Abstract
Extracellular vesicles are lipoproteinaceous membrane-enclosed nanometer-sized structures produced by cells and are thought to mediate cellular communications. Loaded with a specific set of miRNA and protein depending on their tissue of origin, these extracellular vesicles modulate diverse set of biological processes in their target tissues. In recent years, data has gathered on the roles of extracellular vesicles in embryo implantation and pregnancy. Embryo, oviduct, endometrial epithelium and stroma/decidua derived vesicles interact with trophoblast cells and promote their growth and differentiation to aid in embryo implantation. The placental vesicles are detected in maternal circulation that aids in feto-maternal immune tolerance, their levels vary in women with pregnancy-related complications like preeclampsia. Beyond the host, the microbes in the genital tract are also reported to produce extracellular vesicles which are thought to be responsible for inflammation and preterm births. This review focuses on the extracellular vesicular trafficking involved in success of pregnancy.
Keywords: Extracellular vesicles, Exosomes, Embryo, Implantation, Pregnancy, Cross talk, Infection
Introduction
Epidemiological evidences suggest that only 30% of all conceptions get clinically recognized, majority of these are lost spontaneously. Furthermore, in assisted reproduction, despite the fact that morphologically high-quality and genetically normal embryos are being transferred, the pregnancy rates are very low. Clinical observations have suggested that failure of an embryo implantation is the major cause of low take-home baby rates in couples undergoing assisted reproduction.
During embryo implantation, in the uterine cavity there occurs a synchronized change in the endometrium before and after arrival of blastocyst. Traditionally, it has been assumed that the receptive endometrium is a passive tissue that should readily implant an embryo. However, several experimental evidences confirmed that during implantation there occurs an extensive cross talk between the fetal and maternal compartments [1, 2]. Based on these studies, we now suggests that embryo implantation is a three-step process involving 1) gain of a receptive stage endometrium; 2) superimposition of a blastocyst-derived signature onto the receptive endometrium leading to implantation; and finally 3) breaching by the embryo and trophoblast invasion, culminating in decidualization and placentation [3].
Our understanding of the stage 1 of embryo implantation involving receptive endometrium has been extensive and several molecular players have been identified in this process [4, 5]. The stage 2 involves a mutual communication between the blastocyst and the endometrium which is a true sophistication in the sense it involves an elaborate sequence of genetic and cellular interactions that must be executed within a narrow temporal frame for successful implantation. For this communication, the endometrium must receive and respond to signals from the blastocyst and in converse the blastocyst must also receive and react to the signals form the endometrium. Several embryo-derived secretory factors have been identified that alter endometrial morphology and gene expression profiles to endow embryo apposition and invasion [1, 2]. This includes chorionic gonadotropin (CG), granulocyte colony-stimulating factor (GCSF) and Interleukin 1 beta (IL1-β) that alter expression of receptivity-related genes, induce decidualization, improves angiogenesis, and control immunomodulation. Of these, both CG and GCSF, if instilled prior to embryo transfer have been shown to improve pregnancy rates in women undergoing assisted reproduction albeit there are some controversies [6]. From the endometrial side, studies in genetically altered mice have led to identification of several factors that are essential for receptivity, embryo apposition and its further invasion [7]. Further several in vitro experiments have shown that endometrium or decidua derived secretions to improve trophoblast adhesion and invasion [8, 9]. These observations underscore the role of soluble factors in governing embryo-endometrial communication to established embryo implantation and placentation. Subsequent to implantation, the placenta takes a dominant role in maintaining pregnancy and defective placentation can cause pregnancy complications like intrauterine growth restriction, preeclampsia, and even preterm births. Interestingly, there is a distinct maternal control of placentation and any changes in the endometrium/decidua are associated with defective placental functions leading to pregnancy complications [10, 11].
The communication between different cell types is generally considered to be the function of secretory soluble factors like the hormones and morphogens. However, in recent years [26], the role for extracellular vesicles (EVs) in cell-to-cell communication has been established. Produced by a variety of cells including the embryo, endometrium, and placenta, the present review is focused on our current understanding of the EVs in embryo-endometrial communications leading to implantation and successful pregnancy. Figure 1 gives the timeline since the first discovery of EVs and its roles in embryo implantation. As evident, our knowledge in this area is recent and limited. In this review, we have summarized the current knowledge about the bimolecular aspects of the EVs produced by the embryo, maternal tissues and placenta and their physiological roles. Rather than being comprehensive, we have highlighted the biological functions of EVs in embryo-endometrial cross talk during implantation. We have also chosen to review how EVs produced by microbes also affect host physiology and affect pregnancy.
Fig. 1.
Timeline for studies on the role of extracellular vesicles in cell-cell communication during pregnancy
Definition and content of extracellular vesicles
EVs are defined as nano-sized, membrane-enclosed vesicles released by the cells that transport DNA, RNA, and proteins—between cells. The different types of EV include microvesicles (MVs), exosomes, oncosomes, and apoptotic bodies. These are classified based on their biogenesis or release pathways [12]. Microvesicles (MVs) are 100 nm–1 μm size vesicles while exosomes are much smaller than MVs (40–120 nm). Oncosomes as the name suggest are released by cancer cells (1–10 μm) and vesicular apoptotic bodies (50 nm–2 μm) are produced by dying cells. In the context of biogenesis, MVs are released from budding of plasma membrane while exosomes originate in the endosomal compartment that get incorporated in the early to late endosome and multivesicular bodies that are released by fusion with the plasma membrane as exosomes. Presently, our ability to enrich these different types of EVs is improving; however, it is often hard to separate and individually study them and hence most studies are done with an enriched preparation of MVs and exosomes. While some studies have clearly mentioned the types of EVs studied while others have not. Thus for the sake of simplicity, herein, we will collectively refer all vesicles released by cells as EVs unless otherwise stated by the cited studies.
EVs contain an array of molecules, including lipids, proteins and nucleic acids. EV membranes consist of a lipid bilayer alike the plasma membrane. Major constituents in exosomal membrane include sphingomyelin, gangliosides, and disaturated lipids, and their phosphatidylcholine and diacylglycerol proportion was found to be less relative to the membranes of their cells of origin [13]. Some studies have shown that cholesterol is present abundantly in exosomes compared with that in cellular membranes [14] indicative of different origins from the plasma membrane, including lipid rafts or differential sorting of lipids during EV/exosome secretions.
