Abstract
Chemotherapy during childhood damages ovarian reserve and can affect future fertility. However, recent large epidemiological studies showed that the detrimental impact on fertility is less severe if women seek for pregnancy at a younger age. To explain this observation, we hypothesize that the detrimental effects of previous chemotherapy on the ovarian reserve may be attenuated in young adults for two main reasons. Firstly, recent evidence showed that the amount of ovarian reserve is not a critical factor for effective natural conceptions. Provided that the residual ovarian reserve allows regular ovulatory cycles, the chances of pregnancy are similar in women with intact or reduced ovarian reserve. Secondly, ovarian reserve depletion appears to be a phenomenon that is inversely related to the residual ovarian reserve rather than to age. From a mathematical perspective, this kind of regulation intrinsically attenuates the effects of an early loss of a significant amount of primordial follicles. In conclusion, the detrimental effects of chemotherapy on natural fertility may be less severe if women with a history of chemotherapy during childhood seek for pregnancy early. This information should be part of the counseling.
Keywords: Ovarian reserve, Chemotherapy, Infertility, Childhood cancer, Survivor
Introduction
Chemotherapy damages ovarian reserve and may compromise future fertility in childhood cancer survivors (Table 1) [1–9]. The magnitude of the damage depends on the specific agents used, with alkylating agent being the most harmful, and the total doses administered. High doses multi-agents regimens such as those required for bone marrow transplantation almost invariably cause immediate premature ovarian insufficiency (POI). To date, the mechanisms of the chemotherapy-related injury to ovarian reserve have been only partially elucidated and may differ according to the specific agent used [10–12]. They include the accelerated recruitment of primordial follicles (“burn-out” effect) [13], the impairment of the local vascularization [14], and a direct damage to oocytes or granulosa cells [10].
Table 1.
Recent epidemiological studies reporting on fertility in childhood and young adult cancer survivors
| Study | Design | Diagnoses of cases | N. exposed women (age) | Main results |
|---|---|---|---|---|
| Madanat et al., 2008 [1] | Linking of Finnish registers of cancer and the one of central population. Comparisons were made with siblings identified with the second register. | All cancers | 1334 (0–14 years) + 1254 (15–19 years) | RR = 0.62 (95% CI 0.56–0.68) for age 0–14 and RR = 0.64 (95% CI 0.58–0.70) for age 15–19. |
| Reulen et al., 2009 [2] | British cohort study with active follow-up (quesyionnaires). Comparison with the expected pregnancy rates in the general population | All cancers | 10,483 (0–14 years) | O/E 0.64 (96% CI 0.62–0.66) |
| Stansheim et al., 2011 [3] | Linking of Norway registers of cancer and birth. Comparison with matched unexposed women of the National register. | All cancers | not reported (subset of women aged 16–25 years) | HR = 0.67 (95% CI 0.63–0.73) |
| Pivetta et al., 2011 [4] | Multicenter hospital-based Italian cohort study. Comparison with the expected pregnancy rates in the general population | All cancers | 1888 (0–14 years) | O/E 0.57 (96% CI 0.53–0.62) |
| Bramswig et al., 2015 [5] | Prospective German cohort study (patients included in 5 trials). Comparison with the general population. | Hodgkin’s lymphoma | 554 (0–17 years) | No significant difference with the exception of those seeking at 40–44 years (61% vs 78%, p = 0.001). |
| Chow et al., 2016 [6] | Cohort study from 27 Institutions in the USA and Canada (CCSS: Childhood Cancer Survivor Study). Controls were siblings. | All cancers (exclusion of girls requiring brain or pelvis radiotherapy) | 2455 (0–20 years) | HR = 0.82 (95% CI 0.75–0.90) |
| Armuand et al., 2017 [7] | Use of the Sweden national patient register. Comparison with matched unexposed women of the general population (same register). | All cancers | 552 (0–20 years) | HR = 0.82 (95% CI 0.72–0.95) |
| Anderson et al., 2018 [8] | Linking of Scottish registers of cancer, pregnancies and death. Comparison with the expected pregnancy rates in the general population | All cancers | 1638 (0–14 years) + 2674 (15–24 years) | SIR = 0.72 (95% CI 0.66–0.78) for age 0–14 and SIR = 0.69 (95% CI 0.66–0.72) for age 15–24. |
Only studies published during the last decade are included
If identified studies overlapped for study population, only the most recent one was included
SIR standardized incidence ratio, HR hazard ratio, O/E observed/expected ratio
During the last two decades, outstanding progresses have been made to reduce the risk of childlessness in childhood cancer survivors. The cryopreservation of ovarian cortex or oocytes (if post-pubertal) prior to initiate the oncologic treatments have actually improved the future chances of parenthood in these women [11, 15, 16] However, to date, the precise role of fertility preservation procedures remains to be determined [11, 15]. To note, the use of these techniques poses some additional technical and ethical issues in minors [17, 18]. Further evidence on this multifaceted topic and a more in-depth understanding of the mechanisms of damage is needed.
