Abstract
Objective
To evaluate the delivery rate and to characterize patients following ovarian tissue cryopreservation (OTCP) who did not undergo auto-transplantation.
Methods and Materials
All consecutive cancer patients admitted to our IVF unit, from January 2004 to December 2015, who underwent OTCP for the purpose of fertility preservation without autotransplantation were analyzed. The cohort included 338 patients and was linked to the National Live Birth registry of the Israel Ministry of Health in order to determine whether the women delivered following the cancer diagnosis.
Main outcome measures
Delivery rate following OTCP without autotransplantation.
Results
During 6.4 years of follow-up, 30% of the patients delivered, with no differences in gravity, age at first diagnosis of cancer, type of malignancy, or the prevalence of relapse of malignancy between those who delivered and those who did not. Moreover, in multivariate analysis, those undergoing OTCP before the age of 30 and those suffering from breast cancer had significantly higher odds to conceive and deliver following cancer treatment without the need of autotransplantation.
Conclusions
Further studies are required to elucidate the appropriate subgroup of patients with breast cancer under the age of 30 years, who will need OTCP. This information might aid both fertility specialists’ counseling and their oncological patients in pursuing the appropriate fertility preservation strategy.
Keywords: Fertility preservation, Ovarian tissue cryopreservation, Pregnancy after chemotherapy, Gonadotoxic treatment
Introduction
With improvements in long-term survival of girls and young women diagnosed with malignancies has come increased concern for preserving fertility in these patients [1]. Given the availability of new reproductive techniques and a growing interest in quality of life after cancer treatment have, patients should be counseled regarding options for fertility preservation prior to the initiation of gonadotoxic cancer therapies. Some 70% of young female cancer patients stated they were concerned about fertility at the time of diagnosis, and 50% wanted to have children after treatment [2].
The risk of premature ovarian failure (POF) subsequent to cancer treatment varies with patient age, cancer type, treatment regimen, and dose of chemotherapeutic drugs and radiation [3]. Guidelines published by oncological and reproductive societies advocate that young patients treated with gonadotoxic regimens be systematically informed about the risk of infertility and the available options to preserve their fertility [3, 4].
Currently available fertility preservation techniques include controlled ovarian hyperstimulation (COH) or IVF with oocytes or embryo cryopreservation, cryopreservation, and transplantation of ovarian cortex. Ovarian tissue can be extracted by laparoscopy without any significant delay in the initiation of gonadotoxic therapy, and the risk of transplantation of cancer cells remains very low in most types of malignancies [5].
Ovarian cortex cryopreservation is undoubtedly indicated prior to the initiation of gonadotoxic therapy in pre-pubertal girls at risk for premature ovarian failure and in a selected group of patients for whom chemotherapy cannot be withheld for the required period needed to complete an IVF cycle.
A major challenge in patient selection for ovarian cortex cryopreservation is the limited data available on the percentage of patients who actually benefit from this procedure. Not all patients undergoing ovarian tissue preservation procedure will be sterilized following chemotherapy. For this reason, estimates of fertility outcomes following ovarian tissue preservation in patients who did not undergo ovarian tissue autotransplantation are crucial for counseling. Prompted by the aforementioned information, we aim to characterize those patients who chose cryopreservation of ovarian cortex but did not undergo transplantation and to estimate the successful pregnancies in this patient population. This information will help physicians and their patients to choose the appropriate fertility preservation strategy.
Patients and methods
We reviewed the computerized files of all consecutive cancer patients admitted to our IVF unit, from January 2004 to December 2014, who underwent cryopreservation of ovarian cortex for the purpose of fertility preservation. Fertility preservations and ovarian tissue autotransplantations were performed using a uniform technique by our fertility preservation service team, which consistently performed patient selection, pre/post transplantation endocrine monitoring, fertility treatments, and in vitro fertilization (IVF). Patients who underwent autotransplant were excluded for the data file (n = 18).
Data on patient age, type of malignancy, and ovarian cortex cryopreservation-related variables were collected from the files.
