Abstract
[68Ga]Ga-ABY-025/PET-CT targeting human epidermal growth factor receptor type 2 (HER2) has demonstrated its potential clinical value for the detection and quantification of HER2 in a phase I clinical study with breast cancer patients. Previously, the radiopharmaceutical was prepared manually, however larger scale of multicenter clinical trials and routine healthcare requires automation of the production process to limit the operator radiation dose, improve tracer manufacturing robustness, and provide on-line documentation for good manufacturing practice (GMP) compliance. The production of [68Ga]Ga-ABY-025 was implemented on the Modular-Lab PharmTrace synthesis platform (Eckert & Ziegler) and disposable cassettes were developed. Pharmaceutical grade 68Ge/68Ga generator (GalliaPharm®) was used in the study. The active pharmaceutical ingredient starting material ABY-025 (GMP grade) was provided by Affibody AB. The patient examinations were conducted using a Discovery MI PET/CT scanner (20 cm FOV, GE Healthcare). Reproducible and GMP compliant fully automated production of [68Ga]Ga-ABY-025 was developed. The radiochemical purity of the product was 98.7 ± 0.6% with total peptide content of 315 ± 15 µg (n = 3). Radionuclidic purity, sterility, endotoxin content, residual solvent content, and sterile filter integrity were controlled and met acceptance criteria. The product was stable at ambient temperature for at least 2 h. The primary tumor and metastasis were detected with SUVmax values of 8.3 and 16.0, respectively. Automated production of [68Ga]Ga-ABY-025 was established and the process was validated enabling standardized multicenter phase II and III clinical trials and routine clinical use. Patient examinations conformed to the radiopharmaceutical biodistribution observed in the previous phase I study.
Keywords: Affibody, breast cancer, clinical study, HER2, GMP, gallium-68
Introduction
There is an unmet need for a safe and non-invasive whole body accurate quantification of epidermal growth factor receptor type two (HER2) expression in patients with metastatic breast cancer in a routine clinical setting. HER2 is overexpressed in up to 25% cases of breast cancer and is associated with poor survival [1-3]. Anti-HER2 therapy with antibodies and tyrosine kinase inhibitors improves survival of patients, and HER2 expression estimation using immunohistochemistry (IHC) and in situ hybridization (ISH) on biopsy material is golden standard for patient selection and prediction of response in the context of theranostics [1,3-7]. It was demonstrated that the introduction of molecular imaging such as [68Ga]Ga-ABY-025 positron emission tomography ([68Ga]Ga-ABY-025/PET-CT) may allow to overcome disadvantages of samples from biopsies such as challenges to sample from bone and brain lesions, errors related to heterogeneity of receptor expression in lesions as well as patient discomfort and side effects [8]. The use of PET might enable global determination of HER2 expression heterogeneity, and allow multiple examinations to monitor receptor expression changes over time for treatment response monitoring, thereby providing critical information [8] which is currently rarely possible in clinical practice by multiple biopsies [7,9-11].
[68Ga]Ga-ABY-025/PET-CT has the potential for staging, prognosis, patient stratification, quantification of the receptor expression and drug dose estimation, early monitoring of the treatment response and resistance, residual disease, follow-up and relapse [8,12-14]. The phase I clinical study applying [68Ga]Ga-ABY-025 in 16 patients with metastatic breast cancer demonstrated clinically relevant results [8,12-14]. The primary aim of the study was the investigation of the safety, efficacy, side effects as well as organ distribution, pharmacokinetics and dosimetry. The most important scientific goal was investigation of feasibility of HER2 expression quantification and determination of the optimal peptide dose providing the accuracy of the quantification, and high image quality. Ten patients were examined for the peptide dose optimization wherein each of the patients was administered with two peptide doses of 78 ± 8 (n = 13) and 427 ± 19 µg (n = 23) at two occasions one week apart. After the bolus administration the patients were scanned dynamically for 45 min, and at 1, 2 and 4 hour time point whole body examinations were conducted. Six more patients participated in the test-retest study. Higher detection rate and image contrast as well as more favorable organ distribution and lower effective dose and kidney/liver absorbed doses were observed for the radiopharmaceutical with higher peptide content [8,12]. The optimal imaging protocol was set to 2 h post injection of 427 ± 19 µg (n = 23). Accurate measurement of HER2 expression in breast cancer metastases was achieved with small short-term variability of uptake allowing repeated non-invasive mapping of HER2 expression in metastatic breast cancer [8]. Importantly, the HER2 expression degree correlated with biopsy IHC analysis results and a threshold at SUVmax of 6.0 for discriminating HER2-positive and HER2-negative metastases was suggested. These observations were also reinforced by the detection of some lesions by [18F]FDG/PET-CT that were HER2-negative on [68Ga]Ga-ABY-025/PET-CT and IHC.
