748-1716.1960.tb01857.x. [DOI] [PubMed] [Google Scholar]
- 4.Lømo T. Frequency potentiation of excitatory synaptic activity in the dentate area of the hippocampal formation. Acta Physiol Scand. 1966;68(Suppl. 277):128. [Google Scholar]
- 5.Kandel ER, Spencer WA. Cellular neurophysiological approaches in the study of learning. Physiol Rev. 1968;48:65–134. doi: 10.1152/physrev.1968.48.1.65. [DOI] [PubMed] [Google Scholar]
- 6.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skrede KK, Westgaard RH. The transverse hippocampal slice: a well-defined cortical structure maintained in vitro. Brain Res. 1971;35:589–593. doi: 10.1016/0006-8993(71)90508-7. [DOI] [PubMed] [Google Scholar]
- 8.Schwartzkroin PA, Wester K. Long-lasting facilitation of a synaptic potential following tetanization in the in vitro hippocampal slice. Brain Res. 1975;89:107–119. doi: 10.1016/0006-8993(75)90138-9. [DOI] [PubMed] [Google Scholar]
- 9.Andersen P, Sundberg SH, Sveen O, Wigström H. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature. 1977;266:736–737. doi: 10.1038/266736a0. [DOI] [PubMed] [Google Scholar]
- 10.Dunwiddie T, Madison D, Lynch G. Synaptic transmission is required for initiation of long-term potentiation. Brain Res. 1978;150:413–417. doi: 10.1016/0006-8993(78)90293-7. [DOI] [PubMed] [Google Scholar]
- 11.McNaughton BL, Douglas RM, Goddard GV. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978;157:277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- 12.Dunwiddie T, Lynch G. Long-term potentiation and depression of synaptic responses in rat hippocampus—localization and frequency dependency. J Physiol. 1978;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- 14.Watkins JC, Jane DE. The glutamate story. Br J Pharmacol. 2006;147(Suppl 1):S100–S108. doi: 10.1038/sj.bjp.0706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodge D. The history of the pharmacology and cloning of ionotropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology. 2009;56:6–21. doi: 10.1016/j.neuropharm.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Curtis DR, Watkins JC. Analogues of glutamic and gamma-amino-n-butyric acids having potent actions on mammalian neurones. Nature. 1961;191:1010–1011. doi: 10.1038/1911010a0. [DOI] [PubMed] [Google Scholar]
- 17.Curtis DR, Watkins JC. Acidic amino acids with strong excitatory actions on mammalian neurones. J Physiol. 1963;166:1–14. doi: 10.1113/jphysiol.1963.sp007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLennan H, Huffman RD, Marshall KC. Patterns of excitation of thalamic neurones by amino-acids and by acetylcholine. Nature. 1968;219:387–388. doi: 10.1038/219387a0. [DOI] [PubMed] [Google Scholar]
- 19.Duggan AW. The differential sensitivity to L-glutamate and L-aspartate of spinal interneurones and Renshaw cells. Exp Brain Res. 1974;19:522–528. doi: 10.1007/BF00236115. [DOI] [PubMed] [Google Scholar]
- 20.McCulloch RM, Johnston GA, Game CJ, Curtis DR. The differential sensitivity of spinal interneurones and Renshaw cells to Kainate and N-methyl-D-aspartate. Exp Brain Res. 1974;21:515–518. doi: 10.1007/BF00237169. [DOI] [PubMed] [Google Scholar]
- 21.Evans RH, Francis AA, Watkins JC. Selective antagonism by Mg2+ of amino acid-induced depolarization of spinal neurones. Experientia. 1977;33:489–491. doi: 10.1007/BF01922227. [DOI] [PubMed] [Google Scholar]
- 22.Biscoe TJ, Evans RH, Francis AA, et al. D-alpha-Aminoadipate as a selective antagonist of amino acid-induced and synaptic excitation of mammalian spinal neurones. Nature. 1977;270:743–745. doi: 10.1038/270743a0. [DOI] [PubMed] [Google Scholar]
- 23.Biscoe TJ, Davies J, Dray A, et al. Depression of synaptic excitation and of amino acid induced excitatory responses of spinal neurones by D-alpha-aminoadipate, alpha, epsilon-diaminopimelic acid and HA-966. Eur J Pharmacol. 1977;45:315–316. doi: 10.1016/0014-2999(77)90017-6. [DOI] [PubMed] [Google Scholar]
- 24.Lodge D, Headley PM, Curtis DR. Selective antagonism by D-alpha-aminoadipate of amino acid and synaptic excitation of cat spinal neurons. Brain Res. 1978;152:603–608. doi: 10.1016/0006-8993(78)91117-4. [DOI] [PubMed] [Google Scholar]
- 25.Collingridge GL, Davies J. An evaluation of D-alpha-aminoadipate and D-(and DL-)alpha-aminosuberate as selective antagonists of excitatory amino acids in the substantia nigra and mesencephalic reticular formation of the rat. Neuropharmacology. 1979;18:193–199. doi: 10.1016/0028-3908(79)90061-3. [DOI] [PubMed] [Google Scholar]
- 26.McLennan H, Lodge D. The antagonism of amino acid-induced excitation of spinal neurones in the cat. Brain Res. 1979;169:83–90. doi: 10.1016/0006-8993(79)90375-5. [DOI] [PubMed] [Google Scholar]
- 27.Krogsgaard-Larsen P, Honoré T, Hansen JJ, et al. New class of glutamate agonist structurally related to ibotenic acid. Nature. 1980;284:64–66. doi: 10.1038/284064a0. [DOI] [PubMed] [Google Scholar]
- 28.Watkins JC, Evans RH. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- 29.Johnston GA. Glutamate and aspartate as transmitters in the spinal cord. Adv Biochem Psychopharmacol. 1976;15:175–184. [PubMed] [Google Scholar]
- 30.Davies J, Francis AA, Jones AW, Watkins JC. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981;21:77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- 31.Collingridge GL, Kehl SJ, McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris EW, Ganong AH, Cotman CW. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984;323:132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- 33.Wigström H, Gustafsson B. A possible correlate of the postsynaptic condition for long-lasting potentiation in the guinea pig hippocampus in vitro. Neurosci Lett. 1984;44:327–332. doi: 10.1016/0304-3940(84)90044-2. [DOI] [PubMed] [Google Scholar]
