Abstract
Background: Chronic inflammation and infections are associated with increased risk of prostate cancer development. There is considerable evidence that proves the interrelationship between bacterial/viral infections and carcinogenesis. Periodontitis is a chronic inflammatory disease triggered by gram-negative anaerobic bacteria. In this narrative review, we investigate the relationship between periodontal disease and prostate cancer by reviewing previous studies of the association and possible mechanisms that may explain this link. Methods: A comprehensive search for articles published was performed using the key words, “periodontal disease”, “prostate disease”, “prostate cancer”, “prostatic inflammation”. Thorough reviews of each study were conducted and assessed for eligibility, and data was summarized. Results: The role of inflammatory responses in the prostate as drivers of malignancy appears to be predisposed by periodontal pathogens and/or periodontitis inflammatory mediators. Conclusion: Periodontal diseases might be associated with prostate cancer. However, the mechanism(s) explaining this relationship remains unclear and requires further elucidation.
Keywords: Prostate cancer, periodontal diseases, prostatic inflammation, benign prostate hypertrophy
Introduction
Prostate cancer is the most frequent cancer to occur in males and second foremost cause of cancer-related death in the Western world [1]. According to an estimate by GLOBOCAN database, approximately 1.1 million men worldwide were diagnosed with prostate cancer in 2012, accounting for 15% of the cancers diagnosed in men [1,2]. In the United States, prostate cancer is the second leading cause of cancer-related death in men, behind lung cancer [3]. According to an estimate by the American Cancer Society, in 2019 approximately 164,690 new cases of prostate cancer will be diagnosed, and 29,430 deaths will occur from the disease [4]. In recent years, prostate cancer is of significant concern due to the gradual increase in the number of aging men and the existence of more than 3 million men with the disease. Recent observations suggest that prostate cancer is associated with chronic inflammation [5,6]. Several factors might be responsible for prostate inflammation, especially the presence of pathoogens in the urine, and sexual activity [7]. Recent studies report an association between prostate cancer, periodontal diseases, and tooth loss [8,9].
Periodontal diseases are inflammatory conditions that have a local effect on the supporting structure of the teeth, which may lead to tooth loss and contribute to systemic inflammation [10]. Chronic periodontitis predominantly affects the tissues surrounding the teeth in response to accumulation of a subgingival biofilm, which consists of over 700 bacterial species [11,12], many protozoa species [13] and viruses [14]. Of these, the most often recognized periodontal microbes include Actinobacillus actinomycetemcomitans, Prevotella intermedia, Porphyromonas gingivalis, Bacteroides forsythus, Campylobacter rectus, Treponema denticola, Fusobacterium nucleatum, Eubacterium, and Spirochetes (sp) [14,15]. Additionally, various human herpes viruses, such as Epstein-Barr virus (EBV-1), and cytomegalovirus (HCMV) have also been detected [16]. The subgingival biofilm may induce a host release of pro-inflammatory mediators that elicit a chronic inflammatory response, leading to alveolar bone loss [10]. Furthermore, studies have indicated that oral health may influence systemic health [10]. Several reports showed an association between periodontal disease and systemic conditions such as cardiovascular disease [10], type 2 diabetes mellitus [10], preterm low birth weight infants [17-20], osteoporosis [10], rheumatoid arthritis [21,22], Parkinson’s disease [23], Alzheimer’s disease [24], psoriasis [25], respiratory functions [26] and several types of human cancers [27]. In particular, individuals with periodontal disease have a greater risk of cancer overall and site-precise malignancies including oral [28], gastrointestinal [29], lung [9], pancreatic [30], hematologic [31] and prostate [8,9]. It has been reported that chronic inflammatory response to periodontal infection impacts beyond the oral cavity [22] and might increase the risk of various malignancies [23-25]. This association is supported by the effectiveness of anti-inflammatory drugs in reducing the risk of oesophageal, gastric, colorectal, biliary, breast, pancreatic [21], and genitourinary cancers [26,27]. The aim of this review is to advance the hypothesis that periodontal diseases may be significant in its contribution to prostate inflammation and cancer.
Materials and methods
An electronic search was carried out in PubMed database until August 2018 for all relevant publications that explored the association between periodontal diseases and/or periodontal microbes in prostate inflammation and malignancy. The following MeSH terms were used, “Periodontal Disease [All Field]”, “Prostate disease [MeSH terms]”, “Prostate cancer [MeSH terms]”, “Prostatic Inflammation [MeSH terms]”. Literature published in the English language was included.
