Abstract
Under physiological conditions, a finely tuned system of cellular adaptation allows the intestinal mucosa to maintain the gut barrier function while avoiding excessive immune responses to non-self-antigens from dietary origin or from commensal microbes. This homeostatic function is compromised in cystic fibrosis (CF) due to loss-of-function mutations in the CF transmembrane conductance regulator (CFTR). Recently, we reported that mice bearing defective CFTR are abnormally susceptible to a celiac disease-like enteropathy, in thus far that oral challenge with the gluten derivative gliadin elicits an inflammatory response. However, the mechanisms through which CFTR malfunction drives such an exaggerated response to dietary protein remains elusive. Here we demonstrate that the proteostasis regulator/transglutaminase 2 (TGM2) inhibitor cysteamine restores reduced Beclin 1 (BECN1) protein levels in mice bearing cysteamine-rescuable F508del-CFTR mutant, either in homozygosis or in compound heterozygosis with a null allele, but not in knock-out CFTR mice. When cysteamine restored BECN1 expression, autophagy was increased and gliadin-induced inflammation was reduced. The beneficial effects of cysteamine on F508del-CFTR mice were lost when these mice were backcrossed into a Becn1 haploinsufficient/autophagy-deficient background. Conversely, the transfection-enforced expression of BECN1 in human intestinal epithelial Caco-2 cells mitigated the pro-inflammatory cellular stress response elicited by the gliadin-derived P31–43 peptide. In conclusion, our data provide the proof-of-concept that autophagy stimulation may mitigate the intestinal malfunction of CF patients.
Introduction
Cystic fibrosis (CF) is the most frequent monogenic lethal disease affecting more than 85,000 subjects worldwide1–4. CF is caused by loss-of-function mutations in the gene coding for the cystic fibrosis transmembrane conductance regulator (CFTR)5,6, a protein with 1480 amino acid residues that belongs to the ABC transport family and functions as a cyclic AMP-regulated anion channel. CFTR is expressed in, and is relevant to the function of, many tissues, including airways, small and large intestine, pancreas, biliary tree, male reproductive tract and sweat glands3,7, but it is also expressed in central nervous system, leukocytes, smooth muscle and cartilage of the large airways7. Approximately 2000 mutations have been identified in the CFTR gene and are categorized in 6 classes according to their impact on the synthesis (class I), processing (class II), gating (class III), conductance (class IV), quantity (class V) and recycling (class VI) of the CFTR protein8–11. Among, these mutations, the clinically most important one is the F508del-CFTR mutation (class II), which accounts for 70–90% of CFTR cases.
CF is best known for its respiratory phenotype, as the abnormal anion transport results in increased mucin polymer cross-links and mucus viscosity12–14, leading to accumulation of thick, sticky mucus in the lung. These events cause chronic inflammation, persistent and untreatable bacterial colonization and recurrent chest infections, mostly by Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia15. Chronic infection and inflammation ultimately lead to progressive lung disease with bronchiectasis and tissue destruction, culminating in respiratory insufficiency15. Defective CFTR function also frequently leads to intestinal problems16,17, including intestinal obstruction as well as an exaggerated immune response to dietary antigens18–20. Indeed, a constitutive inflammation at both airway and intestinal mucosa, is a feature of CF17,19,20. Moreover, CF patients often show serum antibodies against dietary antigens18,20.
Beyond its function as an ion channel16, CFTR orchestrates proteostasis at respiratory and intestinal epithelial surfaces, thus regulating adaptation to cell-autonomous or external stress21–24. CFTR malfunction causes a maladaptive epithelial stress response with increased generation of reactive oxygen species (ROS) which oxidize and activate tissue transglutaminase (TGM2)25,26. Activated TGM2 targets several substrates, among which Beclin 1 (BECN1), a major pro-autophagic protein that acts as an allosteric activator of phosphatidylinositol 3-kinase catalytic subunit type 3 (PIK3C3)21–23. Transamidation of BECN1 by TGM2 dislodges the BECN1 interactome away from the endoplasmic reticulum (ER), resulting in the functional sequestration of PIK3C3 into intracellular aggregates. This causes inhibition of autophagy, accumulation of the autophagic substrate sequestosome 1 (SQSTM1) and reduced availability of the PIK3C3 product phosphatidyl-inositol-3-phosphate (PtdIns3P) at early endosomes that impairs endosomal maturation and trafficking21,22. Mismanaged proteostasis in epithelial cells consequent to CFTR malfunction also leads to TGM2-mediated crosslinking of the anti-inflammatory peroxisome-proliferator-activated-receptor-γ (PPARγ), as well as increased nuclear translocation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) owing to TGM2 targeting of NF-κB inhibitor alpha (NFKBIA) within histone-deacetylase 6 (HDAC6)+/vimentin+ intracellular aggresomes21,23,25,26. NF-κB activation results in increased levels of pro-inflammatory cytokines, among which interleukin (IL)-17A, IL-21 and IL-15, a master cytokine involved in gut homeostasis18,23,27–29 as well as IL-1β18.
Importantly, CFTR malfunction, TG2 activation and autophagy deficiency are engaged in a self-amplifying feed-forward loop. For this reason, inhibition of TGM2 by cysteamine is sufficient to restore autophagy and to favor the expression of functional CFTR at the epithelial surface. Indeed, treatment of neonatal mice bearing the F508del-CFTR mutation with cysteamine can prevent intestinal obstruction30. Moreover, cysteamine efficiently restores CFTR function and reduces lung inflammation in patients carrying at least one class II CFTR mutation30,31. A combination of two proteostasis regulators, cysteamine and the autophagy inducer epigallocatechin gallate (EGCG), was particularly efficient in restoring the expression and function of the mutant F508del-CFTR protein11,31.