Protein constituents of EVs have been widely studied due to their importance in cell signaling. The idea is that the proteins are delivered from one cell/tissue type to another via MVs and they control the bioactivity of target cells and tissues. Indeed, both MVs and exosomes may contain many ubiquitous proteins, but also unique proteins dependent on the cell type of origin. Exosomes are enriched in major histocompatibility complex class II (MHC class II) and tetraspanins CD37, CD53, CD63, CD81, and CD82, the endosomal sorting complex proteins, Alix, TSG101, and chaperones. Exosomes are also enriched in glycoproteins and transmembrane proteins as compared to its cells of origin. These include integrins, glycoprotein Ib (GPIb), and P-selectin [12].
Nucleic acids are also a notable component within EVs. Beyond the DNA and mRNA, miRNA are considered to be specifically enriched in the exosomes, suggesting that EVs can serve as a pathway for the transfer of genetic information from one cell to another. Further, as miRNAs are enriched in MVs, along with protein signaling, MVs control gene expression in recipient cells and altering its physiology.
Embryo-derived extracellular vesicles and their molecular characteristics
EVs and secreted miRNAs (presumably by the EVs) have been isolated from human and bovine embryo-conditioned media [15, 16]. Both day 3 (D3) and day 5 (D5) in vitro embryo culture medium contain EVs of 50–200 nm (average 100 nm). These EVs are CD9 and CD63 immunoreactives and also for exosome marker protein ALIX. Both D3 and D5 culture media of human embryonic EVs are positive for HLA-G. These EVs are enriched for mRNAs of the pluripotency genes, Oct4, Sox2, Klf4, c-Myc, and Nanog. The embryo-derived EV cargo also consists of a variety of miRNA species that have diverse targets on both epithelial and stromal cells. The target genes of these miRNAs are predicted to mediate cellular activities such as adhesion and migration, suggesting that embryos could potentially modify the endometrial genome.
Embryo-derived extracellular vesicles aid in embryo growth and trophoblast attachment and alter endometrial epithelial physiology
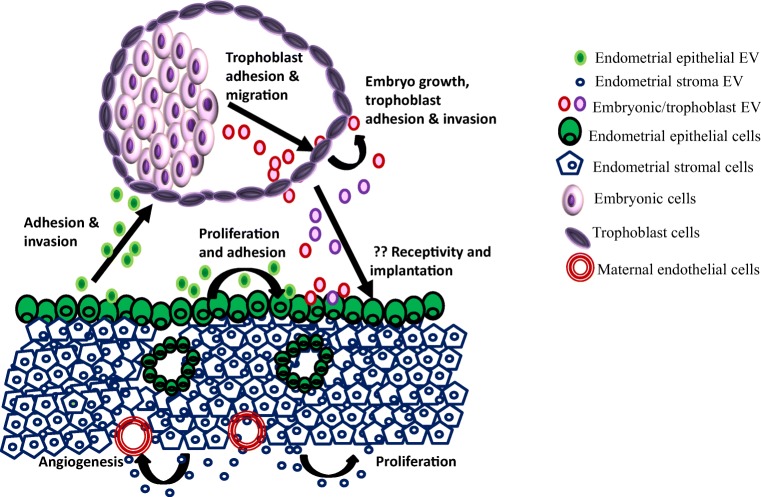
In the porcine, the embryo-derived EVs have been shown to influence their growth, viability, and pregnancy rates [17]. External supplement of embryonic EVs into freshly renewed medium significantly increased blastocyst formation rates and quality in terms of higher ratio of inner cell mass (ICM) and trophectodermal (TE) cells. Furthermore, the embryos supplemented with EVs had higher implantation rates as well as full-term calving rates [17]. These results indicate that embryos secrete exosomes into chemically defined culture medium which in an autocrine/paracrine manner positively influence the blastocyst formation, quality, and development to term (Fig. 2).
Fig. 2.
Extracellular vesicles (EVs) mediated embryo-endometrial cross talk for embryo implantation. EVs are produced by a variety of cell types from the embryo and endometrium. They carry specific cargoes and are internalized by the target tissues. They can have autocrine and paracrine effects. The embryo and endometrial EVs seem to aid in trophoblast adhesion and migration to promote implantation. In addition, the stromal EVs might control proliferation and angiogenesis
While the porcine study indicates the embryonic EVs influence of the blastocyst physiology, another study in the mouse has shown that the EVs from the inner cell mass (ICM) affect the trophectoderm (TE) [18]. It was shown that embryonic stem (ES) cells derived from the ICM generate and shed MVs. These ES cell-derived MVs are taken up by the trophectoderm and promote trophoblast migration. This increased migration was due to the interactions of MV cargo proteins laminin and fibronectin with integrins along the surfaces of the trophoblasts, triggering the activation of two signaling cascades leading to increased invasion [18]. Furthermore, the blastocysts injected with MVs from ES cells when transferred to the uteri of surrogate female implant at higher rates that mock injected blastocysts [18] suggesting that inner cell mass of the embryo controls trophoblast physiology to improve its implantation and stimulating trophoblast migration (Fig. 2). This study has discovered a novel mechanism of communication between the two major compartments of the embryo that promotes implantation. While the study is enticing, a major caveat is the source of MVs. The MVs used in the study are derived from cultured mouse ES cells and their production of the blastocyst ICM has not been demonstrated.
Another mechanism by which the embryonic EVs would aid implantation is by communicating with the maternal endometrium. Indeed, dye-labeled embryo-derived EVs have been observed in in vitro cultured human primary endometrial epithelial and stromal cells [16]. Corroborating the in vitro experiments, in the sheep [19], in vivo intrauterine instillation of labeled conceptus-derived EVs led to its uptake in the luminal epithelium but not the stroma or myometrium. These observations suggest that uptake of EVs occur locally in the endometrial epithelium, whether these EVs altered the cell physiology or gene expression profiles in the endometrial epithelium has not been investigated.
