Abstract
Purpose
Motility of spermatozoa helps not only in planning the type of infertility treatment but also directly reflects the success rate in assisted reproductive technology (ART). Previously, biotin, a water-soluble vitamin, has been shown to increase the motility and longevity of cryopreserved human spermatozoa. The present study was designed to understand the molecular basis of the beneficial effects of presence of biotin in sperm wash medium on early embryo development.
Methods
The effect biotin supplementation to sperm wash medium on the sperm parameters were assessed in swim-up fraction of normozoospermic and asthenozoospermic ejaculates collected from infertile men. Fertilization and early embryo development was studied using Swiss albino mice.
Results
Even though both biotin and pentoxifylline (PTX) enhanced the motility of spermatozoa from normozoospermic and asthenozoospermic samples, biotin group exhibited higher in vitro survival. Using mouse model, we observed that presence of biotin or PTX in sperm wash medium improved the fertilization rate and blastocyst rate compared to control. Blastocysts from these groups had significantly higher total cell number (P < 0.01) and lower apoptotic index. In silico target prediction revealed that GTPase HRas (HRas), tyrosine-protein phosphatase nonreceptor type 1 (PTP1B), and glucokinase are the probable targets for biotin. Solution-state Nuclear Magnetic Resonance (NMR) studies confirmed that biotin interacts both with human HRas and PTP1B.
Conclusion
Our results indicate that presence of biotin in sperm wash medium can improve the fertilization potential and preimplantation embryo development and can be considered as a safe alternate to PTX.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1323-1) contains supplementary material, which is available to authorized users.
Keywords: Sperm motility, Biotin, Pentoxifylline, Fertilization, Blastocyst rate
Introduction
Motility is an important function of spermatozoa which enables them to swim across the female reproductive tract to fertilize the oocyte [1, 2]. Studies have shown that motility is a reliable predictor of fertilization potential of spermatozoa [2, 3]. In assisted reproductive technology (ART), the number of motile spermatozoa that can be extracted from the ejaculate plays a significant role in the treatment plan for the infertile couple. Therefore, enhancing the motility of spermatozoa under in vitro conditions has several advantages in ART. Increase in motility can facilitate extraction of higher number of motile spermatozoa for therapeutic insemination which may help in converting invasive procedures such as intracytoplasmic sperm injection (ICSI) to in vitro fertilization (IVF) and IVF to intra-uterine insemination (IUI). In addition, spermatozoa from absolute asthenozoospermic semen samples or frozen-thawed testicular tissue/epididymal aspirates that are characterized by poor or lack of motility can cause difficulty in identification of viable spermatozoa for ICSI [4]. In such circumstances, triggering the motility not only enables quick selection of viable spermatozoa for the procedure but also improves the fertilization rate.
Progesterone [5], follicular fluid [6], cumulus cells [7], and methylxanthenes [8] are known to trigger the motility in spermatozoa under in vitro conditions. Among these, pentoxifylline (PTX), a phosphodiesterase inhibitor, is the most extensively studied motility enhancer. However, the modulatory effect of PTX on motility has contradictory reports. Few groups reported beneficial effects [9–12], few did not observe any changes [13–15], while a few others even reported its detrimental effect on spermatozoa and embryos [16–19]. Therefore, the concern over using PTX in ART has necessitated the search for an alternate agent that does not have any adverse effect on the gametes and embryos.
Biotin, a B complex (B7) vitamin, plays a significant role in growth and development. Even though deficiency of biotin is very rarely reported in humans, its deficiency in lower animals leads to teratogenesis [20, 21]. In our previous report, we have demonstrated that supplementation of biotin to sperm wash medium can enhance motility and longevity of the frozen-thawed human spermatozoa in vitro [22]. The present study was aimed to assess the effect of biotin on fertilizing ability, preimplantation embryo development, and also to elucidate its mechanism of action.
Materials and methods
Study subjects
The study involving human subjects included 171 normozoospermic and 67 asthenozoospermic infertile men (24–47 years), attending university fertility center for routine semen analysis between October 2014 and May 2016. Study was approved by Institutional Ethics Committee of Kasturba Medical College, Manipal Academy of Higher Education, Manipal (IEC 087/2010), and a written informed consent was taken from subjects willing to provide their semen samples for the study. The semen characteristics of the patients are given in supplementary information (Supplementary Table S1). All the methods were performed in accordance with the institutional guidelines and Helsinki declaration.
Randomly bred Swiss albino mice (8–12 weeks) maintained in controlled conditions (23 ± 2 °C, 12-h light-dark cycle, 50 ± 5% humidity, food and water ad libitum) were used for the study. The study was approved by Institutional Animal Ethics Committee of Kasturba Medical College, Manipal (IAEC/KMC/40/2012). Animal care and handling were conducted according to the institutional guidelines for animal experimentation.
Extraction of motile spermatozoa from ejaculate
Motile spermatozoa were extracted using the swim-up method [22]. In brief, liquefied semen samples were washed with equal volume of Earl’s balanced salt solution (EBSS, E2888, Sigma-Aldrich) and centrifuged for 8 min at 1800 rpm. The pellet obtained was re-suspended in EBSS medium and then equally split into three groups—control, biotin, and PTX groups after which they were again centrifuged at 1200 rpm for 8 min. The pellet from the control group was gently layered with EBSS containing 0.1% bovine serum albumin (BSA, A3311, Sigma-Aldrich). For biotin and PTX groups, the pellet was layered with EBSS containing 10 nM biotin (14400, Sigma-Aldrich) and 1 mM PTX (P1784, Sigma-Aldrich), respectively, and incubated for 60 min at 37 °C and 5% CO2 for motile sperm to swim up. The presence of biotin in the swim-up fraction was confirmed by reversed-phase high-performance liquid chromatography (RP-HPLC, Supplementary Table S2 & Fig. S1). Motility was assessed at various intervals (1, 4, 24, 48, and 72 h) by placing 10 μL of sperm suspension on a slide and observing under microscope (WHO 2010). Kinematic changes were assessed using computer-assisted semen analysis (CASA). Ten microliters of sperm suspension was placed on a clean microscopic slide, covered with a coverslip, and analysis was performed using ISAS software (Proiser, Spain). Curvilinear velocity (VCL), straight line velocity (VSL), average path velocity (VAP), lateral displacement of sperm head (ALH), linearity (LIN), straightness (STR), balancing (WOB), and beat frequency cross (BCF) were assessed at 1- and 4-h intervals.
Effect of biotin supplementation on sperm functional characteristics
Acrosome reaction
Fertilizing ability of motile spermatozoa was estimated using ionophore (A23187)-induced acrosome assay (CIAR) as described by Liu and Baker [23], with minor modifications. Briefly, the motile sperm fraction from various groups was incubated for 30, 60, and 120 min at 37 °C and 5% CO2 with 5 μM of calcium ionophore A23187 (C7522, Sigma-Aldrich). The samples were washed in phosphate-buffered saline (PBS) and smeared on a coverslip. Spermatozoa were permeabilized using methanol (15 min) and stained with 25 μg/mL FITC-conjugated Pisum sativum agglutinin (FITC-PSA, L0770, Sigma-Aldrich) for 30 min in dark. Cells were then washed with Milli-Q water, counter-stained with 7 μg/mL of propidium iodide (PI, P4170, Sigma-Aldrich), and mounted on clean microscopic slide using anti-fade mounting medium (S3023, DAKO). Percentage of acrosome-reacted spermatozoa (without green acrosome cap) was determined by scoring 500 spermatozoa per sample under fluorescent microscope (Imager A1, Carl Zeiss, Germany).