Results and discussion
Association between periodontal disease and prostate cancer
A growing number of reports suggest that periodontal disease is significantly associated with an increased risk of prostate cancer [8,9]. Arora et al. (2010), in a prospective co-twin study, reported an association of prostate cancer and periodontal disease measured by questionnaire-recorded tooth mobility and prostate cancer (hazard ratio 1.47) [8]. In another investigation, Hujoel et al. (2003), in a prospective cohort study, evaluated data from 11,328 individuals and verified a correlation between prostate cancer and periodontal disease (odds ratio 1.81) [9]. Despite the small sample size of (20 cases) of prostate cancer, this study was the only one to include a direct evaluation of the periodontal condition and establish a positive association between both diseases [9]. A study in Taiwan, by Hwang et al. (2014) showed that periodontal treatment consisting of scaling and root planing, subgingival curettage and periodontal flap surgery decreased the risk of cancer of the gastrointestinal tract, lungs, brain, and female reproductive organs [32]. However, the risk of prostate and thyroid cancers was significantly greater even after periodontal treatment [32]. Michaud and Hiraki (2008) reported an inverse association between the number of teeth lost and the risk of prostate cancer [31,33]. However, the tooth loss is not necessarily representative of periodontal disease; for instance, an individual may lose teeth due to caries or fracture. Furthermore, in a more recent study it was found that periodontal disease was associated with a 14% higher risk of prostate cancer [34]. Overall, these studies indicate that, an association probably exists between periodontal diseases and prostate cancer, see Table 1. Joshi et al. (2010) from Case Western Reserve University evaluated serum prostate-specific antigen (PSA) levels as a marker of inflammation in patients with prostatitis and periodontitis and showed that subjects with both diseases have greater levels of PSA compared with either disease alone [35]. Furthermore, Alwithanani et al. (2015) reported the impact of treatment of periodontal disease on clinical symptoms of prostatitis showing that periodontal treatment improves prostate symptoms and lowered serum PSA levels in patients having both periodontitis and prostatitis [36]. Estemalik et al. (2017) further substantiated the association between periodontitis and prostatitis, as evidenced by the presence of similar oral bacteria such as P. gingivalis and T. denticola DNA in both the expressed prostatic secretion (EPS) and dental plaque of the same patient [37]. This finding support the hypothesis that periodontal pathogens may be migrating through the systemic circulation to initiate an infection and inflammatory response in the prostate. The prostate inflammatory responses may in turn induce neoplastic transformations.
Table 1.
Studies on the relationship between periodontal disease and prostate cancer
| Author (Year) | Study Design | Purpose/Criteria | Results/Conclusions |
|---|---|---|---|
| Lee et al. (2017) [34] | Cohort | To investigate the association between PD and PC using records from the National Health Insurance Service-Health Examinee Cohort (NHIS-HEC). | The incidence of PC with PD in men with 40 and above age group was 0.28%. PD was linked with a 14% greater hazard of PC. |
| Ing-ming Hwang et al. (2014) [32] | Cohort | To assess the effect of treatment of PD and the risks for cancers in Taiwan. Therapy included scaling and root planing, subgingival curettage and periodontal flap surgery. | Treatment of PD reduces the risk of overall cancer and individual cancers. |
| Risk of PC was higher in the treatment cohort group. | |||
| Arora et al. (2009) [8] | Prospective co-twin study | Investigation of hereditary risk factors between periodontal disease and cancers. PD measured by questionnaire-recorded tooth mobility. | PD and PC (Hazard ratio 1.47) |
| Hiraki et al. (2008) [33] | Case-control | To determine the significance of tooth loss on the risk of 14 cancers. The teeth loss was categorized into four groups depending on the number of remaining dentition | An inverse association between the number of teeth loss and risk of PC |
| Michaud et al. (2008) [31] | Cohort | To determine if PD or teeth loss is associated with cancer risk. Individuals surveyed on the history of PD with bone loss, number of remaining teeth; status and history of smoking; frequency of food intake; any new diagnose of cancer. Survey obtained at baseline and in all subsequent follow-up. | PD was associated with risk of overall cancer and individual cancers. An inverse association between the number of teeth loss and risk of prostate cancer |
| Hujoel et al. (2003) [9] | Cohort | To investigate PD and cancer relationship. Individuals with PD or gingivitis, a healthy periodontium or edentulous were identified at the commencement of the follow-up. These groups are identified by the teams of dentists and trained recorders evaluated the periodontal status of individual participants. The diagnosis of cancer was determined from death certificates. | Demonstrated a correlation between PC and PD measured by Russell’s periodontal index (odds ratio 1.81). |
| Increased risk of PC in individuals with the PD. |
Acronyms: PD - periodontal disease. PC - prostate cancer.
Chronic inflammation and prostate cancer
Grading and types of prostate inflammation
Several studies have suggested that inflam-mation is common within the prostate [5]. The National Institutes of Health (NIH) consensus classification refers chronic intraprostatic inflammation as chronic prostatitis (CP)/chronic pelvic pain syndrome (CPSS) [38]. According to NIH, prostatitis is classified into four categories: (1) acute bacterial prostatitis (category I); (2) chronic bacterial prostatitis (category II); (3) CP/CPPS (category III); and (4) asymptomatic inflammatory prostatitis (category IV) [38]. CP/CPPS (category III) is the most common prostatitis syndrome, covering approximately 95% of prostatitis cases [38]. This condition is extremely common in adults: 2-10% of adults suffer from symptoms compatible with chronic prostatitis, and approximately 15% of men suffer from symptoms of prostatitis [39]. Subgroup IIIA of this category consists of individuals with white blood cells in their prostatic fluid, post-prostate massage urine/or seminal fluid [39], and subgroup IIIB consist of individuals without leukocytes in any fluid [38]. It has been reported that genitourinary tract pathogens such as Chlamydia trachomatis, Ureaplasma urealyticum, protozoan pathogen Trichomonas vaginalis, Neisseria gonorrhea, herpes simplex types I and II, and cytomegalovirus may be playing a role in CP/CPPS [40-44]. Category IV, asymptomatic inflammatory prostatitis, is present in patients undertaking examination for other urogenital conditions, such as increased PSA [38]. The estimated prevalence of category IV prostatitis is 11.2-32.3% in patients with elevated PSA [45]. It has been reported by Roberts et al. (2004) that prostatitis category III was associated with prostate malignancy whereas category I, II, IV was not [46,47].
