Abstract
The human body is a highly aerobic organism, in which it is necessary to match oxygen supply at tissue levels to the metabolic demands. Along metazoan evolution, an exquisite control developed because although oxygen is required as the final acceptor of electron respiratory chain, an excessive level could be potentially harmful. Understanding the role of the main factors affecting oxygen availability, such as the gradient of pressure of oxygen during normal conditions, and during hypoxia is an important point. Several factors such as anaesthesia, hypoxia, and stress affect the regulation of the atmospheric, alveolar, arterial, capillary and tissue partial pressure of oxygen (PO2). Our objective is to offer to the reader a summarized and practical appraisal of the mechanisms related to the oxygen’s supply within the human body, including a facilitated description of the gradient of pressure from the atmosphere to the cells. This review also included the most relevant measuring methods of PO2 as well as a practical overview of its reference values in several tissues.
Keywords: Hypoxia, gradient of pressure, pressure of oxygen, altitude acclimation, barometric pressure
Introduction
The human body is a highly aerobic organism that consumes oxygen according to its metabolic demand [1]. During aerobic respiration the presence of oxygen in addition to pyruvate, produces adenosine triphosphate (ATP), thus yielding energy to the entire organism [2]. To maintain homeostasis, the amount of oxygen within the tissues should respond to a gradient of pressure that pushes oxygen by diffusion throughout the membranes into the tissues [3]. The amount of dissolved oxygen within the tissues and the cells depends on several factors including: barometric pressure (BP), climatological conditions (temperature, relative humidity, latitude, altitude), as well as physiological, pathological, and physical-chemical processes within the organism itself [4,5].
The composition of gases within the troposphere is constant at approximately the following ratio: 78.08% nitrogen, 20.95% oxygen, 0.93% argon and finally less than 0.038% for carbon dioxide and other gases [6].
Dalton’s law establishes that within a combination of any given gases, the total pressure is the same as the sum of the partial pressures of each individual gas present in that mixture [7]. Thus, the partial pressure of oxygen (PO2) depends mainly on the atmosphere’s barometric pressure (BP) and its fractional concentration [8]. Geographical altitude is an important factor affecting BP, because as altitude increases, the amount of gas molecules in the air decreases, so the air becomes less dense than at sea level. At sea level BP is about 760 mmHg, although can be affected not only by altitude: latitude, humidity, temperature and even the season of the year may also affect BP [9,10]. This changes are normally local, consequently, short-term temporal (time scale of minutes, hours, days and weeks) variations in BP in a same location usually range around 5-15 mmHg [9].
Partial pressure of oxygen
Within the troposphere (lowest region of the atmosphere), PO2 depends on several variables, but mainly on barometric pressure (Figure 1) [4]. Under physiological conditions, this relationship will be affected by any change in elevation or by modifying the fraction of inspired oxygen (FiO2) under controlled circumstances [3,11,12].
Figure 1.
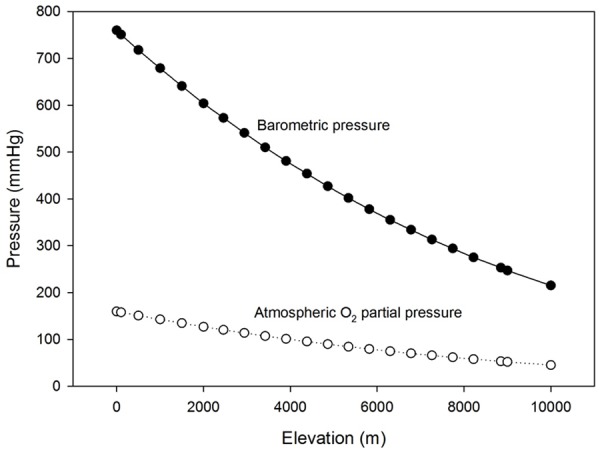
Relationship between elevation and Barometric Pressure (filled circles) and Atmospheric Partial Pressure of Oxygen (hollow circles). *Calculations were based on the standard atmosphere and were done by the authors.
Atmospheric partial pressure of oxygen (AtmPO2)
Humans depend on oxygen for survival, and this gas is acquired from the atmosphere where the partial pressure of oxygen (AtmPO2) within the troposphere depends on BP according to the Dalton’s Law [13]:
AtmPO2 = 0.21 · 760 mmHg = 159 mmHg
Humans are constantly exposed to changes in BP, either artificially or naturally, thus, pressure of inspired oxygen (as well as the other gasses) its inversely proportional reduced among those exposed to hypobaric or normobaric hypoxia [3,14] (Figure 1).
Alveolar partial pressure of oxygen (PAO2)
Once air is warmed and humidified in the nose and upper respiratory tract, the pressure of oxygen decreases while concentration of H2O increases, thus altering effective PO2 in this gas mixture. Therefore, oxygen partial pressure within the upper airway is noted inspired PO2 (PiO2) [15]. The reduction of pressure of oxygen is caused by the addition of water vapour (humidification) to the entire mixture of gases, thus reducing the pressure of the other gases [4]. The pressure of water vapour is constant at 47 mmHg at normal body temperature (37°C), and it is strongly temperature dependent [11]. This results in an effective reduction at the alveolar level in the partial pressure of oxygen (PAO2) from 159 to 149 mmHg that is not likely to be physiologically relevant at sea level, because only represents about 6% of the total AtmPO2 [16]. However, when the BP is already low, such as at the summit of Mount Everest (altitude 8,848 m), a reduction of 47 mmHg (the water vapour pressure) represents almost 20% of the available AtmPO2, making this reduction life threatening [17,18].
Moreover, once the inspired air has been humidified, there is an additional reduction in PO2 from the trachea to the alveolus, due to the dead space and the mixing of inspired and expired gases [19]. This fall in the pressure of oxygen from the upper airways to the alveolus is almost all accounted for by the alveolar pressure of carbon dioxide (PACO2) [10,20]. Since inspired PCO2 is zero and the PACO2 is usually in the range of 40 mmHg, the partial pressure of oxygen must fall [21].
When oxygen is transported into the venous pulmonary capillary, an important gradient of pressure from the upcoming arterial blood pushes the CO2 out to the alveoli [22].
The alveolar partial pressure of oxygen (PAO2) in the alveoli-capillary barrier at sea level is calculated based on the fraction of inspired oxygen (FiO2). At least in the troposphere, air contains a standard 20.95% of oxygen, thus the in order to estimate the alveolar PO2 the following equation is used:
PAO2 = FiO2 (PB-47) - 1/R (PACO2)
Where R is the respiratory exchange ratio and equals 0.8 most of the time and the 47 correspond to the water vapour pressure at normal body temperature (37°) [4].
Arterial partial pressure of oxygen (PaO2)
Once in the lungs, oxygen diffuses across the alveolar-capillary barrier from the alveoli into the arterial circulation. The initial diffusion gradient of pressures in the microcirculation arises when arterial partial pressure of oxygen (PaO2) with a higher pressure is mixed with the pressure of oxygen within the veins (PVO2) [23].
The rate of oxygen diffusion across the alveoli-capillary membrane in addition to a faster and easier elimination of CO2, assures that capillary PaO2 is almost equal to the alveolar PAO2 and during normal conditions (at sea level) it correspond to 75 to 100 mmHg [24].
At sea level, during normal conditions, the partial pressure of oxygen in the arteries is high enough to satisfy the oxygen demands for the entire organism [10]. However, during high altitude exposure (hypobaric hypoxia), as barometric pressure descends, the pressure of oxygen in the arterial circulation is inversely proportion reduced [25,26]. This reduction attributes to the significant reduction in AtmPO2 and determines the actual pressure of oxygen available for tissue and cellular requirements [27,28] (Figure 2).
Figure 2.