New epidemiological evidence
Two recent large cohort studies reporting on long-term fertility in childhood cancer survivors provided some enlightening new information on the role of women age at the time of pregnancy seeking [5, 6].
Specifically, in the first study, Bramswig et al. reported on parenthood of 467 Hodgkin’s lymphoma survivors who received chemotherapy before 18 years of age between 1978 and 1995 [5]. The median length of follow-up was 20 years (Interquartile range-IQR 16–25). Two-hundred twenty-eight women had 406 children (median 1.8 children per mother). The cumulative incidence of parenthood was 67% (95% CI 64–75%) at 28 years of follow-up and 69% (95% CI 61–74%) at 40 years of age. When comparing these data to the local national frequency of parenting and stratifying per class of age, no statistically significant difference emerged up to 40 years. A significant reduction in cumulative pregnancy rate was observed only in cancer survivors aged 40–44 years [5].
The second study reported data from the US Childhood Cancer Survivor Study cohort. It did not exclusively focused on Hodgkin’s lymphoma but, conversely, to all childhood cancer survivors (only women receiving radiotherapy to the pelvis or to the brain were excluded) [6]. The authors actually collected information from 5298 cancer survivors who were treated before age 21 between 1970 and 1999. The median follow-up was 8 years (IQR 4–12). Controls were sisters of survivors. Overall, the hazard ratio (HR) of live birth was 0.82 (95% CI 0.76–0.89). More specifically, it was 0.87 (95% CI 0.80–0.95) in women aged less than 30 years and then dropped to 0.63 (95% CI 0.53–0.76) in those aged 30 to 44 years [6].
Overall, the evidence emerging from these two cohort studies tends to confute the simplistic view suggesting a direct relation between the amount of ovarian reserve and fertility. Ovarian reserve is indisputably damaged by chemotherapy (even if with relevant variations according to the regimens used), but the impact on fertility appears modest in earlier ages. In the majority of cancer survivors, the chances of parenthood may actually be impaired only at later age.
In our opinion, the attenuated detrimental effects of chemotherapy in young age suggests two main considerations that may be clinically useful for physicians involved in the field: (1) the importance of distinguishing the amount of the residual ovarian reserve and the quality of the oocytes and (2) a non-linear vision of the of the rules that guide age and chemotherapy-related loss of ovarian reserve.
Quality of oocytes and ovarian reserve
Natural fertility progressively declines from the early thirties and terminates at about 41 years of age [19, 20]. Thereafter, regular cycles continue for about 5 years but the quality of the ovulated oocytes is hampered and does not allow to achieve live births. Finally, cycles become irregular and frequently anovulatory until menopause that occurs at about 51 years of age [19].
These biological events are accompanied by a progressive decline of the ovarian reserve. The advent of menopause and the preceding irregular cycles are direct consequences of the depletion of the ovarian reserve but the progressive decline in natural fertility in the thirties and early forties has a different explanation. A growing body of biological and clinical evidence actually supports the preponderant role of age rather than residual ovarian reserve as a crucial factor to explain the decline of natural fertility. In other words, the quality of the oocytes rather than the quantity of the residual primordial follicles would determine the chances of motherhood. Even if an initial study suggested some relation between the amount of the residual ovarian reserve and natural fertility in women older than 35 years [21], five subsequent independent studies in the general population failed to show any association between ovarian reserve and natural fertility [22–26]. This evidence is summarized in Table 2.
Table 2.
Studies on ovarian reserve and natural fertility
| Study | Design | Sample size | Outcome | Main findings |
|---|---|---|---|---|
| Navot et al., 1987 [21] | Cohort of women > 35 years with unexplained infertility divided in two groups based on CCCT (day 10 FSH > 26). | 18 women with low ovarian reserve and 35 with normal ovarian reserve | Pregnancy rate | 6% vs 42% (p < 0.05) |
| Hagen et al., 2012 [22] | Cohort of women recruited prior to initiate pregnancy seeking and followed until pregnancy or for 6 menstrual cycles. | 186 divided into low (n = 36), medium (n = 113) and high (n = 37) AMH. | Fecundity rate per month | Low vs medium AMH 0.9 (95% CI 0.5–1.5) (p = ns). High vs medium AMH 0.7 (95% CI 0.4–1.1) (p = ns). |
| Streuli et al., 2014 [23] | Correlation of AMH with time to pregnancy in a cohort of pregnant women. | 86 women, of whom 82% within 6 months. | Time to pregnancy | Correlation between AMH and time to pregnancy: r = − 0.10 (p = ns) |
| Somigliana et al., 2015 [24] | Case-control study of pregnant women comparing those who were subfertile (pregnancy seeking > 1 year) to those who were fertile (pregnancy seeking < 1 year). | 76 subfertile cases and 76 age-matched controls | AMH | 2.6 (1.6–4.0) vs 2.8 (1.4–4.3) (p = ns) |
| Steiner et al., 2017 [25] | Cohort of women seeking pregnancy | 610 women, of whom 487 (80%) conceived. | Hazard ratio of conceiving | Low vs medium AMH 1.2 (95% CI 0.9–1.6) (p = ns). High vs medium AMH 0.9 (95% CI 0.6–1.2) (p = ns). High vs low FSH 1.2 (0.9–1.6) (p = ns) |
| Greenhood et al., 2018 [26] | Case-control study comparing women with unexplained infertility and healthy ovulatory controls not seeking pregnancy | 227 cases and 226 controls | AMH and AFC | AMH 5.9 ± 5.2 vs 5.2 ± 3.9 ng/ml (p = ns). AFC 21 ± 11 vs 17 ± 9 (p = ns) |
AMH is reported as ng/ml, FSH is reported as IU/L.