Chronic inflammation-mediated histological changes in the prostate
Epidemiological, histopathological, and in vivo investigations have determined an association between chronic inflammation and an increased risk of prostate cancer [5,48,49]. However, the significance of inflammation of the prostate gland, and the mechanisms that lead to the development of prostate cancer are still unclear. Numerous meta-analysis and case-control studies have shown that men with prostatitis have a considerable increase risk of having prostate cancer. Daniels et al. (2005) conducted a prospective cohort study of 5821 men, and showed an association between a history of prostatitis and a history of prostate cancer (OR 5.4, 95% CI 4.4-6.6) [50]. Dennis et al. (2002) reported in a meta-analysis of 11 case-control studies that there is an increased risk of prostate malignancy among men with a history of prostatitis (odds ratio = 1.6) [48]. The presence of inflammatory cell infiltrates and proliferative inflammatory atrophy (PIA) are often detected in histologic samples of benign and malignant prostates [51-54]. PIA consists of lesions that might include epithelial atrophy [51,52,55,56] low apoptotic index, and an increased proliferative index [51,55], typically with inflammation characterized by mononuclear infiltrates in the periglandular stroma or by macrophages and neutrophils in the glandular lumina or epithelium. PIA is an inflammatory lesion emerging as a result of infection or cell injury subsequent from free radical toxicity, hypoxia, infection or autoimmunity. These lesions may lead to malignancy through an accumulation of transmutation in rapidly dividing cells. PIA is probably a precursor lesion to cancer and frequently exist near to high-grade prostatic intraepithelial neoplasia (HGPIN) or early cancer. It has been determined that a genetic association between PIA, HGPIN, and cancer exists [51]. MacLennan et al. (2006), in a five years follow-up study, examined prostate biopsy specimens of men with clinically abnormal findings and reported that the majority had chronic inflammation of prostate gland and that 6% of these patients had adenocarcinomas [5]. These studies suggest that the history of chronic inflammation is associated with an increased risk for prostate cancer. Elkahwaji et al. (2009), in a 25 weeks follow-up study, using an animal model of chronic prostatitis induced by E. coli infection, detected the development of prostate hyperplasia and dysplasia [56]. Furthermore, the prostate epithelium exhibited positive nuclear staining for 8-hydroxy-20-deoxyguanosine, a biomarker of DNA injury due to free radical toxicity [56]. This study provides evidence that prostatitis may trigger the development of prostate cancer.
Role of infectious organisms in prostatic cancer
Periodontal microorganisms
Dental biofilm is an organized mass, consisting mainly of microorganisms, that adheres to teeth [57,58]. In the gingival crevice and periodontal pocket, a dysbiotic biofilm is one of the main factors contributing to periodontal diseases [59]. Nearly 50% of the American populations have chronic periodontitis as manifested by alveolar bone resorption [61]. There is an association between periodontal diseases and systemic diseases [10,62,63]. This link may be due to a metastatic, spreading/diffusion of periodontal bacteria and/or bacterial toxins [63] to other parts of the body. Many authors have provided a comprehensible correlation among two or more systemic diseases with periodontitis [62-66].
For instance, oral microbial pathogens are associated with undesirable pregnancy outcomes [67,68]. Periodontal pathogens P. gingivalis, F. nucleatum [68,70,71] and A. actinomycetemcomitans [69,70] have the ability to cross the placental barrier and have been detected in the amniotic fluid of women with preterm low-birth-weight infants [71-73]. Additionally, F. nucleatum DNA is identified in the synovial fluid of individuals who have rheumatoid arthritis [75]. Recent studies link periodontal bacteria to prostate inflammation and cancers [76,77]. In a study conducted in the Department of Periodontics at CWRU, Estemalik et al. (2017) detected DNA of the periodontitis pathogens P. gingivalis, T. denticola, and E. coli in prostatic secretions of men with both periodontal and prostate diseases [37]. Oral microbes migrate to other parts of the body via the bloodstream, causing chronic inflammation that may initiate neoplastic transformation, the first step towards malignancy [71]. A series of studies showed the association of F. nucleatum species to colorectal carcinomas [78-80]. Also, Hwang mentioned that studies showed the presence of fusobacteria in normal tissue next to colon cancer [32]. According to Jacob et al. (2016) individuals with P. gingivalis in their oral cavity had a 59% increased risk of pancreatic cancer than those who did not harbor this microbe [81]. The authors also reported that individuals with A. actinomycetemcomitans in their oral cavity had a 50% greater chance of developing pancreatic cancer [81]. Furthermore, Michaud et al. (2012) showed that individuals with increased levels of antibodies to P. gingivalis are at significant incresed risk of pancreatic cancer [82]. Fan et al. (2018) demonstrated that P. gingivalis and A. actinomycetemcomitans were associated to a higher threat of pancreatic cancer [83]. P. gingivalis and A. actinomycetemcomitans are capable of commencing the Toll-like receptor (TLR) signaling pathways, which are significant for the progression of pancreatic carcinogenesis [84]. Overall, the information presented provides a strong association between periodontal microorganism and the risk of development of several human cancers.