- 34.Larson J, Lynch G. Role of N-methyl-D-aspartate receptors in the induction of synaptic potentiation by burst stimulation patterned after the hippocampal theta-rhythm. Brain Res. 1988;441:111–118. doi: 10.1016/0006-8993(88)91388-1. [DOI] [PubMed] [Google Scholar]
- 35.Nicoll RA, Kauer JA, Malenka RC. The current excitement in long-term potentiation. Neuron. 1988;1:97–103. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- 36.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 37.Errington ML, Lynch MA, Bliss TV. Long-term potentiation in the dentate gyrus: induction and increased glutamate release are blocked by D(-)aminophosphonovalerate. Neuroscience. 1987;20:279–284. doi: 10.1016/0306-4522(87)90019-4. [DOI] [PubMed] [Google Scholar]
- 38.Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- 39.Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- 40.Kleinschmidt A, Bear MF, Singer W. Blockade of “NMDA” receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987;238:355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- 41.Gu QA, Bear MF, Singer W. Blockade of NMDA-receptors prevents ocularity changes in kitten visual cortex after reversed monocular deprivation. Brain Res Dev Brain Res. 1989;47:281–288. doi: 10.1016/0165-3806(89)90183-1. [DOI] [PubMed] [Google Scholar]
- 42.McCabe BJ, Horn G. Learning and memory: regional changes in N-methyl-D-aspartate receptors in the chick brain after imprinting. Proc Natl Acad Sci USA. 1988;85:2849–2853. doi: 10.1073/pnas.85.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCabe BJ, Davey JE, Horn G. Impairment of learning by localized injection of an N-methyl-D-aspartate receptor antagonist into the hyperstriatum ventrale of the domestic chick. Behav Neurosci. 1992;106:947–953. doi: 10.1037//0735-7044.106.6.947. [DOI] [PubMed] [Google Scholar]
- 44.Cline HT, Debski EA, Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci USA. 1987;84:4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scherer WJ, Udin SB. N-Methyl-D-aspartate antagonists prevent interaction of binocular maps in Xenopus tectum. J Neurosci. 1989;9:3837–3843. doi: 10.1523/JNEUROSCI.09-11-03837.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt JT. Long-term potentiation and activity-dependent retinotopic sharpening in the regenerating retinotectal projection of goldfish: common sensitive period and sensitivity to NMDA blockers. J Neurosci. 1990;10:233–246. doi: 10.1523/JNEUROSCI.10-01-00233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearce IA, Cambray-Deakin MA, Burgoyne RD. Glutamate acting on NMDA receptors stimulates neurite outgrowth from cerebellar granule cells. FEBS Lett. 1987;223:143–147. doi: 10.1016/0014-5793(87)80525-2. [DOI] [PubMed] [Google Scholar]
- 48.Balázs R, Jørgensen OS, Hack N. N-Methyl-D-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience. 1988;27:437–451. doi: 10.1016/0306-4522(88)90279-5. [DOI] [PubMed] [Google Scholar]
- 49.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 50.Collingridge GL, Kehl SJ, Loo R, McLennan H. Effects of kainic and other amino acids on synaptic excitation in rat hippocampal slices: 1. Extracellular analysis. Exp Brain Res. 1983;52:170–178. doi: 10.1007/BF00236625. [DOI] [PubMed] [Google Scholar]
- 51.Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 52.Coan EJ, Irving AJ, Collingridge GL. Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neurosci Lett. 1989;105:205–210. doi: 10.1016/0304-3940(89)90038-4. [DOI] [PubMed] [Google Scholar]
- 53.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 55.McNaughton BL. Long-term synaptic enhancement and short-term potentiation in rat fascia dentata act through different mechanisms. J Physiol. 1982;324:249–262. doi: 10.1113/jphysiol.1982.sp014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kauer JA, Malenka RC, Nicoll RA. NMDA application potentiates synaptic transmission in the hippocampus. Nature. 1988;334:250–252. doi: 10.1038/334250a0. [DOI] [PubMed] [Google Scholar]
- 57.Volianskis A, Bannister N, Collett VJ, et al. Different NMDA receptor subtypes mediate induction of long-term potentiation and two forms of short-term potentiation at CA1 synapses in rat hippocampus in vitro. J Physiol. 2013;591:955–972. doi: 10.1113/jphysiol.2012.247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volianskis A, France G, Jensen MS, et al. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 2015;1621:5–16. doi: 10.1016/j.brainres.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thibault O, Joly M, Muller D, et al. Long-lasting physiological effects of bath applied N-methyl-D-aspartate. Brain Res. 1989;476:170–173. doi: 10.1016/0006-8993(89)91553-9. [DOI] [PubMed] [Google Scholar]
- 60.MacDonald JF, Wojtowicz JM. Two conductance mechanisms activated by applications of L-glutamic, L-aspartic, DL-homocysteic, N-methyl-D-aspartic, and DL-kainic acids to cultured mammalian central neurones. Can J Physiol Pharmacol. 1980;58:1393–1397. doi: 10.1139/y80-211. [DOI] [PubMed] [Google Scholar]
- 61.Nowak L, Bregestovski P, Ascher P, et al. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 62.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 63.Coan EJ, Collingridge GL. Magnesium ions block an N-methyl-D-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci Lett. 1985;53:21–26. doi: 10.1016/0304-3940(85)90091-6. [DOI] [PubMed] [Google Scholar]
