Dear Editor,
Infectious bursal disease (IBD) is one of the most important diseases of the poultry. The IBD virus (IBDV), a non-enveloped virus belonging to the Birnaviridae family with a genome consisting of two segments of double-stranded RNA (segments A and B), targets B lymphocytes of bursa of Fabricious leading to immunosuppression. In Pakistan, poultry farming is the second biggest industry and IBD is the second biggest disease threating the poultry sector. However, there is limited genome information of IBDV available in Pakistan (Shabbir et al.2016).
In March 2017, a suspected IBD outbreak was reported in 28-day-old broiler chicken flock in Punjab, which is the most important chicken-farming area of Pakistan. A total of 30% to 50% morbidity and 13% mortality were observed without previous history of the vaccination. Seventeen (n = 17) bursa samples were collected for virus detection. Using RT-PCR with primers of A628U/A1540L (Supplementary Table S1), a specific fragment of IBDV with an expected length of 930 bp was amplified from all 17 samples. Sequencing results showed that all 17 sequences covering the hypervariable region (HVR) of VP2 carry 100% homology. Thereafter, one positive sample was randomly selected for virus isolation and one IBDV strain named PK2 was isolated successfully as described previously (Yuwen et al.2008). Furthermore, the genome cDNA of PK2 was cloned for segment A (primers of AU/A1542L and A1421U2/AL2) or B (BU/B1344L and B1344U/BL) (Supplementary Table S1). Sequencing results showed that the segment A of PK2 strain contained 3,260 nucleotides (nt), including two partially overlapping ORFs (3,073 nt) flanked by 5′-non-coding-region (NCR, 96 nt) and 3′-NCR (91 nt). The large ORF (3,039 nt) of segment A encodes a polyprotein (NH2-VP2-VP4-VP3-COOH) while the small ORF (450 nt) encodes VP5. Segment B consists of VP1 coding ORF (2,637 nt) flanked by 5′-NCR (111 nt) and 3′-NCR (79 nt). The genome sequence of PK2 is submitted to GenBank with the accession number MF996499 (segment A) and MF996500 (segment B), which is the first full-length genome sequence of Pakistan isolated IBDV strain appeared in GenBank.
Then the alignment and phylogenetic analyses based on the nucleotide or amino acid sequences were performed using the Clustal W (version 1.8) (Thompson et al.1997) and the MEGA (version 3.1) (Kumar et al.2004), and the confidence levels were assessed using 1000 bootstrap replications. The sequences of reference IBDV including very virulent, variant, attenuated, and serotype II strains were obtained from GenBank. Phylogenetic analysis based on the amino acid sequence showed that the polyprotein and VP5 coded by segment A was closely related to very virulent IBDV (vvIBDV) strains (Fig. 1A), while segment-B-coded VP1 branched out of the vvIBDV group and was clustered into a unique branch (Fig. 1B). In addition to serotype II, serotype I IBDV was further divided into three wild subtypes including classical, variants, very virulent strains, and one artificially attenuated strain. It has been reported that co-evolution of genome segments is a major evolutionary feature in IBDV (Le Nouen et al.2006). However, recently, it has been reported a few strains exhibited markedly different genetic relatives for segments A and B. (1) vv-A/Att-B (segment A from very virulent strain and segment B from attenuated strain): SH95, GX-NN-L, KZC-104, IBD13HeB01, MB11/ABT/MVC/2016, JBN2011. (2) Att-A/vv-B: ZJ2000, TL2004, and HN04. (3) vv-A/c-B (segment A from vvIBDV and segment B from classic strain): Br/03/DR, CA-S7610. (4) vv-A/var-B (segment A from vvIBDV and segment B from variant strain): 02015.2. (5) vv-A/II-B (segment A from vvIBDV and segment B from serotype II strains): CA-K785, CA-D495, and 100056. It was suggested that the exchange of the double-stranded genomic RNA segments contributed to the generation of new reassortant viruses because of the co-infection with different types of IBDV (Jackwood et al. 2011; Le Nouen et al.2006), which have not been verified in laboratory. In fact, multiple factors may contribute to the gene reassortment, including host immunity, vaccinations, selection pressure, environment, and so on. It is identified that PK2 is a genetically segment-reassortant strain whose segment A was derived from vvIBDV, while segment B was originated from a unique ancestor (vv-A/Uniq-B).
Fig. 1.