CCCT Clomiphene Citrate Challenge Test
The predominant role of age over that of ovarian reserve may partly explain the results of the two above-mentioned epidemiological studies that do not support the notion of a major fertility impairment in cancer survivors until older ages. In fact, one may envisage that in some cases chemotherapy may reduce ovarian reserve and anticipate menopause without affecting fertility. Indeed, since natural fertility ends at a mean age of 41 regardless of the subsequent age of menopause, a clinically relevant impairment of the ovarian reserve that takes place after age 41 may be unremarkable for the sake of childbearing. Moreover, even in women who had a more significant iatrogenic reduction of ovarian reserve and who will enter menopause before 41 years, parenthood may not be affected provided they seek pregnancies early, thus before facing the consequences of ovarian reserve exhaustion (anovulation, irregular menstrual cycles and finally definitive amenorrhea).
Noteworthy, this is very similar to what is observed in women with POI. In fact, the vast majority of these women fulfill their reproductive whishes [27]. Only those who excessively delay pregnancy seeking or who enter menopause at a very young age may ultimately remain childless. Noteworthy, the median time between last conception and amenorrhea was 4 years (IQR 1–8 years), thus remarkably less than the 10-year interval reported for women entering menopause at a normal age (one quarter of studied patients actually entered menopause within 1 year after delivery). To note, this result was observed in a modern Western population with low fertility index, thus leading to over-estimate the time between last conception and menopause. In addition, the study did not also show increased time to pregnancy in POI women [27].
On the other hand, it has to be recognized that even if the available evidence tends to support the idea that fertility and ovarian reserve should be disjointed, specific evidence in the peculiar group of childhood cancer survivors is needed. To note, Letourneau et al. [28] reported high rates of infertility in cancer survivors resuming regular menstrual cycles after chemotherapy (15–27% according to the different oncologic diagnoses). However, the study design was not designed to disentangle the independent effect of age and ovarian exhaustion.
Models of ovarian reserve depletion
The scant relevance of reduced ovarian reserve on the chances of natural pregnancy may explain only in part the attenuation of the impact of previous chemotherapy on fertility documented in the two above-mentioned cohort studies [5, 6]. Other factors may also contribute. To note, even if not fully consistent and inevitably linked to the specific disease and type and doses of chemotherapy, the available literature generally documents that the magnitude of the damage to the ovarian reserve is remarkable, even for non-myeloablative regimens [10, 16]. In vivo xenograft studies using human ovarian cortex showed dramatic toxic effects, with a reduction of the pool of primordial follicles reaching 50–100% according to the agents (or regimen) and doses used [29, 30]. Albeit more limited, there is also some direct histological data on the density of primordial follicles in cancer survivors [10, 31, 32]. In an in vivo study of 26 women treated for different malignancies who subsequently underwent ovarian biopsies, the follicular density appears to be halved [31], but data is controversial [32]. In contrast, evidence from surrogate measurements of ovarian reserve such as serum FSH, serum AMH, and Antral Follicle Count-AFC is larger. Most recent evidence on this issue are presented in Table 3 [33–40]. Even if the magnitude of the estimated damage differs, the available studies consistently support a significant damage. To note, these studies may underestimate the detrimental effects of chemotherapy since women with POI are generally excluded.
Table 3.