Sexually transmitted microorganisms
Pathogens of venereal diseases may affect the prostate by eliciting an inflammatory response. The prostate, like other organs, produces a normal immune response after exposure to an antigen. The etiology of prostatic inflammation may involve an infectious component [48]. The microbial pathogens in urinary tract infections are similar to bacterial prostatitis in category and distribution [85]. Bacterial prostatitis may be an outcome of ascending urinary tract infections, resulting from urethral inoculation during sexual intercourse [85]. In addition, sexually transmitted agents have been identified in the prostate, such as the human papillomavirus, human herpes simplex virus type 2, cytomegalovirus, Neisseria gonorrhea, Chlamydia trachomatis, Treponema pallidum, Trichomonas vaginalis, and some Gram-negative pathogens, such as Escherichia coli [49,86]. Epstein-Barr virus (EBV) and cytomegalovirus’s (CMV) may cause mononucleosis, well-known as ‘kissing disease’ by spreading through direct mouth-to-mouth contact [87,88]. Interestingly, these organisms are risk factors for the development of periodontitis [16,89]. Patients with periodontal conditions associated with the subgingival viruses recover upon antiviral therapy (500 mg valaciclovir, Valtrex, orally twice daily over 10 days), which suggests a clinical role for these viruses in periodontitis [90,91]. Therefore, these organisms may be involved in the development of both diseases.
Role of periodontal and prostate inflammatory mediators on prostate cancer
Periodontitis inflammatory mediators and its expression
Periodontal pathogens and/or microbes harboring LPS endotoxins induce innate immune pathways such as toll-like receptors (TLR). Activation of these pathways causes downstream synthesis and release of pro-inflammatory mediators such as IL-1β, IL-6, TNF-α, PGE2, and matrix metalloproteinases [92-95]. These molecules may enter into systemic circulation leading to an increase in systemic inflammatory markers [94]. The presence of IL-6 in the systemic circulation is associated with the severity of disease [94]. IL-6 is the major procoagulant cytokine that may stimulate cells of the liver to produce acute-phase proteins such as C-reactive protein (CRP), fibrinogen and plasminogen activator inhibitor 1 [94]. The chronic and elevated levels of CRP in the systemic circulation may indicate risk for myocardial infarction, stroke, and peripheral arterial disease even in healthy individuals [96]. In the periodontium, the control of infection/inflammation may inhibit markers of systemic inflammation and dysfunction of endothelial cells within 2-6 months [97]. Locally chronic inflammation results in hypoxia, which leads to the release of nitric oxide and other reactive oxygen species (ROS) [6]. This leads to the conversion of arachidonic acid by cyclooxygenases into prostaglandin, which acts on the regulation of cell proliferation [6]. Also, hypoxia activates the discharge of vascular endothelial growth factors (VEGF), triggering neoangiogenesis and fibroblast differentiation, which are developmental factors in prostatic hyperplasia or neoplasm [98]. Therefore, the pro-inflammatory cells and mediators in periodontal disease result in cellular proliferation, angiogenesis, mutagenesis, decrease adaptation to oxidative stress, and suppression of apoptosis [99].
Inflammatory cytokines induction in neoplastic tissue
Inflammatory mediators generated during prostatitis or in chronic inflammatory diseases in other parts of the body such as in periodontitis may promote neoplastic tissue development [5,27,46,48,50,56,60,100]. These mediators may be involved in the communication between inflammatory cells and prostate tumor cells [100]. For instance, interleukin-1 (IL-1) promotes proliferation [101], growth, and progression of the prostate epithelium and connective tissue stroma [102,103]. IL-6 is capable of manipulating neoplastic developments such as reduction of apoptosis and upturn of angiogenesis [104]. In addition, IL-6 and TNF-alpha enhance proliferation and promote survival signal for different cancer cell types, including neoplastic prostate cell lines [51]. Nelson et al. (2004) suggested that these cells may influence the development of neoplasm [105]. In prostate cancer, both TNF-alpha and IL-6 serum levels are elevated and correlate to advance-stage disease and decreased patient survival [106].
Conclusions and future directions
Periodontal disease appears to be associated with prostate cancer. To date, there is no definite mechanism(s) that can explain this relationship. It is hypothesized that this association might be influenced by periodontal pathogens and/or periodontitis inflammatory mediators. The host response to periodontal pathogens and/or any infectious agents could contribute to an exacerbated inflammatory response and PIA/PIN in the prostate that may progress to cancer. In addition, certain periodontal pathogens are associated with prostatitis. These pathogens may be migrating from the periodontium to the prostate gland through the bloodstream and contributing to prostatic inflammation and cancer development (Figure 1). Further studies are warranted to better characterize the role of periodontal pathogens in initiating malignancy in the prostate gland.
Figure 1.
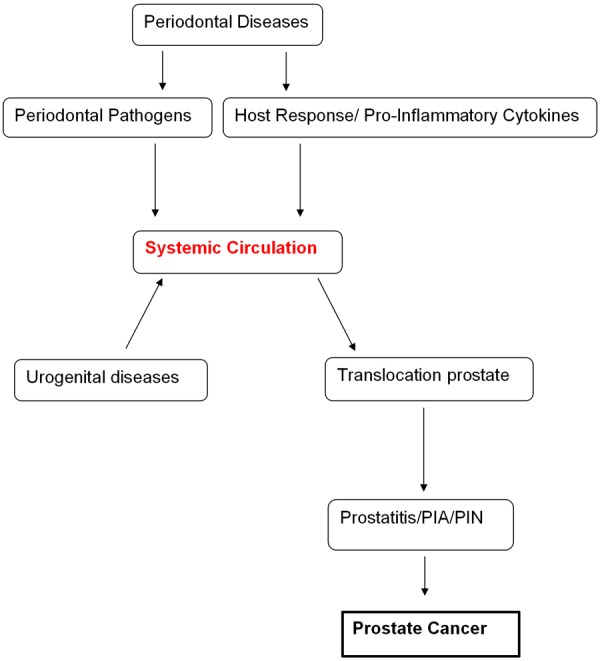
Association of periodontal disease and prostate cancer.