Beyond the EVs, miRNAs have also been identified in culture media of human blastocysts and are detected in biopsied trophectoderm cells suggesting they are released from blastocysts [20–24]. While a formal proof that these miRNAs are EV-derived is yet awaited, since exosomes are enriched with miRNAs, it can be presumed that these blastocysts derived miRs could be secreted via MVs. Interestingly, the levels of the embryo-derived miRs are associated with their implantation potential. Higher levels of certain miRs are observed in media from implanted blastocysts as compared to those that did not implant. Interestingly, the targets of a few of these implantation associated miRs are predicted to be involved in endometrial cell growth and proliferation [20]. Based on these studies, it appears that embryos secrete EVs and/or miRNAs, which target gene-predicted that alter cellular activities such as adhesion and migration, which are required for implantation. However, the pro-implantation effects of embryo derived EVs or miRs have not been experimentally determined.
While the pro-implantation effects of embryo MVs/miRNAs is yet to be established, anti-implantation activity of embryo derived miRNA has been demonstrated [21]. miR-661 is an embryo (but not EV-associated) miRNA found in high abundance in culture media of embryos that did not implant, in assisted reproduction cycles [21], suggesting it to be an anti-implantation factor. Indeed, miR-661 is readily internalized by cultured primary endometrial epithelial cells where it downregulates expression of genes involved in adhesion/invasion (PVRL1 and MTA2) and does not support trophoblast adhesion. Intriguingly, rescuing PVRL1 from being targeted by miR-661 could salvage adhesion [21] suggesting that secretion of miR-661 by embryos is detrimental to implantation. This study is a proof of principle that beyond hCG and other proteins, the miRNA cargo derived from embryos could control implantation. Since EVs are enriched in miRs, it is tempting to suggest that cargo might also influence implantation through their miR cargo. More experimental studies are, however, required to show if the embryo-derived EVs aid in embryo implantation.
Extracellular vesicles secreted by the oviduct
Oviduct is the primary site for fertilization and early embryo development. Secretions of the oviduct influence sperm motility, acrosome reaction and fertilization [25], it also aids in early embryonic development. EVs have been isolated from mouse and bovine oviduct fluid and also in in vitro-cultured bovine oviductal epithelial cells [26–28]. The oviductal EVs contain both exosomes (30–100 nm diameter) and large MVs (> 100 nm). The oviductal EVs express the markers similar to EVs in general, but those isolated from oviductal fluid also contain the oviduct specific protein specifically OVGP1 [27].
Molecular characteristics of oviductal extracellular vesicles
The protein profile of the in vivo- and in vitro-derived bovine oviduct EVs has been well characterized [27]. Proteomic characterization of the EVs identified a total of 315 proteins, from which 97 of them are detected exclusively in the in vivo EVs, 47 were found only in in vitro and 175 were in common to both in vivo- and in vitro-derived EVs. What is the source of the 47 unique proteins in the in vitro-derived EVs is unclear; it is possible that these proteins are derived from media or the serum or as an adaption of the cells to culture conditions. Beyond these specific proteins, the in vitro-derived oviductal EVs have differential expression of several other proteins in comparison to those derived in vivo, the molecular functions/biological processes of the proteins enriched in the in vitro-derived EVs is different from those isolated from the oviductal fluid (in vivo). These observations that highlight extreme caution should be exercised while interpreting the data exclusively from in vitro-derived EVs.
Oviductal extracellular vesicles aid in embryo quality and growth
In vitro, the mouse oviductal EVs have been shown to be taken up by sperm [26], the functional significance of such an uptake is hitherto unknown. Functionally, the protein cargo of the bovine oviduct EVs contains proteins that are involved in diverse functions ranging from regulation of cell metabolism, immunomodulation, and cell death [27]. Interestingly, the in vivo-derived EVs contain embryotrophic factors suggesting its role in early embryo development. Indeed oviduct EVs are capable of passing through the zona pellucida and internalize by most of the embryonic cells. Co-incubation of the oviductal EVs with fertilized eggs while do affect cleavage rates, it significantly improves the numbers of embryos reaching blastocyst stage, these blastocysts have higher numbers of cells but it does not alter hatching rates [27]. Together, the data suggest that oviduct EVs improves blastocyst yield and quality and extends embryo survival overtime in vitro.
Extracellular vesicles secreted by the endometrium
From the above discussions, we see that there exists an EV-mediated embryo to endometrium communication to control implantation. However, the success of pregnancy also requires a communication from the endometrium to the embryo. Several studies have shown that secretory factors from the endometrial cells affect trophoblast functions of the embryo [8, 29, 30]. While the role of soluble secretory molecules is beyond doubt, the EVs-secreted from the endometrium also influence blastocyst implantation [31, 32]. EVs in the size range consistent of both exosomes and MVs are detected in uterine fluid/mucus obtained from fertile women in secretory phase [32]. Using immunostaining for cell surface markers (tetraspanins, CD9 and CD63) EVs are detected on the apical surfaces of endometrial epithelial cells in tissue sections taken across the menstrual cycle [32]. Furthermore, the EV secreted across the menstrual cycle might be different as another EV marker CD63 showed cyclical regulation [32]. These immunopositive bodies are possibly exosomes as they can also be isolated from uterine aspirates, mucus and cultured epithelial cells and their size ranges from 50 to 150 nm. The secretion of EVs has also been demonstrated in the peri-implantation stage bovine endometrium, their levels increase marginally from pre-implantation to post-implantation period [33].
Molecular characteristics of endometrial extracellular vesicles
The EV derived from endometrial epithelial cell line ECC1 contains specific miRNA cargo that has been well characterized [32]. Of the miRNAs profiled in that study, ECC1 cells were shown to contained transcripts of 219 miRNA genes while the EVs contained 227 transcripts of miRNA genes. Of these, 214 miRNA transcripts are common to both EVs and ECC1, while five were unique to only cells. These results indicate that most host miRNAs are sorted in EVs. However, it is intriguing that the EVs contain 13 unique miRNA transcripts that were not detected in the cells. In addition to the miRNAs, the ECC1 derived EVs also contained an interesting class of the non-coding RNAs which include the small nuclear RNA U6 involved in the spliceosome, and RNU44 and RNU48 which are that are involved in chemical modifications of other RNAs. What might be the biological significance of sorting of such non-coding RNA in EVs is difficult to envisage; the EV specific miRNAs are predicted to target genes involved in embryo implantation. These include the adherens junction proteins like cadherins which required for maintaining epithelial and trophoblast layer integrity [34], the ECM-receptor interaction molecules like the integrins which are essential for apposition of the embryo on to endometrial epithelium [34]. In addition, the EV-associated miRNA also target the VEGF, Jak-STAT, and the Toll-like receptor signaling pathways that are modulators of implantation [34]. While these observations are new and enticing, we await experimental and mechanistic data on the role of endometrial EVs on process of embryo implantation.