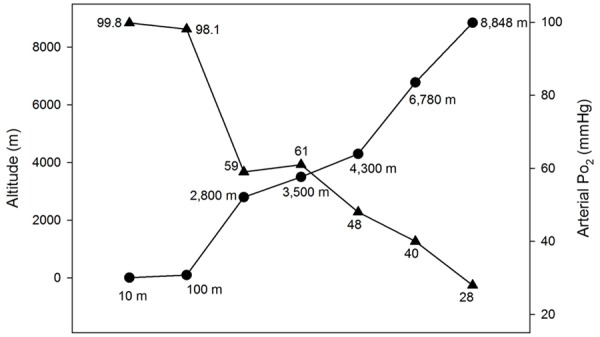
Arterial oxygen tension (PaO2) at different altitudes in humans according to the values given in several reports [3,4,12,17].
Tissue partial pressure of oxygen (PtO2)
Once oxygen has reached the arteries, the difference in pressures (gradient of pressure) between the capillary to the cytosol of surrounding cells results in a steep diffusion gradient, the greatest in the body reaching more than 42% [4]. The average partial pressure in the tissue is called the tissue partial pressure of oxygen (PtO2) [10].
The transport of oxygen from the atmosphere into the entire body is mediated by the rate diffusion as well as the rate of consumption between physiological barriers [29]. Diffusion is based on the kinetic theory that encompasses the rapid movement of molecules, causing a self-generated energy source to rapidly cross membranes [30]. Whereas convective transport refers to the heat transferred and energy-consuming combination of molecules to cause the movement of oxygen in the trachea and the bronchial tree with the surrounding alveoli-capillary circulation [31]. The diffusive transport is the passive movement of oxygen across several barriers, such as the endothelium, the alveolus and the mitochondrial membrane [32]. The amount of diffusive oxygen movement depends on the gradient of partial pressure of oxygen, the available surface area to diffusion, the permeability and thickness of diffusion barriers and the local metabolic demand [33,34].
Tissue partial pressure of oxygen (PtO2) is regulated by the blood flow, the availability of oxygen and the consumption rate from one region to another [3,24,35,36]. The Bohr effect allows that hemoglobin releases more oxygen in response to the metabolic rate of that tissue in highly aerobic tissues [37]. For instance, neurons and cardiac myocytes are largely aerobic and depend on the presence of oxygen for their survival, although some lactate can be produced within the brain, most of them depended on the metabolic rate of oxygen consumption [36,38]. Other cells, such as the bladder myocytes or the skeletal myocytes are more tolerant to hypoxia, and are able to obtain energy without the presence of oxygen for longer periods of time than can neurons in the brain [10].
Intracellular partial pressure of oxygen
Once oxygen reaches the cells, the metabolic demand must to be satisfied. The gradient of partial pressure of oxygen, from the extracellular space into the cell determines the availability of oxygen to the mitochondria [39,40].
In highly aerobic cells, such as the neurons, energy production depends largely on the availability of oxygen supplied to the mitochondria [41]. Inside this organelle, a series of enzyme-catalysed chemical reactions occur, converting metabolites into carbon dioxide and water to generate a form of usable energy in the form of high energy phosphates [42].
Although it has long been reported that the intracellular partial pressure of oxygen (iPO2) drops around the oxygen-consuming organelle, the mitochondrion PO2 must be very small [39]. Various attempts to determine the gradient of oxygen between the mitochondria and the extracellular fluids have led to some incongruous results [40,43,44]. Reported values range from one type of cell to another and ranges from below 1 mmHg measured by indirect methods to 1 to 10 mmHg by intracellular direct methods [45]. The classic insensitivity of mitochondrial respiration to local PO2 has been challenged recently by in vivo [46] and in vitro [47] studies, in which mitochondrial oxygen consumption is dependent on PO2 over the full physiological range.
Partial pressure of oxygen in different tissues
Once the arteries bring O2 to the cells, the difference in pressure between the arterial vascular lumen and the tissue will cause that gases that are at higher pressures diffuse to those tissues with lower pressure, exchanging oxygen and carbon dioxide (CO2) in both directions [29]. The average partial pressure in the tissue along this diffusion gradient is called the tissue partial pressure of oxygen (PtO2) and varies according with oxygen consumption, capillary density, metabolic rate and blood flow [10,48].
While under normal circumstances alveolar PO2 is equal to 104 mmHg, the lungs will transfer this oxygen through the alveolar-capillary barrier, reaching the same PO2 (104 mmHg), however, before reaching the left atria, the pulmonary shunt blood coming from the bronchial veins (40 mmHg) will mix with blood from pulmonary veins, reaching the atria with an arterial PO2 of 95 mmHg. This is known as “pulmonary venous admixture” [10,49].
From the aorta, the amount of oxygen that is released from the hemoglobin will depend upon the metabolic demands from that specific organ, that are usually matched to the arterial oxygen supply and vasomotor sensitivity [50].
In the following section we summarized the range of PO2 according to the type of tissue, describing in more depth those which have more available data in humans. It is important to point out that due to the lack of studies in controlled environments, an specific range mean value is hard to be provided, therefore, we state the reference value according to the lowest-highest range described (Table 1).
Table 1.
References values of PtO2 measurements using different techniques
| PtO2 (mmHg) | Organ and Tissue | Reference | Methods | Species |
|---|---|---|---|---|
| 30-48 | Brain | Meixensberger [51], Hoffman [52], Ortiz-Prado [3] | Positron emission tomography (PET) | Human |
| And rats | ||||
| 104-108 | Alveoulus | Guyton [4] | Polarographic measurements of tissue oxygen tension using gold microelectrodes | Human |
| 8 | Skin epidermis | Wang [35], Carreau [53] | Microelectrodes | Human |
| 24 | Dermal papillae | |||
| 35.2 | Sub-papillary plexus | |||
| 61.2 | Small bowel | Müller [54,55], Carreau [53] | Electron paramagnetic resonance oximetry (EPR) | Human |
| 57.6 | Large bowel | Müller [54,55], Carreau [53] | Electron paramagnetic resonance oximetry (EPR) | Human |
| 55.5 ± 21.3 | Liver | Leary [56] | Electron paramagnetic resonance oximetry (EPR) with Indian ink. | Human |
| 72 ± 20 | Superficial cortex of the kidney | Muller [57], Carreau [53] | Phosphorescence lifetime technique | Human |
| 28.9 ± 3.4 | Muscle fibers | Beerthuizen [58], Carreau [53] | Proton NMR spectra of myoglobin | Human |
| 29.6 ± 1.8 | ||||
| 51.8 ± 14.5 | Bone Marrow | Carreau [53] | The technique of aspiration in a syringe | Human |
| 34 ± 1.6 | Femur Bone | Maurer [59] | Technique of radioactive microspheres in interosseous blood samples and blood flow in the bone | Human |
| 71.4 | Mandibule | |||
| 55 | Suprarenal Gland | Bloom [60] | Phosphorescence lifetime technique | Calf |
| 88 | Ovaries | Fraser [61] | Clark electrode for pO2 | Human |
| 18 | Umbilical Arteries | Gluckman [62], Carreau [53] | Umbilical cord blood gas | Human |
| 29.2 | Umbilical Vein | Guyton [4], Gluckman [62], Carreau [53] | Umbilical cord blood gas | Human |
| 90 ± 5 | Arterial PO2 | Mah and Cheng [20], Guyton [4] | Gasometry | Human |
| 40 ± 5 | Venous PO2 | Mah and Cheng [20], Guyton [4] | Gasometry | Human |
| 48.2 ± 3.1 | Synovial Fluid | Richman [63] | Routine macroscopic and microscopic examination | Human |
| 30.6 ± 3.1 | Cornea | Bonanno [64] | Oxygen sensitive dye, Pd-meso-tetra (4-carboxyphenyl) porphine, bound to bovine serum albumin, was incubated with contact lenses | Human |
| 22 | The Eye | Bonanno [64] | The T1 mapping method was applied | Human |
Partial pressure of oxygen in the brain
The brain is an organ with one of the highest oxygen and glucose requirements, although it is not able to store metabolic products for further use, its blood supply is highly dependent of vasoactive substances, arterial blood gases and metabolic demand allowing the availability of these nutrients [3,65,66].