CF patients exhibit a three-fold increase in the prevalence of celiac disease (CD)18,32,33 an extremely frequent permanent intolerance to gluten/gliadin proteins that occurs in a proportion of susceptible individuals bearing the human leukocyte antigen (HLA) DQ2/DQ834–36. Accordingly, CFTR defective mice exhibit an increased susceptibility to the enteropathogenic effects of gliadin, a common dietary protein present in gluten from wheat, rye, and barley18. Gliadin inhibited the function of CFTR in human enterocytes and mouse models of CD through a direct molecular interaction involving a specific gliadin-derived peptide (P31–43) with the nucleotide-binding domain-1 (NBD1) of CFTR18. Indeed, the effects of gliadin on enterocyte proteostasis are reminiscent of those observed in CFTR defective mice. Given the pivotal role of BECN1 and autophagy in orchestrating proteostasis in CF epithelia, we investigated whether the increased responsiveness to gliadin in CF mice may be due to defective autophagy and whether re-establishing BECN1 levels and autophagy by means of cysteamine would protect the CF intestine against the detrimental effects of gliadin.
Results
Cysteamine restores CFTR function in CftrF508del mice after gliadin challenge
Cysteamine is reportedly effective in rescuing CFTR at the intestinal epithelial surface of mice homozygous for the F508del-CFTR mutation30,31. To investigate whether rescuing CFTR function by means of cysteamine would abrogate the pathogenic response to gliadin, we orally administered cysteamine for 5 consecutive days (60 µg/kg in 100 µl saline/day) to knock-in mice harboring the most common loss-of-function F508del-CFTR mutation (Cftrtm1EUR, F508del, FVB/129, CftrF508del/F508del), CFTR knock-out mice (B6.129P2-KOCftrtm1UNC, Cftr−/−), knock-in mice harbouring one F508del-CFTR allele in combination with one null-CFTR allele (CftrF508del/−) or their wild-type (WT) littermates (FVB/129 or B6.129P2). After or without cysteamine pre-treatment (60 µg/kg in 100 µl saline/day), mice were orally challenged with gliadin (5 mg/daily for 1 week and then 5 mg/daily thrice a week for 3 weeks) or vehicle for the following 4 weeks (n = 10 per group of treatment), according to established procedures18.
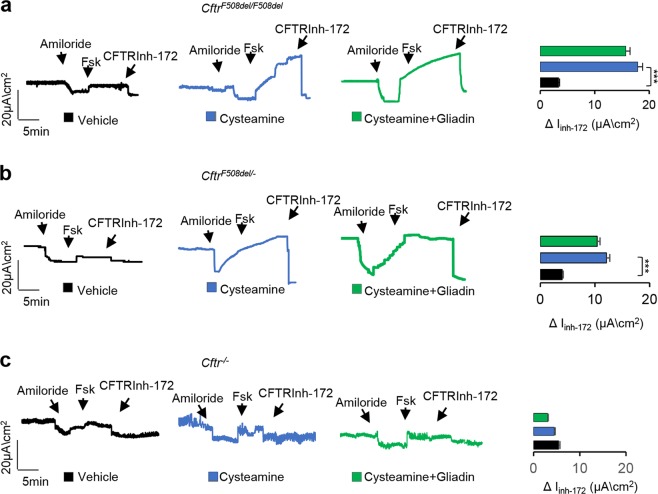
Since gliadin is capable of impairing CFTR function in gliadin-sensitive CFTR-sufficient mice18, we first assessed whether the cysteamine-mediated rescue of F508del-CFTR function would persist after gliadin administration. To this aim, the small intestine from CftrF508del/F508del mice exposed to cysteamine and/or gliadin (n = 10 per group of treatment) were mounted in Ussing chambers and CFTR function was assessed as the forskolin (Fsk)-induced increase of chloride current (Isc (μA/cm2)). In CftrF508del/F508del and CftrF508del/− mice, the 5-day pre-treatment with cysteamine enhanced intestinal CFTR function, as expected31. This CFTR rescuing ability of cysteamine was not compromised by gliadin challenge (Fig. 1a, b). Of note, cysteamine restored CFTR function in gliadin-treated or vehicle-treated CftrF508del/F508del and CftrF508del/− mice, whereas it failed to do so in Cftr−/− mice (Fig. 1c), in line with the idea that the positive effect of cysteamine requires the presence of ‘rescuable’ F508del-CFTR protein.
Fig. 1. Cysteamine restores CFTR function in CftrF508del mice after gliadin challenge.