The protein cargo of the ECC1-derived EVs has also been characterized [31]. Like in the in vivo condition, the EVs secreted by the ECC1 are also found to be hormonally regulated; the protein cargo sorted in these EVs also differed in response to steroid hormone [31]. This information although is novel, the hormonal regulation of endosomal recycling in the endometrium has been previously demonstrated and the altered expression of endometrial recycling protein is associated with infertility [35, 36]. Nevertheless, a total of 1043 protein cargo are reported in ECC1 EVs of which 254 protein cargos are uniquely packaged within EVs from estrogen treated cells and 126 within those EVs derived from cells challenged with both estrogen and progesterone. In general, most EV-associated proteins are involved in exosome biogenesis, endosomal-sorting complex required for transport (ESCRT) machinery and tetraspanin. These proteins are characteristic features of exosomes from most biological fluids and tissues. However, 190 proteins identified are unique to endometrial epithelial exosomes. This include enzyme like ligases, oxidoreductases, transferases, lyases, isomerases, phosphatases, kinases, metalloproteinases, and hydrolases. The endometrial epithelial cell protein cargo is highly complex but yet the proteins enriched in EVs have specific role in embryo implantation.
Endometrial extracellular vesicles have a role in receptivity and implantation
The endometrial EVs during the course of embryo implantation vary in their protein cargo. Several proteins are differentially abundant in the EVs isolated from preimplantation stage bovine endometrial epithelium as compared to post-implantation stages [33]. These preimplantation-stage endometrial EVs have higher abundance of proteins involved in cell apoptosis while the EVs derived from post-implantation stage endometrium have higher abundance of proteins involved in cell adhesion [33]. While these changes might reflect the physiological alterations occurring in the epithelium, treatment of epithelial cells with EVs from pre-implantation stage endometrium activate genes in the apoptotic pathways, the post-implantation stage EVs activate genes in cell adhesion [33] suggesting a paracrine effect of endometrial EVs in regulation of receptivity and implantation (Fig. 2).
Since EV contains cargo that has roles in embryo implantation, the effects of ECC1-derived EVs on trophoblast invasion have been studied [31]. The in vitro adhesive capacity of trophoblast cell line HTR8 cells was examined in presence of EVs derived from hormone-treated ECC1 cells. The results revealed that EVs derived from both estrogen alone or that with progesterone treated epithelial cells led to a very rapid increase in the adhesive capacity of the trophoblast cells [31]. This is paralleled by a significant increase in levels of focal adhesion kinase (FAK), phosphorylation of FAK (Tyr397) and levels of the matrix protein fibronectin in trophoblast cells [31]. Together the data suggest that endometrial EVs have a potential to control trophoblast physiology to aid in implantation (Fig. 2).
Extracellular vesicles from endometrial stromal cells
The decidua is an epithelioid transformation of the stromal cells of the endometrium at the time of embryo implantation. This decidua plays a key role in controlling trophoblast invasion and placentation [8, 9]. While the production of MVs by the decidua has not been reported, MVs have been detected in the culture supernatant of bovine endometrial stromal cells [37] and primary cultures of endometrial stromal cells from women with and without endometriosis [38, 39].
The protein cargo of the bovine endometrial stromal cells has been defined, the proteome content has been shown to alter in response to hypoxia [37] suggesting the modulation of EV biosynthesis or differential protein sorting in response to external stimuli. The miRNA cargo of the human endometrial stromal cells from healthy and women with endometriosis has been characterized [38, 39] and its profile is distinct from the endometrial epithelial cells [39]. Furthermore, there is about 75% concordance in miRNA levels in cells versus their EV counterparts, the cellular exosome expression ratios varied substantially with some miRNAs were very abundant in exosomes, but rare in cells; the converse has also been observed [39]. These observations was found to be comparable with the selective sorting of miRNAs observed in many cancer cell lines earlier [40].
Functionally, the stromal cell-derived MVs can be internalized by stromal cells themselves and also into endothelial cells in vitro suggesting an autocrine/paracrine mode of action [37]. As compared to controls; the stromal cells from endometriosis women contain altered abundance of miRNA species that control angiogenesis [37]. Indeed, treatment of endothelial cells (HUVEC) with EVs from stromal cells of women with endometriosis had greater tube formation compared with those treated with EVs extracted from normal women [38]. It is possible that the stromal EVs might regulate angiogenesis (Fig. 2) that is required at the time of implantation [2].
While the biological significance of the above findings are yet not very clear, but both these studies imply that release of EVs and/or its cargo from the endometrial stromal cells gets altered when subjected to adverse stimuli.
Extracellular vesicles of the placenta and their roles in pregnancy
The release of membranous material by the syncytiotrophoblasts of the placenta into the maternal circulation during pregnancy is long known [41] and not surprisingly the EVs of placental origin has been the most well characterized and is a subject of excellent reviews [42–46], and hence will not be detailed here.
In general, syncytiotrophoblasts the primary source of placenta-derived EV and is a major signaling mechanism between fetus and mother. EVs and exosomes have been isolated from trophoblastic cell lines, placental cultures and also in vivo from maternal circulation. The concentrations of both exosomes and EVs in increase maternal plasma as gestation progresses, their release and bioactivity are preferentially increased by low oxygen tensions and d-glucose concentrations [43]. The pregnancy-associated exosomes contain a number of membrane-bound protein markers, such as NKGD2 ligands, FasL, TRAIL, and syncytin suggesting their role in feto-maternal tolerance. The other specific pregnancy-associated exosomal proteins include the placental alkaline phosphatase and TGF-β. The placental exosomes also contain mRNA and miRNA cargo that has diverse biological functions [42, 47].