Mitochondrial membrane potential and sperm DNA integrity
Mitochondrial membrane potential and DNA integrity of spermatozoa were assessed at 24 and 48 h post-incubation using rhodamine-123 and sperm chromatin dispersion (SCD) test, respectively [24]. Based on the fluorescence intensity in the mid-piece region, spermatozoa were categorized as intact (bright), partial damage (faint), and damaged mitochondria (no fluorescence). A minimum of 500 spermatozoa were scored and the results were expressed in percentage. In SCD test, depending on the halo size, the sperm heads were categorized as no halo, small, and large halo by observing under fluorescent microscope. Spermatozoa with no or small halo were considered to have DNA damage and results were expressed in percentage.
Measurement of intracellular cyclic AMP level
Intracellular cyclic AMP (cAMP) level in spermatozoa was measured using ELISA kit (581001, Cayman chemical). Approximately five million spermatozoa were treated with 0.5 mL of 0.1 M HCl, incubated for 20 min at room temperature, and centrifuged at 3000 rpm for 10 min. The supernatant was collected and stored at − 80 °C until further analysis. The samples and cAMP standards were acetylated using 4 M KOH and acetic anhydride. Fifty microliters of acetylated samples was added to each well along with blank, total activity, non-specific binding, and maximum binding wells. After adding acetyl cholinesterase-cAMP tracer and cAMP antiserum (50 μL each), the plate was incubated for 18 h at 4 °C, washed five times with wash buffer, and color was developed by adding Ellman’s reagent (200 μL). Plate was incubated for 90 min in the dark on a micro-plate shaker after which absorbance was recorded at 405 nm. Data was analyzed by using a computer spread sheet of Cayman chemical (MI, USA, www.caymanchem.com/analysis/elisa). The assay was performed in duplicates.
In vitro fertilization and preimplantation embryo development
Effect of biotin on fertilizing ability of spermatozoa and subsequent preimplantation embryo development was determined by performing in vitro fertilization using a murine model. Oocyte cumulus complex (OCC) was collected from superovulated female mice as described earlier [25]. Caudal spermatozoa collected from male mice were released into EBSS medium containing 0.1% BSA and incubated for 2 h at 37 °C and 5% CO2 followed by centrifugation at 1200 rpm for 8 min. The pellet was layered with EBSS medium (containing 2.5% BSA) with or without 10 nM biotin or 1 mM PTX. After 45 min, motile spermatozoa were collected from all the three groups and sperm density was adjusted to 3–5 millions/mL to prepare insemination droplet of 80 μL and covered with pre-incubated paraffin oil. At 13 h post-hCG, the OCCs were collected and washed gently in EBSS with 0.1% BSA. OCCs from each mouse were divided into three groups and inseminated with spermatozoa from control, biotin, and PTX groups and incubated at 37 °C, 5% CO2. At 12 h post-insemination, the OCCs were gently pipetted to strip off the cumulus cells and observed under inverted microscope (Olympus IX71, Tokyo, Japan). Oocytes with 2 pronuclei and 2 polar bodies (2PN/2PB) were cultured further until blastocyst stage in potassium simplex optimization medium (KSOM, homemade). The developmental potential was assessed by calculating the blastocyst rate and hatching rate. The apoptotic index in blastocysts was assessed by terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling (TUNEL) assay and the total cell number (TCN) was assessed by staining the nuclei with 4′,6′-diamidino-2-phenylindole (DAPI) assay as described earlier by Kalthur et al. [25].
qRT PCR analysis of mRNA expression in blastocyst
RNA extraction, cDNA synthesis, and real-time quantitative polymerase chain reaction (RT-PCR) were performed according to manufacturer’s instructions. Briefly, 30–35 expanded embryos per group were lysed in lysis buffer and eluted from the RNA aqueous column using 8 μL pre-warmed (95 °C) elution buffer (AM1931, Ambion). The concentration and purity of the RNA was determined using Nanodrop analyzer (HellmaTrayCell; Hellma GmbH & Co. K.G, Mullheim, Germany). cDNA was synthesized from 35 ng total RNA using a Superscript first-strand cDNA synthesis kit for reverse transcription-polymerase chain reaction (E6300; New England Biolabs, Ipswich, MA, USA). Real-time quantitative polymerase chain reaction (real-time qPCR) for pluripotency genes was performed using predesigned TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). Triplicate amplification reactions were set up for each sample. The thermal cycler used was a StepOne Real-Time PCR System (Applied Biosystems, USA). The program set for the real-time qPCR was as follows: 20-s incubation at 95 °C, amplification at 95 °C for 1 s, and annealing at 60 °C for 20 s, for 40 cycles. Expression of Oct4, Sox2, Nanog, Cdx2, Bmp4, p53, Caspase3, and Rad51 was normalized against that of the housekeeping gene b-Actin (Actb). The primer details are provided in Supplementary Tables S4 & S5.
In silico target prediction
PharmMapper, a freely accessed web-server designed to identify potential protein targets for a given probe, small molecule (drugs, natural products, or other newly discovered compounds with binding targets unidentified) using an integrated pharmacophore mapping approach with statistical method, was employed to carry out the bioinformatics study, the methodology of which is given in the Supplementary file.
The major objective of our bioinformatics study was to predict potential protein targets of biotin in human sperm sample. Subsequently, Nuclear Magnetic Resonance (NMR) studies were carried out on human sperm samples to confirm the findings of our bioinformatics study. Hence, only human protein targets were selected for the screening purpose. Sequence similarity searching, using the most reliable and widely used strategy, BLAST, identified that 100% and 83% sequences are conserved for GTPase HRas and tyrosine-protein phosphatase nonreceptor type 1, between human and mouse.
Protein ligand interaction [protein tyrosine phosphatase nonreceptor type 1 and HRas interaction with biotin]
The human ejaculate was centrifuged at 1200 rpm for 10 min and the pellet obtained was washed with cold PBS thrice. The supernatant was discarded and the pellet was treated with 100 μL RIPA buffer, incubated in vortex shaker for 30 min at room temperature, and centrifuged to remove undigested spermatozoa. Resultant supernatant was collected and the protein concentration was estimated (32 mg/mL) at 280 nm using Nanodrop and then stored at − 80 °C until they were purified using SDS page. To this, 100 μL Laemmli buffer was added and incubated at 99 °C for 10 min. The proteins were then subjected to SDS-PAGE and using the protein molecular weight markers, the specific protein bands were collected at 18.4 kDa and 21 kDa for protein tyrosine phosphatase nonreceptor type 1 (PTP1B) and HRas, respectively. The polyacrylamide protein bands were cut and crushed in Tris-HCl buffer (pH 7.4), vortexed at room temperature for 30 min. They were then centrifuged at 14,000 rpm for 20 min at 4 °C. The supernatant was collected, lyophilized, and later subjected to 1H-NMR to study the protein ligand interactions.
In vitro ligand detection using 1H-NMR methods based on change in chemical shift and line width was employed to experimentally confirm the interaction of PTP1B and HRas with biotin. A brief introduction to chemical shift and line width-based NMR method to decipher ligand-protein interaction is discussed in the subsequent section.