In order to enable dissemination of this diagnostic methodology for clinical use and to facilitate standardized multicenter trials and clinical routine, it is necessary to provide data evaluation methods that are independent on the variation of PET scanner characteristics. Intra-image normalization such as tumor-to-reference tissue ratio (T/R) was investigated and spleen was found the most accurate approach providing a simple and robust semi-quantification of HER2 expression [14]. The spleen T/R ratio was associated with high test-retest reproducibility and correlated well to SUVs and invasive HER2-scores. It met the selection criteria such as correlation with biopsy analysis results, low variation of radioactivity uptake, and low probability of hosting metastases from breast cancer on spleen. The suggested cut off for the discrimination between HER2-positive and HER2-negative lesions was set to 6.5. Intra-image normalization of tumor-to-spleen uptake provides a simple and robust semi-quantification of HER2 expression. However, in rare cases extraordinarily high HER2 receptor shedding after therapy may result in high serum-HER2 level and altered distribution of [68Ga]Ga-ABY-025 to the normal tissues. Such possibility should be taken into consideration when using semi-quantitative techniques. Reliable and quantitative assessment of HER2-receptor expression is crucial for the identification of patients with the HER2-positive tumors that can be expected to benefit from HER2-targeted treatment. It is also important to avoid unnecessary cost and potential risk of serious adverse effects of treatment for patients with HER2-negative tumors. Individual therapeutic dose determination, treatment response monitoring and early therapy correction would result in personalized medicine providing possibility to optimize therapeutic response and avoid futile treatments, minimize risks and toxicity as well as reduce cost and patient distress [15]. [68Ga]Ga-ABY-025/PET-CT was demonstrated to fulfil these criteria and semi-quantitative methodology for the HER2 expression estimation independent on the variability of the PET instruments was developed [8,12-14]. The next step towards the routine clinical use is a multicenter phase II/III clinical trial with a larger patient cohort.
PET radiopharmaceuticals have a short shelf-life and are usually manufactured in-house. Their production and availability are some of the most critical aspects in the execution of clinical trials and implementation of new clinical diagnostic methods. [68Ga]Ga-ABY-025 was prepared manually [13] during the phase I study with 16 patients and total 32 examinations. However, a multicenter phase II/III study and subsequent clinical practice will present demand on a larger production scale as well as standardization and dissemination of the production method to multiple medical centers. An automated production of [68Ga]Ga-ABY-025 may accelerate the fulfillment of such demand. We report herein the development and validation of an automated method for the production of [68Ga]Ga-ABY-025 using a commercially available synthesis platform as well as the first clinical results using the radiopharmaceutical.
Material and methods
Facilities, equipment, and materials
The active pharmaceutical ingredient starting material ABY-025 (GMP grade drug product) produced synthetically was provided by Affibody AB. The manufacturer followed GMP guidelines, i. e. the EU directive 2003/94/EC and ICH Q7 guideline for APIs. Safety, pharmacology, and toxicology studies in animals revealed no ABY-025 related toxicity. Certificates of compliance and certificates of analysis were obtained from the manufacturer. The drug product ABY-025 (Molecular weight: 7.6 kDa) was supplied packed in Ph.Eur. Type I glass vials with fluoropolymer coated bromobutyl rubber stoppers and aluminium overseals with flip-off white plastic caps. Each vial contained a solution of 500 µg ABY-025 in 0.5 mL of 0.2 M sodium acetate (pH 5.3). The product was sterile, non-pyrogenic, and without bacteriostatic preservatives. The purchased chemicals were used without further purification: HCl (ultrapure, Merck), sodium acetate buffer (pH 4.6, Sigma-Aldrich), sterile water (Fresenius Kabi), phosphate buffer (Apoteket AB), NaOH (10 M, Sigma-Aldrich), ethanol (APL), water (Fluka, TraceSelect), trifluoroacetic acid (Merck, Darmstadt, Germany).
The aseptic production of [68Ga]Ga-ABY-025 was conducted in a GMP grade A workstation (unidirectional laminar airflow workbench (LAFW)) situated in a cleanroom with GMP grade B air quality. The 68Ge/68Ga generator (GalliaPharm®, Eckert & Ziegler Radiopharma GmbH, Berlin, Germany) and Modular-Lab PharmTracer synthesis platform (Eckert & Ziegler Eurotope GmbH, Berlin, Germany) were placed in the LAFW. A high-performance liquid chromatography system (LaChrom, Hitachi, VWR) consisting of an L-2130 pump, UV detector (L-2400), and a radiation flow detector (Bioscan) coupled in series was used for product quality control. Separation of the analytes was accomplished using an analytical column with stationary reversed phase (C-4; Vydac-C4; 50×4.6 mm; particle size: 3 µm). The conditions were as follows: A = 10 mM TFA; B = 70% acetonitrile (MeCN), 30% H2O, 10 mM TFA with UV-detection at 220 nm; linear gradient elution: 0-2 min at 25% B, 2-7 min at 25 to 100% B, 7-10 min at 100% B; flow rate was 1.0 mL/min. Data acquisition and handling were performed using the EZChrom Elite Software Package.