Disclosure of conflict of interest
None.
Abbreviations
- PD
Periodontal Disease, Prostate disease
- PIA
Proliferative Inflammatory Atrophy
- PC
Prostate cancer
References
- 1.Gaudreau PO, Stagg J, Soulières D, Saad F. The present and future of biomarkers in prostate cancer: proteomics, genomics, and immunology advancements. Biomark Cancer. 2016;8:15–33. doi: 10.4137/BIC.S31802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Xie T, Dong B, Yan Y, Hu G, Xu Y. Association between MMP-2 expression and prostate cancer: a meta-analysis. Biomed Rep. 2016;4:241–5. doi: 10.3892/br.2015.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 5.MacLennan GT, Eisenberg R, Fleshman RL, Taylor JM, Fu P, Resnick MI, Gupta S. The influence of chronic inflammation in prostatic carcinogenesis: a 5-year followup study. J Urol. 2006;176:1012–6. doi: 10.1016/j.juro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, Nelson WG. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–81. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 7.Dennis LK, Dawson DV. Meta-analysis of measures of sexual activity and prostate cancer. Epidemiology. 2002;13:72–9. doi: 10.1097/00001648-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Arora M, Weuve J, Fall K, Pedersen NL, Mucci LA. An exploration of shared genetic risk factors between periodontal disease and cancers: a prospective co-twin study. Am J Epidemiol. 2010;171:253–9. doi: 10.1093/aje/kwp340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hujoel PP, Drangsholt M, Spiekerman C, Weiss NS. An exploration of the periodontitis-cancer association. Ann Epidemiol. 2003;13:312–6. doi: 10.1016/s1047-2797(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore W, Moore LV. The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 12.Paes Batista da Silva A, Barros SP, Moss K, Preisser J, Marchesan JT, Aspiras M, Ward M, Offenbacher S. Microbial profiling in experimentally induced biofilm overgrowth among patients with various periodontal states. J Periodontol. 2016;87:27–35. doi: 10.1902/jop.2015.150328. [DOI] [PubMed] [Google Scholar]
- 13.Ghabanchi J, Zibaei M, Afkar MD, Sarbazie A. Prevalence of oral entamoeba gingivalis and trichomonas tenax in patients with periodontal disease and healthy population in Shiraz, southern Iran. Indian J Dent Res. 2010;21:89–91. doi: 10.4103/0970-9290.62821. [DOI] [PubMed] [Google Scholar]
- 14.Saygun I, Şahin S, Özdemir A, Kurtiş B, Yapar M, Kubar A, Özcan G. Detection of human viruses in patients with chronic periodontitis and the relationship between viruses and clinical parameters. J Periodontol. 2002;73:1437–43. doi: 10.1902/jop.2002.73.12.1437. [DOI] [PubMed] [Google Scholar]
- 15.Socransky S, Haffajee A, Cugini M, Smith C, Kent R. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 16.Slots J, Kamma J, Sugar C. The herpesvirus-Porphyromonas gingivalis-periodontitis axis. J Periodontal Res. 2003;38:318–23. doi: 10.1034/j.1600-0765.2003.00659.x. [DOI] [PubMed] [Google Scholar]
- 17.Jeffcoat MK, Hauth JC, Geurs NC, Reddy MS, Cliver SP, Hodgkins PM, Goldenberg RL. Periodontal disease and preterm birth: results of a pilot intervention study. J Periodontol. 2003;74:1214–8. doi: 10.1902/jop.2003.74.8.1214. [DOI] [PubMed] [Google Scholar]
- 18.Davenport E, Williams C, Sterne J, Murad S, Sivapathasundram V, Curtis M. Maternal periodontal disease and preterm low birthweight: case-control study. J Dent Res. 2002;81:313–8. doi: 10.1177/154405910208100505. [DOI] [PubMed] [Google Scholar]
- 19.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72:2272–9. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG, Mauriello SM, Moss KL, Beck JD. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006;107:29–36. doi: 10.1097/01.AOG.0000190212.87012.96. [DOI] [PubMed] [Google Scholar]
- 21.Mercado F, Marshall R, Klestov A, Bartold P. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–87. doi: 10.1902/jop.2001.72.6.779. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz P, Bissada N, Palomo L, Han Y, Al-Zahrani M, Panneerselvam A, Askari A. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol. 2009;80:535–40. doi: 10.1902/jop.2009.080447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller T, Palluch R, Jackowski JJ. Caries and periodontal disease in patients with Parkinson’s disease. Spec Care Dentist. 2011;31:178–81. doi: 10.1111/j.1754-4505.2011.00205.x. [DOI] [PubMed] [Google Scholar]
- 24.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimer’s Dement. 2008;4:242–50. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Preus HR, Khanifam P, Kolltveit K, Mørk C, Gjermo P. Periodontitis in psoriasis patients. A blinded, case-controlled study. Acta Odontol Scand. 2010;68:165–70. doi: 10.3109/00016350903583678. [DOI] [PubMed] [Google Scholar]
- 26.Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8:54–69. doi: 10.1902/annals.2003.8.1.54. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick SG, Katz J. The association between periodontal disease and cancer: a review of the literature. J Dent. 2010;38:83–95. doi: 10.1016/j.jdent.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Yao QW, Zhou DS, Peng HJ, Ji P, Liu DS. Association of periodontal disease with oral cancer: a meta-analysis. Tumor Biol. 2014;35:7073–7. doi: 10.1007/s13277-014-1951-8. [DOI] [PubMed] [Google Scholar]