a CftrF508del/F508del b CftrF508del/−, and c Cftr−/− mice orally treated with vehicle or cysteamine (60 µg/kg in 100 µl saline/day for 5 days) and then challenged with gliadin for consecutive 4 weeks (5 mg/daily for 1 week and then 5 mg/daily thrice a week for 3 weeks) in the presence or absence of cysteamine (60 µg/kg in 100 µl saline/day) (n = 10 mice per group of treatment). The CFTR-dependent Cl− secretion was measured by forskolin-induced (Fsk) increase of the chloride current (Isc (μA/cm2) in small intestines mounted in Ussing chambers; quantification of the peak CFTR Inhibitor 172 (CFTRinh172)-sensitive Isc (∆Isc). ***p < 0.001 versus cysteamine (ANOVA, Bonferroni post hoc test)
Cysteamine protects CftrF508del mice from the effects of gliadin in vivo
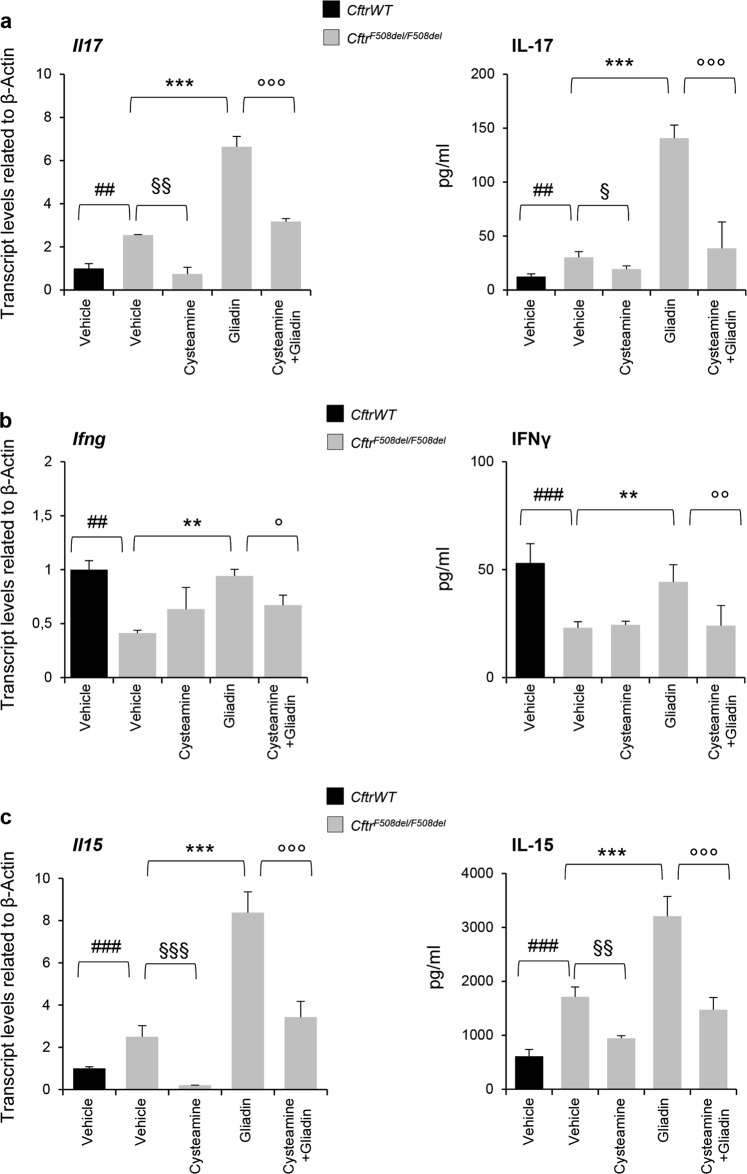
Next, we investigated whether cysteamine would control the increased mucosal immune response that occurred in gliadin exposed CftrF508del/F508del mice. To this aim, we measured the levels of proinflammatory cytokines in small intestine homogenates from mice fed with gliadin for 4 weeks in the presence or absence of cysteamine. Cysteamine was effective in preventing the increased production of IL-17A and IFN-γ induced by gliadin (p < 0.01 and p < 0.001) (Fig. 2a, b). In addition, cysteamine controlled the production of IL-15, a master pro-inflammatory cytokine pivotal for driving the gliadin-induced enteropathy37–41 (Fig. 2c). Indeed, IL-15 is constitutively upregulated in mouse CF intestine and is significantly induced by oral gliadin challenge18. Again, the anti-inflammatory effect of cysteamine against gliadin-induced cytokine producton was observed in CftrF508del/F508del, CftrF508del/− but not in Cftr−/− mice (Supplementary Figure 1), supporting the hypothesis that cysteamine controls the gliadin-induced inflammation through restoring F508del-CFTR function.
Fig. 2. Cysteamine protects CftrF508del mice from the effects of gliadin in vivo.
a IL-17A, b IFN-γ, and c IL-15 mRNA (left) and protein (right) levels in small intestine homogenates from CftrF508del/F508del or their CftrWT littermates treated with vehicle or cysteamine (60 µg/kg in 100 µl saline/day for 5 days) and then challenged with gliadin for consecutive 4 weeks (5 mg/daily for 1 week and then 5 mg/daily thrice a week for 3 weeks) in the presence or absence of cysteamine (60 µg/kg in 100 µl saline/day) (n = 10 per group). Means ± SD of pooled samples assayed in triplicates. ##p < 0.01 or ### p < 0.001 CftrWT versus CftrF508del/F508del; §p < 0.05 or §§p < 0.01 or §§§p < 0.001 versus cysteamine treatment; **p < 0.01, ***p < 0.001 versus gliadin challenge; °p < 0.05 or °°p < 0.01 or °°°p < 0.001 versus cysteamine + gliadin (ANOVA, Bonferroni post hoc test)
Cysteamine protects CftrF508del mice in vivo from the increased responsiveness to gliadin through restoring BECN1 and autophagy
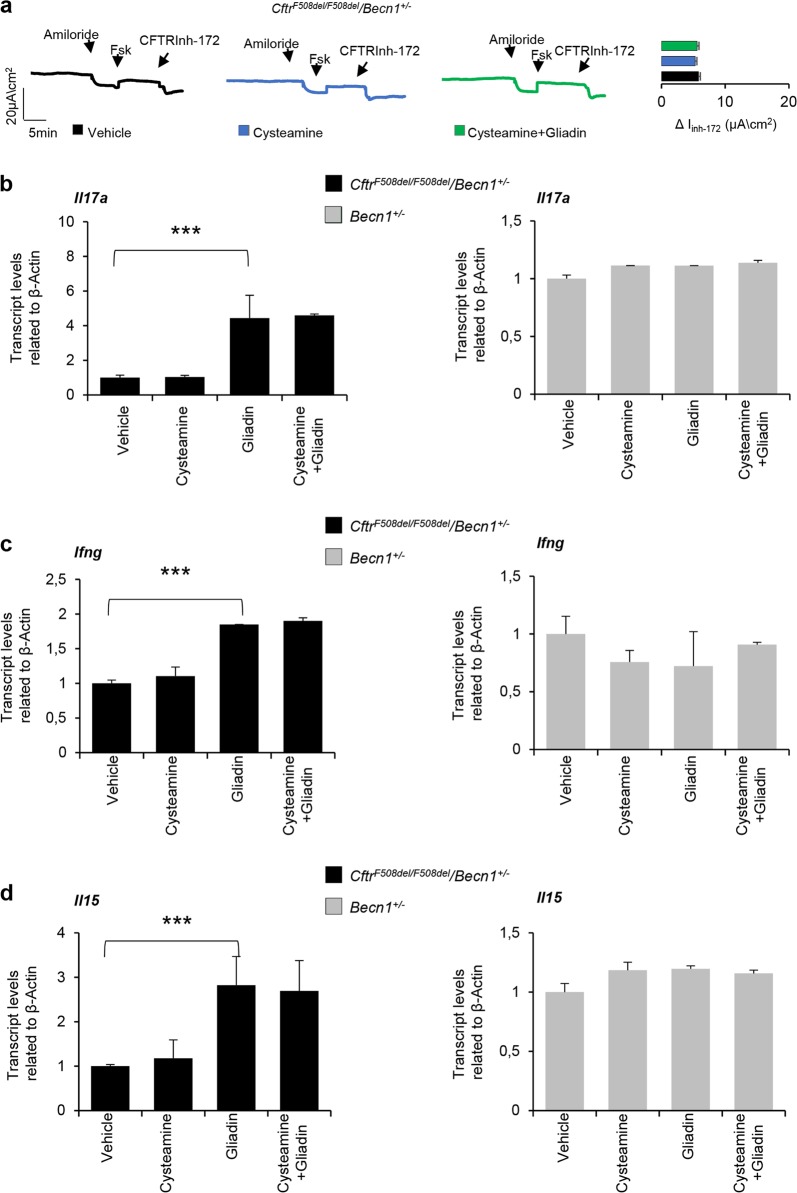