Controlled studies on the impact of chemotherapy on ovarian reserve
| Study | N. cases | Age (years) | N. controls | Outcome | Main findingsa |
|---|---|---|---|---|---|
| Lie Fong et al., 2009 [32] | 182 | 25 (17–47) | 42 | AMH | 1.7 (< 0.1–19.9) vs 2.1 (0.1–7.4): p = ns |
| Gracia et al., 2012 [33] | 71 | 25.7 (24.2–27.2) | 67 | FSH | 11.1 [9.5–13.1] vs 7.2 [6.0–8.8]: p = 0.001 |
| AMH | 0.8 [0.6–1.1] vs 2.8 [2.1–4.0]: p < 0.001 | ||||
| AFC | 15 [11–18] vs 27 [23–31]: p < 0.001 | ||||
| Dillon et al., 2013 [34] | 84 | 26 [24–27] | 98 | FSH | 13.9 [10.4–16.4] vs 7.2 [6.6–8.0]: p < 0.001 |
| AMH | 0.7 [0.5–1.] vs 2.4 [2.0–2.7]: p < 0.001 | ||||
| AFC | 11 [9–14] vs 23 [20–26]: p < 0.001 | ||||
| El-Shalakany et al., 2013 [35] | 30 | 19.1 ± 4.6 | 30 | FSH | 8.4 ± 1.5 vs 3.3 ± 0.4: p = 0.001 |
| AMH | 1.5 ± 0.7 vs 2.0 ± 1.0: p = 0.02 | ||||
| Krawczuk-Rybak et al., 2013 [36] | 83 | 18.9 ± 5.0 | 38 | FSH | 12.2 ± 19.4 vs 5.4 ± 1.9: p = 0.001 |
| AMH | 2.3 ± 2.0 vs 3.8 ± 1.7: p = 0.001 | ||||
| Johnson et al., 2014 [37] | 84 | 25 (15–39) | 115 | AMH | 0.7 [0.5–0.9] vs 2.3 [2.0–2.6]: p < 0.05 |
| AFC | 16 [14–19] vs 27 [25–30]: p < 0.05 | ||||
| Akar et al., 2015 [38] | 41 | 15.0 ± 2.4 | 44 | FSH | 13.5 ± 16.2 vs 7.3 ± 2.7: p = 0.017 |
| AMH | 1.6 ± 0.4 vs 1.7 ± 0.3: p = ns | ||||
| AFC | 3.4 ± 3.3 vs 8.6 ± 3.5: p < 0.001 | ||||
| Thomas-Teinturier et al., 2015 [39] | 105 | 25 [17–49] | 20 | AMH | 1.5 [0–13.7] vs 3.1 [0.5–6.6]: p = 0.003 |
Only studies in childhood or young women cancer survivors and comparing data to a control group were included
Data is reported as median (range) or median [5–95th centile] or mean ± SD
aData was reported firstly for cases and secondly for controls
AMH was reported as ng/ml and FSH as IU/L
AFC was reported only if collected by transvaginal ultrasound
To explain this apparent paradox (i.e., a relevant biological damage but a modest clinical effect), one may speculate on the mathematical rules that regulate the exhaustion of the pool of primordial follicles. The rate of decline of the ovarian reserve has been a topic of controversy [41–47]. Differences in the outcome studied (FSH, AMH, antral follicle count or histological count of the primordial follicles) could explain at least in part this controversy. Of utmost relevance here is that the loss of primordial follicles (i.e., the real physiological unit of the ovarian reserve) is not linear [47]. A systematic review on this issue showed that the absolute rate of loss per unit of time is higher at younger ages (the vast majority of the ovarian reserve is actually lost before menarche) and then progressively decreases [46]. In this context, two different models of ovarian reserve depletion may be hypothesized: (1) age-dependent loss and (2) ovarian reserve-dependent loss. In the first model, the rate of primordial follicles loss would be exclusively guided by age. The crude loss would decrease with age, but this rate would be fixed for every specific age. Conversely, in the second model, the loss would be guided by the amount of the residual pool. The crude loss of primordial follicles would be inversely related to the remnant pool rather than to a specific age.
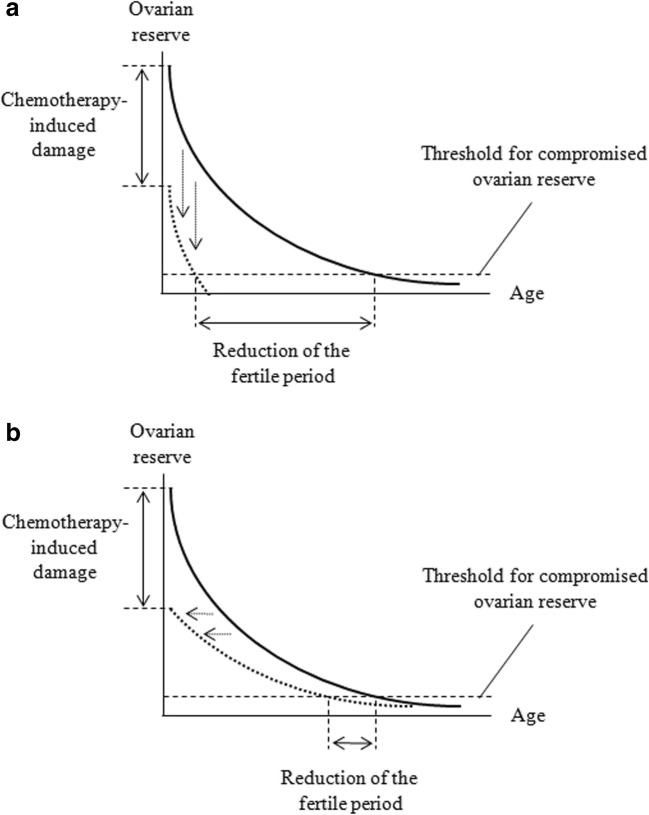
The long-term detrimental effects of chemotherapy on ovarian reserve are expected to be markedly different according to the postulated model of primordial follicle loss. If the loss is exclusively driven by age, the impact of a damage to the ovarian reserve would be dramatic, while, in the second case, it would be significantly attenuated. From a mathematical point of view, the two models can be simplistically presented as follows:
where Y is the residual ovarian reserve, X is time (age), and A is a constant indicating the amount of chemotherapy-related damage.