- 29.Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol. 2005;34:467–74. doi: 10.1093/ije/dyh375. [DOI] [PubMed] [Google Scholar]
- 30.Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99:171–5. doi: 10.1093/jnci/djk021. [DOI] [PubMed] [Google Scholar]
- 31.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9:550–8. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang IM, Sun LM, Lin CL, Lee CF, Kao CH. Periodontal disease with treatment reduces subsequent cancer risks. QJM. 2014;107:805–12. doi: 10.1093/qjmed/hcu078. [DOI] [PubMed] [Google Scholar]
- 33.Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008;17:1222–7. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Kweon HH, Choi JK, Kim YT, Choi SH. Association between periodontal disease and prostate cancer: results of a 12-year longitudinal cohort study in South Korea. J Cancer. 2017;8:2959. doi: 10.7150/jca.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi N, Bissada NF, Bodner D, MacLennan GT, Narendran S, Jurevic R, Skillicorn R. Association between periodontal disease and prostate-specific antigen levels in chronic prostatitis patients. J Periodontol. 2010;81:864–9. doi: 10.1902/jop.2010.090646. [DOI] [PubMed] [Google Scholar]
- 36.Alwithanani N, Bissada NF, Joshi N, Bodner D, Demko C, MacLennan GT, Skillicorn R, Ponsky L, Gupta S. Periodontal treatment improves prostate symptoms and lowers serum PSA in men with high PSA and chronic periodontitis. Dentistry. 2015;5:1. [Google Scholar]
- 37.Estemalik J, Demko C, Bissada NF, Joshi N, Bodner D, Shankar E, Gupta S. Simultaneous detection of oral pathogens in subgingival plaque and prostatic fluid of men with periodontal and prostatic diseases. J Periodontol. 2017;88:823–829. doi: 10.1902/jop.2017.160477. [DOI] [PubMed] [Google Scholar]
- 38.Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–7. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 39.Nickel JC, Alexander RB, Anderson R, Berger R, Comiter CV, Datta NS, Fowler JE, Krieger JN, Landis JR, Litwin MS, McNaughton-Collins M. Category III chronic prostatitis/chronic pelvic pain syndrome: insights from the national institutes of health chronic prostatitis collaborative research network studies. Curr Urol Rep. 2008;9:320–7. doi: 10.1007/s11934-008-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasini M, Kotarski V, Škerk V, Markotić A, Andrašević AT, Lepej SŽ, Maleković G, Sternak SL, Škerk V. The significance of Chlamydia trachomatis in prostatitis syndrome. J Chemother. 2014;26:382–4. doi: 10.1179/1973947814Y.0000000165. [DOI] [PubMed] [Google Scholar]
- 41.Badalyan R, Fanarjyan S, Aghajanyan I. Chlamydial and ureaplasmal infections in patients with nonbacterial chronic prostatitis. Andrologia. 2003;35:263–5. [PubMed] [Google Scholar]
- 42.Skerk V, Schönwald S, Granić J, Krhen I, Barsić B, Mareković I, Roglić S, Desnica B, Zeljko Z. Chronic prostatitis caused by Trichomonas vaginalis-diagnosis and treatment. J Chemother. 2002;14:537–8. doi: 10.1179/joc.2002.14.5.537. [DOI] [PubMed] [Google Scholar]
- 43.Kim TH, Kim HR, Myung SC. Detection of nanobacteria in patients with chronic prostatitis and vaginitis by reverse transcriptase polymerase chain reaction. Korean J Urol. 2011;52:194–9. doi: 10.4111/kju.2011.52.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leskinen MJ, Vainionp R, Syrjnen S, Leppilahti M, Marttila T, Kylml T, Tammela TL. Herpes simplex virus, cytomegalovirus, and papillomavirus DNA are not found in patients with chronic pelvic pain syndrome undergoing radical prostatectomy for localized prostate cancer. Urology. 2003;61:397–401. doi: 10.1016/s0090-4295(02)02166-0. [DOI] [PubMed] [Google Scholar]
- 45.Carver BS, Bozeman CB, Williams B, Venable DD. The prevalence of men with National Institutes of Health category IV prostatitis and association with serum prostate specific antigen. J Urol. 2003;169:589–91. doi: 10.1097/01.ju.0000042720.98483.08. [DOI] [PubMed] [Google Scholar]
- 46.Roberts RO, Bergstralh EJ, Bass SE, Lieber MM, Jacobsen SJ. Prostatitis as a risk factor for prostate cancer. Epidemiology. 2004;15:93–9. doi: 10.1097/01.ede.0000101022.38330.7c. [DOI] [PubMed] [Google Scholar]
- 47.Doluoglu OG, Ceylan C, Kilinc F, Gazel E, Resorlu B, Odabas O. Is there any association between national institute of health category IV prostatitis and prostate-specific antigen levels in patients with low-risk localized prostate cancer? Int Braz J Urol. 2016;42:346–50. doi: 10.1590/S1677-5538.IBJU.2015.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 49.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daniels NA, Ewing SK, Zmuda JM, Wilt TJ, Bauer DC. Correlates and prevalence of prostatitis in a large community-based cohort of older men. Urology. 2005;66:964–70. doi: 10.1016/j.urology.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 51.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–92. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–32. doi: 10.1016/s0090-4295(00)00776-7. [DOI] [PubMed] [Google Scholar]