We previously reported that the inhibition of TGM2 with the subsequent restoration of BECN1 protein levels and autophagy are pivotal for allowing cysteamine to rescue F508del-CFTR at the epithelial surface11,18,21,31. For this reason, we investigated whether the protective effects of cysteamine against gliadin induced immune activation would be linked to its capacity to restore autophagy. To this aim, CftrF508del/F508del mice were backcrossed into a Becn1 haploinsufficient background (to generate CftrF508del/F508del/Becn1+/− mice)31, and gliadin was orally administered upon optional pretreatment with cysteamine. Cysteamine was unable to restore the function of the intestinal CFTR (determined in Ussing chambers) from CftrF508del/F508del/Becn1+/− mice, either before or after gliadin challenge (Fig. 3a). Gliadin triggered an inflammatory response in CftrF508del/F508del/Becn1+/− mice, similarly to that observed in CftrF508del/F508del mice (Fig. 3b–d). Of note, cysteamine failed to mitigate the gliadin-elicited production of IL-15, IL-17A, and IFN-γ in CftrF508del/F508del/Becn1+/− mice (Fig. 3b–d). In conclusion, it appears that the haploinsufficiency of Becn1 (genotype: Becn1+/−) abrogates the anti-inflammatory effects of cysteamine that is normally seen in CftrF508del/F508del mice.
Fig. 3. Cysteamine protects CftrF508del mice in vivo from the increased responsiveness to gliadin through restoring BECN1 and autophagy.
a CftrF508del/F508del/Becn1+/− mice treated with cysteamine (60 µg/kg in 100 µl saline/day for 5 days) and then challenged with gliadin for consecutive 4 weeks (5 mg/daily for 1 week and then 5 mg/daily thrice a week for 3 weeks) in the presence or absence of cysteamine (60 µg/kg in 100 µl saline/day) (n = 10 mice per group of treatment). Assessment of CFTR-dependent Cl− secretion measured by forskolin-induced (Fsk) increase of the chloride current (Isc (μA/cm2) in small intestines mounted in Ussing chambers; quantification of the peak CFTR Inhibitor 172 (CFTRinh172)-sensitive Isc (∆Isc). b IL-17A, c IFN-γ, and d IL-15 mRNA levels in small intestine homogenates from CftrF508del/F508del/Becn1+/− (left) or Becn1+/− (right) mice treated with vehicle or cysteamine (60 µg/kg in 100 µl saline/day for 5 days) and then challenged with gliadin for consecutive 4 weeks (5 mg/daily for 1 week and then 5 mg/daily thrice a week for 3 weeks) in the presence or absence of cysteamine (60 µg/kg in 100 µl saline/day) (n = 10 per group of treatment). Means ± SD of pooled samples assayed in triplicates. ***p < 0.001 versus gliadin challenge; (ANOVA, Bonferroni post hoc test)
Restoring BECN1 protects intestinal epithelial cells from the detrimental effects of gliadin
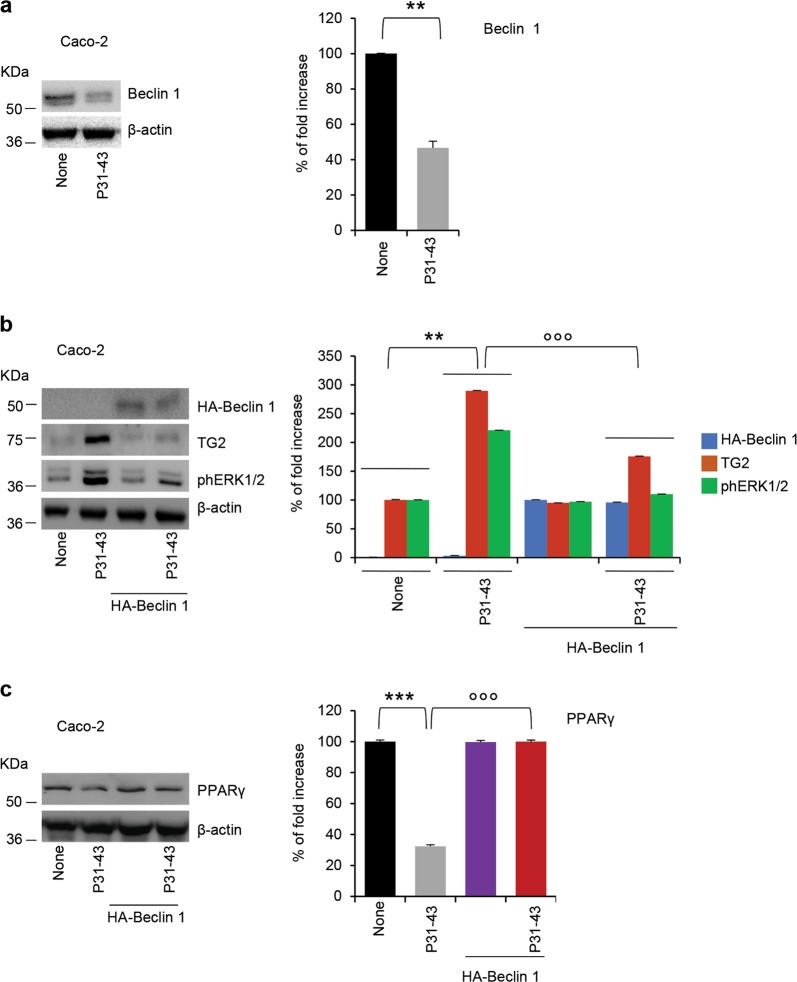
To complete our demonstration that BECN1 and autophagy are crucial for the inflammatory effects of gliadin in intestinal epithelial cells, we resorted to human intestinal epithelial Caco-2 cells, which are reportedly sensitive to gliadin or gliadin-derived peptides40. When confluent Caco-2 cells were challenged for 3 h with a peptic-tryptic digest of gliadin from bread wheat (PT gliadin; 500 μg/ml)40,41 or the gliadin-derived peptide LGQQQPFPPQQPY (P31–43), CFTR function is inhibited18. Moreover, gliadin treatment of Caco-2 cells caused a reduction in BECN1 protein levels, as compared to unchallenged controls (Fig. 4a). Next, we transfected Caco-2 cells with HA-Beclin 1 and challenged them for 3 h with gliadin or P31–43. Of note, the enforced expression of BECN1 (which enhances the generation of autophagosomes and autophagy21), prevented signs of gliadin-induced inflammation, as it avoided the upregulation of TGM2, the activating phosphorylation of ERK 1/2 and the downregulation of PPARγ that were induced by gliadin (Fig. 4b, c). These results suggest that BECN1 plays an active role in mitigating the gliadin-induced inflammatory response of intestinal epithelial cells.