Changes in tissue brain Partial Pressure of Oxygen depends on the cerebral metabolic rate (CMR), the local cerebral blood flow (CBF) and the systemic exposure of hypoxia [3,36,67,68]. Brain PtO2 can change due to several factors like CMR, hypoxia, exercise, angiogenesis, stress and Anesthesia [3]. In general and considering that humans are in constant activity and many cofounders cannot be controlled, the available evidence suggest that cortical PtO2 ranges from 20-25 mmHg in rest and low altitude and reach up to 48 mmHg in high altitudes or intense physical activity [51,52,69].
Partial pressure of oxygen in the liver
The liver receives more than 6% of the cardiac output per minute and more than 26% of the cardiac output when considering the portal venous system [10]. This organ seems to be highly oxygenated, however, during sympathetic vascular tone changes, anesthesia, restraining and also depending of the method of measurement, liver tissue PO2 fluctuates [56]. The liver can survive with less than 60% of the total liver blood supply due to sympathetic electric nerve stimulation, resulting in an important reduction of tissue PO2, however under normal conditions the very few reports available in humans refer that PO2 ranges from 50-55 mmHg [56,70].
Partial pressure of oxygen in skeletal muscle
The muscle is a highly effective oxygen consuming tissue that responds to blood flow requirements and oxygen availability [71]. The local tissue oxygenation of the skeletal muscle is highly variable, being skeletal muscle one of the most tolerant tissues to hypoxia and metabolic acidosis [72]. Tissue oxygenation level depends on the rate of oxygen supply and the rate of oxygen consumption per tissue [73]. The critical level in which the muscle will suffer ischemia has not been explored, however, muscle PO2 and its relationship with systemic factors such as sepsis and infections have been reported several times [58,74]. Considering the reports available, skeletal muscle oxygenation ranges from 7.5 to 31 mmHg [74].
Partial pressure of oxygen in the skin
The skin is one of the most vasoactive tissue within the body, reacting strongly to sympathetic, thermic and metabolic changes [10]. At rest and in neutral thermal conditions, less than 2% of the total cardiac output goes to the skin [75], however, fluctuations in skin blood flow are always occurring due to sympathomimetic variability [76]. The oxygen availability measured locally depends on the influence of the microcirculation and the skin PtO2 ranges according to the skin layers. The more external layer ranges from 3.2 to 8 mmHg, the papillary dermis from 6.4 to 24 mmHg and below the subcutaneous fat, the skin PtO2 ranges from 8 to 38 mmHg [53,75].
Methods to measure tissue partial pressure of oxygen
Several methods have been used to measure the availability of oxygen within the tissues (PtO2). In Table 2 we summarize the methods that are available nowadays with some technical specifications such as the mechanism of measurement, the site of data collection and minimum sample volume needed (Table 2).
Table 2.
Adapted from Harold M. Swartz; Jeff F. Dunn * Minimum Volume Sampled
| Method | Parameter measured | Mechanism of measurement | Site of measurement | *Volume sampled |
|---|---|---|---|---|
| Microelectrode | pO2 | Current generated by the electrolytic decomposition of dioxygen | Interstitial volume in contact with the tip | μl |
| Near infrared monitoring of haemoglobin and myoglobin | Physiological parameter relative or absolute changes in saturation | Amount or fraction of haemoglobin (Hb) or myoglobin (Mb) and its relative oxygen saturation | Location of the proteins. In the vascular system by non-linear weighting of Hb related to vessel diameter. Idem in muscle for Mb. | ml’s |
| Near infrared monitoring or cytochromes | Physiological parameter relative changes in cytochrome oxidation | Redox state of cytochoromes | Intracellular cytochromes | 5 ml’s |
| Phosphorescent and fluorescent methods based on redox states of intermediates | Physiological parameter based on redox potential | Ratio of reduced and oxidized states of redox couples | Sites of the redox intermediates (usually intracellular) | μl’s |
| Phosphorescent and fluorescent methods based on quenching by oxygen | O2 | Change in lifetimes of the excited states | Sites of the introduced probe molecules, intravascular or at a catheter tip | μl’s |
| NMR perfluorocarbon relaxation | O2 | Effect on relaxation rates of fluonne nuclei | Sites of the introduced emulsion | μl-ml’s |
| Substances that localize in hypoxic areas | Physiological parameter | Amount of material that localizes in the tissue, related to perfusion and O2 at time of administration | Tissues where substances localize | <10 μ in biopsy |
| EPR oximetry based on soluble materials | pO2 | Effect on linewidth of EPR spectrum | Sites of the particles (usually interstitial) | 100 μl |
| EPR oximetry based on soluble materials | O2 | Effect on linewidth of EPR spectrum or relaxation rates | Sites of the soluble molecules (usually throughout the tissues) | -1 ml |
| NMR spectroscopy | Physiological parameter metabolic correlates with oxygen | Concentrations of metabolites which change with oxidative status of cells | Sites of metabolites | -1 ml |
| 25 μl-ml’s | ||||
| Proton NMR spectra of myoglobin | Physiological parameter relative or absolute change in oxymyoglobin | Relative concentrations of deoxy and oxymyoglobin | Muscle (myoglobin) | -1 ml |
| μl-ml’s | ||||
| NMR overhauser effect | O2 | Relaxation rates of protons that couple to free radicals | Sites of the soluble free radicals (usually throughout the tissues) | Potential resolution of MRI |
| NMR bold effect | Physiological parameter | Amount of deoxyhemoglobin in the voxels | Vascular system with a non-uniform weighting to vascular diameters | <0.2 ml |
| μl-ml’s |
The minimum volume of tissue that was sampled for theoretical rather than practical interest.
Qualitative methods to measure tissue PtO2
The most common qualitative methods available to measure brain PtO2 include, but are not limited, to positron emission tomography (PET), near-infrared spectroscopy (NIR) and magnetic resonance imaging (MRI) or nuclear magnetic resonance (NMR) [77,78].
Positron emission tomography (PET)
Positron emission tomography (PET) is an imaging technique that uses positron emitting isotopes which are injected into the tissue to provide a three-dimensional image or picture of functional processes in the body [79]. The parameters used to measure brain oxygenation are based on the oxygen extraction fraction (OEF) or the cerebral metabolic rate for oxygen (CMRO2). The use of PET in brain oxygenation studies has been reported several times, although its use is reduced in the clinical setting due to its high cost and technical complexity [77,80].
Near infrared spectroscopy (NIR)
Near infrared spectroscopy (NIR) is a technology based on light absorption in the near infra-red spectrum (700-1000 nm) [81]. It is characterized for its ability to scatter through skin, bone and other tissues, thus detecting low resolution but real time changes in regional hemoglobin content and rarely with brain cerebral perfusion [82,83].
Blood oxygenation level dependent MRI (BOLD MRI)
Oxyhemoglobin has diamagnetic properties whereas deoxyhemoglobin is a paramagnetic molecule [84]. These magnetic properties can be used as an endogenous source of contrast to visualize tissue oxygenation [85-87]. This technology can be used to measure brain oxygenation based on the concept that changes in deoxyhemoglobin modulate the MRI signal intensity. For example, an increase in regional cerebral blood flow caused by neural activity is accompanied by a local reduction in deoxyhemoglobin content [88].