These two possibilities are illustrated in Fig. 1. In the first scenario (age-dependent loss), one would observe definitive ovarian reserve impairment within a very short period of time. Conversely, in the second scenario (ovarian reserve-dependent loss), despite the similar immediate loss, clinically significant impairment of ovarian reserve would occur much later and the advent of the clinical events associated to ovarian reserve impairment (irregular cycles, menopause and infertility) would be only marginally anticipated.
Fig. 1.
Impact of chemotherapy on ovarian reserve according to the hypothesized mathematical model guiding the loss of the primordial follicle pool. The model based on the crucial role of age is represented in the upper panel (a). The model based on the crucial role of the remnant ovarian reserve is represented in the lower panel (b). The plain curves represent the decline in ovarian reserve in basal situation (referral lines). The dotted curves represent the decline in ovarian reserve in girls exposed to chemotherapy: for simplicity, we postulated a halving of the residual ovarian reserve at the starting point. In a, the dotted curve actually corresponds to a vertical shift (downwards) of the referral curve. In b, the dotted curve actually corresponds to a lateral shift (to the left) of the referral curve. These shifts are represented with dotted arrows. The dashed horizontal lines represent the threshold of residual ovarian reserve that is required to ensure ovulatory regular cycles. Even if the notion that the decrease in ovarian reserve follows exponential rules is based on a published model [46], all the curves represented in these figures are theoretical and, therefore, no precise units are reported. However, the scales of the two axes have to be considered linear
Overall, the second model appears to better fit with the available epidemiological evidence and would explain the above emphasized contrast between the remarkable impact of chemotherapy on ovarian reserve and the relatively mild clinical impact on fertility that becomes evident only at older ages.
Comment
Oocyte quality and thus age are more important than ovarian reserve for natural conception, provided there is a sufficient amount of residual primordial follicles to ensure regular menstrual cycles. Moreover, loss in ovarian reserve with age follows a complex non-linear model that appears to depend on residual ovarian reserve and that actually attenuates the impact of chemotherapy-related injury. These two observations may explain the contrast between the relevant crude amount of primordial follicle loss associated with chemotherapy and the relatively milder clinical impact. Even if these considerations are mainly theoretical and lack sufficient evidence to elaborate a precise mathematical model, they provide a reasonable explanation for the available epidemiological evidence and may have research and clinical implications.
Firstly, they highlight the importance of improving our capacity to predict fertility impairment at the time of cancer diagnosis in children and adolescent. The availability of a reliable and validated tool to predict future fertility would consent a more accurate counseling and would improve the shared decision-making process. To date, the only validated criteria are the so-called “Edinburgh criteria” [48]. The authors reported a risk of POI in women who do and do not fulfill the criteria of 35% and 1%, respectively (p < 0.001). These results are of particular relevance considering that only 8% of young girls with cancer fulfilled these criteria and should be scheduled for fertility preservation. On the other hand, these criteria lack external validation and provide evidence on the rate of POI rather than on childbearing. Not recommending fertility preservation procedures exclusively based on the Edinburg criteria may be questionable.
Secondly, our considerations highlight the importance of an early reproductive counseling of childhood cancer survivors. Postponing motherhood is a diffuse demographic phenomenon of Western countries that may impact on the ultimate chances of pregnancy in women in general [49]. This social trend may be even more detrimental for childhood cancer survivors whose period of fecundity may be shortened. In fact, if on one hand, this reduction may be modest on average, on the other side, it can greatly impair the reproductive performance if affected women delay pregnancy seeking. In fact, more and more women are currently seeking pregnancy in the upper boundary of the fertility window [49]. Cancer survivors who do so may be inevitably exposed to a major risk of childlessness. On the other hand, even if there is evidence that women with a history of cancer may face fertility problems in their own way [50], one cannot oblige them to behave differently than the general population and to anticipate pregnancy seeking. For this reason, the considerations expressed in this opinion paper should not be used per sé to argue against the use of fertility preservation techniques in girls or adolescent planned for chemotherapy. Educating to look for a pregnancy at a younger age is wise, but one has to accept that this counseling will be followed in a limited percentage of women.