Fig. 4. Restoring BECN1 protects intestinal epithelial cells from the detrimental effects of gliadin peptides.
a Caco-2 cells treated with gliadin-derived P31–43 peptide or with vehicle for 3 h. Immunoblot with anti-Beclin 1 or anti-β-actin (left), as loading control, in whole lysates and relative densitometric analysis (right) of immunoblot. Means ± SD of pooled samples assayed in triplicates; **p < 0.01 versus P31–43 (Student’s t test). b, c Caco-2 cells transfected with HA-Beclin 1 and then challenged for 3 h with P31–43. b Immunoblot of TGM2, phospho-ERK 1/2 (phERK 1/2) and with anti-HA tag for transfection control. Densitometric analysis of protein levels relative to β-actin (right). Means ± SD of pooled samples assayed in triplicates; **p < 0.01 versus P31–43, °°°p < 0.001 versus HA-Beclin1 + P31–43 (ANOVA, Bonferroni post hoc test). c Immunoblot of PPARγ (left) and densitometric analysis of protein levels relative to β-actin (right). Means ± SD of pooled samples assayed in triplicates; ***p < 0.001 versus P31–43, °°°p < 0.001 versus HA-Beclin1 + P31–43 (ANOVA, Bonferroni post hoc test)
Discussion
The proteostasis network is a system of cellular adaptation to endogenous or environmental stress. Mismanaged proteostasis contributes to a number of diseases arising as a result of inherited or stress-induced defects in protein conformation42. Autophagy is a major player of the proteostasis network as it regulates the turnover of large protein aggregates and even entire organelles. In addition, several components of the autophagy machinery dynamically interact with multiple signalling pathways to ensure intracellular homeostasis43–45. CF is the quintessential example of a disease characterized by major alterations of the proteostasis network11,21,23,46. Defective CFTR function highly compromises the capacity of cells to adequately respond to endogenous stress signals as well as to external challenges arising within the respiratory and gastrointestinal tracts11.
The intestine from CF patients is exposed to a particularly high antigenic load due to the frequent insufficiency of the exocrine pancreas17. Moreover, the local overactivation of the innate immune system compromises the handling of dietary molecules, thus favouring inadequate cellular and humoral immune responses to food components. Accordingly, CF patients often exhibit increased levels of antibodies against alimentary antigens, including anti-gliadin IgA antibodies, shifts in the intestinal microbiota, elevated fecal calprotectin levels and increased intestinal permeability17,20,47. Moreover, the prevalence of autoantibodies against TGM2 is four times higher than in the general population, even in the absence of histological evidence of intestinal lesions18,32,33. Indeed, when CFTR is disabled, the intracellular milieu undergoes major pathogenic changes. CFTR inhibition results in a ROS-mediated increase in the abundance and activity of TGM225,26 with consequent functional sequestration of BECN1 complex and inhibition of autophagy21–23. Thus, CFTR inhibition, TGM2 activation and BECN1 inactivation act in concert to compromise proteostasis in the small intestine of CF mice, driving constitutive pro-inflammatory reactions that involves the activation of the NF-κB pathway and the NLRP3 inflammasome.
Here we demonstrate that functional BECN1 and autophagy are required to prevent the increased susceptibility of CF intestine to the gluten component gliadin. Indeed, the proteostasis regulator cysteamine was capable of reducing the pro-inflammatory effects of gliadin in mice bearing the most common F508del-CFTR mutant, either in homozygosity or in compound heterozygosity. Apparently, cysteamine abrogates the susceptibility of mice to oral gliadin challenge by acting as TGM2 inhibitor, thus preventing the BECN1 sequestration and autophagy impairement that normally result from CFTR inhibition. However, cysteamine fails to prevent gliadin-induced inflammation in CFTR KO mice, meaning that its effects are mediated by its ability to rescue CFTR function, as reported23,31. Importantly, the beneficial effects of cysteamine on F508del-CFTR mice are lost when these mice are backcrossed in an autophagy-deficient (Becn1 haploinsufficient mice) background, indicating that the restoration of BECN1 levels and autophagy are indeed required to avoid the enteropathic effects of gliadin.