The cohort database was linked with the database Israel National Cancer Registry in order to verify the cancer diagnosis using the patient’s personal identification number. The Israel National Cancer Registry was established in 1960 and maintains data on all malignancies in Israel, including borderline tumors (with the exception of basal cell and squamous cell carcinomas of the skin) and benign tumors of the brain and the central nervous system. The registry receives notifications of all incident malignancies from pathology reports, hospital discharge summaries, patient listings from oncology and hematology institutes, and death certificates. Depending on the cancer site, completeness of the data was found to be 90–95% [6]. At the time that this study was conducted, the registry was complete through December 31, 2014. Cases of cancer are codes in the registry according to the International Classification of Diseases for Oncology, Version 3 (ICD-O-3) [7]. The database was also linked to the National Live Birth registry of the Israel Ministry of Health in order to determine whether the women delivered following the cancer diagnosis until December 31, 2016, to allow at least 2 years of follow-up.
Start of follow-up for each subject was defined as date of cancer diagnosis or the subject’s twentieth birthday, in the event that the cancer diagnosis occurred prior to that date (girls under age 20 were defined as being under aged for desired pregnancy). End of follow-up was defined as the earliest of the following dates: first delivery after malignancy, death, or December 31, 2016. (If women delivered more than once during follow-up, the first birth was the end of follow-up.)
Statistical analysis
Women characteristics were compared between women who delivered and those who did not deliver after malignancy diagnosis by using the chi-square test for categorical variables and by independent samples t test for continuous variables. All p values below 0.05 were considered statistically significant.
Logistic multivariate regression analysis was calculated to predict the likelihood of a live birth adjusted for age at first cancer diagnosis, parity before first malignancy, cancer type, and recurrence of malignancy. Adjusted odds ratios (OR) are presented with 95% confidence intervals (CI). Goodness of the fitted model was checked by the Hosmer and Lemeshow test.
Statistical analyses were performed using SAS software, version 9.4 (SAS institute, Inc. Cary, NC).
Ethical approval
This study was approved by the Institutional Review board (No. 2951-16-SMC).
Results
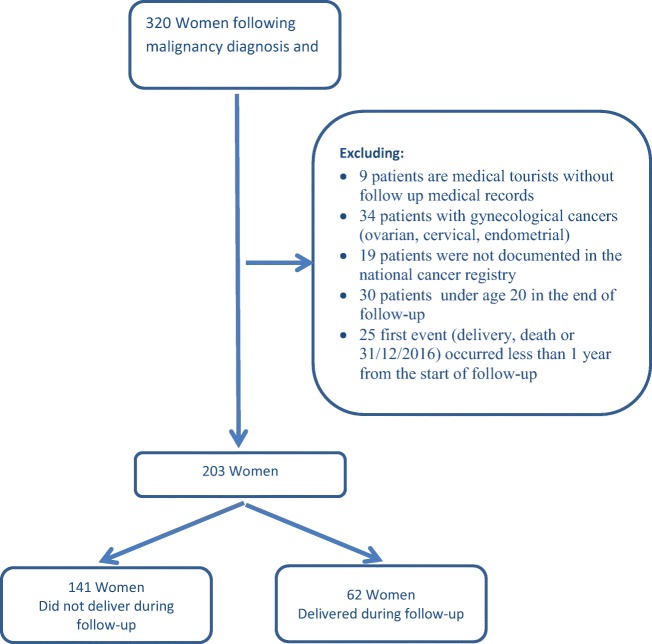
The study cohort consisted of 338 patients who underwent fertility preservation at the Sheba Medical Center during the period from 2004 to 2015. Of these, 18 patients were excluded since they underwent ovarian tissue autotransplantation. Of the remaining 320 patients, 117 were excluded for the reasons detailed in Fig. 1.
Fig. 1.
Details of the exclusion criteria following malignancy diagnosis
Overall, 203 patients were included in our analysis. For 62 (30.5%) patients in the study cohort, the National Live Birth Registry contained a record following malignancy diagnosis (30 women delivered once and 32 delivered more than once during the follow-up). Patients who delivered did not differ from those who did not for the following characteristics: gravidity/parity, the age at first diagnosis of cancer, type of malignancy, and recurrence of malignancy (Table 1). Of the 31 women diagnosed with breast cancer, 17 (54.8%) were older than 30 years at the time of diagnosis. Of the 126 women diagnosed with hematological cancer, 17 (13.5) were older than 30 years at the time of cancer diagnosis.