- 64.Blake JF, Brown MW, Collingridge GL. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neurosci Lett. 1988;89:182–186. doi: 10.1016/0304-3940(88)90378-3. [DOI] [PubMed] [Google Scholar]
- 65.Blake JF, Yates RG, Brown MW, Collingridge GL. 6-Cyano-7-nitroquinoxaline-2,3-dione as an excitatory amino acid antagonist in area CA1 of rat hippocampus. Br J Pharmacol. 1989;97:71–76. doi: 10.1111/j.1476-5381.1989.tb11925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davies SN, Collingridge GL. Role of excitatory amino acid receptors in synaptic transmission in area CA1 of rat hippocampus. Proc R Soc Lond B Biol Sci. 1989;236:373–384. doi: 10.1098/rspb.1989.0028. [DOI] [PubMed] [Google Scholar]
- 67.Andreasen M, Lambert JD, Jensen MS. Effects of new non-N-methyl-D-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. J Physiol. 1989;414:317–336. doi: 10.1113/jphysiol.1989.sp017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collingridge GL, Herron CE, Lester RA. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collingridge GL, Herron CE, Lester RA. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herron CE, Lester RA, Coan EJ, Collingridge GL. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986;322:265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- 71.MacDermott AB, Mayer ML, Westbrook GL, et al. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- 72.Alford S, Frenguelli BG, Schofield JG, Collingridge GL. Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J Physiol. 1993;469:693–716. doi: 10.1113/jphysiol.1993.sp019838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunwiddie TV, Lynch G. The relationship between extracellular calcium concentrations and the induction of hippocampal long-term potentiation. Brain Res. 1979;169:103–110. doi: 10.1016/0006-8993(79)90377-9. [DOI] [PubMed] [Google Scholar]
- 74.Lynch G, Larson J, Kelso S, et al. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- 75.Baudry M, Lynch G. Regulation of glutamate receptors by cations. Nature. 1979;282:748–750. doi: 10.1038/282748a0. [DOI] [PubMed] [Google Scholar]
- 76.Kauer JA, Malenka RC, Nicoll RA. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988;1:911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- 77.Muller D, Joly M, Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988;242:1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- 78.Davies SN, Lester RA, Reymann KG, Collingridge GL. Temporally distinct pre- and post-synaptic mechanisms maintain long-term potentiation. Nature. 1989;338:500–503. doi: 10.1038/338500a0. [DOI] [PubMed] [Google Scholar]
- 79.Finn RC, Browning M, Lynch G. Trifluoperazine inhibits hippocampal long-term potentiation and the phosphorylation of a 40,000 Dalton protein. Neurosci Lett. 1980;19:103–108. doi: 10.1016/0304-3940(80)90264-5. [DOI] [PubMed] [Google Scholar]
- 80.Lovinger DM, Wong KL, Murakami K, Routtenberg A. Protein kinase C inhibitors eliminate hippocampal long-term potentiation. Brain Res. 1987;436:177–183. doi: 10.1016/0006-8993(87)91573-3. [DOI] [PubMed] [Google Scholar]
- 81.Reymann KG, Brödemann R, Kase H, Matthies H. Inhibitors of calmodulin and protein kinase C block different phases of hippocampal long-term potentiation. Brain Res. 1988;461:388–392. doi: 10.1016/0006-8993(88)90274-0. [DOI] [PubMed] [Google Scholar]
- 82.Malinow R, Madison DV, Tsien RW. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988;335:820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- 83.Lisman JE. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci USA. 1985;82:3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 85.Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- 86.Frey U, Krug M, Brödemann R, et al. Long-term potentiation induced in dendrites separated from rat’s CA1 pyramidal somata does not establish a late phase. Neurosci Lett. 1989;97:135–139. doi: 10.1016/0304-3940(89)90152-3. [DOI] [PubMed] [Google Scholar]
- 87.Lynch G, Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984;224:1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- 88.Linden DJ, Routtenberg A. The role of protein kinase-C in long-term potentiation—a testable model. Brain Res Brain Res Rev. 1989;14:279–296. doi: 10.1016/0165-0173(89)90004-0. [DOI] [PubMed] [Google Scholar]
- 89.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 90.Park P, Volianskis A, Sanderson TM, et al. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130131–20130131. doi: 10.1098/rstb.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sacktor TC, Hell JW. The genetics of PKM zeta and memory maintenance. Sci Signal. 2017 doi: 10.1126/scisignal.aao2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Collingridge GL, Bliss TVP. NMDA receptors - their role in long-term potentiation. Trends Neurosci. 1987;10:288–293. [Google Scholar]
- 93.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 94.Bliss TVP, Collingridge GL. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol Brain. 2013;6:5. doi: 10.1186/1756-6606-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dingledine R, Hynes MA, King GL. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wigström H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- 97.Wigström H, Gustafsson B. Large long-lasting potentiation in the dentate gyrus in vitro during blockade of inhibition. Brain Res. 1983;275:153–158. doi: 10.1016/0006-8993(83)90428-6. [DOI] [PubMed] [Google Scholar]