Quantitative methods to measure brain PtO2
The physical and chemical characteristics of oxygen can be measured according to its specific interaction with determined oxygen-reactive molecules [89]. The measurement of tissue partial pressure of oxygen (PtO2) is expressed in mmHg, kPa or Torr and is one of the main “direct” measurements of oxygenation in the tissue [77].
Polarographic microelectrodes
Molecules of oxygen are electron acceptors and this oxidative reaction can be measured using microelectrodes [90]. This oxygen reduction reaction allows a signal that creates a potential difference which is recorded by the electrode [91]. The use of this type of electrodes has allowed the measurement of brain PtO2 during various conditions, including head trauma, brain surgery, hypothermia and hibernation [92-96].
Electron paramagnetic resonance oximetry
Electron paramagnetic resonance oximetry (EPR) is a spectroscopic technique that detects chemical species that have unpaired electrons [97]. EPR oximetry is a relatively non-invasive method for monitoring tissue partial pressure of oxygen (PtO2) using paramagnetic oxygen sensitive materials including perchlorotriphenylmethyl molecules or lithium phthalocyanine (LiPc) crystals [85,97-100].
The fundamental mechanism of this technique is the detection of unpaired electron species which react with the implanted materials (i.e. LiPc crystals) [101]. The identification of these chemical species co-existing in the determined paramagnetic spectrum can be observed and interpreted as oxygen tensions [100,102-104].
The use of EPR oximetry for the study of tissue oxygenation allows multiple measurements to be performed through the use of crystals that are highly sensitive to low PtO2 [98]. The advantages of this method are stable calibration and relative unresponsiveness to changes in pH or redox reactions [104,105].
Mass spectrometry and brain PtO2 measurements
Mass spectrometry (MS) is a technique that make it possible to obtain analytical information of the molecular mass and its elemental composition of a sample or molecule [106]. For this it is necessary to ionize molecules using different techniques such as chromatographic separation in order to measure the mass to charge ratio caused by external electric and magnetic fields [83,106].
Mass spectrometry is a complicated technology to use, Atoms are very reactive and they have a short live, thus, manipulation must be performed in a vacuum environment, with very low barometric pressures that ranges from ~10-5 to 10-8 Torr [106]. These factors, plus the greater degree of invasively, and the response time and delay of mass spectrometers, make mass spectrometry less favourable as a method [83].
Fluorescence and phosphorescence-based probes
The optical methods of oxygen detection are based on the recognition of an atom or molecule which has been electronically excited by the absorption of a photon [3]. This excitation facilitates the transitions of a species from high excitation state or activation, to a ground or low excitation state, this molecular reaction involves the emission of a photon of light [3].
Fiber optic optodes can be used to measure brain PtO2 in awake and unanesthetized subjects, however its availability in human studies is limited. This technology is based on short pulses of light that are transmitted along a fiber optic sensor, exciting the platinum (new version) or ruthenium (older version) based tip, producing a photon-molecular reaction that is quenched by the presence of oxygen [3,45,107,108].
One of the most important physiological advantages of this optical technique is that it is very sensitive during hypoxia [3]. This feature is clinically relevant when studying tumour growth which depends on oxygenation as well as when studying ischemia or brain injuries [109]. Another important feature of this technology is its insensitivity to magnetic fields. This technology allows us to measure brain PtO2 while applying simultaneously other exploration or imaging techniques, such as MRI or EPR. This feature can be used to validate two or more methods [110].
The effects of acute and chronic hypoxia on Tissue PO2
The effects of hypoxia (acute or chronic) and the presence of oxygen deprivation in different tissues have been reported as early as the 1950’s [111]. The hypoxic environment was simulated using different fractions of inspired oxygen (normobaric hypoxia) or by exposing the subject to lower barometric pressure (hypobaric hypoxia), either by using low pressure chambers, or taking the subject to high altitude [8,112].
Although oxygen levels are critical parameters in order to asses tissue survival, monitoring the level of oxygen at a tisular level remains a challenge [3,52,68,110]. Real time, in vivo measurements during acute inflammation, hypoxia or hyperoxia have been done very few times and is not widely available [80].
Measuring tissue oxygenation during acute or chronic is a difficult task, especially due to the presence of cofounders like exercise, anesthesia, time of exposure or restraining the animal model [113,114]. In humans, acclimation to high altitude exposure or controlled normobaric hypoxia will cause different readings in terms of PtO2 [68]. Adaption on the other hand will cause differences between populations, making extrapolation a difficult task [115]. Obtaining reference values in such conditions is very difficult due to the implications of such a challenge and the ethical limitations of these type of technologies in humans.
Discussion
This practical review of the available literature about the gradient of pressure of oxygen revealed complex, varied and often not conclusive results. We tried to summarize the most relevant information to present it as friendly as possible for educational purposes. A more profound analysis of cellular and molecular hypoxia and normoxia signalling we recommend Keeley and Mann review [116].
The usefulness of understanding the gradient of PO2 among healthcare providers is essential. Understanding how the gradient of pressure works and how oxygen is delivered is related to an entire spectrum of clinical uses. Some of the most important results come from athletes performance [117], forecasting mortality due to prevalent diseases [118], wound healing evaluation [119], treatment effectiveness in ulcers, burns, cancer or cerebral and cardio vascular disease [120-125].
In this sense, we have exposed the physiological mechanisms, the methods for measuring and the pressures values reported in different organs from the atmosphere to the mitochondria. Tissue partial pressure of oxygen reflects a balance between arterial blood flow and tissue oxygen consumption rate [92]. Due to technical limitations and confounding factors such as anesthesia, inflammation, restraint and hypoxia, an appraisal of partial pressure of oxygen during normal conditions is very difficult. However, in vivo and clinical data available have been included to offer the reader a better perspective of how partial pressure of oxygen behaves within the human body.
Conclusions
The human body is a complex living organism, which has developed mechanisms to keep oxygen levels in a suitable level as to cover the metabolic demand, while avoiding excessive oxygen pressure.
The partial pressure of oxygen varies in the different structures of the organism. Each organ and tissue have its own requirements in order to correctly function. For example, the partial pressure of oxygen in the lungs for carrying out the gas exchange is different from the partial pressure of oxygen within the pulmonary tissue. We have emphasized that the organism has been able to develop physiological mechanisms that allow it to respond to short-term and long-term changes not only of the oxygen partial pressure, but also of the different gases in the atmosphere. This fascinating response capacity is responsible of how the human body manages to function correctly when it finds itself in different climates and altitudes.
Acknowledgements
This study was funded by Universidad de Las Americas with academic purposes only.
Disclosure of conflict of interest
None.