- 53.Vral A, Magri V, Montanari E, Gazzano G, Gourvas V, Marras E, Perletti G. Topographic and quantitative relationship between prostate inflammation, proliferative inflammatory atrophy and low-grade prostate intraepithelial neoplasia: a biopsy study in chronic prostatitis patients. Int J Oncol. 2012;41:1950–8. doi: 10.3892/ijo.2012.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perletti G, Montanari E, Vral A, Gazzano G, Marras E, Mione S, Magri V. Inflammation, prostatitis, proliferative inflammatory atrophy: ‘Fertile ground’for prostate cancer development? Mol Med Rep. 2010;3:3–12. doi: 10.3892/mmr_00000211. [DOI] [PubMed] [Google Scholar]
- 55.Ruska KM, Sauvageot J, Epstein JI. Histology and cellular kinetics of prostatic atrophy. Am J Surg Pathol. 1998;22:1073–7. doi: 10.1097/00000478-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Elkahwaji J, Hauke R, Brawner C. Chronic bacterial inflammation induces prostatic intraepithelial neoplasia in mouse prostate. Br J Cancer. 2009;101:1740. doi: 10.1038/sj.bjc.6605370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Listgarten MA. The structure of dental plaque. Periodontol 2000. 1994;5:52–65. doi: 10.1111/j.1600-0757.1994.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 58.Bowen WH, Burne RA, Wu H, Koo H. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–42. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 61.Eke PI, Dye B, Wei L, Thornton-Evans G, Genco R. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–20. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 62.Scannapieco FA. Position paper of the American academy of periodontology: periodontal disease as a potential risk factor for systemic diseases. J Periodontol. 1998;69:841–50. [PubMed] [Google Scholar]
- 63.Dyke TE, Winkelhoff AJ. Infection and inflammatory mechanisms. J Clin Periodontol. 2013;40(Suppl 14):S1–7. doi: 10.1111/jcpe.12088. [DOI] [PubMed] [Google Scholar]
- 64.Al-Katma MK, Bissada NF, Bordeaux JM, Sue J, Askari AD. Control of periodontal infection reduces the severity of active rheumatoid arthritis. J Clin Rheumatol. 2007;13:134–7. doi: 10.1097/RHU.0b013e3180690616. [DOI] [PubMed] [Google Scholar]
- 65.Ozmeric N, Bissada N, Paes B da Silva A. The association between inflammatory bowel disease and periodontal conditions: is there a common bacterial etiology? J Int Acad Periodontol. 2018;20/2:40–51. [PubMed] [Google Scholar]
- 66.Paes Batista da Silva A, Bissada NF. Arthritis and periodontitis: an association debated for two centuries. Curr Rheumatol Rev. 2016;12:202–207. doi: 10.2174/1573397111666151026223058. [DOI] [PubMed] [Google Scholar]
- 67.Ebersole JL, Novak MJ, Michalowicz BS, Hodges JS, Steffen MJ, Ferguson JE, DiAngelis A, Buchanan W, Mitchell DA, Papapanou PN. Systemic immune responses in pregnancy and periodontitis: relationship to pregnancy outcomes in the obstetrics and periodontal therapy (OPT) study. J Periodontol. 2009;80:953–60. doi: 10.1902/jop.2009.080464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.León R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, Martinez M, Gamonal J. Detection of porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2007;78:1249–55. doi: 10.1902/jop.2007.060368. [DOI] [PubMed] [Google Scholar]
- 69.Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol. 2007;78:670–6. doi: 10.1902/jop.2007.060362. [DOI] [PubMed] [Google Scholar]
- 70.Swati P, Thomas B, Vahab SA, Kapaettu S, Kushtagi P. Simultaneous detection of periodontal pathogens in subgingival plaque and placenta of women with hypertension in pregnancy. Arch Gynecol Obstet. 2012;285:613–9. doi: 10.1007/s00404-011-2012-9. [DOI] [PubMed] [Google Scholar]
- 71.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72:2272–9. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han YW, Wang X. Mobile microbiome oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485–91. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tateishi F, Hasegawa-Nakamura K, Nakamura T, Oogai Y, Komatsuzawa H, Kawamata K, Douchi T, Hatae M, Noguchi K. Detection of Fusobacterium nucleatum in chorionic tissues of high risk pregnant women. J Clin Periodontol. 2012;39:417–24. doi: 10.1111/j.1600-051X.2012.01855.x. [DOI] [PubMed] [Google Scholar]
- 74.Cahill R, Tan S, Dougan G, O’Gaora P, Pickard D, Kennea N, Sullivan MH, Feldman RG, Edwards AD. Universal DNA primers amplify bacterial DNA from human fetal membranes and link Fusobacterium nucleatum with prolonged preterm membrane rupture. Mol Hum Reprod. 2005;11:761–6. doi: 10.1093/molehr/gah234. [DOI] [PubMed] [Google Scholar]
- 75.Témoin S, Chakaki A, Askari A, El-Halaby A, Fitzgerald S, Marcus RE, Han YW, Bissada NF. Identification of oral bacterial DNA in synovial fluid of arthritis patients with native and failed prosthetic joints. J Clin Rheumatol. 2012;18:117–21. doi: 10.1097/RHU.0b013e3182500c95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagenlehner FM, Elkahwaji JE, Algaba F, Bjerklund-Johansen T, Naber KG, Hartung R, Weidner W. The role of inflammation and infection in the pathogenesis of prostate carcinoma. BJU Int. 2007;100:733–7. doi: 10.1111/j.1464-410X.2007.07091.x. [DOI] [PubMed] [Google Scholar]