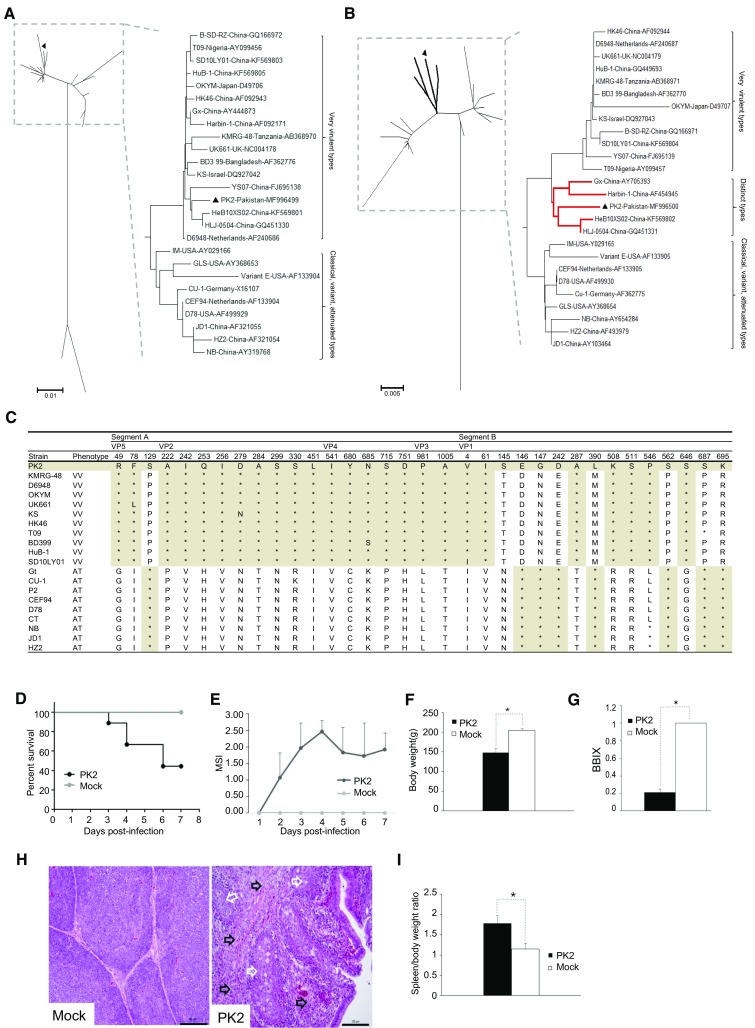
Genome analysis. Phylogenetic tree analysis of amino acid sequence of polyprotein (A) and VP1 (B). Trees were generated by the neighbor-joining method using MEGA 6 with 1000 replications. Two styles of trees were shown. The reassortant virus PK2 isolated in this study is highlighted with solid triangle. For serotype I strains, polyprotein consisted of two main branches, while VP1 were divided into three branches and the unique branch was highlighted with red bold lines. (C) Unique amino acid substitutions in VP5, polyprotein, and VP1 of vvIBDV and attenuated strains. VV, very virulent strain; AT, attenuated strain. Asterisks indicate residues identical to the sequence of PK2. (D–I) Pathogenic analyses of PK2 strain in SPF chickens. Two-week-old SPF chickens were infected with PK2 strain while the control group were inoculated with phosphate buffer solution. Chickens were monitored daily for clinical signs. At 7 days post-infection, all surviving chickens were weighted and euthanized to examine for pathological changes. (D) Survival rate. (E) The mean symptomatic index (MSI). (F) Body weight. (G) The bursa: body-weight index (BBIX) [BBIX = (bursa: body-weight ratios)/(bursa: body-weight ratios in the negative group)]. (H) Histopathological slide of bursa (hematoxylin and eosin). The histopathological bursal lesions including follicle atrophy, macrophages infiltration, lymphocytic necrosis (white arrows), and connective tissue hyperplasia (black arrows) are shown. The scale bar indicates 100 μm. (I) The spleen/body weight ratio. Average titers and standard deviations (error bars) from three independent samples are shown. The star “*” means the treatments differ significantly at a confidence level (P < 0.05).
In segment A, genetic distances derived from the amino acid sequences revealed that the polyprotein of PK2 shared 98.4%–99.8% identity with those of very virulent strains, while it has only 89.5%–97.9% identity with non-vvIBDV strains including classical, variant, attenuated, and serotype II strains. Sixteen amino acid residues in VP2 (222A, 242I, 253Q, 256I, 279D, 284A, 299S, 330S, 451L), VP4 (541I, 680Y, 685 N, 715S, 751D), and VP3 (981P, 1005A), which are usually conserved in vvIBDV strains, were also present in PK2 (Fig. 1C). In addition, N-terminus quadruplet (MLSL) and the conserved amino acid residues at positions 49R, 78F in VP5 of vvIBDV (Lu et al.2015) were also observed in PK2 (Fig. 1C). However, the amino acid residue at position 129 is P, which is the same with attenuated IBDV. PK2 VP5 has the amino acid sequence identity of 94.5%–96.6% with those of vv strains, while it has 93.2%–95.2% identity with non-vv strains.