Thirdly, one may also consider to perform fertility preservation techniques after the end of the oncologic treatments, once survivors reach the legal age of majority. In childhood survivors, this option was shown to be feasible and deserves consideration [18]. Young women with substantial but not definitive damage to the ovarian reserve may benefit from this approach. It has some indisputable advantages over fertility procedures done at the time of cancer diagnosis, including some ethical issues. However, the use of post-chemotherapy fertility preservation techniques for adult women with reduced but not compromised ovarian reserve should be currently considered a second-line option and needs validation. Notably, eligible women are also expected to be those collecting a low number of eggs. Unfortunately, even if a reduced ovarian reserve may be unremarkable for natural conception, it may hamper ART success in general (and thus also the success of oocyte storage programs) [51]. The number of stored oocytes is actually an important determinant of success and women previously exposed to chemotherapy are at higher risk of impaired egg retrieval. The possibility to perform several rather than only 1–2 cycles of ovarian hyper-stimulation cannot be expected to fully overcome a condition of impaired ovarian reserve.
Our general reasoning is not definitive and should be viewed as speculative. The utility of fertility preservation cannot be questioned based on the available evidence. In particular, there is the need for further and more informative studies on the impact of chemotherapy. The findings emerging from the two large cohort studies [5, 6] are exposed to a bias of selection (only survivors were included) and were obtained in selected populations (Hodgkin’s lymphoma in the first study and cancers not requiring radiotherapy to the pelvis or the brain in the second study). Moreover, the attitude of cancer survivors towards motherhood may be influenced by their personal oncologic history and this could impact on the propensity to seek for pregnancy [50]. Both studies did not address this possible confounder. Finally, it is noteworthy that our interpretation of the evidence is exclusively based on age and the pool of remnant primordial follicles. The complete figure is presumably more complicated [52, 53]. For instance, it has recently been reported that, despite being normally fertile, women with very low serum AMH (< 0.4 ng/ml) face a higher risk of miscarriage [54]. Moreover, not all evidence concord on the most reliable model to describe the rate of follicle primordial loss over the years [51, 52].
In conclusion, the advent of large cohort studies on the long-term impact of chemotherapy in childhood cancer survivors is opening new perspectives. Disentangling the mechanisms linking chemotherapy, damage to the ovarian reserve and subsequent natural fertility may open new avenues of research and may influence clinical practice.
Compliance with ethical standards
Conflict of interest
Edgardo Somigliana received during the last 3 years grants of research from Ferring and Merck-Serono. All the other authors do not have any financial disclosure to declare.
References
- 1.Madanat LM, Malila N, Dyba T, Hakulinen T, Sankila R, Boice JD, Jr, Lähteenmäki PM. Probability of parenthood after early onset cancer: a population-based study. Int J Cancer. 2008;123(12):2891–2898. doi: 10.1002/ijc.23842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reulen RC, Zeegers MP, Wallace WH, Frobisher C, Taylor AJ, Lancashire ER, Winter DL, Hawkins MM, British Childhood Cancer Survivor Study Pregnancy outcomes among adult survivors of childhood cancer in the British Childhood Cancer Survivor Study. Cancer Epidemiol Biomark Prev. 2009;18(8):2239–2247. doi: 10.1158/1055-9965.EPI-09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stensheim H, Cvancarova M, Møller B, Fosså SD. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer. 2011;129(5):1225–1236. doi: 10.1002/ijc.26045. [DOI] [PubMed] [Google Scholar]
- 4.Pivetta E, Maule MM, Pisani P, Zugna D, Haupt R, Jankovic M, Aricò M, Casale F, Clerico A, Cordero di Montezemolo L, Kiren V, Locatelli F, Palumbo G, Pession A, Pillon M, Santoro N, Terenziani M, Valsecchi MG, Dama E, Magnani C, Merletti F, Pastore G, Italian Association of Pediatric Hematology and Oncology (AIEOP) Group Marriage and parenthood among childhood cancer survivors: a report from the Italian AIEOP Off-Therapy Registry. Haematologica. 2011;96(5):744–751. doi: 10.3324/haematol.2010.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brämswig JH, Riepenhausen M, Schellong G. Parenthood in adult female survivors treated for Hodgkin’s lymphoma during childhood and adolescence: a prospective, longitudinal study. Lancet Oncol. 2015;16:667–675. doi: 10.1016/S1470-2045(15)70140-3. [DOI] [PubMed] [Google Scholar]