Table 1.
Patient different characteristics (who delivered and did not deliver) during follow-up
| Variable | All women | No birth | Birth | p value | |||
|---|---|---|---|---|---|---|---|
| during follow-up | during follow-up | ||||||
| N = 203 | n = 141 | n = 62 | |||||
| n | % | n | % | n | % | ||
| Age at 1 cancer diagnosis (start follow) | 0.23 | ||||||
| 20 | 58 | 28.6 | 40 | 28.4 | 18 | 29.0 | |
| 20.1 to < = 25 | 60 | 29.6 | 39 | 27.7 | 21 | 33.9 | |
| 25.1 to < = 30 | 45 | 22.2 | 29 | 20.6 | 16 | 25.8 | |
| > 30 | 40 | 19.7 | 33 | 23.4 | 7 | 11.3 | |
| Cancer type | 0.35 | ||||||
| Breast | 31 | 15.3 | 19 | 13.5 | 12 | 19.4 | |
| Hematology | 126 | 62.1 | 87 | 61.7 | 39 | 62.9 | |
| Bone and connective | 26 | 12.8 | 18 | 12.8 | 8 | 12.9 | |
| Other | 20 | 9.9 | 17 | 12.1 | 3 | 4.8 | |
| Cancer recurrence | 0.52 | ||||||
| Yes | 13 | 6.4 | 8 | 5.7 | 5 | 8.1 | |
| No | 190 | 93.6 | 133 | 94.3 | 57 | 91.9 | |
| Delivered before cancer | 0.42 | ||||||
| Yes | 18 | 8.9 | 11 | 7.8 | 7 | 11.3 | |
| No | 185 | 91.3 | 130 | 92.2 | 55 | 88.7 | |
There were no significant differences in follow-up time between the two groups (mean 6.4 and 6.4 years, median 5.4 and 4.7 years for women who delivered and did not deliver, respectively).
The mean age at the end of follow-up was 30.7 and 32.0 years, for those who delivered and those who did not, respectively. The above differences were not statistically significant.
During the follow-up period, 46 women died of cancer (22.7%).
In a multivariate analysis, patients who were diagnosed with cancer after the age of 30 and who underwent ovarian tissue cryopreservation were significantly less likely to deliver (OR = 0.24; 95%CI 0.07–0.85). Patients diagnosed with breast cancer were significantly more likely to deliver following cancer treatment than women with other cancer diagnoses (OR = 5.50; 95%CI 1.13–26.80) (Table 2).
Table 2.
Data on patients diagnosed with cancer who significantly more likely to deliver following cancer treatment compared to patients with other cancer diagnoses
| Adjusteda odds ratio | p value | |||
|---|---|---|---|---|
| n/N | % | |||
| Birth | 62/203 | 30.5 | ||
| Age at 1 cancer diagnosis | ||||
| 20 | 18/58 | 31.0 | Ref | |
| 20.1 to < = 25 | 21/60 | 35.0 | 1.14 [0.51–2.55] | 0.74 |
| 25.1 to < = 30 | 16/45 | 35.6 | 0.85 [0.34–2.13] | 0.73 |
| > 30 | 7/40 | 17.5 | 0.24 [0.07–0.85] | 0.02 |
| Cancer group | ||||
| Breast | 12/31 | 38.7 | 5.50 [1.13–26.80] | 0.03 |
| Hematology | 39/126 | 30.9 | 2.43 [0.66–9.01] | 0.18 |
| Bone and connective | 8/26 | 30.8 | 2.30 [0.50–10.50] | 0.28 |
| Other | 3/20 | 15.0 | Ref | |
| Cancer recurrence | ||||
| Yes | 5/13 | 38.5 | 1.22 [0.34–4.4] | 0.77 |
| No | 57/190 | 30.0 | Ref | |
| Delivered before follow-up | ||||
| Yes | 7/18 | 38.9 | 1.9 [0.62–5.99] | 0.26 |
| No | 55/185 | 29.7 | Ref | |
aLogistic regression model, odds for giving birth
Discussion
This study reports on the fertility potential of 203 patients who underwent ovarian tissue cryoperservation (OTCP) without autotransplantation of ovarian cortex during a mean of 6.4 years of follow-up. Thirty percent of the patients delivered, with no differences in gravidity, the age at first diagnosis of cancer, type of malignancy, or relapse of malignancy between those who delivered and those who did not. Moreover, those undergoing ovarian tissue cryopreservation who were diagnosed with cancer before the age of 30 and those suffering from breast cancer had significantly higher odds to deliver following cancer treatment without the of autotransplantaion.