1D 1H-NMR studies for ligand-protein interaction
NMR is one of the most powerful tools to study the ligand-macromolecule interactions in solution [26]. The proton chemical shift is sensitive to the chemical environment as well as intermolecular interactions between ligand-protein systems. Change in the chemical environment due to complexation or due to intermolecular interaction with neighbors in solution will be reflected as a shift either to the higher frequency (downfield) or to the low frequency (upfield) depending on the magnetic shielding properties of the new environment. Besides changes in the NMR chemical shift, the mobility of the molecule also exhibits drastic change due to complexation or formation of intermolecular adducts. Complex formation with the protein leads to a more macromolecule-like behavior for the small molecule, viz., decrease in molecular motion and increase in molecular correlation time (τc) [26–28] (Fig. S2). Consequently, a cumulative effect of complexation is observed as NMR line broadening revealing a much smaller transverse relaxation time (T2) of the small molecule. Hence, detectable change in NMR chemical shift and line width of a ligand in absence and presence of protein is a preliminary indicator of binding interaction involving the both in solution [29].
Equation 1 depicts the relation between line width and apparent transverse relaxation time (T2*).
| 1 |
where ∆ν1/2 = observed linewidth at half height. The apparent transverse relaxation time (T2*) that determines the line width is a direct manifestation of the motional correlation time (τc) that in turn depends on the molecular weight of the chemical species. Hence, any change in molecular weight due to complexation will be correctly identified by analyzing the changes observed in case of line width of the molecule.
Materials
Biotin, D2O, and DMSO at 99.9% purity were procured from Sigma-Aldrich. The lyophilized protein samples (PTP1B 18 kDa and HRas 21 kDa) were used and quantified by NanoDrop™. All other reagents were of analytical grade. Double-distilled water was used throughout the experiments and Tris-HCL (tris(hydroxyl methyl)aminomethane hydrochloride) buffer (pH 7.5) was prepared in double-distilled water.
Preparation of stock solutions
Both protein stock solutions were prepared in Tris-HCL buffer (PB: 0.05 M; pH 7.5). The stock concentrations were determined to be 25 μM for both proteins. Ten-millimolar biotin stock solution was prepared in DMSO and final concentration of biotin was 1 mM and 10% DMSO volume was maintained throughout experiments for each sample. Ten percent D2O was used as the lock solvent. The reaction mixture was then transferred to a NMR sample tube and a series of 1H-NMR spectra were acquired under standard observation conditions. Final concentrations for both proteins were 10 μM with 1 mM biotin concentration.
NMR spectroscopy
NMR measurements were carried out on Bruker 500 MHz WB NMR spectrometer using a BBFO probehead at 300 K over a spectral width of 9.00 ppm. A total of 24 k data points (TD) were collected for each spectrum with a 1H 90° pulse width of 14.15 μs, and a relaxation delay (d1) of 5 s. A total of 16 scans (NS) were accumulated for each experiment. Chemical shifts were referenced to the residual solvent signal of HDO at 4.69 ppm. Multiple solvent suppression technique involving a phase-modulated sinc pulse was employed to achieve suppression of 4.69 ppm water peak and 3.56 ppm DMSO peak simultaneously. All spectra were processed using TopSpin 3.2 software.
Statistical analysis
All the data are presented as mean and standard error (mean ± SEM), except embryo developmental parameters which are given as percentage data. The statistical significance level of mean ± SEM was calculated using one-way analysis of variance (ANOVA) and percentage data was analyzed by chi-square test using GraphPad InStat 3.0 statistical package (GraphPad Inc., USA). P value < 0.05 was considered as statistically significant. All the graphs were plotted using Origin 6.0 (Origin Lab Corporation, Northampton, MA, USA).
Results
Effect on sperm motility and functional characteristics
Normozoospermic semen samples
At 1 h after incubation, a significant increase in total motility (progressive and non-progressive) was observed in both biotin (P < 0.05) and PTX (P < 0.01) groups which then started declining gradually with incubation time, in all the groups (Table 1). Both at 24 and 48 h, total motility was higher in biotin compared to other groups. PTX group had the lowest percentage of motility at 48 h (P < 0.05 vs control and P < 0.01 vs biotin). Even at 72 h, the biotin group had higher percentage of motile spermatozoa compared to PTX (P < 0.05).
Table 1.
Effect of biotin and pentoxifylline on the sperm motility pattern at various time intervals after processing by swim-up technique
| Sample | Time in hours | Sample size (n) | Total motility (%) | Progressive motility (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Biotin | Pentoxifylline | Control | Biotin | Pentoxifylline | |||
| Normozoospermic | 1 | 171 | 87.88 ± 0.74 | 90.11 ± 0.66 a | 90.65 ± 0.60 b | 72.55 ± 1.20 | 77.46 ± 1.04 b | 78.74 ± 0.94 c |
| 4 | 171 | 85.72 ± 0.92 | 87.64 ± 0.75 | 88.17 ± 0.76 | 71.67 ± 1.20 | 74.99 ± 1.03 | 74.76 ± 1.11 | |
| 24 | 165 | 69.42 ± 1.70 | 73.20 ± 1.48 | 67.62 ± 1.91 | 51.29 ± 1.82 | 56.25 ± 1.80 | 49.61 ± 2.00 d | |
| 48 | 105 | 49.30 ± 2.57 | 53.26 ± 2.42 | 40.23 ± 2.63 a,e | 32.19 ± 2.40 | 37.08 ± 2.43 | 25.44 ± 2.34 e | |
| 72 | 43 | 42.16 ± 3.64 | 43.95 ± 3.60 | 31.84 ± 3.50 d | 24.84 ± 3.01 | 27.79 ± 3.34 | 15.65 ± 2.68 d | |
| Asthenozoospermic | 1 | 67 | 71.56 ± 3.04 | 77.84 ± 2.40 | 79.29 ± 2.37 | 53.13 ± 2.96 | 63.90 ± 2.47 a | 63.78 ± 2.50 a |
| 4 | 67 | 67.58 ± 3.17 | 75.03 ± 2.44 | 73.05 ± 2.87 | 48.20 ± 3.07 | 59.90 ± 2.51 a | 55.60 ± 2.91 | |
| 24 | 67 | 47.52 ± 3.88 | 54.52 ± 3.31 | 43.50 ± 3.68 | 31.11 ± 3.63 | 35.82 ± 3.26 | 25.64 ± 3.30 | |
aP < 0.05
bP < 0.01
cP < 0.001 vs control
dP < 0.05
eP < 0.01 vs biotin
A similar trend was observed in progressive motility (Table 1). Both biotin and PTX groups had significantly higher percentage of progressively motile spermatozoa (P < 0.01 in biotin; P < 0.001 in PTX) compared to control until the 4-h interval. Biotin maintained significantly higher percentage of spermatozoa with progressive motility until 72 h of incubation when compared to PTX (P < 0.05 at 24 and 72 h, P < 0.01 at 48 h).
Asthenozoospermic semen samples
At 1-h interval, even though the percentage of total motile spermatozoa did not increase significantly in biotin and PTX groups, progressive motility was significantly higher than control (P < 0.05, Table 1). As observed in normozoospermic samples, the progressive motility was higher in biotin (P < 0.05 vs control and P > 0.05 vs PTX) at 4 h. At 24 h, biotin group had the highest percentage (35.82 ± 3.26) of spermatozoa with progressive motility compared to control (31.11 ± 3.63) and PTX (25.64 ± 3.30). Beyond 24 h, none of the groups possessed any motility indicating the poor in vitro survival of asthenozoospermic samples.