- 77.De Marzo AM, Nakai Y, Nelson WG. Inflammation, atrophy, and prostate carcinogenesis. Urol Oncol. 2007;25:398–400. doi: 10.1016/j.urolonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7:e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA, Holt RA. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, Baselga J. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–8. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacob JA. Study links periodontal disease bacteria to pancreatic cancer risk. JAMA. 2016;315:2653–4. doi: 10.1001/jama.2016.6295. [DOI] [PubMed] [Google Scholar]
- 82.Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjønneland A, Dahm CC, Overvad K, Jenab M, Fedirko V, Boutron-Ruault MC. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62:1764–70. doi: 10.1136/gutjnl-2012-303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Abnet CC, Stolzenberg-Solomon R, Miller G, Ravel J. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67:120–127. doi: 10.1136/gutjnl-2016-312580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch M, Jonnadula S. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med. 2015;212:2077–94. doi: 10.1084/jem.20142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meares E Jr. Infectious diseases. 2nd edtion. Philadelphia: WB Saunders Company; 1998. Urethritis, prostatitis, epididymitis, and orchitis; pp. 954–61. [Google Scholar]
- 86.De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schröder F, Sciarra A, Tubaro A. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60:106–17. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 87.Grinde B, Olsen I. The role of viruses in oral disease. J Oral Microbiol. 2010;2:2127. doi: 10.3402/jom.v2i0.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bravender T. Epstein-Barr virus, cytomegalovirus, and infectious mononucleosis. Adolesc Med State Art Rev. 2010;21:251–64. [PubMed] [Google Scholar]
- 89.Kamma JJ, Slots J. Herpesviral-bacterial interactions in aggressive periodontitis. J Clin Periodontol. 2003;30:420–6. doi: 10.1034/j.1600-051x.2003.20002.x. [DOI] [PubMed] [Google Scholar]
- 90.Sunde PT, Olsen I, Enersen M, Grinde B. Patient with severe periodontitis and subgingival Epstein-Barr virus treated with antiviral therapy. J Clin Virol. 2008;42:176–8. doi: 10.1016/j.jcv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Miller CS, Avdiushko SA, Kryscio RJ, Danaher RJ, Jacob RJ. Effect of prophylactic valacyclovir on the presence of human herpesvirus DNA in saliva of healthy individuals after dental treatment. J Clin Microbiol. 2005;43:2173–80. doi: 10.1128/JCM.43.5.2173-2180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salvi GE, Beck JD, Offenbacher S. PGE2, IL-1β, and TNF-α responses in diabetics as modifiers of periodontal disease expression. Ann Periodontol. 1998;3:40–50. doi: 10.1902/annals.1998.3.1.40. [DOI] [PubMed] [Google Scholar]
- 93.Emingil G, Tervahartiala T, Mãntylã P, Määttä M, Sorsa T, Atilla G. Gingival crevicular fluid matrix metalloproteinase (MMP)-7, extracellular MMP inducer, and tissue inhibitor of MMP-1 levels in periodontal disease. J Periodontol. 2006;77:2040–50. doi: 10.1902/jop.2006.060144. [DOI] [PubMed] [Google Scholar]
- 94.Moutsopoulos NM, Madianos PN. Low grade inflammation in chronic infectious diseases. Ann N Y Acad Sci. 2006;1088:251–64. doi: 10.1196/annals.1366.032. [DOI] [PubMed] [Google Scholar]
- 95.Joshipura K, Wand H, Merchant A, Rimm E. Periodontal disease and biomarkers related to cardiovascular disease. J Dent Res. 2004;83:151–5. doi: 10.1177/154405910408300213. [DOI] [PubMed] [Google Scholar]
- 96.Torres JL, Ridker PM. High sensitivity C-reactive protein in clinical practice. Am Heart Hosp J. 2003;1:207–11. doi: 10.1111/j.1541-9215.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- 97.Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–20. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 98.Ficarra V, Sekulovic S, Zattoni F, Zazzera M, Novara G. Why and how to evaluate chronic prostatic inflammation. European Urology Supplements. 2013;12:110–5. [Google Scholar]
- 99.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100:2645–50. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Robert G, Descazeaud A, Allory Y, Vacherot F, de la Taille A. Should we investigate prostatic inflammation for the management of benign prostatic hyperplasia? European Urology Supplements. 2009;8:879–86. [Google Scholar]
- 103.De Nunzio C, Albisinni S, Gacci M, Tubaro A. The role of inflammation in the progression of benign prostatic hyperplasia. Curr Bladder Dysfunct Rep. 2013;8:142–9. [Google Scholar]
- 104.Culig Z, Steiner H, Bartsch G, Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem. 2005;95:497–505. doi: 10.1002/jcb.20477. [DOI] [PubMed] [Google Scholar]
- 105.Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol. 2004;172:S6–12. doi: 10.1097/01.ju.0000142058.99614.ff. [DOI] [PubMed] [Google Scholar]
- 106.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-α correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]