References
- 1.Bylund-Fellenius AC, Walker PM, Elander A, Holm S, Holm J, Schersten T. Energy metabolism in relation to oxygen partial pressure in human skeletal muscle during exercise. Biochem J. 1981;200:247–255. doi: 10.1042/bj2000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinopoulos C, Kiss G, Kawamata H, Starkov AA. Measurement of ADP-ATP exchange in relation to mitochondrial transmembrane potential and oxygen consumption. Methods Enzymol. 2014;542:333. doi: 10.1016/B978-0-12-416618-9.00017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortiz-Prado E, Natah S, Srinivasan S, Dunn JF. A method for measuring brain partial pressure of oxygen in unanesthetized unrestrained subjects: the effect of acute and chronic hypoxia on brain tissue PO(2) J Neurosci Methods. 2010;193:217–25. doi: 10.1016/j.jneumeth.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall J. Guyton and Hall textbook of medical physiology (Guyton Physiology) [Internet] Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 5.Mellemgaard K. The alveolar-arterial oxygen difference: its size and components in normal man. Acta Physiol Scand. 1966;67:10–20. doi: 10.1111/j.1748-1716.1966.tb03281.x. [DOI] [PubMed] [Google Scholar]
- 6.United States Committee on Extension to the Standard Atmosphere, United States. National Oceanic and Atmospheric Administration, United States. National Aeronautics and Space Administration, United States. Air Force. U.S. standard atmosphere, 1976. National Oceanic and Amospheric [sic] Administration: for sale by the Supt. of Docs., US Govt. Print. Off. 1976. [Google Scholar]
- 7.Gill AL, Bell CN. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97:385–395. doi: 10.1093/qjmed/hch074. [DOI] [PubMed] [Google Scholar]
- 8.Grocott MP, Martin DS, Levett DZ, McMorrow R, Windsor J, Montgomery HE. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009;360:140–149. doi: 10.1056/NEJMoa0801581. [DOI] [PubMed] [Google Scholar]
- 9.West JB, Schoene RB, Milledge JS, Ward MP. High altitude medicine and physiology [Internet] London: Hodder Arnold; 2007. [Google Scholar]
- 10.John E. Guyton and Hall textbook of medical physiology, 13E. 2016 [cited 2016 Sep 8] [Google Scholar]
- 11.West JB. Acclimatization and tolerance to extreme altitude. J Wilderness Med. 1993;4:17–26. doi: 10.1580/0953-9859-4.1.17. [DOI] [PubMed] [Google Scholar]
- 12.West JB. Human responses to extreme altitudes. Integr Comp Biol. 2006;46:25–34. doi: 10.1093/icb/icj005. [DOI] [PubMed] [Google Scholar]
- 13.Bassett BE, Bennett PB. Introduction to the physical and physiological bases of hyperbaric therapy. Hyperb Oxyg Ther. 1977:11–14. [Google Scholar]
- 14.Savourey G, Launay JC, Besnard Y, Guinet A, Travers S. Normo-and hypobaric hypoxia: are there any physiological differences? Eur J Appl Physiol. 2003;89:122–126. doi: 10.1007/s00421-002-0789-8. [DOI] [PubMed] [Google Scholar]
- 15.Tucker VA. Respiratory exchange and evaporative water loss in the flying budgerigar. J Exp Biol. 1968;48:67–87. [Google Scholar]
- 16.Vleck D. Measurement of O2 consumption, CO2 production, and water vapor production in a closed system. J Appl Physiol. 1987;62:2103–2106. doi: 10.1152/jappl.1987.62.5.2103. [DOI] [PubMed] [Google Scholar]
- 17.Boussuges A, Molenat F, Burnet H, Cauchy E, Gardette B, Sainty JM, Jammes Y, Richalet JP. Operation Everest III (Comex’97): modifications of cardiac function secondary to altitude-induced hypoxia. An echocardiographic and Doppler study. Am J Respir Crit Care Med. 2000;161:246–270. doi: 10.1164/ajrccm.161.1.9902096. [DOI] [PubMed] [Google Scholar]
- 18.Mu Y, Nepal S. High mountain adventure tourism: trekkers’ perceptions of risk and death in Mt. Everest region, nepal. Asia Pac J Tour Res. 2016;21:500–511. [Google Scholar]
- 19.Möller W, Celik G, Feng S, Bartenstein P, Meyer G, Eickelberg O, Tatkov S, Nilius G. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol. 2015;118:1525–1532. doi: 10.1152/japplphysiol.00934.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mah KK, Cheng HM. Learn Teach Tools Basic Clin Respir Physiol [Internet] Springer; 2015. Diffusion, blood O2, CO2 content and transport; pp. 15–26. [Google Scholar]
- 21.Krogh A, Lindhard J. The volume of the dead space in breathing and the mixing of gases in the lungs of man. J Physiol. 1917;51:59–90. doi: 10.1113/jphysiol.1917.sp001785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheid P, Piiper J. Blood/gas equilibrium of carbon dioxide in lungs. A critical review. Respir Physiol. 1980;39:1–31. doi: 10.1016/0034-5687(80)90011-0. [DOI] [PubMed] [Google Scholar]
- 23.Mayer K, Trzeciak S, Puri NK. Assessment of the adequacy of oxygen delivery. Curr Opin Crit Care. 2016;22:437–443. doi: 10.1097/MCC.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 24.Scheufler KM. Tissue oxygenation and capacity to deliver O2 do the two go together? Transfus Apher Sci. 2004;31:45–54. doi: 10.1016/j.transci.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Sutton JR, Bryan AC, Gray GW, Horton ES, Rebuck AS, Woodley W, Rennie ID, Houston CS. Pulmonary gas exchange in acute mountain sickness. Aviat Space Environ Med. 1976;47:1032–1037. [PubMed] [Google Scholar]
- 26.Frisancho AR. Functional adaptation to high altitude hypoxia. Science. 1975;187:313–319. doi: 10.1126/science.1089311. [DOI] [PubMed] [Google Scholar]
- 27.West JB. High-altitude medicine. Am J Respir Crit Care Med. 2012;186:1229–1237. doi: 10.1164/rccm.201207-1323CI. [DOI] [PubMed] [Google Scholar]
- 28.Naeije R. Physiological adaptation of the cardiovascular system to high altitude. Prog Cardiovasc Dis. 2010;52:456–466. doi: 10.1016/j.pcad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Nikinmaa M. Oxygen and carbon dioxide transport in vertebrate erythrocytes: an evolutionary change in the role of membrane transport. J Exp Biol. 1997;200:369–380. doi: 10.1242/jeb.200.2.369. [DOI] [PubMed] [Google Scholar]
- 30.Kreuzer F. Facilitated diffusion of oxygen and its possible significance; a review. Respir Physiol. 1970;9:1–30. doi: 10.1016/0034-5687(70)90002-2. [DOI] [PubMed] [Google Scholar]
- 31.Ellsworth ML, Pittman RN. Arterioles supply oxygen to capillaries by diffusion as well as by convection. Am J Physiol. 1990;258:H1240–H1243. doi: 10.1152/ajpheart.1990.258.4.H1240. [DOI] [PubMed] [Google Scholar]
- 32.Peacock AJ. ABC of oxygen: oxygen at high altitude. BMJ. 1998;317:1063–6. doi: 10.1136/bmj.317.7165.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamer J, Wiedemann K, Berlet H, Weinhardt F, Hoyer S. Cerebral glucose and energy metabolism, cerebral oxygen consumption, and blood flow in arterial hypoxaemia. Acta Neurochir (Wien) 1978;44:151–160. doi: 10.1007/BF01402057. [DOI] [PubMed] [Google Scholar]