Sperm kinematics
At 1 h after incubation, in normozoospermic samples, VSL (P < 0.05), ALH (P < 0.01), and STR (P < 0.05) were significantly higher in PTX than control, while WOB was significantly higher in biotin group compared to PTX (P < 0.05). While in asthenozoospermic samples, non-significantly higher values were observed both in biotin and PTX groups compared to control. At 4 h after incubation, in normozoospermic semen samples, there was no significant difference between the groups (except ALH in PTX, P < 0.05 compared to control). In biotin group, except VCL and ALH, other parameters were non-significantly higher than control in asthenozoospermic cohort, whereas in PTX group, except LIN, STR, and WOB, other parameters were non-significantly higher than control (Table 2).
Table 2.
Sperm kinematics at 1 and 4 h after incubation using computer-assisted semen analysis (CASA)
| Sample | Parameter | 1 h | 4 h | ||||
|---|---|---|---|---|---|---|---|
| Control | Biotin | Pentoxifylline | Control | Biotin | Pentoxifylline | ||
| Normozoospermic (N = 79) | VCL (μm/s) | 81.53 ± 1.59 | 80.11 ± 1.63 | 85.30 ± 1.54 | 88.46 ± 1.59 | 88.39 ± 1.63 | 90.03 ± 1.65 |
| VSL (μm/s) | 24.03 ± 0.53 | 24.95 ± 0.62 | 26.25 ± 0.53a | 28.44 ± 0.67 | 28.61 ± 0.64 | 28.97 ± 0.62 | |
| VAP (μm/s) | 39.06 ± 0.61 | 39.00 ± 0.69 | 40.62 ± 0.64 | 42.50 ± 0.66 | 42.68 ± 0.65 | 42.85 ± 0.67 | |
| ALH (μm) | 2.60 ± 0.08 | 2.59 ± 0.07 | 2.91 ± 0.07 b,d | 3.07 ± 0.09 | 3.13 ± 0.09 | 3.39 ± 0.08 a | |
| LIN (%) | 29.73 ± 0.62 | 31.47 ± 0.70 | 31.06 ± 0.58 | 32.40 ± 0.75 | 33.19 ± 0.83 | 32.50 ± 0.79 | |
| STR (%) | 61.28 ± 0.85 | 62.85 ± 1.22 | 64.81 ± 0.87a | 66.42 ± 0.88 | 66.76 ± 0.94 | 67.31 ± 0.95 | |
| WOB (%) | 48.20 ± 0.49 | 49.16 ± 0.45c | 47.59 ± 0.37 | 48.11 ± 0.51 | 48.32 ± 0.51 | 47.74 ± 0.53 | |
| BCF (beats/s) | 8.84 ± 0.14 | 9.11 ± 0.12 | 9.10 ± 0.12 | 9.14 ± 0.15 | 9.32 ± 0.13 | 9.01 ± 0.17 | |
| Asthenozoospermic (N = 15) | VCL (μm/s) | 55.17 ± 5.57 | 58.50 ± 4.77 | 58.33 ± 4.57 | 52.08 ± 5.19 | 53.83 ± 3.74 | 59.25 ± 4.82 |
| VSL (μm/s) | 17.50 ± 1.69 | 19.00 ± 1.29 | 19.08 ± 1.55 | 18.17 ± 1.42 | 21.92 ± 2.21 | 21.17 ± 1.56 | |
| VAP (μm/s) | 27.83 ± 3.13 | 31.08 ± 2.11 | 30.75 ± 1.98 | 28.08 ± 2.01 | 31.00 ± 2.15 | 32.83 ± 1.71 | |
| ALH (μm) | 1.50 ± 0.31 | 1.58 ± 0.19 | 1.58 ± 0.26 | 1.67 ± 0.28 | 1.67 ± 0.33 | 1.92 ± 0.31 | |
| LIN (%) | 33.17 ± 2.83 | 33.92 ± 2.16 | 33.42 ± 2.18 | 37.33 ± 3.31 | 41.08 ± 3.22 | 36.75 ± 2.53 | |
| STR (%) | 61.58 ± 3.40 | 62.25 ± 2.97 | 62.00 ± 2.88 | 65.42 ± 3.63 | 69.83 ± 3.02 | 66.00 ± 3.07 | |
| WOB (%) | 52.92 ± 2.46 | 54.33 ± 1.39 | 53.42 ± 1.58 | 55.58 ± 2.22 | 57.83 ± 2.30 | 55.17 ± 2.03 | |
| BCF (beats/s) | 5.17 ± 0.96 | 5.67 ± 0.58 | 5.50 ± 0.72 | 5.58 ± 0.78 | 6.67 ± 0.90 | 6.42 ± 0.87 | |
aP < 0.05
bP < 0.01 vs control
cP < 0.05 vs PTX
dP < 0.01 vs biotin
Calcium ionophore-induced acrosome reaction
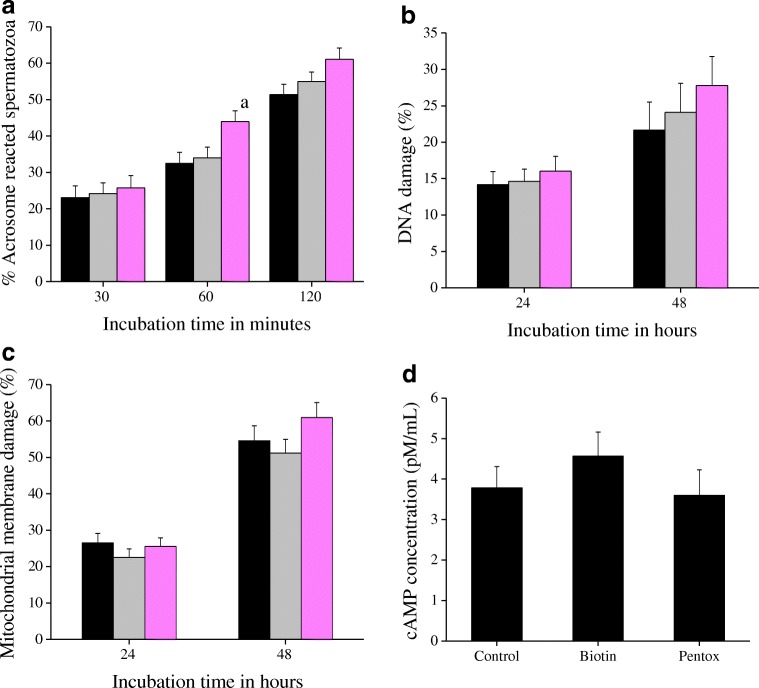
At 30 min after incubation, the percentage of acrosome-reacted spermatozoa was almost similar in all the groups which increased with incubation time (Fig. 1a). PTX group had significantly higher percentage of acrosome-reacted spermatozoa (43.95 ± 2.97%, P < 0.05 vs control) at 60 min. However, at 120 min, there was no significant difference between the groups (51.37 ± 2.83, 54.95 ± 2.62, and 61.05 ± 3.15% in control, biotin, and PTX, respectively).