In segment B, the homology analysis revealed that VP1 of PK2 had identity of 97.5%–98.3% with vvIBDV, while the identity with non-vv was 97.4%–98.7%. As was shown in Fig. 1C, among fifteen characteristic amino acid residues of VP1, PK2 has eight amino acids (4V, 61I, 145S, 287A, 508K, 511S, 546P, 646S) as same as vvIBDV, while the other seven amino acids (146E, 147G, 242D, 390L, 562S, 687S, and 695K) are the same as attenuated IBDV. The amino acid triplets at positions 145/146/147 of VP1 are important virulent sites through influencing the RNA dependant RNA polymerase (RdRp). It has been reported that there were three types of the triplet, TDN, TEG, NEG, which were the characteristic of very virulent, HLJ0504-like, and attenuated IBD (Gao et al.2014). Interestingly, VP1 of PK2 has the fourth kind of triplet (SEG), as being discovered in some Uruguay isolates (Hernández et al.2015). To be mentioned, VP1 of PK2 has 98%–98.8% identity with HLJ-0504, Gx, Harbin-1, and HeB10XS02, which was named as HLJ0504-like strain (He et al.2014; Qi et al.2011). In the group, PK2 shared 95.8%–96.6% amino acid sequence identity of polyprotein and 98%–98.8% of VP1 with other strains. It was reported that HLJ0504-like strains were the popular strains in China (He et al.2014; Qi et al.2011). Recently, this type of IBDV were also observed in Nigeria (Nwagbo et al.2016) and Algeria (Abed et al.2018). Segment B was reported to play key role in IBDV evolution through enhancing the virulence of the virus synergistically with its existing genome segment A (Hon et al.2006). However, the origin of segment B is unknown. Maybe the unique B was from other reservoir hosts like other poultry or wild birds (Jackwood et al.2005).
Furthermore, the serious pathogenicity of PK2 was evaluated. The 2 week-old SPF chickens housed separately in negative pressure isolators were randomly divided into two groups. The 9 chickens in the first group were individually infected with 1.26 × 103 ELD50 (0.2 mL) of PK2 strain via eye and intranasal routes. The 3 chickens in the second group were provided with 0.2 mL of phosphate buffer solution as the negative control. Chickens were monitored daily for clinical signs for 7 days. The clinical signs were evaluated and showed as mean symptomatic index (MSI) as described previously (Le Nouen et al.2006). PK2 caused 100% morbidity and 55.6% mortality, whereas neither clinical signs nor mortality were observed in the PBS control group (Fig. 1D, 1E). From 2 days post-infection (d p.i.), chickens in PK2-infected group began showing typical signs of acute IBD (ruffled feathers, dehydration, and prostration), and the highest MSI (2.46 ± 0.35) was observed at 4 d p.i. (Fig. 1E). In PK2 group, the mortality peak (33.3%) occurred from 3 to 4 d p.i., and there were 2 chickens died on 6 d p.i. (Fig. 1D). At 7 d p.i., all surviving chickens were weighed and euthanized. The body weight of PK group (147.38 ± 11.32 g) was significantly lower than the control (204.43 ± 5.31 g) (P < 0.05) (Fig. 1F). IBDV was detected in the infected bursa with 6.4 × 108 viral RNA copies/g. PK2 caused severe histopathological bursal lesions including follicle atrophy, connective tissue hyperplasia, macrophages infiltration, and lymphocytic necrosis (Fig. 1H) with the bursa: body-weight index (BBIX) of 0.21 ± 0.04 (Fig. 1G). Meanwhile, the spleen/body weight ratio of PK group (1.15 ± 0.14) also had significant difference from the control (1.78 ± 0.20) (Fig. 1I). It is valuable to further study the pathogenic mechanism in more detail.
In conclusion, a naturally occurring reassortant strain of IBDV, carrying segment A from a very virulent strain and segment B from a unique ancestor, was first identified in Pakistan. For the first time, the full-length genome and the virulence of Pakistan IBDV was analyzed. The genomic and biological characterization of the strain clearly highlights the nature and dynamics of the IBDV in poultry sector of Pakistan. The present study therefore provides useful data for further understanding of the nature and evolution of IBDV, which will support a better prevention and control of the disease in Pakistan.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Nos. 2016YFE0203200, 2017YFD0500704), the Major Project of National Natural Science Foundation of China (No. 31430087), the Modern Agro-industry Technology Research System (No. CARS-41-G15).
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
Animal experiments in this study were approved by the Ethics Committees of Harbin Veterinary Research Institute (HVRI), Chinese Academy of Agricultural Sciences (CAAS) (Approval Number: SQ-2017-080).