- 34.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Côté D, Vinogradov SA, Scadden DT, Lin CP. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–73. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Winlove CP, Michel CC. Oxygen partial pressure in outer layers of skin of human finger nail folds. J Physiol. 2003;549:855–863. doi: 10.1113/jphysiol.2002.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortiz-Prado E, León AB, Unigarro L, Santillan P. Oxigenación y flujo sanguíneo cerebral, revisión comprensiva de la literatura. Brain oxygenation and cerebral blood flow, a comprehensive literature review. Rev Ecuat Neurol. 2018:27. [Google Scholar]
- 37.Malte H, Lykkeboe G. The Bohr/Haldane effect: a model based uncovering of the full extent of its impact on O2 delivery to and CO2 removal from tissues. J Appl Physiol. 2018;125:916–922. doi: 10.1152/japplphysiol.00140.2018. [DOI] [PubMed] [Google Scholar]
- 38.Vlassenko AG, Raichle ME. Brain aerobic glycolysis functions and Alzheimer’s disease. Clin Transl Imaging. 2015;3:27–37. doi: 10.1007/s40336-014-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodmer SI, Balestra GM, Harms FA, Johannes T, Raat NJ, Stolker RJ, Mik EG. Microvascular and mitochondrial PO2 simultaneously measured by oxygen-dependent delayed luminescence. J Biophotonics. 2012;5:140–151. doi: 10.1002/jbio.201100082. [DOI] [PubMed] [Google Scholar]
- 40.Mik EG. Measuring mitochondrial oxygen tension: from basic principles to application in humans. Anesth Analg. 2013;117:834–846. doi: 10.1213/ANE.0b013e31828f29da. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman WE, Charbel FT, Gonzalez-Portillo G, Ausman JI. Measurement of ischemia by changes in tissue oxygen, carbon dioxide, and pH. Surg Neurol. 1999;51:654–658. doi: 10.1016/s0090-3019(99)00011-7. [DOI] [PubMed] [Google Scholar]
- 42.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fercher A, Borisov SM, Zhdanov AV, Klimant I, Papkovsky DB. Intracellular O2 sensing probe based on cell-penetrating phosphorescent nanoparticles. ACS Nano. 2011;5:5499–5508. doi: 10.1021/nn200807g. [DOI] [PubMed] [Google Scholar]
- 44.Zhdanov AV, Ogurtsov VI, Taylor CT, Papkovsky DB. Monitoring of cell oxygenation and responses to metabolic stimulation by intracellular oxygen sensing technique. Integr Biol. 2010;2:443–451. doi: 10.1039/c0ib00021c. [DOI] [PubMed] [Google Scholar]
- 45.Dmitriev RI, Papkovsky DB. Optical probes and techniques for O2 measurement in live cells and tissue. Cell Mol Life Sci. 2012;69:2025–2039. doi: 10.1007/s00018-011-0914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golub AS, Pittman RN. Oxygen dependence of respiration in rat spinotrapezius muscle in situ. Am J Physiol Heart Circ Physiol. 2012;303:H47–H56. doi: 10.1152/ajpheart.00131.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson DF, Harrison DK, Vinogradov SA. Oxygen, pH, and mitochondrial oxidative phosphorylation. J Appl Physiol. 2012;113:1838–1845. doi: 10.1152/japplphysiol.01160.2012. [DOI] [PubMed] [Google Scholar]
- 48.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 49.Sandoval J, Long GR, Skoog C, Wood LD, Oppenheimer L. Independent influence of blood flow rate and mixed venous PO2 on shunt fraction. J Appl Physiol. 1983;55:1128–1133. doi: 10.1152/jappl.1983.55.4.1128. [DOI] [PubMed] [Google Scholar]
- 50.Jain V, Langham MC, Floyd TF, Jain G, Magland JF, Wehrli FW. Rapid magnetic resonance measurement of global cerebral metabolic rate of oxygen consumption in humans during rest and hypercapnia. J Cereb Blood Flow Metab. 2011;31:1504–1512. doi: 10.1038/jcbfm.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meixensberger J, Dings J, Kuhnigk H, Roosen K. Monit Cereb Blood Flow Metab Intensive Care [Internet] Springer; 1993. Studies of tissue PO2 in normal and pathological human brain cortex; pp. 58–63. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman WE, Charbel FT, Edelman G. Brain tissue oxygen, carbon dioxide, and pH in neurosurgical patients at risk for ischemia. Anesth Analg. 1996;82:582–586. doi: 10.1097/00000539-199603000-00027. [DOI] [PubMed] [Google Scholar]
- 53.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15:1239–53. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Müller M, Schindler E, Roth S, Schürholz A, Vollerthun M, Hempelmann G. Effects of desflurane and isoflurane on intestinal tissue oxygen pressure during colorectal surgery. Anaesthesia. 2002;57:110–115. doi: 10.1046/j.0003-2409.2001.02363.x. [DOI] [PubMed] [Google Scholar]
- 55.Müller M, Schück R, Erkens U, Sticher J, Haase C, Hempelmann G. Effects of lumbar peridural anesthesia on tissue pO2 of the large intestine in man. Anasthesiol Intensivmed Notfallmed Schmerzther. 1995;30:108–110. doi: 10.1055/s-2007-996457. [DOI] [PubMed] [Google Scholar]
- 56.Leary TS, Klinck JR, Hayman G, Friend P, Jamieson NV, Gupta AK. Measurement of liver tissue oxygenation after orthotopic liver transplantation using a multiparameter sensor. Anaesthesia. 2002;57:1128–1133. doi: 10.1046/j.1365-2044.2002.02782_5.x. [DOI] [PubMed] [Google Scholar]
- 57.Muller M, Padberg W, Schindler E, Sticher J, Osmer C, Friemann S, Hempelmann G. Renocortical tissue oxygen pressure measurements in patients undergoing living donor kidney transplantation. Anesth Analg. 1998;87:474–476. doi: 10.1097/00000539-199808000-00045. [DOI] [PubMed] [Google Scholar]
- 58.Beerthuizen GI, Goris RJ, Kreuzer FJ. Is skeletal muscle PO2 related to the severity of multiple organ failure and survival in critically ill patients? A preliminary study. Prog Clin Biol Res. 1989;308:137–42. [PubMed] [Google Scholar]
- 59.Maurer P, Meyer L, Eckert AW, Berginski M, Schubert J. Measurement of oxygen partial pressure in the mandibular bone using a polarographic fine needle probe. Int J Oral Maxillofac Surg. 2006;35:231–236. doi: 10.1016/j.ijom.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Bloom SR, Edwards AV, Hardy RN. Adrenal and pancreatic endocrine responses to hypoxia and hypercapnia in the calf. J Physiol. 1977;269:131–154. doi: 10.1113/jphysiol.1977.sp011896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fraser IS, Baird DT, Cockburn F. Ovarian venous blood PO2, PCo2 and pH in women. J Reprod Fertil. 1973;33:11–17. doi: 10.1530/jrf.0.0330011. [DOI] [PubMed] [Google Scholar]
- 62.Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 63.Richman AI, Su EY, Ho G. Reciprocal relationship of synovial fluid volume and oxygen tension. Arthritis Rheumatol. 1981;24:701–705. doi: 10.1002/art.1780240512. [DOI] [PubMed] [Google Scholar]
- 64.Bonanno JA, Stickel T, Nguyen T, Biehl T, Carter D, Benjamin WJ, Soni PS. Estimation of human corneal oxygen consumption by noninvasive measurement of tear oxygen tension while wearing hydrogel lenses. Invest Ophthalmol Vis Sci. 2002;43:371–376. [PubMed] [Google Scholar]
- 65.Zauner A, Daugherty WP, Bullock MR, Warner DS. Brain oxygenation and energy metabolism: part I-biological function and pathophysiology. Neurosurgery. 2002;51:289–302. [PubMed] [Google Scholar]