Fig. 1.
a Effect of biotin (10 nM) and PTX (1 mM) on kinetics of acrosome reaction estimated using calcium ionophore (A23187)-induced acrosome reaction assay (CIAR). The swim-up suspension was incubated with ionophore (5 μM) for 30, 60, and 120 min and stained with FITC-PSA for visualization of acrosome sperm head. A total of 500 spermatozoa were assessed for each sample and the data represented as percentage of the difference in the number of ionophore-induced acrosome-reacted spermatozoa to the baseline acrosome-reacted spermatozoa. The results correspond to mean ± SE and the statistical significance value is represented as (a): P < 0.05 vs control. b DNA integrity of the spermatozoa processed with sperm wash medium containing biotin (10 nM) or pentoxifylline (1 mM) at 24 and 48 h after in vitro incubation. The extent of DNA damage in the spermatozoa was assessed by sperm chromatin dispersion (SCD) test. The results correspond to mean ± SE of spermatozoa and the statistical significance value. c Mitochondrial membrane integrity of the spermatozoa processed with sperm wash medium containing biotin (10 nM) or pentoxifylline (1 mM) at 24 and 48 h after in vitro incubation. The spermatozoa were stained with Rhodamine 123 to assess the mitochondrial membrane potential. The results correspond to mean ± SE of percentage of spermatozoa with completely damaged mitochondria. d Effect of biotin (10 nM) and PTX (1 mM) on intracellular cAMP concentration of spermatozoa was measured using cAMP ELISA kit, at 1 h post-swim-up. A total of 15 sets (n = 5 per group) of sample were assessed among the groups and the data is represented as mean ± SE. No statistical significance was found. Black: control; gray: biotin; pink: PTX
Sperm DNA integrity
Control samples had 21.30 ± 3.80% of spermatozoa with DNA damage at 24 h after incubation, which was similar in biotin and PTX groups (Fig. 1b). At 48 h, DNA damage increased by ~ 15% in all the groups studied. However, no significant difference was observed between the groups.
Mitochondrial membrane integrity
In control group at 24 h, 25.84 ± 2.56% of spermatozoa showed complete mitochondrial damage which further increased to 54.83 ± 5.92% at 48 h (Fig. 1c). Processing the semen samples either with biotin or PTX did not have any significant effect on mitochondrial membrane potential. However, the damage was higher in PTX group at 48 h (60.96 ± 5.03, 54.83 ± 5.92, 52.04 ± 4.47% in PTX, control, and biotin, respectively).
Intracellular cyclic AMP level
As depicted in Fig. 1d, non-significantly higher intracellular cAMP level was observed in spermatozoa processed with biotin (4.57 ± 0.60 pM/mL) compared to control (3.78 ± 0.53) and PTX (3.60 ± 0.63) at 1 h after swim-up procedure.
Effect on murine in vitro fertilization and preimplantation embryo development
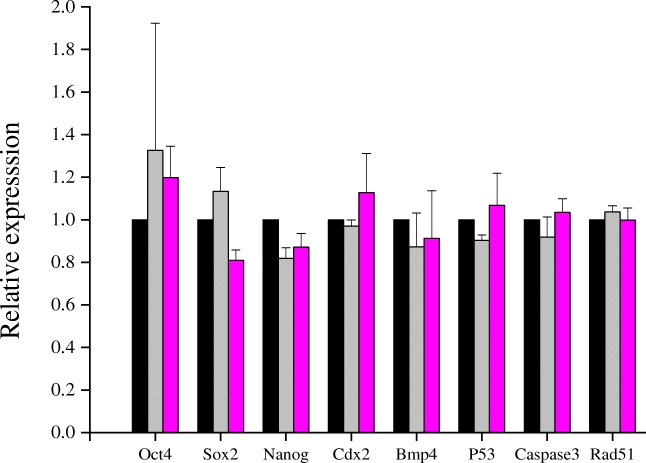
At 16 h post insemination, biotin group had significantly higher fertilization rate (92.90%) compared to control (84.54%, P < 0.001) and PTX (87.71%, P < 0.05, Table 3). Significantly higher percentage of zygotes progressed to two-cell stage in both biotin (P < 0.001) and PTX groups (P < 0.05) compared to control. In general, embryos derived from biotin-treated sperm suspension were cleaving faster than other groups. At 72 h, 95.52% of embryos in biotin group were compacted which was lower in control (89.33%, P < 0.01) and PTX groups (93.09%, P > 0.05). At 96 h, biotin group exhibited higher blastocyst rate (88.66%) compared to control (80.00%, P < 0.01) and PTX (81.91%, P < 0.05). Similarly, 46.87% of the blastocysts were hatched in biotin group by this time which was significantly higher than control (34.22%, P < 0.01) and PTX (34.53%, P < 0.01) indicating that the embryos from biotin group developed faster than other groups. The blastocysts of biotin and PTX group had significantly higher TCN (P < 0.05) and non-significantly lower apoptotic index compared to control (Table 3). Blastocysts obtained in vitro in presence of biotin (10 nM) or PTX (1 mM) did not show any significant difference in the gene expression pattern compared to control embryos (Fig. 2).
Table 3.
Effect of biotin and pentoxifylline on the fertilization and embryo developmental potential in vitro
| Control | Biotin (10 nM) | PTX (1 mM) | |
|---|---|---|---|
| Number of oocytes inseminated | 291 | 366 | 358 |
| Fertilization rate (%) | 84.54 | 92.90 c | 87.71 d |
| 2-cell rate (%) | 91.87 | 98.53 c | 96.82 a |
| 4-cell rate at 48 h post-insemination (%) | 92.89 | 94.33 | 93.09 |
| Compaction rate at 72 h post-insemination (%) | 89.33 | 95.52 b | 93.09 |
| Blastocyst rate at 96 h post-insemination (%) | 80.00 | 88.66 b | 81.91 d |
| Blastocyst rate at 120 h post-insemination (%) | 86.22 | 90.45 | 91.45 |
| Hatching rate at 96 h post-insemination (%) | 34.22 | 46.87 b | 35.53 e |
| Hatching rate at 120 h post-insemination (%) | 49.33 | 62.09 b | 57.89 |
| Total cell number (mean ± SE) | 72.96 ± 3.63 | 91.05 ± 3.26 b | 89.20 ± 3.08 b |
| Apoptotic index (mean ± SE) | 6.12 ± 1.06 | 4.21 ± 0.38 | 4.07 ± 0.50 |
aP < 0.05
bP < 0.01
cP < 0.001 vs control
dP < 0.05
eP < 0.01 vs biotin
Fig. 2.
Expression level of transcripts in blastocysts derived by in vitro fertilization using spermatozoa treated with biotin (10 nM) or pentoxifylline (1 mM) in Swiss albino mice. The fertilized embryos were cultured in vitro in KSOM medium until blastocyst stage. The RNA extracted from pooled blastocysts in each group were estimated for mRNA levels of Oct4, Sox2, Nanog, Cdx2, Bmp4, p53, Caspase3, and Rad51 using real-time RT-PCR
Nuclear Magnetic Resonance spectroscopy
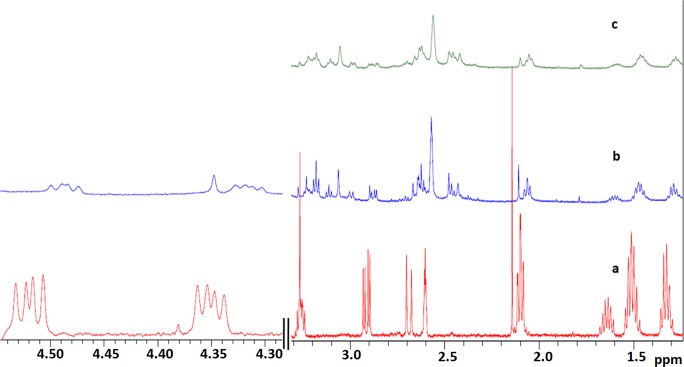
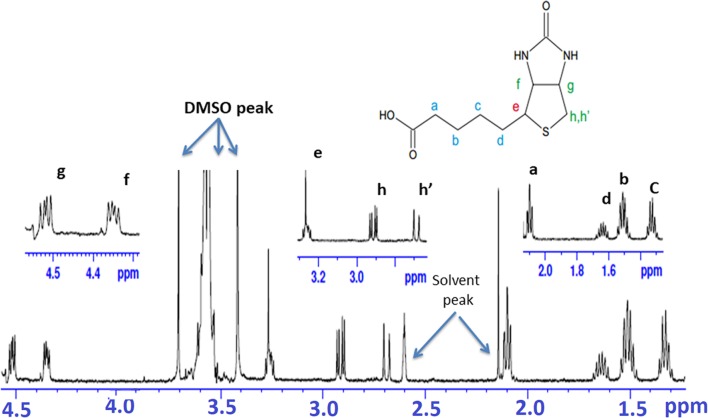
The docking studies performed helped in predicting possible interaction of biotin with protein tyrosine phosphatase nonreceptor type 1 (PTP1B) and HRas interaction with biotin. To confirm this interaction, we performed NMR spectroscopy. Figure 3 represents a stack plot of the relevant spectral regions of biotin in its free state and in presence of the protein. The resonance assignment for free biotin is given in Fig. 4 while Table 4 documents the chemical shift, line width, and apparent transverse relaxation time of all the biotin 1H-NMR peaks in the free as well as in the bound state.