- 6.Chow EJ, Stratton KL, Leisenring WM, Oeffinger KC, Sklar CA, Donaldson SS, Ginsberg JP, Kenney LB, Levine JM, Robison LL, Shnorhavorian M, Stovall M, Armstrong GT, Green DM. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2016;17:567–576. doi: 10.1016/S1470-2045(16)00086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armuand G, Skoog-Svanberg A, Bladh M, Sydsjö G. Reproductive patterns among childhood and adolescent cancer survivors in Sweden: a population-based matched-cohort study. J Clin Oncol. 2017;35(14):1577–1583. doi: 10.1200/JCO.2016.71.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson RA, Brewster DH, Wood R, Nowell S, Fischbacher C, Kelsey TW, Wallace WHB. The impact of cancer on subsequent chance of pregnancy: a population-based analysis. Hum Reprod. 2018;33(7):1281–1290. doi: 10.1093/humrep/dey216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dorp W, Haupt R, Anderson RA, Mulder RL, van den Heuvel-Eibrink MM, van Dulmen-den Broeder E, Su HI, Falck Winther J, Hudson MM, Levine JM, Wallace WH. Reproductive function and outcomes in female survivors of childhood, adolescent, and young adult cancer: a review. J Clin Oncol. 2018;36(21):2169–2180. doi: 10.1200/JCO.2017.76.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18:525–535. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 11.Wallace WH, Kelsey TW, Anderson RA. Fertility preservation in pre-pubertal girls with cancer: the role of ovarian tissue cryopreservation. Fertil Steril. 2016;105:6–12. doi: 10.1016/j.fertnstert.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Oktem O, Kim SS, Selek U, Schatmann G, Urman B. Ovarian and Uterine functions in female survivors of childhood cancers. Oncologist. 2018;23:214–224. doi: 10.1634/theoncologist.2017-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:185ra62. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 14.Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, Raanani H, Levron J, Fridman E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22(6):1626–1633. doi: 10.1093/humrep/dem027. [DOI] [PubMed] [Google Scholar]
- 15.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377:1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 17.Mertes H. Let’s not forget that many prepubertal girls do have other options besides ovarian tissue cryopreservation. Hum Reprod. 2015;30:2011–2013. doi: 10.1093/humrep/dev176. [DOI] [PubMed] [Google Scholar]
- 18.Filippi F, Meazza C, Paffoni A, Raspagliesi F, Terenziani M, Somigliana E. Egg freezing in childhood and young adult cancer survivors. Pediatrics. 2016;138:e20160291. doi: 10.1542/peds.2016-0291. [DOI] [PubMed] [Google Scholar]
- 19.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 20.Eijkemans MJ, van Poppel F, Habbema DF, Smith KR, Leridon H, te Velde ER. Too old to have children? Lessons from natural fertility populations. Hum Reprod. 2014;29:1304–1312. doi: 10.1093/humrep/deu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navot D, Rosenwaks Z, Margalioth EJ. Prognostic assessment of female fecundity. Lancet. 1987;2:645–647. doi: 10.1016/S0140-6736(87)92439-1. [DOI] [PubMed] [Google Scholar]
- 22.Hagen CP, Vestergaard S, Juul A, Skakkebæk NE, Andersson AM, Main KM, Hjøllund NH, Ernst E, Bonde JP, Anderson RA, Jensen TK. Low concentration of circulating antimullerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertil Steril. 2012;98:1602–1608. doi: 10.1016/j.fertnstert.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Streuli I, de Mouzon J, Paccolat C, Chapron C, Petignat P, Irion OP, de Ziegler D. AMH concentration is not related to effective time to pregnancy in women who conceive naturally. Reprod BioMed Online. 2014;28:216–224. doi: 10.1016/j.rbmo.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Somigliana E, Lattuada D, Colciaghi B, Filippi F, La Vecchia I, Tirelli A, Baffero GM, Paffoni A, Persico N, Bolis G, Fedele L. Serum anti-Mullerian hormone in subfertile women. Acta Obstet Gynecol Scand. 2015;94:1307–1312. doi: 10.1111/aogs.12761. [DOI] [PubMed] [Google Scholar]
- 25.Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, Baird DD. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318:1367–1376. doi: 10.1001/jama.2017.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenwood EA, Cedars MI, Santoro N, Eisenberg E, Kao CN, Haisenleder DJ, Diamond MP, Huddleston HG, National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Cooperative Reproductive Medicine Network Antimüllerian hormone levels and antral follicle counts are not reduced compared with community controls in patients with rigorously defined unexplained infertility. Fertil Steril. 2017;108(6):1070–1077. doi: 10.1016/j.fertnstert.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Daan NM, Hoek A, Corpeleijn E, Eijkemans MJ, Broekmans FJ, Fauser BC, Koster MP. Reproductive characteristics of women diagnosed with premature ovarian insufficiency. Reprod BioMed Online. 2016;32:225–232. doi: 10.1016/j.rbmo.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Letourneau JM, Ebbel EE, Katz PP, Oktay KH, McCulloch CE, Ai WZ, Chien AJ, Melisko ME, Cedars MI, Rosen MP. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118:1933–1939. doi: 10.1002/cncr.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oktem O, Oktay K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007;67:10159–10162. doi: 10.1158/0008-5472.CAN-07-2042. [DOI] [PubMed] [Google Scholar]