In aggregate, stimulating autophagy might represent a novel option to prevent intestinal manifestations of CF. In favor of this notion, it appears that enforced expression of BECN1 in gliadin-sensitive human intestinal epithelial cells18,40, effectively opposes the capability of the gliadin-derived P31–43 peptide18,40,41 to induce an epithelial stress response. In this perspective, and in line with the evidence that the best option to stimulate autophagy is to interfere with the function of its endogenous inhibitors48, it might be attempted to neutralize BECN1 inhibitory proteins. Druggable endogenous BECN1 antagonist include proteins from the BCL2 family (which can be targeted with so-called BH3 mimetics including ABT737, navitoclax, and venetoclax)49, the mechanistic target of rapamycin complex-1 (mTORC1) (which are inhibited by rapamycin, everolimus or tacrolimus)48, as well as the acetyltransferase EP300 (which is inhibited by aspirin, epigallocatechine gallate, or spermidine)31,46,50,51. Moreover, there is the option to directly inhibit TGM2 by cysteamine31,52 and to combine cysteamine with other autophagy stimulators such as EGCG31.
In conclusion, our data highlight the implication of CFTR in the suppression of diet-induced inflammation. CFTR may be viewed as a major stress sensor that alerts the autophagy machinery when a potentially harmful perturbation risks to perturb mucosal homeostasis. Once activated, autophagy then orchestrates the proper handling of luminal triggers by the intestinal mucosa. Pharmacological autophagy enhancement may be harnessed to prevent intestinal inflammation and to improve the nutritional status of CF patients.
Materials and methods
Cells and treatments
Human colon adenocarcinoma-derived Caco-2 were obtained from the ATCC. Cells were maintained in T25 flask in Modified Eagle Medium (MEM) supplemented with 10% fetal bovine serum (FBS), 2 mM Glutamine + 1% Non-Essential-Amino-Acids (NEAA) and the antibiotics penicillin/streptomycin (100 units/ml) (all reagents from Lonza)18. Cells were treated with 20 µg/ml of α-gliadin peptide P31–43 for 3 h synthesized by Inbios (Napoli, Italy). Cells were also treated with Pepsin-trypsin-gliadin (PT-gliadin) (500 μg/ml)40,41,53. Caco-2 cells were also transfected with HA-beclin 1 and then treated with α-gliadin peptide P31–43 for 3 h.
Plasmids and transfection
The pcDNA3-HA–beclin 1 expression vector (a gift from N. Mizushima) was used for transfection experiments. Cells were transfected with pcDNA3-HA–beclin 1 by means of Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s instructions.
Mice and treatments
CF mice homozygous for the F508del-CFTR in the FVB/129 outbred background (Cftrtm1EUR, F508del, FVB/129, abbreviated CftrF508del/F508del) were obtained from Bob Scholte, Erasmus Medical CenterRotterdam, The Netherlands, CF coordinated action program EU FP6LSHMCT-2005-018932.50. Transgenic KO Cftr mice (B6.129P2-KOCftrtm1UNC, abbreviated Cftr−/−), were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The heterozygous CftrF508del/+ males were backcrossed with heterozygous Cftr+/− females to obtain F508del/null CFTR heterozygous mice (abbreviated CftrF508del/−).
Female CftrF508del/+ mice were backcrossed to the C57BL/6J background Becn1+/– male mice (generous gift from Beth Levine, Center for Autophagy Research, Department of Internal Medicine, UT Southwestern Medical Center, Dallas, USA and Francesco Cecconi, University of Tor Vergata, Rome, Italy) to obtain at the first generation Becn1 haplo-insufficient F508del heterozygous mice (abbreviated CftrF508del/+/Becn1+/−). These CftrF508del/+/Becn1+/− mice were crossbred to obtain Becn1 haplo-insufficient F508del homozygous mice (abbreviated CftrF508del/F508del/Becn1+/−). The newly generated CftrF508del/− and the CftrF508del/F508del/Becn1+/– were housed at the San Raffaele Scientific Institute SOPF animal house (Milan, Italy).
Mice were challenged with vehicle alone or cysteamine (60 µg/kg in 100 µl saline/day) for 5 days. Mice were also challenged via gavage for 4 weeks with vehicle alone or gliadin (Sigma-Aldrich, G3375) (5 mg/daily for one week and then 5 mg/daily thrice a week for 3 weeks)18 in the presence or absence of cysteamine (60 µg/kg in 100 µl saline/day) challenge, (n = 10 mice per group of treatment).
Mice were anesthetized with Avertine (tribromoethanol, 250 mg/kg, Sigma Aldrich, Milan, Italy, T48402) and then killed and small intestines were collected. These studies and procedures were approved by the local Ethics Committee for Animal Welfare (IACUC No 849, 713) in compliance with European Community regulations for animal use in research (2010/63 UE).
Ussing chamber
Chambers for mounting mouse tissue biopsies were obtained from Physiologic Instruments (model P2300, San Diego, CA, USA). Chamber solution was buffered by bubbling with identical Ringer solution on both sides and were maintained at 37 °C, vigorously stirred, and gassed with 95%O2/5%CO2. Tissues were short circuited using Ag/AgCl agar electrodes. A basolateral-to-apical chloride gradient was established by replacing NaCl with Na-gluconate in the apical (luminal) compartment to create a driving force for CFTR-dependent Cl− secretion. To measure stimulated Isc, the changed sodium gluconate solution, after stabilization, was supplied with 100 µM amiloride. Agonists (forskolin) were added to the bathing solutions as indicated (for a minimum 5 min of observation under each condition) to activate CFTR channels present at the apical surface of the epithelium (either cell surface or lumen side of the tissue) and CFTRInh-172 (10 µM) was added to the mucosal bathing solution to block CFTR-dependent Isc. Short-circuit current (expressed as Isc (μA/cm2)) and resistance were acquired or calculated using the VCC-600 transepithelial clamp from Physiologic Instruments and the Acquire &Analyze2∙3 software for data acquisition (Physiologic Instruments), as previously described18,54.