Fig. 3.
1H-NMR spectral region (a) free biotin (red), (b) biotin in presence of PTP1B (blue), and (c) biotin in presence of HRas (green) recorded in Tris-HCl buffer (10% DMSO) at 300 K. The area from 4.30 to 4.6 ppm for free biotin and biotin in presence of PTP1B has been magnified while the same for HRas is not shown due to phase distortion in biotin peaks in this area
Fig. 4.
1H-NMR spectra of 1 mM biotin in Tris-HCL buffer (pH 7.5) at 300 K. [peak e (multiplet) merges with buffer peak]
Table 4.
Chemical shift and line width values of biotin protons in absence and presence of protein tyrosine phosphatases and HRAS
| Free biotin protons | Biotin-tyrosine phosphatases | Biotin-HRAS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Proton position | Chemical shift (ppm) | Line width# (Hz) | T2* (s) | Chemical shift (ppm) | Line width (Hz) | T2* (s) | Chemical shift (ppm) | Line width (Hz) | T2* (s) |
| Hc | 1.33 | 3.90 | 0.081 | 1.29 | 4.56 | 0.069 | 1.28 | 5.37 | 0.059 |
| Hb | 1.51 | 4.46 | 0.071 | 1.47 | 6.94 | 0.045 | 1.46 | 24.68 | 0.012 |
| Hd | 1.64 | 3.30 | 0.096 | 1.61 | 4.95 | 0.064 | 1.59 | 39.28 | 0.008 |
| Ha | 2.09 | 3.47 | 0.091 | 2.06 | 3.89 | 0.081 | 2.05 | 7.25 | 0.043 |
| Hh’ | 2.69 | 2.18 | 0.146 | 2.64 | 2.49 | 0.127 | 2.63 | 7.52 | 0.042 |
| Hh | 2.91 | 1.27 | 0.250 | 2.88 | 1.87 | 0.170 | 2.87 | 5.31 | 0.059 |
| He | 3.26 | 1.51 | 0.210 | 3.23 | 3.92 | 0.081 | 3.22 | 13.46 | 0.023 |
| Hf | 4.52 | 2.07 | 0.153 | 4.48 | 3.19 | 0.099 | 4.47 | 13.41 | 0.023 |
| Hg | 4.35 | 2.90 | 0.109 | 4.32 | 3.98 | 0.080 | 4.31 | 14.99 | 0.021 |
#LB was 1 Hz in each case
A close inspection of Fig. 3 reveals upfield shift of all the chemical shifts of biotin in presence of both the proteins indicating complex formation causing magnetic shielding of biotin protons. The chemical shift change (Δδ) for biotin protons in free and bound state was calculated using data from Table 4 that exhibited variation from 0.03 to 0.05 ppm in both cases. Furthermore, due to the formation of biotin-protein complex undergoing a continuous exchange between free and bound state, a significant change in transverse relaxation times of all the biotin protons was observed. In case of PTP1B, the maximum change in apparent transverse relaxation time is 61.4% for He of biotin whereas for HRas, the change for the same proton was 89% (Table 4). In case of HRas, the maximum change observed is 91.6% for Hd proton. On an average, the change in T2* in presence of PTP1B and HRas is 29.3% and 73%, respectively. The results clearly exhibit that the biotin-HRas interaction is c.a. 2.5 times stronger than the biotin-PTP1B.
Discussion
In the present study, we have observed that biotin can enhance the motility and longevity of human spermatozoa under in vitro conditions. The motility enhancing effect was comparable between asthenozoospermic and normozoospermic semen samples, which is in accordance with earlier reports on PTX [30] and biotin [22].
Sperm longevity is a reliable predictor of fertilization potential [31] and determines the outcome of IUI and IVF procedures. The rapid surge and quick drop in progressive motility observed in PTX group in our study is in accordance with earlier reports [22, 32, 33]. Results of mitochondrial function and DNA integrity assessment suggest that the poor survival of spermatozoa is not due to these two factors (Fig. 1). On the contrary, biotin helped in maintaining a higher percentage of progressive motility and in vitro survival compared to PTX which was also documented in our earlier study with frozen-thawed semen samples [22]. Even though non-significant, the elevated intracellular cAMP level in spermatozoa from biotin-treated group could have led to the increase in motility observed in this group. An earlier study by Jayaprakash et al. [34] has shown that PTX can cause premature acrosome reaction, thereby leading to poor fertilization. However, in our study, even though PTX group showed higher percentage of spermatozoa undergoing acrosome reaction as early as 60 min, there was no significant difference in the fertilization rate. It is possible that the acrosome reaction kinetics induced by PTX in human spermatozoa may be different from mouse spermatozoa which needs further investigation.
One of the major concerns of using PTX is its adverse effects on oocyte and embryo development [17–19, 35]. However, this was not reflected in our study as we observed better blastocyst and hatching rate in PTX compared to control. This disparity between our results and earlier reports with respect to oocyte and embryo toxicity could be attributed to lower concentration of PTX (1 mM) used in our study. Earlier studies in the literature have employed varying concentrations of PTX ranging from 1 to 5 mM [9–19, 22, 34, 35]. In the present study, we intended to use optimal concentration of PTX that enhanced the sperm motility without having any toxic effect on the embryo development. In our earlier study, we have reported motility enhancement of frozen-thawed human spermatozoa at 1 mM concentration [22]. In addition, when two-cell stage mouse embryos were cultured in different concentrations of PTX, it resulted in drastic reduction in blastocyst rate at and above 2 mM concentration (Supplementary Table S6). Therefore, based on these findings, we chose a lower concentration of PTX (1 mM) in this study.
With the current findings, it is difficult to understand the exact mechanism by which the embryos derived from spermatozoa processed with biotin exhibit improved cleavage rate, better hatching potential, significantly higher total cell number, and non-significantly lower apoptotic index. Earlier studies have shown that biotin is an important regulator of chromatin organization [36, 37]. The lower apoptotic index observed in blastocysts of IVF-derived embryos further emphasizes the significance of biotin in maintaining the chromatin integrity. The gene expression pattern in the expanded blastocysts indicated marginal upregulation of Oct4 and Sox2 genes which regulate pluripotency (Fig. 2). In addition, biotin, even at higher concentrations (up to 100 nM) in medium, did not seem to affect the embryo development (Supplementary Table S7) indicating that the presence of biotin in embryo culture medium also can exert beneficial effects on preimplantation embryo development. Therefore, presence of trace amounts of biotin in the sperm suspension can exert further beneficial effect on embryo development. Earlier findings on biotin deficiency causing teratogenic effect [20, 21] give a possible clue for the nutritional significance of biotin during early embryo development.