Changes in circulating levels and cargo content placental EVs has been reported in pregnancy disorders. A significantly high numbers of placental EVs are detected in maternal serum in preeclampsia, these EVs have pro-inflammatory, anti-angiogenic and procoagulant activity, which is expected to lead to systemic inflammation, endothelial dysfunction and activation of the clotting system [44, 48]. The role of exosomes as biomarker of preeclampsia and other gestational disorders including diabetes has been proposed [45, 49].
Endothelial and adipose-derived extracellular vesicles in pregnancy
While the placental-derived EVs have been extensively investigated in terms of preeclampsia, a role of endothelial and adipose-derived EVs in pregnancy has been proposed. Exosomes are released by endothelial cells and its cargo is altered with hyperglycemia and oxidative stress. Such EVs/cargo change leads to dysfunction of the fetoplacental endothelium [50]. It is suggested that hyperglycemia and oxidative stress during pregnancy would affect exosomal cargo in the fetoplacental vasculature leading to endothelial dysfunction resulting in adverse pregnancy outcome such as hypertension, edema, thrombosis, and infarction [51].
Maternal obesity is a risk factor for several pregnancy complications including gestational diabetes mellitus. Adipose tissue is also known to produce EVs and hypertrophy of adipose tissue or metabolic stress alters MV biogenesis and its cargo, mainly the miRNA [52, 53]. Interestingly, exosomes derived from the adipose tissue macrophages of obese mice cause glucose intolerance and insulin resistance in lean mice [52] suggesting that adipose tissue-derived exosomes might also contribute to the systemic inflammation and insulin resistance seen in obese gestational diabetic pregnancies. Such EVs may also alter placental physiology and deregulating the placental nutrient signaling pathways leading to pregnancy complications associated with obesity [49].
Microbial extracellular vesicles in pregnancy
The initiation and successful maintenance of pregnancy requires a complex interplay between several maternal compartments. However in recent years, the involvement of the microbiome in regulation of host physiology and diseases is gaining interest. The female reproductive tract, specifically the cervix and vagina is rich in bacteria; microbial communities have also been isolated from the fallopian tubes, placenta and endometrium [54] .This microbiome influence various aspects maternal health including gynecological cancers [55] and also the mother-child microbial transfer which is a determinant of infant health [56] Furthermore, alterations in the maternal microbiome are associated with preterm births [57], a major cause of fetal and neonatal mortality.
Like the eukaryotic cells, bacterial cells facilitate cell-cell communication via secreted vesicles. These bacterial vesicles (termed as microvesicles) transfer of biologically active molecules that induce phenotypic changes in the recipient cells to coordinate diverse cellular processes and control of their population density [58]. Furthermore, the vesicles also are involved in pathogen-host communication and manipulate host responses for their own benefits and organisms in the reproductive tract are no exception [59].
In the context of pregnancy, vaginal colonization with Group B Streptococcus (GBS), opportunistic gram positive pathogenic bacteria, is associated with premature rupture of amniotic membrane and preterm birth [60]. We have previously demonstrated that GBS produces membrane vesicles (MVs) that are loaded with virulence factors including the proteases and pore forming toxin [61]. These GBS MVs can undergo anterograde transport from the vagina in to the uterus and intra-amniotic administration of GBS MVs led to amniotic membranes degradation and mechanical weakening resembling preterm premature rupture membrane (PPROM). Interestingly, instillation of MVs in the amniotic sac also resulted and caused intrauterine fetal death and preterm delivery. These observations indicate that, like the EVs from the reproductive tract, the MVs from the vaginal microbes can also affect the feto-placental tissues and resulting in pregnancy complications. Whether the commensal flora of the female tract also produces EVs and if it has any effect on embryo implantation or pregnancy is yet not explored.
Summary and future directions
Table 1 summarizes the different sources of EVs reported from feto-maternal tissues. The secretion of EVs by the endometrial epithelial cells and the unique protein and miRNA cargoes suggest that these should have biological functions. Further, the specific sorting of proteins and miRNAs whose targets have role in embryo implantation and the observation that trophoblast cells treated with EVs have higher adhesive potential are evidences to suggest that EV-mediated cross talk exist and might aid in embryo implantation, placentation, maintenance of pregnancy and also its complications. While the evidences are obviously tantalizing, there several caveats. Most of the data on EV cargo is derived from immortalized or long-term passaged cell lines and very few from the primary tissue. Thus,their in vivo relevance is unclear. The experimental data on pro-implantation effects of endometrial epithelial cell-derived EVs is largely based on in vitro trophoblast adhesion/invasion assays and its in vivo evidence is not established. It has been shown that EVs of different sizes contain different cargos and these vesicles can elicit distinct biological effects on host cells [62]. This fact has been completely disregarded in studies pertaining to pregnancy. There is reasonable evidence that the media components such as protein supplements could be miRNA carriers [22, 24]. Therefore the data from spent media should therefore need to take into account possible contaminants from the media itself. Presently, there is only limited data on EVs and its cargo in embryo culture fluid. Considering the fact that the media might alter stability and sequestering of secreted RNA, such data could vary depending on media formulations. Presently data suggesting that EVs contain protein and RNA cargo that have roles in embryo implantation; the lipid cargo of the endometrial EVs is unknown and needs to be established. Further, the role of steroid hormones on EV sorting, EV cargo loading, and its biological effects need to be studied.
Table 1.