- 98.Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 100.Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- 101.Muller D, Lynch G. N-Methyl-D-aspartate receptor-mediated component of synaptic responses to single-pulse stimulation in rat hippocampal slices. Synapse. 1988;2:666–668. doi: 10.1002/syn.890020614. [DOI] [PubMed] [Google Scholar]
- 102.Wigström H, Gustafsson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol Scand. 1985;125:159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]
- 103.Gustafsson B, Wigström H, Abraham WC, Huang YY. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987;7:774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abraham WC, Gustafsson B, Wigström H. Single high strength afferent volleys can produce long-term potentiation in the hippocampus in vitro. Neurosci Lett. 1986;70:217–222. doi: 10.1016/0304-3940(86)90466-0. [DOI] [PubMed] [Google Scholar]
- 105.Huang YY, Wigström H, Gustafsson B. Facilitated induction of hippocampal long-term potentiation in slices perfused with low concentrations of magnesium. Neuroscience. 1987;22:9–16. doi: 10.1016/0306-4522(87)90193-x. [DOI] [PubMed] [Google Scholar]
- 106.Peet MJ, Leah JD, Curtis DR. Antagonists of synaptic and amino acid excitation of neurones in the cat spinal cord. Brain Res. 1983;266:83–95. doi: 10.1016/0006-8993(83)91311-2. [DOI] [PubMed] [Google Scholar]
- 107.Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- 108.Davies SN, Lodge D. Evidence for involvement of N-methylaspartate receptors in “wind-up” of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987;424:402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- 109.Dawbarn D, Pycock CJ. Motor effects following application of putative excitatory amino acid antagonists to the region of the mesencephalic dopamine cell bodies in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1981;318:100–104. doi: 10.1007/BF00508833. [DOI] [PubMed] [Google Scholar]
- 110.Crunelli V, Forda S, Kelly JS. Blockade of amino acid-induced depolarizations and inhibition of excitatory post-synaptic potentials in rat dentate gyrus. J Physiol. 1983;341:627–640. doi: 10.1113/jphysiol.1983.sp014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brown DA, Docherty RJ, Halliwell JV. Chemical transmission in the rat interpeduncular nucleus in vitro. J Physiol. 1983;341:655–670. doi: 10.1113/jphysiol.1983.sp014831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kimura H, Okamoto K, Sakai Y. Pharmacological evidence for L-aspartate as the neurotransmitter of cerebellar climbing fibres in the guinea-pig. J Physiol. 1985;365:103–119. doi: 10.1113/jphysiol.1985.sp015761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fox K, Armstrong-James M. The role of the anterior intralaminar nuclei and N-methyl D-aspartate receptors in the generation of spontaneous bursts in rat neocortical neurones. Exp Brain Res. 1986;63:505–518. doi: 10.1007/BF00237474. [DOI] [PubMed] [Google Scholar]
- 114.Davies J, Miller AJ, Sheardown MJ. Amino acid receptor mediated excitatory synaptic transmission in the cat red nucleus. J Physiol. 1986;376:13–29. doi: 10.1113/jphysiol.1986.sp016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salt TE. Excitatory amino acid receptors and synaptic transmission in the rat ventrobasal thalamus. J Physiol. 1987;391:499–510. doi: 10.1113/jphysiol.1987.sp016752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mo N, Dun NJ. Excitatory postsynaptic potentials in neonatal rat sympathetic preganglionic neurons: possible mediation by NMDA receptors. Neurosci Lett. 1987;77:327–332. doi: 10.1016/0304-3940(87)90522-2. [DOI] [PubMed] [Google Scholar]
- 117.Davies J, Evans RH, Jones AW, et al. Differential activation and blockade of excitatory amino acid receptors in the mammalian and amphibian central nervous systems. Comp Biochem Physiol C Comp Pharmacol. 1982;72:211–224. doi: 10.1016/0306-4492(82)90086-7. [DOI] [PubMed] [Google Scholar]
- 118.Dale N, Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Corradetti R, King AE, Nistri A, et al. Pharmacological characterization of D-aminophosphonovaleric acid antagonism of amino acid and synaptically evoked excitations on frog motoneurones in vitro: an intracellular study. Br J Pharmacol. 1985;86:19–25. doi: 10.1111/j.1476-5381.1985.tb09430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brodin L, Grillner S, Rovainen CM. N-Methyl-D-aspartate (NMDA), kainate and quisqualate receptors and the generation of fictive locomotion in the lamprey spinal cord. Brain Res. 1985;325:302–306. doi: 10.1016/0006-8993(85)90328-2. [DOI] [PubMed] [Google Scholar]
- 121.Dale N, Grillner S. Dual-component synaptic potentials in the lamprey mediated by excitatory amino acid receptors. J Neurosci. 1986;6:2653–2661. doi: 10.1523/JNEUROSCI.06-09-02653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lukasiewicz PD, McReynolds JS. Synaptic transmission at N-methyl-D-aspartate receptors in the proximal retina of the mudpuppy. J Physiol. 1985;367:99–115. doi: 10.1113/jphysiol.1985.sp015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Massey SC, Miller RF. N-Methyl-D-aspartate receptors of ganglion cells in rabbit retina. J Neurophysiol. 1990;63:16–30. doi: 10.1152/jn.1990.63.1.16. [DOI] [PubMed] [Google Scholar]