Through the bioinformatics analysis performed to predict the target molecules for biotin in spermatozoa and embryo developmental potential, we obtained three best fit molecules such as GTPase HRas, glucokinase, and PTP1B, which are known to have specific role in spermatozoa. Among the top three hits, HRas, designated as p21, is present in the membrane of most eukaryotic cells which binds to guanine nucleotides and has GTPase activity in vitro and in vivo [38, 39]. The presence of c-ras proteins in the acrosomal region of human spermatozoa and its possible role in capacitation and/or acrosome reaction was demonstrated by Naz et al. [40]. Similarly, PTP1B was shown to have a role in acrosome reaction [41, 42]. Glucokinase, a key enzyme in glycolysis pathway, is known to be involved in deriving energy for motility. An earlier study has shown that biotin enhances the glucokinase activity by increasing the cGMP level in hepatocytes [43]. The bioinformatics data (Supplementary Table S3) obtained in the present study supports the possible role of these proteins in sperm function and its possible interaction with biotin. The result of NMR spectroscopic study confirms that biotin interacts with both HRas and PTP1B. However, a stronger interaction was observed with HRas. Therefore, the increase in fertilization rate and developmental potential of embryos derived from spermatozoa processed with biotin seems to be mediated through the action of biotin on these two proteins present in the spermatozoa. However, further studies are necessary to confirm these findings.
In conclusion, biotin supplementation to the sperm wash medium appears to be beneficial in enhancing the in vitro sperm survival without affecting the fertilizing ability. Since biotin is an essential micronutrient, it may be a safer sperm motility enhancing agent than pentoxifylline. However, further studies are necessary to confirm that biotin can exert similar effects on development of human embryos when used in sperm preparation medium.
Electronic supplementary material
(DOCX 182 kb)
Funding information
The financial assistance from Indian Council of Medical Research (ICMR) is thankfully recognized (ICMR/5/10/10/2009-RHN).
Compliance with ethical standards
Study was approved by Institutional Ethics Committee of Kasturba Medical College, Manipal Academy of Higher Education, Manipal (IEC 087/2010), and a written informed consent was taken from subjects willing to provide their semen samples for the study. All the methods were performed in accordance with the institutional guidelines and Helsinki declaration. The study was approved by Institutional Animal Ethics Committee of Kasturba Medical College, Manipal (IAEC/KMC/40/2012). Animal care and handling were conducted according to the institutional guidelines for animal experimentation.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Irvine DS. Computer assisted semen analysis systems: sperm motility assessment. Hum Reprod. 1995;1:53–59. doi: 10.1093/humrep/10.suppl_1.53. [DOI] [PubMed] [Google Scholar]
- 2.Shulman A, Hauser R, Lipitz S, Frenkel Y, Dor J, Bider D, Mashiach S, Yogev L, Yavetz H. Sperm motility is a major determinant of pregnancy outcome following intrauterine insemination. J Assist Reprod Genet. 1998;15:381–385. doi: 10.1023/A:1022585000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnelly ET, Lewis SE, McNally JA, Thompson W. In vitro fertilization and pregnancy rates: the influence of sperm motility and morphology on IVF outcome. Fertil Steril. 1998;70:305–314. doi: 10.1016/S0015-0282(98)00146-0. [DOI] [PubMed] [Google Scholar]
- 4.Nagy ZP, Joris H, Verheyen G, Tournaye H, Devroey P, Van Steirteghem AC, Correlation between motility of testicular spermatozoa, testicular morphology and the outcome of intracytoplasmic sperm injection. Hum Reprod 1998; 13:890–895. [DOI] [PubMed]
- 5.Oehninger S, Sueldo C, Lanzendorf S, Mahony M, Burkman LJ, Alexander NJ, Hodgen GD. A sequential analysis of the effect of progesterone on specific sperm functions crucial to fertilization in vitro in infertile patients. Hum Reprod. 1994;9:1322–1327. doi: 10.1093/oxfordjournals.humrep.a138702. [DOI] [PubMed] [Google Scholar]
- 6.Yanagimachi R. In vitro capacitation of hamster spermatozoa by follicular fluid. J Reprod Fertil. 1969;18:275–286. doi: 10.1530/jrf.0.0180275. [DOI] [PubMed] [Google Scholar]
- 7.Kalthur G, Kumar P, Adiga SK. Enhancement in motility of sperm co-incubated with cumulus oocyte complex (COC) in vitro. Eur J Obstet Gynecol Reprod Biol. 2009;145:167–171. doi: 10.1016/j.ejogrb.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Schill WB. Caffeine- and kallikrein-induced stimulation of human sperm motility: a comparative study. Andrologia. 1975;7:229–236. doi: 10.1111/j.1439-0272.1975.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 9.Yovich JM, Edirisinghe WR, Cummins JM, Yovich JL. Preliminary results using pentoxifylline in a pronuclear stage tubal transfer (PROST) program for severe male factor infertility. Fertil Steril. 1988;50:179–181. doi: 10.1016/S0015-0282(16)60030-4. [DOI] [PubMed] [Google Scholar]
- 10.Yovich JM, Edirisinghe WR, Cummins JM, Yovich JL. Influence of pentoxifylline in severe male factor infertility. Fertil Steril. 1990;53:715–722. doi: 10.1016/S0015-0282(16)53470-0. [DOI] [PubMed] [Google Scholar]
- 11.Tasdemir M, Tasdemir I, Kodama H, Tanaka T. Pentoxifylline-enhanced acrosome reaction correlates with fertilization in vitro. Hum Reprod. 1993;8:2102–2107. doi: 10.1093/oxfordjournals.humrep.a137990. [DOI] [PubMed] [Google Scholar]
- 12.Tesarik J, Mandoza C. Sperm treatment with pentoxifylline improves the fertilizing ability in patients with acrosome reaction insufficiency. Fertil Steril. 1993;60:141–148. doi: 10.1016/S0015-0282(16)56052-X. [DOI] [PubMed] [Google Scholar]
- 13.Tournaye H, Janssens R, Camus M, Staessen C, Devroey P, Van Steirteghem A. Pentoxifylline is not useful in enhancing sperm function in cases with previous in vitro fertilization failure. Fertil Steril 1993; 59:210–215. [DOI] [PubMed]
- 14.Tournaye H, Janssens R, Devroey P, Van Steirteghem A. The influence of pentoxifylline on motility and viability of spermatozoa from normozoospermic semen samples. Int J Androl 1994; 17:1–8. [DOI] [PubMed]
- 15.Dimitriadou F, Rizos D, Mantzavinos T, Arvaniti K, Voutsina K, Prapa A, Kanakas N. The effect of pentoxifylline on sperm motility, oocyte fertilization, embryo quality, and pregnancy outcome in an in vitro fertilization program. Fertil Steril. 1995;63:880–886. doi: 10.1016/S0015-0282(16)57497-4. [DOI] [PubMed] [Google Scholar]