Molecular and functional characteristics of extracellular vesicles derived from feto-maternal tissues to aid in pregnancy
| Source of EVs | Key characteristics | Functions | References | ||
|---|---|---|---|---|---|
| Size | Markers | Contents | |||
| Embryo/embryonic stem cells | 50–200 nm | CD9, CD63, ALIX, HLA-G | mRNAs of the pluripotency genes such as Oct4, Sox2, Klf4, c-Myc, Nanog and mi RNAs | Mediates embryo growth Promotes embryo implantation |
[17–21] |
| Oviduct epithelium | 30–100 nm, > 100 nm | OVGP1 | Total of 315 proteins with varying distribution among the in vivo and in vitro derived EVs | Promotes early embryonic development | [27] |
| Endometrial epithelial cells | 40–150 nm | Tetraspanins, CD9, CD63 | 254 proteins and 227 miRNAs | Control trophoblast physiology which can promote embryo implantation | [32–34] |
| Endometrial stromal cells | 30–120 nm | Tetraspanin-6, disintegrin, metalloproteinase domain-containing protein 10 | 250 proteins and several miRNAs including miR-21, miR-126 | Control angiogenesis Promote Cell proliferation Crucial role in embryonic and fetal survival |
[37–39] |
| Placenta | 50–150 nm | NKGD2 ligands, FasL, TRAIL, syncytin, placental alkaline phosphatase TGF-β | mRNA, miRNA and proteins | Feto-maternal tolerance Pro-inflammatory, anti-angiogenic and procoagulant activity |
[41–49] |
| Endothelial and adipose tissue | 150–200 nm | TNF-α, CD63, HSP-TNF-α HPS70 CD63 | 231 proteins and miRNAs and proteins | Contribute to the systemic inflammation and insulin resistance seen in obese gestational diabetic pregnancies | [50–52] |
| Microbial (group B Streptococcus) | < 50 nm, 150–300 nm | Unknown | cAMP factor, Hyaluronate lyase, PcsB protein, DNA, RNA | Degradation of amniotic membranes Mechanical weakening resembling preterm premature rupture membrane (PPROM) Fetal death and preterm delivery |
[61] |
While these are technical issues pertaining to EV biology in general, the idea that EVs have a definitive role in implantation is at best speculative. There is only frugal in vitro data where EVs have been shown to aid in trophoblast adhesion and the in vivo evidence to this notion is yet not available. Further, there is no data demonstrating that EV-mediated cargo actually alters implantation related genes in the host cells and mechanistic evidence of the same need to be generated. Targeting EV biogenesis in the endometrium and embryos would be an essential experimental proof to define its role in implantation. However, considering that the mechanism of EV biogenesis involves proteins having key roles in endosomal physiology, it might be an impossible to task to achieve such targeted animals and such data if any would be hard to interpret in terms of its specificity.
In context of pregnancy, the placental EVs have been best characterized and its alterations in maternal serum with pregnancy complications have been demonstrated. However, its use as biomarkers needs clinical validation. Further, a completely unexplored area is the host pathogen interactions with the use of EVs. Data is emerging where the pathogen EVs can affect the host, the vice versa has not been investigated.
Given that EVs are secreted and its levels (or that of its cargo) are predictive of their implantation potential of embryo, progression of gestation and pregnancy complications this could be transformed into a powerful noninvasive testing method for various conditions. For example, EV measurements in the medium of in vitro cultured embryos can aid in a more consistent objective selection method in contrast to current approaches which are highly subjective and have limited clinical value. Given that EVs are secreted differentially by the receptive and non-receptive endometrium and levels of endosomal proteins are altered in endometria of women with infertility, measurement of EVs in the uterine/cervical/vaginal mucosa might be of utility in developing non-invasive/liquid-biopsy test for diagnosis of uterine health and receptivity. Finally, the pregnancy-associated maternal serum EVs has high potential to develop noninvasive testing of placental health and gestational complications.
Funding information
The manuscript bears the NIRRH ID:REV/652/07-2017. DM lab and is supported by grants from the Indian Council of Medical Research, Department of Science and Technology, Department of Biotechnology, Government of India. NK is a recipient of the Kerala State Council for Science, Technology & Environment Post-Doctoral Fellowship.
References
- 1.Modi DN, Godbole G, Suman P, Gupta SK. Endometrial biology during trophoblast invasion. Front Biosci (Schol Ed) 2012;4:1151–1171. doi: 10.2741/s323. [DOI] [PubMed] [Google Scholar]
- 2.Modi DN, Bhartiya P. Physiology of embryo-endometrial cross talk. Biomed Res J. 2015;2(1):83–104. [Google Scholar]
- 3.Ashary N, Tiwari A, Modi D. Embryo implantation: war in times of love. Endocrinology. 2018;159(2):1188–1198. doi: 10.1210/en.2017-03082. [DOI] [PubMed] [Google Scholar]
- 4.Bhusane K, Bhutada S, Chaudhari U, Savardekar L, Katkam R, Sachdeva G. Secrets of endometrial receptivity: some are hidden in uterine secretome. Am J Reprod Immunol. 2016;75(3):226–236. doi: 10.1111/aji.12472. [DOI] [PubMed] [Google Scholar]
- 5.Evans J, Salamonsen LA, Winship A, Menkhorst E, Nie G, Gargett CE, Dimitriadis E. Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol. 2016;12(11):654–667. doi: 10.1038/nrendo.2016.116. [DOI] [PubMed] [Google Scholar]
- 6.Shah J, Gangadharan A, Shah V. Effect of intrauterine instillation of granulocyte colony-stimulating factor on endometrial thickness and clinical pregnancy rate in women undergoing in vitro fertilization cycles: an observational cohort study. Int J Infertil Fetal Med. 2014;5(3):100–106. [Google Scholar]
- 7.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godbole G, Suman P, Malik A, Galvankar M, Joshi N, Fazleabas A, Gupta SK, Modi D. Decrease in expression of HOXA10 in the decidua after embryo implantation promotes trophoblast invasion. Endocrinology. 2017;158(8):2618–2633. doi: 10.1210/en.2017-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol. 2016;75(3):341–350. doi: 10.1111/aji.12466. [DOI] [PubMed] [Google Scholar]