- 124.Garthwaite J. Cellular uptake disguises action of L-glutamate on N-methyl-D-aspartate receptors. With an appendix: diffusion of transported amino acids into brain slices. Br J Pharmacol. 1985;85:297–307. doi: 10.1111/j.1476-5381.1985.tb08860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Olverman HJ, Jones AW, Watkins JC. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature. 1984;307:460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- 126.Monaghan DT, Yao D, Olverman HJ, et al. Autoradiography of D-2-[3H]amino-5-phosphonopentanoate binding sites in rat brain. Neurosci Lett. 1984;52:253–258. doi: 10.1016/0304-3940(84)90170-8. [DOI] [PubMed] [Google Scholar]
- 127.Baudry M. Long-term potentiation and kindling: similar biochemical mechanisms? Adv Neurol. 1986;44:401–410. [PubMed] [Google Scholar]
- 128.Cain DP. Long-term potentiation and kindling: how similar are the mechanisms? Trends Neurosci. 1989;12:6–10. doi: 10.1016/0166-2236(89)90146-x. [DOI] [PubMed] [Google Scholar]
- 129.Slater NT, Stelzer A, Galvan M. Kindling-like stimulus patterns induce epileptiform discharges in the guinea pig in vitro hippocampus. Neurosci Lett. 1985;60:25–31. doi: 10.1016/0304-3940(85)90376-3. [DOI] [PubMed] [Google Scholar]
- 130.Mody I, Heinemann U. NMDA receptors of dentate gyrus granule cells participate in synaptic transmission following kindling. Nature. 1987;326:701–704. doi: 10.1038/326701a0. [DOI] [PubMed] [Google Scholar]
- 131.Coutinho-Netto J, Abdul-Ghani AS, Collins JF, Bradford HF. Is glutamate a trigger factor in epileptic hyperactivity? Epilepsia. 1981;22:289–296. doi: 10.1111/j.1528-1157.1981.tb04112.x. [DOI] [PubMed] [Google Scholar]
- 132.Croucher MJ, Collins JF, Meldrum BS. Anticonvulsant action of excitatory amino acid antagonists. Science. 1982;216:899–901. doi: 10.1126/science.7079744. [DOI] [PubMed] [Google Scholar]
- 133.Meldrum BS, Croucher MJ, Badman G, Collins JF. Antiepileptic action of excitatory amino acid antagonists in the photosensitive baboon, Papio papio. Neurosci Lett. 1983;39:101–104. doi: 10.1016/0304-3940(83)90172-6. [DOI] [PubMed] [Google Scholar]
- 134.Herron CE, Williamson R, Collingridge GL. A selective N-methyl-D-aspartate antagonist depresses epileptiform activity in rat hippocampal slices. Neurosci Lett. 1985;61:255–260. doi: 10.1016/0304-3940(85)90473-2. [DOI] [PubMed] [Google Scholar]
- 135.Anderson WW, Swartzwelder HS, Wilson WA. The NMDA receptor antagonist 2-amino-5-phosphonovalerate blocks stimulus train-induced epileptogenesis but not epileptiform bursting in the rat hippocampal slice. J Neurophysiol. 1987;57:1–21. doi: 10.1152/jn.1987.57.1.1. [DOI] [PubMed] [Google Scholar]
- 136.Stelzer A, Slater NT, Bruggencate ten G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987;326:698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- 137.Kleckner NW, Dingledine R. Selectivity of quinoxalines and kynurenines as antagonists of the glycine site on N-methyl-D-aspartate receptors. Mol Pharmacol. 1989;36:430–436. [PubMed] [Google Scholar]
- 138.Horne AL, Harrison NL, Turner JP, Simmonds MA. Spontaneous paroxysmal activity induced by zero magnesium and bicuculline: suppression by NMDA antagonists and GABA mimetics. Eur J Pharmacol. 1986;122:231–238. doi: 10.1016/0014-2999(86)90107-x. [DOI] [PubMed] [Google Scholar]
- 139.Aram JA, Lodge D. Epileptiform activity induced by alkalosis in rat neocortical slices: block by antagonists of N-methyl-D-aspartate. Neurosci Lett. 1987;83:345–350. doi: 10.1016/0304-3940(87)90112-1. [DOI] [PubMed] [Google Scholar]
- 140.Ashwood TJ, Wheal HV. Extracellular studies on the role of N-methyl-D-aspartate receptors in epileptiform activity recorded from the kainic acid-lesioned hippocampus. Neurosci Lett. 1986;67:147–152. doi: 10.1016/0304-3940(86)90388-5. [DOI] [PubMed] [Google Scholar]
- 141.Avoli M, Olivier A. Bursting in human epileptogenic neocortex is depressed by an N-methyl-D-aspartate antagonist. Neurosci Lett. 1987;76:249–254. doi: 10.1016/0304-3940(87)90724-5. [DOI] [PubMed] [Google Scholar]
- 142.Armstrong-James M, Fox K. Evidence for a specific role for cortical NMDA receptors in slow-wave sleep. Brain Res. 1988;451:189–196. doi: 10.1016/0006-8993(88)90763-9. [DOI] [PubMed] [Google Scholar]
- 143.Aanonsen LM, Wilcox GL. Nociceptive action of excitatory amino acids in the mouse: effects of spinally administered opioids, phencyclidine and sigma agonists. J Pharmacol Exp Ther. 1987;243:9–19. [PubMed] [Google Scholar]
- 144.Cahusac PM, Evans RH, Hill RG, et al. The behavioural effects of an N-methylaspartate receptor antagonist following application to the lumbar spinal cord of conscious rats. Neuropharmacology. 1984;23:719–724. doi: 10.1016/0028-3908(84)90102-3. [DOI] [PubMed] [Google Scholar]
- 145.Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990;518:218–226. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- 146.Mendell LM. Modifiability of spinal synapses. Physiol Rev. 1984;64:260–324. doi: 10.1152/physrev.1984.64.1.260. [DOI] [PubMed] [Google Scholar]
- 147.Thompson SW, King AE, Woolf CJ. Activity-dependent changes in rat ventral horn neurons in vitro; summation of prolonged afferent evoked postsynaptic depolarizations produce a d-2-amino-5-phosphonovaleric acid sensitive windup. Eur J Neurosci. 1990;2:638–649. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 148.Coderre TJ, Melzack R. Central neural mediators of secondary hyperalgesia following heat injury in rats: neuropeptides and excitatory amino acids. Neurosci Lett. 1991;131:71–74. doi: 10.1016/0304-3940(91)90339-u. [DOI] [PubMed] [Google Scholar]
- 149.Barnes CA. Aging and the physiology of spatial memory. Neurobiol Aging. 1988;9:563–568. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- 150.Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- 152.Danysz W, Wroblewski JT, Costa E. Learning impairment in rats by N-methyl-D-aspartate receptor antagonists. Neuropharmacology. 1988;27:653–656. doi: 10.1016/0028-3908(88)90189-x. [DOI] [PubMed] [Google Scholar]