- 66.Jespersen SN, Østergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32:264–277. doi: 10.1038/jcbfm.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brugniaux JV, Hodges AN, Hanly PJ, Poulin MJ. Cerebrovascular responses to altitude. Respir Physiol Neurobiol. 2007;158:212–223. doi: 10.1016/j.resp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Dunn JF, Wu Y, Zhao Z, Srinivasan S, Natah SS. Baron JC, editor. Training the brain to survive stroke. PLoS One. 2012;7:e45108. doi: 10.1371/journal.pone.0045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muir ER, Cardenas DP, Duong TQ. MRI of brain tissue oxygen tension under hyperbaric conditions. NeuroImage. 2016;133:498–503. doi: 10.1016/j.neuroimage.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carneiro JJ, Donald DE. Change in liver blood flow and blood content in dogs during direct and reflex alteration of hepatic sympathetic nerve activity. Circ Res. 1977;40:150–8. doi: 10.1161/01.res.40.2.150. [DOI] [PubMed] [Google Scholar]
- 71.Parežnik R, Knezevic R, Voga G, Podbregar M. Changes in muscle tissue oxygenation during stagnant ischemia in septic patients. Intensive Care Med. 2006;32:87–92. doi: 10.1007/s00134-005-2841-8. [DOI] [PubMed] [Google Scholar]
- 72.Esau SA. Hypoxic, hypercapnic acidosis decreases tension and increases fatigue in hamster diaphragm muscle in Vifro1-3. Am Rev Respir Dis. 1989;139:1410–1417. doi: 10.1164/ajrccm/139.6.1410. [DOI] [PubMed] [Google Scholar]
- 73.Gerard I, Beerthuizen JM, Jan R, Goris A, Bredee JJ, Mashhour YA, Kimmich HP, van der Kley AJ, Kreuzer F. Muscle oxygen tension, hemodynamics, and oxygen transport after extracorporeal circulation. Crit Care Med. 1988;16:748–750. doi: 10.1097/00003246-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 74.Beerthuizen GI, Goris RJ, Kreuzer FJ. Skeletal muscle Po2 during imminent shock. Arch Emerg Med. 1989;6:172. doi: 10.1136/emj.6.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pardo Ríos M. La presión transcutánea de oxígeno como factor pronóstico en la angioplastia transluminal percutánea: una solución a las limitaciones del índice tobillo brazo. Proy Investig [Internet] 2013 [Google Scholar]
- 76.Hori M, Inoue M, Kitakaze M, Koretsune Y, Iwai K, Tamai J, Ito H, Kitabatake A, Sato T, Kamada T. Role of adenosine in hyperemic response of coronary blood flow in microembolization. Am J Physiol. 1986;250:H509–H518. doi: 10.1152/ajpheart.1986.250.3.H509. [DOI] [PubMed] [Google Scholar]
- 77.Hori Y, Hirano Y, Koshino K, Moriguchi T, Iguchi S, Yamamoto A, Enmi J, Kawashima H, Zeniya T, Morita N, Nakagawara J, Casey ME, Iida H. Validity of using a 3-dimensional PET scanner during inhalation of 15O-labeled oxygen for quantitative assessment of regional metabolic rate of oxygen in man. Phys Med Biol. 2014;59:5593–609. doi: 10.1088/0031-9155/59/18/5593. [DOI] [PubMed] [Google Scholar]
- 78.Carlier PG, Bertoldi D, Baligand C, Wary C, Fromes Y. Muscle blood flow and oxygenation measured by NMR imaging and spectroscopy. NMR Biomed. 2006;19:954–967. doi: 10.1002/nbm.1081. [DOI] [PubMed] [Google Scholar]
- 79.Jerusalem G, Hustinx R, Beguin Y, Fillet G. PET scan imaging in oncology. Eur J Cancer. 2003;39:1525–1534. doi: 10.1016/s0959-8049(03)00374-5. [DOI] [PubMed] [Google Scholar]
- 80.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9:1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bülow J, Kjaer M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports. 2001;11:213–22. doi: 10.1034/j.1600-0838.2001.110404.x. [DOI] [PubMed] [Google Scholar]
- 82.Highton D, Ghosh A, Tachtsidis I, Panovska-Griffiths J, Elwell CE, Smith M. Monitoring cerebral autoregulation after brain injury: multimodal assessment of cerebral slow-wave oscillations using near-infrared spectroscopy. Anesth Analg. 2015;121:198. doi: 10.1213/ANE.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ndubuizu O, LaManna JC. Brain tissue oxygen concentration measurements. Antioxid Redox Signal. 2007;9:1207–1220. doi: 10.1089/ars.2007.1634. [DOI] [PubMed] [Google Scholar]
- 84.Pauling L, Coryell CD. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936;22:210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dunn JF, Wadghiri YZ, Meyerand ME. Regional heterogeneity in the brain’s response to hypoxia measured using BOLD MR imaging. Magn Reson Med. 1999;41:850–854. doi: 10.1002/(sici)1522-2594(199904)41:4<850::aid-mrm27>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 86.Zhao D, Jiang L, Hahn EW, Mason RP. Comparison of 1H blood oxygen level-dependent (BOLD) and 19F MRI to investigate tumor oxygenation. Magn Reson Med. 2009;62:357–364. doi: 10.1002/mrm.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Egred M, Waiter GD, Redpath TW, Semple SK, Al-Mohammad A, Walton S. Blood oxygen level-dependent (BOLD) MRI: a novel technique for the assessment of myocardial ischemia as identified by nuclear imaging SPECT. Eur J Intern Med. 2007;18:581–586. doi: 10.1016/j.ejim.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 88.Rajagopalan P, Krishnan KR, Passe TJ, Macfall JR. Magnetic resonance imaging using deoxyhemoglobin contrast versus positron emission tomography in the assessment of brain function. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:351–366. doi: 10.1016/0278-5846(95)00017-p. [DOI] [PubMed] [Google Scholar]
- 89.Wen B, Urano M, Humm JL, Seshan VE, Li GC, Ling CC. Comparison of helzel and oxylite systems in the measurements of tumor partial oxygen pressure (pO2) Radiat Res. 2008;169:67–75. doi: 10.1667/RR0888.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Subbaroyan J, Martin DC, Kipke DR. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. J Neural Eng. 2005;2:103. doi: 10.1088/1741-2560/2/4/006. [DOI] [PubMed] [Google Scholar]
- 91.Clark LC, Wolf R, Granger D, Taylor Z. Continuous recording of blood oxygen tensions by polarography. J Appl Physiol. 1953;6:189–193. doi: 10.1152/jappl.1953.6.3.189. [DOI] [PubMed] [Google Scholar]
- 92.Ma Y, Wu S. Simultaneous measurement of brain tissue oxygen partial pressure, temperature, and global oxygen consumption during hibernation, arousal, and euthermy in non-sedated and non-anesthetized Arctic ground squirrels. J Neurosci Methods. 2008;174:237–244. doi: 10.1016/j.jneumeth.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weiss HR, Cohen JA, McPherson LA. Blood flow and relative tissue PO2 of brain and muscle: effect of various gas mixtures. Am J Physiol Content. 1976;230:839–844. doi: 10.1152/ajplegacy.1976.230.3.839. [DOI] [PubMed] [Google Scholar]
- 94.Ma Y, Wu S, Rasley B, Duffy L. Adaptive response of brain tissue oxygenation to environmental hypoxia in non-sedated, non-anesthetized arctic ground squirrels. Comp Biochem Physiol A Mol Integr Physiol. 2009;154:315–322. doi: 10.1016/j.cbpa.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bingmann D, Kolde G. PO 2-profiles in hippocampal slices of the guinea pig. Exp Brain Res. 1982;48:89–96. doi: 10.1007/BF00239575. [DOI] [PubMed] [Google Scholar]
- 96.Yonekura M, Austin G. Microelectrode for measuring local cortical oxygen tension and blood flow in the same microareas of cat cortex. Neurol Res. 1985;7:89–92. doi: 10.1080/01616412.1985.11739706. [DOI] [PubMed] [Google Scholar]
- 97.O’Hara JA, Hou H, Demidenko E, Springett RJ, Khan N, Swartz HM. Simultaneous measurement of rat brain cortex PtO2 using EPR oximetry and a fluorescence fiber-optic sensor during normoxia and hyperoxia. Physiol Meas. 2005;26:203. doi: 10.1088/0967-3334/26/3/006. [DOI] [PubMed] [Google Scholar]