Real-time and reverse transcription analysis
The analysis was performed as previously described18,21,30,31,55. Total RNA was extracted with the RNeasy Mini Kit (Qiagen, 74104) from mouse intestin homogenates. The mRNA was reverse transcribed with Onetranscript plus cDNA sintesis kit (abm good). The sequences of mouse primers were:18
IL-15: forward 5′-ACCAGCCTACAGGAGGCC-3′
reverse 5′- TGAGCAGCAGGTGGAGGTAC-3′
IL-17: forward 5′-ACCGCAATGAAGACCCTGAT-3′
reverse 5′- TCCCTCCGCATTGACACA-3′
INF-ϒ: forward5′-AGAGGATGGTTTGCATCTGGGTCA-3′
reverse5′- ACAACGCTATGCAGCTTGTTCGTG-3′
Expression levels of genes were normalized to β-actin (primer design HK-sy-mo600) levels in the same samples.
Immunoblot
The whole lysate of cell lines and mice intestine homogenates were obtained from treated and untreated cells as described21–26,55. Equal amounts of protein were resolved by SDS-PAGE gel and blotted with antibodies against: PPARγ (Santa Cruz Biotechnology, sc7273) 1:500, BECN1 (Abcam, ab58878) 1:1000, HA (BD Bioscience) 1:5000, TG2 (CUB7402 Neomarker) 1:1000, phospho-ERK1/2 (phERK1/2, Cell Signaling Technology, #91101) 1:1000, Normalization was performed by probing the membrane with anti-β-actin (Cell Signaling, #4970) 1:1000.
ELISA
ELISA analysis was performed on tissue samples using standard ELISA kits (R&D Systems) for IL-15, IL-17A, INF-γ, according to the manufacturer’s instructions. Samples were read in triplicate at 450 nm in a Microplate Reader (BioRad, Milan, Italy) using Microplate Manager 5.2.1 software. Values were normalized to protein concentration evaluated by Bradford analysis.
Statistical analysis
GraphPad Prism software 6.01 (GraphPad Software) was used for analysis. Data are expressed as means ± SD. Statistical significance was calculated by ANOVA (Bonferroni’s post hoc test) for multiple comparisons and by Student’s t-test for single comparisons. We considered all P values 0.05 to be significant. The in vivo groups consisted of ten mice/group. The data reported are either representative of at least three experiments.
Supplementary information
Acknowledgements
We thank Dr Bob Scholte, Erasmus Medical Center Rotterdam, The Netherlands, who provided Cftrtm1EUR (F508del (FVB/129) mice (European Economic Community European Coordination Action for Research in Cystic Fibrosis program EU FP6 SHM-CT-2005-018932), and Dr Francesco Cecconi, University of Tor Vergata, Rome, Italy, who provided C57BL/6J background Becn1+/–mice. This study was supported by The European Institute for Research in Cystic Fibrosis (IERFC) non-profit foundation, E-Rare (Rescue CFTR preclinic) (to L.M. and G.K.; G.K. is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085 and GDW20181100051), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM) (all to G.K.). This work was supported in part by grants from AIRC (n. 15244 to M.P.), Fondazione Fibrosi Cistica (FFC#10/2018 to M.P.), Regione Lazio (E56C18000460002 to M.P.). The authors also acknowledge the support of the grant from the Russian Government Programme for the Recruitment of the leading scientists into the Russian Institutions of Higher Education 14.W03.31.0029.
Conflict of interest
V.R., L.M., and G.K. are listed as inventors on a patent application describing the use of cysteamine for the treatment of CF. G.K. is a scientific co-founder of Samsara therapeutics.
Footnotes
Edited by G. Raschellà
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally.: Valeria Raia, Guido Kroemer, Luigi Maiuri
Deceased: Luigi Maiuri
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-019-1500-x).
References
- 1.Sosnay PR, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat. Genet. 2013;45:1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopes-Pacheco M. CFTR Modulators: shedding light on precision medicine for cystic fibrosis. Front. Pharmacol. 2016;7:275. doi: 10.3389/fphar.2016.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratjen F, Döring G. Cystic fibrosis. Lancet. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 4.De Boeck K, Zolin A, Cuppens H, Olesen HV, Viviani L. The relative frequency of CFTR mutation classes in European patients with cystic fibrosis. J. Cyst. Fibros. 2014;13:403–409. doi: 10.1016/j.jcf.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Riordan JR, et al. ldentification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:166–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 6.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 7.Spielberg DR, Clancy JP. Cystic fibrosis and its management through established and emerging therapies. Annu. Rev. Genom. Hum. Genet. 2016;17:155–175. doi: 10.1146/annurev-genom-090314-050024. [DOI] [PubMed] [Google Scholar]
- 8.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinica application. Nat. Rev. Genet. 2015;16:45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quon BS, Rowe SM. New and emerging targeted therapies for cystic fibrosis. BMJ. 2016;352:i859. doi: 10.1136/bmj.i859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir. Med. 2016;4:662–674. doi: 10.1016/S2213-2600(16)00023-0. [DOI] [PubMed] [Google Scholar]
- 11.Maiuri L, Raia V, Kroemer G. Strategies for the etiological therapy of cystic fibrosis. Cell Death Differ. 2017;11:1825–1844. doi: 10.1038/cdd.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang XX, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. lnvest. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan S, et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci. Transl. Med. 2015;7:276ra27. doi: 10.1126/scitranslmed.3010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah VS, et al. Relationships among CFTR expression, HC03- secretion, and host defense may inform gene- an d cellbased cystic fibrosis therapies. Proc. Natl Acad. Sci. USA. 2016;113:5382–5387. doi: 10.1073/pnas.1604905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N. Engl. J. Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ooi CY, Durie PR. Cystic fibrosis from the gastroenterologist’s perspective. Nat. Rev. Gastroenterol. Hepatol. 2016;13:175–185. doi: 10.1038/nrgastro.2015.226. [DOI] [PubMed] [Google Scholar]