- 153.Tonkiss J, Morris RG, Rawlins JN. Intra-ventricular infusion of the NMDA antagonist AP5 impairs performance on a non-spatial operant DRL task in the rat. Exp Brain Res. 1988;73:181–188. doi: 10.1007/BF00279671. [DOI] [PubMed] [Google Scholar]
- 154.Staubli U, Thibault O, DiLorenzo M, Lynch G. Antagonism of NMDA receptors impairs acquisition but not retention of olfactory memory. Behav Neurosci. 1989;103:54–60. doi: 10.1037//0735-7044.103.1.54. [DOI] [PubMed] [Google Scholar]
- 155.Olney JW, Labruyere J, Collins JF, Curry K. D-aminophosphonovalerate is 100-fold more powerful than D-alpha-aminoadipate in blocking N-methylaspartate neurotoxicity. Brain Res. 1981;221:207–210. doi: 10.1016/0006-8993(81)91076-3. [DOI] [PubMed] [Google Scholar]
- 156.Garthwaite G, Hajós F, Garthwaite J. Ionic requirements for neurotoxic effects of excitatory amino acid analogues in rat cerebellar slices. Neuroscience. 1986;18:437–447. doi: 10.1016/0306-4522(86)90164-8. [DOI] [PubMed] [Google Scholar]
- 157.Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 158.Scharfman HE, Schwartzkroin PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science. 1989;246:257–260. doi: 10.1126/science.2508225. [DOI] [PubMed] [Google Scholar]
- 159.Watson GB, Rader RK, Lanthorn TH. Epileptiform activity in vitro can produce long-term synaptic failure and persistent neuronal depolarization. Brain Res. 1989;498:81–88. doi: 10.1016/0006-8993(89)90401-0. [DOI] [PubMed] [Google Scholar]
- 160.Furshpan EJ, Potter DD. Seizure-like activity and cellular damage in rat hippocampal neurons in cell culture. Neuron. 1989;3:199–207. doi: 10.1016/0896-6273(89)90033-0. [DOI] [PubMed] [Google Scholar]
- 161.Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43:1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 162.Hagberg H, Lehmann A, Sandberg M, et al. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J Cereb Blood Flow Metab. 1985;5:413–419. doi: 10.1038/jcbfm.1985.56. [DOI] [PubMed] [Google Scholar]
- 163.Engelsen B, Westerberg E, Fonnum F, Wieloch T. Effect of insulin-induced hypoglycemia on the concentrations of glutamate and related amino acids and energy metabolites in the intact and decorticated rat neostriatum. J Neurochem. 1986;47:1634–1641. doi: 10.1111/j.1471-4159.1986.tb00806.x. [DOI] [PubMed] [Google Scholar]
- 164.Simon RP, Swan JH, Griffiths T, Meldrum BS. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984;226:850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- 165.Wieloch T. Hypoglycemia-induced neuronal damage prevented by an N-methyl-D-aspartate antagonist. Science. 1985;230:681–683. doi: 10.1126/science.2996146. [DOI] [PubMed] [Google Scholar]
- 166.Collins RC, Dobkin BH, Choi DW. Selective vulnerability of the brain: new insights into the pathophysiology of stroke. Ann Intern Med. 1989;110:992–1000. doi: 10.7326/0003-4819-110-12-992. [DOI] [PubMed] [Google Scholar]
- 167.Watkins JC. The synthesis of some acidic amino acids possessing neuropharmacological activity. J Med Pharm Chem. 1962;91:1187–1199. doi: 10.1021/jm01241a010. [DOI] [PubMed] [Google Scholar]
- 168.Lunn WH, Schoepp DD, Calligaro DO, et al. DL-tetrazol-5-ylglycine, a highly potent NMDA agonist: its synthesis and NMDA receptor efficacy. J Med Chem. 1992;35:4608–4612. doi: 10.1021/jm00102a015. [DOI] [PubMed] [Google Scholar]
- 169.Allan RD, Hanrahan JR, Hambley TW, et al. Synthesis and activity of a potent N-methyl-D-aspartic acid agonist, trans-1-aminocyclobutane-1,3-dicarboxylic acid, and related phosphonic and carboxylic acids. J Med Chem. 1990;33:2905–2915. doi: 10.1021/jm00172a036. [DOI] [PubMed] [Google Scholar]
- 170.Lanthorn TH, Hood WF, Watson GB, et al. Cis-2,4-methanoglutamate is a potent and selective N-methyl-D-aspartate receptor agonist. Eur J Pharmacol. 1990;182:397–404. doi: 10.1016/0014-2999(90)90036-6. [DOI] [PubMed] [Google Scholar]
- 171.Shinozaki H, Ishida M, Shimamoto K, Ohfune Y. Potent NMDA-like actions and potentiation of glutamate responses by conformational variants of a glutamate analogue in the rat spinal cord. Br J Pharmacol. 1989;98:1213–1224. doi: 10.1111/j.1476-5381.1989.tb12667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Monahan JB, Hood WF, Compton RP, et al. Characterization of D-3,4-cyclopropylglutamates as N-methyl-D-aspartate receptor agonists. Neurosci Lett. 1990;112:328–332. doi: 10.1016/0304-3940(90)90225-x. [DOI] [PubMed] [Google Scholar]
- 173.Kawai M, Horikawa Y, Ishihara T, et al. 2-(Carboxycyclopropyl)glycines: binding, neurotoxicity and induction of intracellular free Ca2+ increase. Eur J Pharmacol. 1992;211:195–202. doi: 10.1016/0014-2999(92)90529-d. [DOI] [PubMed] [Google Scholar]
- 174.Jane DE, Olverman HJ, Watkins JC. Agonists and competitive antagonists: structure-activity and molecular modelling studies. In: Collingridge GL, Watkins JC, editors. The NMDA receptor. 2. Oxford: Oxford University Press; 1994. pp. 31–104. [Google Scholar]
- 175.Fagg GE, Olpe HR, Pozza MF, et al. CGP 37849 and CGP 39551: novel and potent competitive N-methyl-D-aspartate receptor antagonists with oral activity. Br J Pharmacol. 1990;99:791–797. doi: 10.1111/j.1476-5381.1990.tb13008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lehmann J, Hutchison AJ, McPherson SE, et al. CGS 19755, a selective and competitive N-methyl-D-aspartate-type excitatory amino acid receptor antagonist. J Pharmacol Exp Ther. 1988;246:65–75. [PubMed] [Google Scholar]