Contributor Information
Xiaomei Wang, Phone: +86-451-51051690, Email: wangxiaomei@caas.cn.
Xiaole Qi, Phone: +86-451-51051690, Email: qixiaole@caas.cn.
References
- Abed M, Soubies S, Courtillon C, Briand FX, Allée C, Amelot M, De Boisseson C, Lucas P, Blanchard Y, Belahouel A. Infectious bursal disease virus in algeria: detection of highly pathogenic reassortant viruses. Infect Genet Evol. 2018;60:48–57. doi: 10.1016/j.meegid.2018.01.029. [DOI] [PubMed] [Google Scholar]
- Gao L, Li K, Qi X, Gao H, Gao Y, Qin L, Wang Y, Shen N, Kong X, Wang X. Triplet amino acids located at positions 145/146/147 of the RNA polymerase of very virulent infectious bursal disease virus contribute to viral virulence. J Gen Virol. 2014;95:888–897. doi: 10.1099/vir.0.060194-0. [DOI] [PubMed] [Google Scholar]
- He X, Xiong Z, Yang L, Guan D, Yang X, Wei P. Molecular epidemiology studies on partial sequences of both genome segments reveal that reassortant infectious bursal disease viruses were dominantly prevalent in southern china during 2000–2012. Arch Virol. 2014;159:3279–3292. doi: 10.1007/s00705-014-2195-z. [DOI] [PubMed] [Google Scholar]
- Hernández M, Tomás G, Marandino A, Iraola G, Maya L, Mattion N, Hernández D, Villegas P, Banda A, Panzera Y. Genetic characterization of south american infectious bursal disease virus reveals the existence of a distinct worldwide-spread genetic lineage. Avian Pathol. 2015;44:212–221. doi: 10.1080/03079457.2015.1025696. [DOI] [PubMed] [Google Scholar]
- Hon CC, Lam TY, Drummond A, Rambaut A, Lee YF, Yip CW, Zeng F, Lam PY, Ng PT, Leung FC. Phylogenetic analysis reveals a correlation between the expansion of very virulent infectious bursal disease virus and reassortment of its genome segment b. J Virol. 2006;80:8503–8509. doi: 10.1128/JVI.00585-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood D, Gough R, Sommer S. Nucleotide and amino acid sequence analysis of a birnavirus isolated from penguins. Vet Rec. 2005;156:550–552. doi: 10.1136/vr.156.17.550. [DOI] [PubMed] [Google Scholar]
- Jackwood DJ, Sommer-Wagner SE, Crossley BM, Stoute ST, Woolcock PR, Charlton BR. Identification and pathogenicity of a natural reassortant between a very virulent serotype 1 infectious bursal disease virus (IBDV) and a serotype 2 IBDV. Virology. 2011;420:98–105. doi: 10.1016/j.virol.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. Mega 3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Le Nouen C, Rivallan G, Toquin D, Darlu P, Morin Y, Beven V, de Boisseson C, Cazaban C, Comte S, Gardin Y. Very virulent infectious bursal disease virus: reduced pathogenicity in a rare natural segment-b-reassorted isolate. J Gen Virol. 2006;87:209–216. doi: 10.1099/vir.0.81184-0. [DOI] [PubMed] [Google Scholar]
- Lu Z, Zhang L, Wang N, Chen Y, Gao L, Wang Y, Gao H, Gao Y, Li K, Qi X. Naturally occurring reassortant infectious bursal disease virus in northern china. Virus Res. 2015;203:92–95. doi: 10.1016/j.virusres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Nwagbo IO, Shittu I, Nwosuh CI, Ezeifeka GO, Odibo FJ, Michel LO, Jackwood DJ. Molecular characterization of field infectious bursal disease virus isolates from nigeria. Vet World. 2016;9:1420. doi: 10.14202/vetworld.2016.1420-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Gao L, Qin L, Deng X, Wu G, Zhang L, Yu F, Ren X, Gao Y, Gao H. Genomic sequencing and molecular characteristics of a very virulent strain of infectious bursal disease virus isolated in china. Agr Sci Technol. 2011;12:1946–1949. [Google Scholar]
- Shabbir MZ, Ali M, Abbas M, Chaudhry UN, Munir M. Molecular characterization of infectious bursal disease viruses from pakistan. Arch Virol. 2016;161:2001–2006. doi: 10.1007/s00705-016-2869-9. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The clustal_x windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuwen Y, Gao Y, Gao H, Qi X, Li T, Liu W, Wang X. Sequence analysis of the VP2 hypervariable region of eight very virulent infectious bursal disease virus isolates from the northeast of china. Avian Dis. 2008;52:284–290. doi: 10.1637/8175-111707-Reg.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