- 18.Villella VR, et al. A pathogenic role for cystic fibrosis transmembrane conductance regulator in celiac disease. EMBO J. 2019;38:e100101. doi: 10.15252/embj.2018100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Lisle RC, Borowitz D. The cystic fibrosis intestine. Cold Spring Harb. Perspect. Med. 2013;3:a009753. doi: 10.1101/cshperspect.a009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raia V, et al. Evidence of chronic inflammation in morphologically normal small intestine of cystic fibrosis patients. Pediatr. Res. 2000;47:344–350. doi: 10.1203/00006450-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Luciani A, et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 2010;12:863–875. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- 22.Villella VR, et al. Disease-relevant proteostasis regulation of cystic fibrosis transmembrane conductance regulator. Cell Death Differ. 2013;20:1101–1115. doi: 10.1038/cdd.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villella VR, et al. Targeting the intracellular environment in cystic fibrosis: restoring autophagy as a novel strategy to circumvent the CFTR defect. Front. Pharmacol. 2013;4:1–9. doi: 10.3389/fphar.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrari E, et al. Cysteamine re-establishes the clearance of Pseudomonas aeruginosa by macrophages bearing the cystic fibrosis-relevant F508del-CFTR mutation. Cell Death Dis. 2017;8:e2544. doi: 10.1038/cddis.2016.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiuri L, et al. Tissue transglutaminase activation modulates inflammation in cystic fibrosis via PPARgamma down-regulation. J. Immunol. 2008;180:7697–7705. doi: 10.4049/jimmunol.180.11.7697. [DOI] [PubMed] [Google Scholar]
- 26.Luciani A, et al. SUMOylation of tissue transglutaminase as link between oxidative stress and inflammation. J. Immunol. 2009;183:2775–2778. doi: 10.4049/jimmunol.0900993. [DOI] [PubMed] [Google Scholar]
- 27.Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr. Pulmonol. 2015;50(Suppl 40):S39–S56. doi: 10.1002/ppul.23242. [DOI] [PubMed] [Google Scholar]
- 28.Stone KP, Kastin AJ, Pan W. NFĸB is an unexpected major mediator of interleukin-15 signaling in cerebral endothelia. Cell. Physiol. Biochem. 2011;28:115–124. doi: 10.1159/000331720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat. Rev. Immunol. 2015;15:771–783. doi: 10.1038/nri3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Stefano D, et al. Restoration of CFTR function in patients with cystic fibrosis carrying the F508del-CFTR mutation. Autophagy. 2014;10:2053–2074. doi: 10.4161/15548627.2014.973737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tosco A, et al. A novel treatment of cystic fibrosis acting on-target: cysteamine plus epigallocatechin gallate for the autophagy-dependent rescue of class II-mutated CFTR. Cell Death Differ. 2016;23:1380–1393. doi: 10.1038/cdd.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fluge G, et al. Co-morbidity of cystic fibrosis and celiac disease in Scandinavian cystic fibrosis patients. J. Cyst. Fibros. 2009;8:198–202. doi: 10.1016/j.jcf.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Walkowiak JA, et al. Cystic fibrosis is a risk factor for celiac disease. Acta Biochim. Pol. 2010;57:115–118. [PubMed] [Google Scholar]
- 34.Meresse B, Malamut G, Cerf-Bensussan N. Celiac disease: an immunological jigsaw. Immunity. 2012;36:907–919. doi: 10.1016/j.immuni.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Sollid LM, Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat. Rev. Immunol. 2013;13:294–302. doi: 10.1038/nri3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meresse B, Ripoche J, Heyman M, Cerf-Bensussan N. Celiac disease: from oral tolerance to intestinal inflammation, autoimmunity and lymphomagenesis. Mucosal Immunol. 2009;2:8–23. doi: 10.1038/mi.2008.75. [DOI] [PubMed] [Google Scholar]
- 37.DePaolo RW, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerf-Bensussan N, Meresse B. Coeliac disease & gluten sensitivity: epithelial stress enters the dance in coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2015;12:491–497. doi: 10.1038/nrgastro.2015.120. [DOI] [PubMed] [Google Scholar]
- 39.Setty M, et al. Distinct and synergistic contributions of epithelial stress and adaptive immunity to functions of intraepithelial killer cells and active celiac disease. Gastroenterology. 2015;149:681–91. e10. doi: 10.1053/j.gastro.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barone MV, Troncone R, Auricchio S. Gliadin peptides as triggers of the proliferative and stress/innate immune response of the celiac small intestinal mucosa. Int. J. Mol. Sci. 2014;15:20518–20537. doi: 10.3390/ijms151120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maiuri L, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–37. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- 42.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015;8:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine, B. & Kroemer, G. Biological functions of autophagy genes: a disease perspective. Cell176, 11–42 (2019). [DOI] [PMC free article] [PubMed]
- 45.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 46.Izzo V, et al. Metabolic interactions between cysteamine and epigallocatechin gallate. Cell Cycle. 2017;16:271–279. doi: 10.1080/15384101.2016.1249550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Troncone R, et al. Increased serum antibody levels to dietary antigens in cystic fibrosis. Acta Paediatr. 1994;83:440–441. doi: 10.1111/j.1651-2227.1994.tb18139.x. [DOI] [PubMed] [Google Scholar]
- 48.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 2017;16:487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maiuri MC, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pietrocola F, et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015;22:509–516. doi: 10.1038/cdd.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pietrocola F, et al. Aspirin recapitulates features of caloric restriction. Cell Rep. 2018;22:2395–2407. doi: 10.1016/j.celrep.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossin, F. et al. TG2 regulates the heat-shock response by the post-translational modification of HSF1. EMBO Rep. 19, pii: e45067 (2018). [DOI] [PMC free article] [PubMed]
- 53.Silano M, et al. Early tissue transglutaminase-mediated response underlies K562(S)-cell gliadin-dependent agglutination. Pediatr. Res. 2012;71:532–538. doi: 10.1038/pr.2012.4. [DOI] [PubMed] [Google Scholar]
- 54.Romani L, et al. Thymosin α1 represents a potential potent single-molecule-based therapy for cystic fibrosis. Nat. Med. 2017;23:590–600. doi: 10.1038/nm.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gavina M, et al. Nebulized hyaluronan ameliorates lung inflammation in cystic fibrosis mice. Pediatr. Pulmonol. 2013;48:761–771. doi: 10.1002/ppul.22637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.