- 98.Sakata Y, Grinberg O, Grinberg S, Springett R, Swartz H. Simultaneous NIR-EPR spectroscopy of rat brain oxygenation. Adv Exp Med Biol. 2005;566:357–362. doi: 10.1007/0-387-26206-7_47. [DOI] [PubMed] [Google Scholar]
- 99.Liu S, Timmins GS, Shi H, Gasparovic CM, Liu KJ. Application of in vivo EPR in brain research: monitoring tissue oxygenation, blood flow, and oxidative stress. NMR Biomed. 2004;17:327–334. doi: 10.1002/nbm.899. [DOI] [PubMed] [Google Scholar]
- 100.Sostaric JZ, Pandian RP, Bratasz A, Kuppusamy P. Encapsulation of a highly sensitive EPR active oxygen probe into sonochemically prepared microspheres. J Phys Chem B. 2007;111:3298–3303. doi: 10.1021/jp0682356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dinguizli M, Jeumont S, Beghein N, He J, Walczak T, Lesniewski PN, Hou H, Grinberg OY, Sucheta A, Swartz HM, Gallez B. Development and evaluation of biocompatible films of polytetrafluoroethylene polymers holding lithium phthalocyanine crystals for their use in EPR oximetry. Biosens Bioelectron. 2006;21:1015–22. doi: 10.1016/j.bios.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 102.Dunn JF, O’Hara JA, Zaim-Wadghiri Y, Lei H, Meyerand ME, Grinberg OY, Hou H, Hoopes PJ, Demidenko E, Swartz HM. Changes in oxygenation of intracranial tumors with carbogen: a BOLD MRI and EPR oximetry study. J Magn Reson Imaging. 2002;16:511–521. doi: 10.1002/jmri.10192. [DOI] [PubMed] [Google Scholar]
- 103.Dunn JF, Grinberg O, Roche M, Nwaigwe CI, Hou HG, Swartz HM. Noninvasive assessment of cerebral oxygenation during acclimation to hypobaric hypoxia. J Cereb Blood Flow Metab. 2000;20:1632–1635. doi: 10.1097/00004647-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 104.Rolett EL, Azzawi A, Liu KJ, Yongbi MN, Swartz HM, Dunn JF. Critical oxygen tension in rat brain: a combined 31 P-NMR and EPR oximetry study. Am J Physiol Regul Integr Comp Physiol. 2000;279:R9–R16. doi: 10.1152/ajpregu.2000.279.1.R9. [DOI] [PubMed] [Google Scholar]
- 105.Swartz HM. Vivo EPR ESR [Internet] Springer; 2003. The measurement of oxygen in vivo using EPR techniques; pp. 403–440. [DOI] [PubMed] [Google Scholar]
- 106.Owens G, Belmusto L, Woldring S. Experimental intracerebral pO2 and pCO2 monitoring by mass spectrography. J Neurosurg. 1969;30:110–115. doi: 10.3171/jns.1969.30.2.0110. [DOI] [PubMed] [Google Scholar]
- 107.Baudelet C, Gallez B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med. 2002;48:980–986. doi: 10.1002/mrm.10318. [DOI] [PubMed] [Google Scholar]
- 108.Seddon BM, Honess DJ, Vojnovic B, Tozer GM, Workman P. Measurement of tumor oxygenation: in vivo comparison of a luminescence fiber-optic sensor and a polarographic electrode in the p22 tumor. Radiat Res. 2001;155:837–846. doi: 10.1667/0033-7587(2001)155[0837:motoiv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 109.Vaupel P, Schlenger K, Knoop C, Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- 110.Nwaigwe CI, Roche MA, Grinberg O, Dunn JF. Effect of hyperventilation on brain tissue oxygenation and cerebrovenous PO2 in rats. Brain Res. 2000;868:150–156. doi: 10.1016/s0006-8993(00)02321-0. [DOI] [PubMed] [Google Scholar]
- 111.Opitz E. Increased vascularization of the tissue due to acclimatization to high altitude and its significance for the oxygen transport. Exp Med Surg. 1950;9:389–403. [PubMed] [Google Scholar]
- 112.Rodríguez FA, Casas H, Casas M, Pagés T, Rama R, Ricart A, Ventura JL, Ibáñez J, Viscor G. Intermittent hypobaric hypoxia stimulates erythropoiesis and improves aerobic capacity. Med Sci Sports Exerc. 1999;31:264–8. doi: 10.1097/00005768-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 113.Liu KJ, Bacic G, Hoopes PJ, Jiang J, Du H, Ou LC, Dunn JF, Swartz HM. Assessment of cerebral pO2 by EPR oximetry in rodents: effects of anesthesia, ischemia, and breathing gas. Brain Res. 1995;685:91–98. doi: 10.1016/0006-8993(95)00413-k. [DOI] [PubMed] [Google Scholar]
- 114.Lei H, Grinberg O, Nwaigwe CI, Hou HG, Williams H, Swartz HM, Dunn JF. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res. 2001;913:174–179. doi: 10.1016/s0006-8993(01)02786-x. [DOI] [PubMed] [Google Scholar]
- 115.Moore LG. Human genetic adaptation to high altitude. High Alt Med Biol. 2001;2:257–279. doi: 10.1089/152702901750265341. [DOI] [PubMed] [Google Scholar]
- 116.Keeley TP, Mann GE. Defining physiological normoxia for improved translation of cell physiology to animal models and humans. Physiol Rev. 2018;99:161–234. doi: 10.1152/physrev.00041.2017. [DOI] [PubMed] [Google Scholar]
- 117.Fallon S, Belcoe A, Shawcross C, May A, Monteverde C, McCann D. Elite female athletes’ ventilatory compensation to decreased inspired O2 during the wingate test. Res Q Exerc Sport. 2015;86:182–189. doi: 10.1080/02701367.2014.983448. [DOI] [PubMed] [Google Scholar]
- 118.Asher SR, Curry P, Sharma D, Wang J, O’Keefe GE, Daniel-Johnson J, Vavilala MS. Survival advantage and PaO2 threshold in severe traumatic brain injury. J Neurosurg Anesthesiol. 2013;25:168–173. doi: 10.1097/ANA.0b013e318283d350. [DOI] [PubMed] [Google Scholar]
- 119.McPhail IR, Cooper LT, Hodge DO, Cabanela ME, Rooke TW. Transcutaneous partial pressure of oxygen after surgical wounds. Vasc Med. 2004;9:125–127. doi: 10.1191/1358863x04vm539oa. [DOI] [PubMed] [Google Scholar]
- 120.Vella A, Carlson LA, Blier B, Felty C, Kuiper JD, Rooke TW. Circulator boot therapy alters the natural history of ischemic limb ulceration. Vasc Med. 2000;5:21–25. doi: 10.1177/1358836X0000500104. [DOI] [PubMed] [Google Scholar]
- 121.Höckel M, Knoop C, Schlenger K, Vorndran B, Baussmann E, Mitze M, Knapstein PG, Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993;26:45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- 122.Swartz HM, Williams BB, Hou H, Khan N, Jarvis LA, Chen EY, Schaner PE, Ali A, Gallez B, Kuppusamy P, Flood AB. Direct and repeated clinical measurements of pO2 for enhancing cancer therapy and other applications. Adv Exp Med Biol. 2016;923:95–104. doi: 10.1007/978-3-319-38810-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ferdinand P, Roffe C. Hypoxia after stroke: a review of experimental and clinical evidence. Exp Transl Stroke Med. 2016;8:9. doi: 10.1186/s13231-016-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Epstein BS, Hardy DL, Harrison HN, Teplitz C, Villarreal Y, Mason AD Jr. Hypoxemia in the burned patient a clinical-pathologic study. Ann Surg. 1963;158:924. doi: 10.1097/00000658-196312000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Soussi S, Vallée F, Roquet F, Bevilacqua V, Benyamina M, Ferry A, Cupaciu A, Chaussard M, De Tymowski C, Boccara D, Mimoun M, Chaouat M, Anstey J, Mebazaa A, Legrand M PRONOBURN study group. Measurement of oxygen consumption variations in critically ill burns patients: are the fick method and indirect calorimetry interchangeable? Shock. 2017;48:532–538. doi: 10.1097/SHK.0000000000000885. [DOI] [PubMed] [Google Scholar]