- 10.James-Allan LB, Whitley GS, Leslie K, Wallace AE, Cartwright JE. Decidual cell regulation of trophoblast is altered in pregnancies at risk of pre-eclampsia. J Mol Endocrinol. 2018;60(3):239–246. doi: 10.1530/JME-17-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrido-Gomez T, Dominguez F, Quiñonero A, Diaz-Gimeno P, Kapidzic M, Gormley M, Ona K, Padilla-Iserte P, McMaster M, Genbacev O, Perales A, Fisher SJ, Simón C. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc Natl Acad Sci U S A. 2017;114(40):E8468–E8477. doi: 10.1073/pnas.1706546114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MikoŁaj P, Balaj ZL, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65(8):783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laulagnier K, Motta C, Hamdi S, Sébastien ROY, Fauvelle F, Pageaux JF, et al. Mast cell-and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380(1):161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llorente A, Skotland T, Sylvänne T, Kauhanen D, Róg T, Orłowski A, Vattulainen I, Ekroos K, Sandvig K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta. 2013;1831:1302–1309. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Mellisho EA, Velásquez AE, Nuñez MJ, Cabezas JG, Cueto JA, Fader C, Castro FO, Rodríguez-Álvarez L. Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro. PLoS One. 2017;12(5):e0178306. doi: 10.1371/journal.pone.0178306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacomini E, Vago R, Sanchez AM, Podini P, Zarovni N, Murdica V, Rizzo R, Bortolotti D, Candiani M, Viganò P. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci Rep. 2017;7(1):5210. doi: 10.1038/s41598-017-05549-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu P, Qing S, Liu R, Qin H, Wang W, Qiao F, Ge H, Liu J, Zhang Y, Cui W, Wang Y. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS One. 2017;12(3):e0174535. doi: 10.1371/journal.pone.0174535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desrochers LM, Bordeleau F, Reinhart-King CA, Cerione RA, Antonyak MA. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat Commun. 2016;7:11958. doi: 10.1038/ncomms11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns GW, Brooks KE, Spencer TE. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol Reprod. 2016;94:56. doi: 10.1095/biolreprod.115.134973. [DOI] [PubMed] [Google Scholar]
- 20.Capalbo A, Ubaldi FM, Cimadomo D, Noli L, Khalaf Y, Farcomeni A, Ilic D, Rienzi L. MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril. 2016;105:225–235. doi: 10.1016/j.fertnstert.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Cuman C, Van Sinderen M, Gantier MP, Rainczuk K, Sorby K, Rombauts L, Osianlis T, Dimitriadis E. Human blastocyst secreted microRNA regulate endometrial epithelial cell adhesion. EBioMedicine. 2015;2:1528–1535. doi: 10.1016/j.ebiom.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kropp J, Salih SM, Khatib H. Expression of microRNAs in bovine and human pre-implantation embryo culture media. Front Genet. 2014;5:91. doi: 10.3389/fgene.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noli L, Capalbo A, Dajani Y, Cimadomo D, Bvumbe J, Rienzi L, Ubaldi FM, Ogilvie C, Khalaf Y, Ilic D. Human embryos created by embryo splitting secrete significantly lower levels of miRNA-30c. Stem Cells Dev. 2016;25:1853–1862. doi: 10.1089/scd.2016.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbluth EM, Shelton DN, Wells LM, Sparks AE, Van Voorhis BJ. Human embryos secrete microRNAs into culture media–a potential biomarker for implantation. Fertil Steril. 2014;101:1493–1500. doi: 10.1016/j.fertnstert.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Cerezales S, Ramos-Ibeas P, Acuña OS, Avilés M, Coy P, Rizos D, Gutiérrez-Adán A. The oviduct: from sperm selection to the epigenetic landscape of the embryo. Biol Reprod. 2017;98(3):262–276. doi: 10.1093/biolre/iox173. [DOI] [PubMed] [Google Scholar]
- 26.Al-Dossary AA, Strehler EE, Martin-DeLeon PA. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS One. 2013;8(11):e80181. doi: 10.1371/journal.pone.0080181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almiñana C, Corbin E, Tsikis G, Alcântara-Neto AS, Labas V, Reynaud K, Galio L, Uzbekov R, Garanina AS, Druart X, Mermillod P. Oviduct extracellular vesicles protein content and their role during oviduct–embryo cross-talk. Reproduction. 2017;154(3):253–268. doi: 10.1530/REP-17-0054. [DOI] [PubMed] [Google Scholar]
- 28.Lopera-Vásquez R, Hamdi M, Fernandez-Fuertes B, Maillo V, Beltrán-Breña P, Calle A, Redruello A, López-Martín S, Gutierrez-Adán A, Yañez-Mó M, Ramirez MÁ, Rizos D. Extracellular vesicles from BOEC in in vitro embryo development and quality. PLoS One. 2016;11(2):e0148083. doi: 10.1371/journal.pone.0148083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godbole G, Suman P, Gupta SK, Modi D. Decidualized endometrial stromal cell derived factors promote trophoblast invasion. Fertil Steril. 2011;95(4):1278–1283. doi: 10.1016/j.fertnstert.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 30.Zhu XM, Han T, Sargent IL, Wang YL, Yao YQ. Conditioned medium from human decidual stromal cells has a concentration-dependent effect on trophoblast cell invasion. Placenta. 2009;30(1):74–78. doi: 10.1016/j.placenta.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Greening DW, Nguyen HP, Elgass K, Simpson RJ, Salamonsen LA. Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: insights into endometrial-embryo interactions. Biol Reprod. 2016;94:38. doi: 10.1095/biolreprod.115.134890. [DOI] [PubMed] [Google Scholar]
- 32.Ng YH, Rome S, Jalabert A, Forterre A, Singh H, Hincks CL, Salamonsen LA. Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One. 2013;8:e58502. doi: 10.1371/journal.pone.0058502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusama K, Nakamura K, Bai R, Nagaoka K, Sakurai T, Imakawa K. Intrauterine exosomes are required for bovine conceptus implantation. Biochem Biophys Res Commun. 2018;495(1):1370–1375. doi: 10.1016/j.bbrc.2017.11.176. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Matic LP, et al. Extracellular vesicles secreted by atherogenic macrophages transfer microRNA to inhibit cell migration. Arterioscler Thromb Vasc Biol. 2018;38(1):49–63. doi: 10.1161/ATVBAHA.117.309795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patil VS, Sachdeva G, Modi DN, Katkam RR, Manjramkar DD, Hinduja I, Puri CP. Rab coupling protein (RCP): a novel target of progesterone action in primate endometrium. J Clin Mol Endocrinol. 2005;35(2):357–372. doi: 10.1677/jme.1.01807. [DOI] [PubMed] [Google Scholar]