- 177.Evans RH, Francis AA, Jones AW, et al. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol. 1982;75:65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Perkins MN, Stone TW, Collins JF, Curry K. Phosphonate analogues of carboxylic acids as aminoacid antagonists on rat cortical neurones. Neurosci Lett. 1981;23:333–336. doi: 10.1016/0304-3940(81)90021-5. [DOI] [PubMed] [Google Scholar]
- 179.Davies J, Evans RH, Herrling PL, et al. CPP, a new potent and selective NMDA antagonist. Depression of central neuron responses, affinity for [3H]D-AP5 binding sites on brain membranes and anticonvulsant activity. Brain Res. 1986;382:169–173. doi: 10.1016/0006-8993(86)90127-7. [DOI] [PubMed] [Google Scholar]
- 180.Harris EW, Ganong AH, Monaghan DT, et al. Action of 3-((+/-)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP): a new and highly potent antagonist of N-methyl-D-aspartate receptors in the hippocampus. Brain Res. 1986;382:174–177. doi: 10.1016/0006-8993(86)90128-9. [DOI] [PubMed] [Google Scholar]
- 181.Aebischer B, Frey P, Haerter H-P, et al. Synthesis and NMD A antagonistic properties of the enantiomers of 4-(3-phosphonopropyl)piperazine-2-carboxylic acid (CPP) and of the unsaturated analogue (E)-4-(3-phosphonoprop-2-enyl)piperazine-2-carboxylic acid (CPP-ene) Helv Chim Acta. 1989;72:1043–1051. [Google Scholar]
- 182.Ornstein PL, Klimkowski VJ. Competitive NMDA receptor antagonists. In: Krogsgaard-Larsen P, Hansen JJ, editors. Excitatory amino acid receptors. Design of agonists and antagonists. Chichester: Ellis Horwood Ltd; 1992. pp. 183–201. [Google Scholar]
- 183.Müller W, Lowe DA, Neijt H, et al. Synthesis and N-Methyl-D-aspartate (NMDA) antagonist properties of the enantiomers of α-amino-5-(phosphonomethyl)[1,1′-biphenyl]-3-propanoic acid. Use of a new chiral glycine derivative. Helv Chim Acta. 2004;75:855–864. [Google Scholar]
- 184.Olverman HJ, Monaghan DT, Cotman CW, Watkins JC. [3H]CPP, a new competitive ligand for NMDA receptors. Eur J Pharmacol. 1986;131:161–162. doi: 10.1016/0014-2999(86)90533-9. [DOI] [PubMed] [Google Scholar]
- 185.Murphy DE, Hutchison AJ, Hurt SD, et al. Characterization of the binding of [3H]-CGS 19755: a novel N-methyl-D-aspartate antagonist with nanomolar affinity in rat brain. Br J Pharmacol. 1988;95:932–938. doi: 10.1111/j.1476-5381.1988.tb11723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sills MA, Fagg G, Pozza M, et al. [3H]CGP 39653: a new N-methyl-D-aspartate antagonist radioligand with low nanomolar affinity in rat brain. Eur J Pharmacol. 1991;192:19–24. doi: 10.1016/0014-2999(91)90063-v. [DOI] [PubMed] [Google Scholar]
- 187.Fagg GE, Matus A. Selective association of N-methyl aspartate and quisqualate types of L-glutamate receptor with brain postsynaptic densities. Proc Natl Acad Sci USA. 1984;81:6876–6880. doi: 10.1073/pnas.81.21.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Monaghan DT, Cotman CW. Identification and properties of N-methyl-D-aspartate receptors in rat brain synaptic plasma membranes. Proc Natl Acad Sci USA. 1986;83:7532–7536. doi: 10.1073/pnas.83.19.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wong EH, Knight AR, Ransom R. Glycine modulates [3H]MK-801 binding to the NMDA receptor in rat brain. Eur J Pharmacol. 1987;142:487–488. doi: 10.1016/0014-2999(87)90095-1. [DOI] [PubMed] [Google Scholar]
- 190.Herrling PL. Excitatory amino acids: clinical results with antagonists. London: Academic; 1997. [Google Scholar]
- 191.Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 192.Stringer JL, Guyenet PG. Elimination of long-term potentiation in the hippocampus by phencyclidine and ketamine. Brain Res. 1983;258:159–164. doi: 10.1016/0006-8993(83)91244-1. [DOI] [PubMed] [Google Scholar]
- 193.Stringer JL, Greenfield LJ, Hackett JT, Guyenet PG. Blockade of long-term potentiation by phencyclidine and sigma opiates in the hippocampus in vivo and in vitro. Brain Res. 1983;280:127–138. doi: 10.1016/0006-8993(83)91180-0. [DOI] [PubMed] [Google Scholar]
- 194.Lodge D, Anis NA. Effects of phencyclidine on excitatory amino acid activation of spinal interneurones in the cat. Eur J Pharmacol. 1982;77:203–204. doi: 10.1016/0014-2999(82)90022-x. [DOI] [PubMed] [Google Scholar]
- 195.Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Berry SC, Dawkins SL, Lodge D. Comparison of sigma- and kappa-opiate receptor ligands as excitatory amino acid antagonists. Br J Pharmacol. 1984;83:179–185. doi: 10.1111/j.1476-5381.1984.tb10133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.MacDonald JF, Miljkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987;58:251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- 198.Lodge D, Mercier MS. Ketamine and phencyclidine: the good, the bad and the unexpected. Br J Pharmacol. 2015;172:4254–4276. doi: 10.1111/bph.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.White PF, Way WL, Trevor AJ. Ketamine—its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119–136. doi: 10.1097/00000542-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 200.Thomson AM, West DC, Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985;313:479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- 201.Aram JA, Martin D, Tomczyk M, et al. Neocortical epileptogenesis in vitro: studies with N-methyl-D-aspartate, phencyclidine, sigma and dextromethorphan receptor ligands. J Pharmacol Exp Ther. 1989;248:320–328. [PubMed] [Google Scholar]