- 16.Tesarik J, Mendoza C, Carreras A. Effects of phopshodiesterase inhibitors caffeine and pentoxifylline on spontaneous and stimulus-induced acrosome reaction in human sperm. Fertil Steril. 1992;58:1185–1190. doi: 10.1016/S0015-0282(16)55567-8. [DOI] [PubMed] [Google Scholar]
- 17.Tournaye H, Janssens R, Verheyen G, Devroey P, Van Steirteghem A. An indiscriminate use of pentoxifylline does not improve in-vitro fertilization in poor fertilizers. Hum Reprod 1994; 9:1289–1292. [DOI] [PubMed]
- 18.Lacham-Kaplan O, Trounson AO. Embryo development capacity of oocytes fertilized by immature sperm and sperm treated with motility stimulants. Reprod Fertil Dev. 1994;6:113–116. doi: 10.1071/RD9940113. [DOI] [PubMed] [Google Scholar]
- 19.Scott L, Smith S. Human sperm motility-enhancing agents have detrimental effects on mouse oocytes and embryos. Fertil Steril. 1995;63:166–175. doi: 10.1016/S0015-0282(16)57313-0. [DOI] [PubMed] [Google Scholar]
- 20.Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J Nutr. 2003;133:2519–2525. doi: 10.1093/jn/133.8.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe T, Dakshinamurti K, Persaud TV. Biotin influences palatal development of mouse embryos in organ culture. J Nutr. 1995;125:2114–2121. doi: 10.1093/jn/125.8.2114. [DOI] [PubMed] [Google Scholar]
- 22.Kalthur G, Salian SR, Keyvanifard F, Sreedharan S, Thomas JS, Kumar P, Adiga SK. Supplementation of biotin to sperm preparation medium increases the motility and longevity in cryopreserved human spermatozoa. J Assist Reprod Genet. 2012;29:631–635. doi: 10.1007/s10815-012-9760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu DY, Baker HW. Calcium ionophore-induced acrosome reaction correlates with fertilization rates in vitro in patients with teratozoospermic semen. Hum Reprod. 1998;13:905–910. doi: 10.1093/humrep/13.4.905. [DOI] [PubMed] [Google Scholar]
- 24.Kotdawala AP, Kumar S, Salian SR, Thankachan P, Govindraj K, Kumar P, Kalthur G, Adiga SK. Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function. J Assist Reprod Genet. 2012;29:1447–1453. doi: 10.1007/s10815-012-9894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalthur G, Salian SR, Nair R, Mathew J, Adiga SK, Kalthur SG, Zeegers D, Hande MP. Distribution pattern of cytoplasmic organelles, spindle integrity, oxidative stress, octamer-binding transcription factor 4 (Oct4) expression and developmental potential of oocytes following multiple superovulation. Reprod Fertil Dev. 2016;28:2027–2038. doi: 10.1071/RD15184. [DOI] [PubMed] [Google Scholar]
- 26.Fielding L. NMR methods for the determination of protein-ligand dissociation constants. Curr Top Med Chem. 2003;3:39–53. doi: 10.2174/1568026033392705. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig C, Guenther UL. Ligand based NMR methods for drug delivery. Front Biosci. 2009;14:4565–4574. doi: 10.2741/3549. [DOI] [PubMed] [Google Scholar]
- 28.Cala O, Guillière F, Krimm I. NMR-based analysis of protein–ligand interactions. Anal Bioanal Chem. 2014;406:943–956. doi: 10.1007/s00216-013-6931-0. [DOI] [PubMed] [Google Scholar]
- 29.Bakkialakshmi S, Chandrakala D. A spectroscopic investigations of anticancer drugs binding to bovine serum albumin. Spectrochim Acta Mol Biomol Spectrosc. 2012;88:2–9. doi: 10.1016/j.saa.2011.10.076. [DOI] [PubMed] [Google Scholar]
- 30.Tesarik J, Thebault A, Testart J. Effect of pentoxifylline on sperm movement characteristics in normozoospermic and asthenozoospermic specimens. Hum Reprod. 1992;7:1257–1263. doi: 10.1093/oxfordjournals.humrep.a137837. [DOI] [PubMed] [Google Scholar]
- 31.Coccia ME, Becattini C, Criscuoli L, Fuzzi B, Scarselli G. A sperm survival test and in-vitro fertilization outcome in the presence of male factor infertility. Hum Reprod. 1997;12:1969–1973. doi: 10.1093/humrep/12.9.1969. [DOI] [PubMed] [Google Scholar]
- 32.Tournaye H, Van der Linden M, Van den Abbeel E, Devroey P, Van Steirteghem A. The effect of pentoxifylline on mouse in-vitro fertilization and early embryonic development. Hum Reprod 1994; 9:1903–1908. [DOI] [PubMed]
- 33.Calogero AE, Fishel S, Hall J, Ferrara E, Vicari E, Green S, Hunter A, Burrello N, Thornton S, D’Agata R. Correlation between intracellular cAMP content, kinematic parameters and hyperactivation of human spermatozoa after incubation with pentoxifylline. Hum Reprod. 1998;13:911–915. doi: 10.1093/humrep/13.4.911. [DOI] [PubMed] [Google Scholar]
- 34.Jayaprakash D, Kumar KS, Shivaji S, Seshagiri PB. Pentoxifylline induces hyperactivation and acrosome reaction in spermatozoa of golden hamsters: changes in motility kinematics. Hum Reprod. 1997;12:2192–2199. doi: 10.1093/humrep/12.10.2192. [DOI] [PubMed] [Google Scholar]
- 35.Rupasri R, Jayaprakash D, Peter AT, Sreenivasa MS, Kumar M, Seshagiri PB. Pentoxifylline improves sperm capacitation and in vitro fertilization of oocytes in the golden hamster. Theriogenol. 1995;44:553–562. doi: 10.1016/0093-691X(95)00226-X. [DOI] [PubMed] [Google Scholar]
- 36.Zempleni J, Teixeira DC, Kuroishi T, Cordonier EL, Baier S. Biotin requirements for DNA damage prevention. Mutat Res. 2012;733:58–60. doi: 10.1016/j.mrfmmm.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dakshinamurti K. Biotin—a regulator of gene expression. J Nutr Biochem. 2005;16:419–423. doi: 10.1016/j.jnutbio.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Barbacid M. Ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 39.Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Oncogenes and signal transduction. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-G. [DOI] [PubMed] [Google Scholar]
- 40.Naz RK, Ahmad K, Kaplan P. Expression and function of ras proto-oncogene proteins in human sperm cells. J Cell Sci. 1992;102:487–494. doi: 10.1242/jcs.102.3.487. [DOI] [PubMed] [Google Scholar]
- 41.Shi L, Zhang Q, Xu B, Jiang X, Dia Y, Zhang CY, Zen K. Sustained high protein-tyrosine phosphatase 1B activity in the sperm of obese males impairs the sperm acrosome reaction. J Biol Chem. 2014;289:8432–8441. doi: 10.1074/jbc.M113.517466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruete MC, Lucchesi O, Bustos MA, Tomes CN. Epac, Rap and Rab3 act in concert to mobilize calcium from sperm’s acrosome during exocytosis. Cell Commun Signal. 2014;12:43. doi: 10.1186/s12964-014-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez-Novell JM, Ballester J, Medrano A, Otaegui PJ, Guinovart JJ, Rodriguez-Hil JE. The presence of a high-Km hexokinase activity in dog, but not in boar, sperm. FEBS Lett. 2004;570:211–216. doi: 10.1016/j.febslet.2004.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 182 kb)