- 36.Rosas C, Gabler F, Vantman D, Romero C, Vega M. Levels of Rabs and WAVE family proteins associated with translocation of GLUT4 to the cell surface in endometria from hyperinsulinemic PCOS women. Hum Reprod. 2010;25(11):2870–2877. doi: 10.1093/humrep/deq232. [DOI] [PubMed] [Google Scholar]
- 37.Koh YQ, Peiris HN, Vaswani K, Reed S, Rice GE, Salomon C, Mitchell MD. Characterization of exosomal release in bovine endometrial intercaruncular stromal cells. Reprod Biol Endocrinol. 2016;14(1):78. doi: 10.1186/s12958-016-0207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harp D, Driss A, Mehrabi S, Chowdhury I, Xu W, Liu D, Garcia-Barrio M, Taylor RN, Gold B, Jefferson S, Sidell N, Thompson W. Exosomes derived from endometriotic stromal cells have enhanced angiogenic effects in vitro. Cell Tissue Res. 2016;365(1):187–196. doi: 10.1007/s00441-016-2358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maida Y, Takakura M, Nishiuchi T, Yoshimoto T, Kyo S. Exosomal transfer of functional small RNAs mediates cancer-stroma communication in human endometrium. Cancer Med. 2016;5(2):304–314. doi: 10.1002/cam4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13(1):17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burton GJ, Jones CJ. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan J Obstet Gynecol. 2009;48(1):28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 42.Salomon C, Yee SW, Mitchell MD, Rice GE. The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions. Biomed Res Int. 2014;2014:1–10. doi: 10.1155/2014/693157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adam S, Elfeky O, Kinhal V, Dutta S, Lai A, Jayabalan N, Nuzhat Z, Palma C, Rice GE, Salomon C. Fetal-maternal communication via extracellular vesicles–implications for complications of pregnancies. Placenta. 2017;54:83–88. doi: 10.1016/j.placenta.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Tannetta D, Collett G, Vatish M, Redman C, Sargent I. Syncytiotrophoblast extracellular vesicles–circulating biopsies reflecting placental health. Placenta. 2017;52:134–138. doi: 10.1016/j.placenta.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tannetta D, Masliukaite I, Vatish M, Redman C, Sargent I. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J Reprod Immunol. 2017;119:98–106. doi: 10.1016/j.jri.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Pillay P, Moodley K, Moodley J, Mackraj I. Placenta-derived exosomes: potential biomarkers of preeclampsia. Int J Nanomedicine. 2017;12:8009–8023. doi: 10.2147/IJN.S142732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Truong G, Guanzon D, Kinhal V, Elfeky O, Lai A, Longo S, Nuzhat Z, Palma C, Scholz-Romero K, Menon R, Mol BW, Rice GE, Salomon C. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells–liquid biopsies for monitoring complications of pregnancy. PLoS One. 2017;12(3):e0174514. doi: 10.1371/journal.pone.0174514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salomon C, Guanzon D, Scholz-Romero K, Longo S, Correa P, Illanes SE, Rice GE. Placental exosomes as early biomarker of preeclampsia: potential role of exosomal microRNAs across gestation. J Clin Endocrinol Metab. 2017;102(9):3182–3194. doi: 10.1210/jc.2017-00672. [DOI] [PubMed] [Google Scholar]
- 49.Jayabalan N, Nair S, Nuzhat Z, Rice GE, Zuñiga FA, Sobrevia L, Leiva A, Sanhueza C, Gutiérrez JA, Lappas M, Freeman DJ, Salomon C. Cross talk between adipose tissue and placenta in obese and gestational diabetes mellitus pregnancies via exosomes. Front Endocrinol. 2017;8:239. doi: 10.3389/fendo.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sáez T, Salsoso R, Leiva A, Toledo F, de Vos P, Faas M, Sobrevia L. Human umbilical vein endothelium-derived exosomes play a role in foetoplacental endothelial dysfunction in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis. 2018;1864(2):499–508. doi: 10.1016/j.bbadis.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Sáez T, de Vos P, Sobrevia L, Faas MM. Is there a role for exosomes in foetoplacental endothelial dysfunction in gestational diabetes mellitus? Placenta. 2018;61:48–54. doi: 10.1016/j.placenta.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372–384. doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue. Cell Proliferat. 2016;49(1):3–13. doi: 10.1111/cpr.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pelzer ES, Willner D, Buttini M, Hafner LM, Theodoropoulos C, Huygens F. The fallopian tube microbiome: implications for reproductive health. Oncotarget. 2018;9(30):21541. doi: 10.18632/oncotarget.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Champer M, Wong AM, Champer J, Brito IL, Messer PW, Hou JY, et al. The role of the vaginal microbiome in gynecological cancer: a review. BJOG. 2017. [DOI] [PubMed]
- 56.Younes JA, Lievens E, Hummelen R, van der Westen R, Reid G, Petrova MI. Women and their microbes: the unexpected friendship. Trends Microbiol. 2017. [DOI] [PubMed]
- 57.Vinturache AE, Gyamfi-Bannerman C, Hwang J, Mysorekar IU, Jacobsson B. Maternal microbiome–a pathway to preterm birth. Semin Fetal Neonatal Med. 2016;21(2):94–99. doi: 10.1016/j.siny.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Ofir-Birin Y, Heidenreich M, Regev-Rudzki N. Pathogen-derived extracellular vesicles coordinate social behaviour and host manipulation. Semin Cell Dev Biol. 2017;67:83–90. doi: 10.1016/j.semcdb.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Nudel K, Massari P, Genco CA. Neisseria gonorrhoeae modulates cell death in human endocervical epithelial cells through export of exosome-associated cIAP2. Infect Immun. 2015;83(9):3410–3417. doi: 10.1128/IAI.00732-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bianchi-Jassir F, Seale AC, Kohli-Lynch M, Lawn JE, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Ip M, le Doare K, Madhi SA, Saha SK, Schrag S, Sobanjo-ter Meulen A, Vekemans J, Rubens CE. Preterm birth associated with group B Streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S133–S142. doi: 10.1093/cid/cix661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Surve MV, Anil A, Kamath KG, Bhutda S, Sthanam LK, Pradhan A, Srivastava R, Basu B, Dutta S, Sen S, Modi D, Banerjee A . Membrane vesicles of group B streptococcus disrupt feto-maternal barrier leading to preterm birth. PLoS Pathog 2016; 12(9): e1005816. [DOI] [PMC free article] [PubMed]
- 62.Vyas N, Walvekar A, Tate D, Lakshmanan V, Bansal D, Cicero AL, et al. Vertebrate hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci Rep. 2014;4:7357. doi: 10.1038/srep07357. [DOI] [PMC free article] [PubMed] [Google Scholar]