- 202.Chapman AG, Meldrum BS. Non-competitive N-methyl-D-aspartate antagonists protect against sound-induced seizures in DBA/2 mice. Eur J Pharmacol. 1989;166:201–211. doi: 10.1016/0014-2999(89)90060-5. [DOI] [PubMed] [Google Scholar]
- 203.Rauschecker JP, Hahn S. Ketamine-xylazine anaesthesia blocks consolidation of ocular dominance changes in kitten visual cortex. Nature. 1987;326:183–185. doi: 10.1038/326183a0. [DOI] [PubMed] [Google Scholar]
- 204.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 205.Fletcher EJ, Lodge D. Glycine reverses antagonism of N-methyl-D-aspartate (NMDA) by 1-hydroxy-3-aminopyrrolidone-2 (HA-966) but not by D-2-amino-5-phosphonovalerate (D-AP5) on rat cortical slices. Eur J Pharmacol. 1988;151:161–162. doi: 10.1016/0014-2999(88)90711-x. [DOI] [PubMed] [Google Scholar]
- 206.Foster AC, Kemp JA. HA-966 antagonizes N-methyl-D-aspartate receptors through a selective interaction with the glycine modulatory site. J Neurosci. 1989;9:2191–2196. doi: 10.1523/JNEUROSCI.09-06-02191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kemp JA, Foster AC, Leeson PD, et al. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci USA. 1988;85:6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 209.Ingram R, Kang H, Lightman S, et al. Some distorted thoughts about ketamine as a psychedelic and a novel hypothesis based on NMDA receptor-mediated synaptic plasticity. Neuropharmacology. 2018 doi: 10.1016/j.neuropharm.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 210.Perkins MN, Stone TW. Quinolinic acid: regional variations in neuronal sensitivity. Brain Res. 1983;259:172–176. doi: 10.1016/0006-8993(83)91084-3. [DOI] [PubMed] [Google Scholar]
- 211.Lehmann J, Schaefer P, Ferkany JW, Coyle JT. Quinolinic acid evokes [3H]acetylcholine release in striatal slices: mediation by NMDA-type excitatory amino acid receptors. Eur J Pharmacol. 1983;96:111–115. doi: 10.1016/0014-2999(83)90536-8. [DOI] [PubMed] [Google Scholar]
- 212.Monaghan DT, Beaton JA. Quinolinate differentiates between forebrain and cerebellar NMDA receptors. Eur J Pharmacol. 1991;194:123–125. doi: 10.1016/0014-2999(91)90134-c. [DOI] [PubMed] [Google Scholar]
- 213.Monaghan DT, Olverman HJ, Nguyen L, et al. Two classes of N-methyl-D-aspartate recognition sites: differential distribution and differential regulation by glycine. Proc Natl Acad Sci USA. 1988;85:9836–9840. doi: 10.1073/pnas.85.24.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Monaghan DT. Differential stimulation of [3H]MK-801 binding to subpopulations of NMDA receptors. Neurosci Lett. 1991;122:21–24. doi: 10.1016/0304-3940(91)90183-t. [DOI] [PubMed] [Google Scholar]
- 215.Reynolds IJ, Palmer AM. Regional variations in [3H]MK801 binding to rat brain N-methyl-D-aspartate receptors. J Neurochem. 1991;56:1731–1740. doi: 10.1111/j.1471-4159.1991.tb02074.x. [DOI] [PubMed] [Google Scholar]
- 216.O’Shea RD, Manallack DT, Conway EL, et al. Evidence for heterogenous glycine domains but conserved multiple states of the excitatory amino acid recognition site of the NMDA receptor: regional binding studies with [3H]glycine and [3H]L-glutamate. Exp Brain Res. 1991;86:652–662. doi: 10.1007/BF00230539. [DOI] [PubMed] [Google Scholar]
- 217.Beaton JA, Stemsrud K, Monaghan DT. Identification of a novel N-methyl-D-aspartate receptor population in the rat medial thalamus. J Neurochem. 1992;59:754–757. doi: 10.1111/j.1471-4159.1992.tb09433.x. [DOI] [PubMed] [Google Scholar]
- 218.Buller AL, Larson HC, Schneider BE, et al. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci. 1994;14:5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Moriyoshi K, Masu M, Ishii T, et al. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 220.Monyer H, Seeburg PH. Constituents involved in glutamate receptor signaling. Hippocampus. 1993;3:125–129. [PubMed] [Google Scholar]
- 221.Pachernegg S, Strutz-Seebohm N, Hollmann M. GluN3 subunit-containing NMDA receptors: not just one-trick ponies. Trends Neurosci. 2012;35:240–249. doi: 10.1016/j.tins.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 222.Hrabetova S, Sacktor TC. Long-term potentiation and long-term depression are induced through pharmacologically distinct NMDA receptors. Neurosci Lett. 1997;226:107–110. doi: 10.1016/s0304-3940(97)00252-8. [DOI] [PubMed] [Google Scholar]
- 223.Hrabetova S, Serrano P, Blace N, et al. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci. 2000;20:RC81. doi: 10.1523/JNEUROSCI.20-12-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liu L, Wong TP, Pozza MF, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 225.Massey PV, Johnson BE, Moult PR, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Berberich S, Punnakkal P, Jensen V, et al. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bartlett TE, Bannister NJ, Collett VJ, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 228.Morishita W, Malenka RC. Mechanisms underlying dedepression of synaptic NMDA receptors in the hippocampus. J Neurophysiol. 2008;99:254–263. doi: 10.1152/jn.01011.2007. [DOI] [PubMed] [Google Scholar]
- 229.Bartlett TE, Lu J, Wang YT. Slice orientation and muscarinic acetylcholine receptor activation determine the involvement of N-methyl D-aspartate receptor subunit GluN2B in hippocampal area CA1 long-term depression. Mol Brain. 2011;4:41. doi: 10.1186/1756-6606-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.France G, Fernández-Fernández D, Burnell ES, et al. Multiple roles of GluN2B-containing NMDA receptors in synaptic plasticity in juvenile hippocampus. Neuropharmacology. 2017;112:76–83. doi: 10.1016/j.neuropharm.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]