The risk of developing POF is related to the age of the patient, the agents being used, and type of cancer. The younger the patient, the lower the risk of developing POF. In one study, the rate of secondary amenorrhea for patients with Hodgkin lymphoma receiving chemotherapy with an escalated dosage of BEACOPP (cyclophosphamide, doxorubicin, etoposide, procarbazine, prednisone, vincristine, and bleomycin) was 40.4% in those treated prior to the age of 30 years, and 70.4% among those over the age of 30 at diagnosis [8]. The risk of POF also increases with the total dose of chemotherapy agents applied, especially if alkylating agents are used. The risk of developing secondary amenorrhea is also very high in the case of pelvic radiation; thus, 50% of oocytes are destroyed if two Gray are applied. If around ten Gray are applied, the risk of amenorrhea increases to > 50% [9]. Our study demonstrated that in patients who underwent ovarian cortex cryoperservation without autotransplantation, age over 30 at the time of treatment was an independent risk factor for reduced fertility.
In a recent 12-year analysis [10], breast cancer and hematological disease were the most frequent indications for ovarian tissue cryopreservation. Overall, 90% of post-pubertal patients were diagnosed with poor ovarian reserve (AMH < = 0.5 ng/ml) after a mean of 50 months of follow-up including 30% with POF (FSH > 40 IU/ml). Ovarian function returned in 70% of post-pubertal patients without the need for grafts of cryopreserved tissue. Spontaneous pregnancies were reported in almost half of them. Among the 13 pre-pubertal patients who were included in the analysis and who reached puberty during the follow-up period, ten had POF. Eight patients received cryopreserved ovarian grafts to reverse POF, and three of them have already become pregnant. The authors of this analysis have suggested that ovarian tissue cryopreservation indications should be extended to patients whose treatments have a medium risk of ovarian insufficiency. In our study of young women diagnosed with malignancies and undergoing cryopreservation, those with a breast cancer diagnosis were most likely to give birth without undergoing ovarian autotransplantation. Moreover, 38.7% of our study patients with breast cancer gave birth without the need for ovarian autotransplantation.
Safety of cryopreservation of ovarian tissue has been demonstrated in a large cohort of 476 patients (Dolmans et al. 2013a) and did not report any severe adverse events during laparoscopy. However, in another cohort of 92 patients who completed a questionnaire 18 months after removal of an entire ovary for cryopreservation, 27% described complications from the operation. Three of them required additional surgeries for cutaneous infections or bladder lesions, and for one, laparoscopy was converted to laparotomy (Rosendahl et al. 2008). There has also been a report of a death in a patient undergoing laparoscopic ovarian tissue cryopreservation procedure due to a severe anesthesia complication [11]. Moreover, any wedge or cortical resection of the ovary has a negetive affect on ovarian reserve as does any surgery involving the removal of ovarian tissue, and even to a greater extent if thermal hemostasis is used [11].
A previous publication by Meirow et al. [12] from our unit has also demontrated that OTCP is a highly effective technique for fertility preservation. In addition, OTCP may also provide a good chance for future pregnancy by preserving the possiblity of ovarian tissue autotransplantation. In this study [12], one third (7 of 20) of graft recipients (following ovarian tissue autotrasplantation) fulfilled their wish and delivered their own biological child. Some managed to conceive and deliver several times, some spantanously, and some using IVF. Of notice, one patient had a spontaneous pregnancy already at the time of ovarian cortex transplantation, further demonstrating the limitations of patient selection.