- 30.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging. 2011;3:782–793. doi: 10.18632/aging.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007;110:2222–2229. doi: 10.1002/cncr.23071. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin M, Kelsey TW, Wallace WH, Anderson RA, Telfer EE. Non-growing follicle density is increased following adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) chemotherapy in the adult human ovary. Hum Reprod. 2017;32:165–174. doi: 10.1093/humrep/dew260. [DOI] [PubMed] [Google Scholar]
- 33.Lie Fong S, Laven JS, Hakvoort-Cammel FG, Schipper I, Visser JA, Themmen AP, de Jong FH, van den Heuvel-Eibrink MM. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Hum Reprod. 2009;24:982–990. doi: 10.1093/humrep/den487. [DOI] [PubMed] [Google Scholar]
- 34.Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, Vance A, Ginsberg JP. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97:134–140. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dillon KE, Sammel MD, Ginsberg JP, Lechtenberg L, Prewitt M, Gracia CR. Pregnancy after cancer: results from a prospective cohort study of cancer survivors. Pediatr Blood Cancer. 2013;60:2001–2006. doi: 10.1002/pbc.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Shalakany AH, Ali MS, Abdelmaksoud AA, Abd El-Ghany S, Hasan EA. Ovarian function in female survivors of childhood malignancies. Pediatr Hematol Oncol. 2013;30:328–335. doi: 10.3109/08880018.2013.778927. [DOI] [PubMed] [Google Scholar]
- 37.Krawczuk-Rybak M, Leszczynska E, Poznanska M, Zelazowska-Rutkowska B, Wysocka J. Anti-müllerian hormone as a sensitive marker of ovarian function in young cancer survivors. Int J Endocrinol. 2013;2013:125080. doi: 10.1155/2013/125080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson LN, Sammel MD, Dillon KE, Lechtenberg L, Schanne A, Gracia CR. Antimüllerian hormone and antral follicle count are lower in female cancer survivors and healthy women taking hormonal contraception. Fertil Steril. 2014;102:774–781. doi: 10.1016/j.fertnstert.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akar B, Doğer E, Çakıroğlu Y, Çorapçıoğlu F, Sarper N, Çalışkan E. The effect of childhood cancer therapy on ovarian reserve and pubertal development. Reprod BioMed Online. 2015;30:175–180. doi: 10.1016/j.rbmo.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Thomas-Teinturier C, Allodji RS, Svetlova E, Frey MA, Oberlin O, Millischer AE, Epelboin S, Decanter C, Pacquement H, Tabone MD, Sudour-Bonnange H, Baruchel A, Lahlou N, De Vathaire F. Ovarian reserve after treatment with alkylating agents during childhood. Hum Reprod. 2015;30:1437–1446. doi: 10.1093/humrep/dev060. [DOI] [PubMed] [Google Scholar]
- 41.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 42.Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845–851. doi: 10.1016/S0015-0282(99)00396-9. [DOI] [PubMed] [Google Scholar]
- 43.Hanson B. Questioning the construction of maternal age as a fertility problem. Health Care Women Int. 2003;24:166–176. doi: 10.1080/07399330390178459. [DOI] [PubMed] [Google Scholar]
- 44.Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- 45.Rosen MP, Sternfeld B, Schuh-Huerta SM, Reijo Pera RA, McCulloch CE, Cedars MI. Antral follicle count: absence of significant midlife decline. Fertil Steril. 2010;94:2182–2185. doi: 10.1016/j.fertnstert.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5(1):e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelsey TW, Anderson RA, Wright P, et al. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod. 2012;18:79–87. doi: 10.1093/molehr/gar059. [DOI] [PubMed] [Google Scholar]
- 48.Wallace WH, Smith AG, Kelsey TW, Nelson SM, Wallace WH. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol. 2014;15:1129–1136. doi: 10.1016/S1470-2045(14)70334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mills M, Rindfuss RR, McDonald P, te Velde E, ESHRE Reproduction and Society Task Force Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17:848–860. doi: 10.1093/humupd/dmr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dijk M, van den Berg MH, Overbeek A, Lambalk CB, van den Heuvel-Eibrink MM, Tissing WJ, Kremer LC, van der Pal HJ, Loonen JJ, Versluys B, Bresters D, GJL K, van Leeuwen FE, van Dulmen-den Broeder E, DCOG LATER-VEVO study group Reproductive intentions and use of reproductive health care among female survivors of childhood cancer. Hum Reprod. 2018;33(6):1167–1174. doi: 10.1093/humrep/dey058. [DOI] [PubMed] [Google Scholar]
- 51.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26:1768–1774. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 52.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-müllerian hormone from conception to menopause. PLoS One. 2011;6(7):e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zamah AM, Stephenson MD. Antimüllerian hormone and miscarriage: fifty shadesof gray. Fertil Steril. 2018;109:1008–1009. doi: 10.1016/j.fertnstert.2018.02.140. [DOI] [PubMed] [Google Scholar]
- 54.Lyttle Schumacher BM, Jukic AMZ, Steiner AZ. Antimüllerian hormone as a risk factor for miscarriage in naturally conceived pregnancies. Fertil Steril. 2018;109(6):1065–1071. doi: 10.1016/j.fertnstert.2018.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]