In the present study, while evaluating 203 patients following OTCP, but did not undergo subsequent auto-transplantation, we could demonstrate at least one live birth occurred in 30% of these patients, a birth rate similar to those in the population undergoing autotransplantation in the same center [12]. Furthemore, since we followed the entire study cohort, rather than those who actually desired a pregnancy in our study population, it is reasonable to assume that the rate of live births reported here is an under-estimate. Moreover, since we could not validate that all our patients remained residents in Israel during follow-up, their deliveries, if any, would have not appear in the national birth rate, actually supporting our statement of live birth underestimation.
In the present study, we did not collect information regarding the hormonal function and menstrual characteristics of patient who conceived without ovarian transplantation, assuming that patients with amenorrhea who wished to conceived would have sought evaluation and treatment. Regardless, we recognize the bias in our patient selection as 16 of the 20 patients that underwent OTCP autotransplantation lacked any endocrine activity before transplantation; 15 regained their menses (93.7%) [12]. Moreover, in our study, we focused only on births rather than on other indicators of ovarian function, especially since the issue of fertility is our primary concern, and premature manpause can be medically treated in most cases.
Although OTCP is a very important and useful tool, patient selection remains a challenge. Our research demonstrates that at least 30% of patients did not actually need the proceedure and were exposed to unecessary surgery that carried not only risks but also reduced the patient ovarian reserve making future spontaneous pregnancy less likely. We demonstrated that breast cancer patients and patients under 30 years of age were less likely to need OTCP autotransplantation. Further studies are required to define the appropriate subgroup of patients with breast cancer under the age of 30 years, who will need OTCP. This information will be of value to fertility specialists’ counseling and their oncologic patients determining the appropriate fertility preservation strategy.
Compliance with ethical standards
This study was approved by the Institutional Review board (No. 2951-16-SMC).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Kim H, Kim H, Ku SY. Fertility preservation in pediatric and young adult female cancer patients. Ann Pediatr Endocrinol Metab. 2018;23(2):70–74. doi: 10.6065/apem.2018.23.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letourneau JM, Melisko ME, Cedars MI, Rosen MP. A changing perspective: improving access to fertility preservation. Nat Rev Clin Oncol. 2011;8(1):56–60. doi: 10.1038/nrclinonc.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 4.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Wolff M, Donnez J, Hovatta O, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy--a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45(9):1547–1553. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Fishler Y, Chetrit A, Brachana M and Modan B. Estimation of completeness of The Cancer Registry in Israel. Israel Center for Disease Control, Ministry of Health (Hebrew): publication. 2003 #230.
- 7.International classification of diseases for oncology, Third edition, first revision. Geneva: World Health Organization, 2013. http://www.who.int/classifications/icd/adaptations/oncology/en/. Accessed 06 Aug 2018
- 8.Behringer K, Breuer K, Reineke T, May M, Nogova L, Klimm B, Schmitz T, Wildt L, Diehl V, Engert A, German Hodgkin's Lymphoma Study Group Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2005;23(30):7555–7564. doi: 10.1200/JCO.2005.08.138. [DOI] [PubMed] [Google Scholar]
- 9.Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin’s Lymphoma Study Group. Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18(1):117–121. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]
- 10.Imbert R, Moffa F, Tsepelidis S, et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod. 2014;29(9):1931–1940. doi: 10.1093/humrep/deu158. [DOI] [PubMed] [Google Scholar]
- 11.Mohamed AA, Al-Hussaini TK, Fathalla MM, El Shamy TT, Abdelaal II, Amer SA. The impact of excision of benign nonendometriotic ovarian cysts on ovarian reserve: a systematic review. Am J Obstet Gynecol. 2016;215(2):169–176. doi: 10.1016/j.ajog.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 12.Meirow D, Ra'anani H, Shapira M, Brenghausen M, Derech Chaim S, Aviel-Ronen S, Amariglio N, Schiff E, Orvieto R, Dor J. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril. 2016;106(2):467–474. doi: 10.1016/j.fertnstert.2016.04.031. [DOI] [PubMed] [Google Scholar]