The starting material, 68Ga (t1/2 = 68 min, β+ = 89%, and EC = 11%), used for the production was obtained from the pharmaceutical grade 68Ge/68Ga-generator (1850 MBq, GalliaPharm®) by elution with 0.1 M hydrochloric acid. The amount of detected metal impurities as provided by the manufacturer was less than the defined limit in the European Pharmacopeia monograph [16]. The appearance of the 68Ga eluate was clear and colorless. The 68Ge breakthrough in the eluate as a percentage of the eluted 68Ga radioactivity was calculated by measuring aliquots of the generator eluate and counting the radioactivity content using an ionization chamber with NaI(Tl) scintillation detector immediately after elution and in the well-type NaI(Tl) scintillation counter after 48 h post elution.
Production of [68Ga]Ga-ABY-025
The production was conducted using a commercial fully automated platform for labelling synthesis (Modular-Lab PharmTracer) with sterile, disposable dedicated cassette system (AS-GA-ABY025-C4-1.0). The process included cassette pressure test, labelling synthesis, product purification, formulation, and sterile filtration as well as sterile filter integrity test. Such parameters as reaction time, temperature, and radioactivity were monitored in real time. The process included a step for the fractionation of the generator eluate. For the product purification step an Oasis HLB SPE cartridge was used, and eluting the final product was performed with 1 mL of 50% ethanol.
The cassette was loaded with reaction mixture containing the reaction buffer (250 µL of 1 M sodium acetate buffer and 200 µL of 50% ethanol), and precursor (300 µL (39 nmol) of ABY-025 solution) was added to the reactor positioned in the heating module. The reaction mixture was heated for 15 min at 80°C. After completion of the reaction, the crude product was cooled down by adding 3 mL of water and then purified on a SPE cartridge eluting the final product with 1 mL of 50% ethanol. The resulting product was diluted to 7.1 ± 0.3 mL with phosphate buffered saline containing 200 µl (26 nmol) of ABY-025 solution for the formulation, and the final product was passed through a 0.22 µm sterile filter into a sterile 10 mL sterile capped glass bottle. The sterile filter integrity was controlled on the platform applying pressurized air. A sample was taken for the determination of the identity, radiochemical purity, peptide concentration, and pH. The total radioactivity of the product was then measured in a dose calibrator.
The chemical purity, radiochemical purity and amount of the peptide were determined by HPLC. A sample of the product was kept for subsequent determination of 68Ge content, residual solvent content as well as sterility and endotoxin content. The stability of the product at room temperature was monitored by UV-Radio-HPLC for 2 hours. The recovery of radioactivity from the analytical column was investigated earlier [13], to confirm that no radioactive product or impurities were left on the column, by collecting HPLC effluent with and without analytical column and measuring the radioactivity. The tests were performed both for the product ([68Ga]Ga-ABY-025) and free 68Ga(III). Specificity, linearity, and precision as repeatability were re-qualified for the UV-detector. The investigational medicinal product [68Ga]Ga-ABY-025 was delivered to the clinic for the administration of the total volume.
Clinical examination
The study was approved by the Swedish Medical Products Agency (EudraCT 2017-002115-34; Dnr: 5.1-2018-30296; ClinicalTrials.gov: NCT03655353), and the regional board of medical ethics. The patients signed informed consent. The [68Ga]Ga-ABY-025/PET-CT examinations from skull to mid-thigh were conducted at 2 and 3 h post administration of 96 MBq radiopharmaceutical containing 262 µg of the peptide. The examinations were conducted using a Discovery MI PET/CT scanner (20 cm FOV, GE Healthcare, Milwaukee, MI, USA). Attenuation correction was acquired by a 140 kV, Auto mA 10-80 mA CT examination. The [18F]FDG/PET-CT examination using standard clinical protocol was conducted on the following day 1 h post administration of 262 MBq in order to locate viable lesions. Biopsies were based on [68Ga]Ga-ABY-025/PET-CT findings and guided by ultrasound or CT. HER2 immunohistochemistry and in-situ hybridization analyses were performed as described previously [8].
Results
Generator qualification
The qualification of the 68Ge/68Ga generator was conducted to control appearance, elution efficiency, and 68Ge breakthrough as well as performing labelling synthesis of a validated tracer. The appearance of the eluate was colorless and clear. The elution efficiency was estimated at the time of secular equilibrium as percentage of the eluted radioactivity of the total radioactivity expected theoretically with decay correction for 68Ge, and it corresponded to 71 ± 2%. The 68Ge breakthrough was determined by measuring the radioactivity of the 68Ga eluate directly and 48 hours after elution resulting in < 0.00005%.
Production of [68Ga]Ga-ABY-025
The fully automated production of [68Ga]Ga-ABY-025 was conducted on a commercial synthesis platform, Modular-Lab PharmTracer, according to the flow chart presented in Figure 1. The procedure consisted of four distinct steps wherein the start of each step required the initialization by the operator. The first step was the control of the intactness of the disposable cassette and absence of any leakage. The second step was the pre-conditioning of the SPE cartridge for the product purification. The third step included the labelling synthesis, product purification and formulation, and sterile filtration. The fourth step was dedicated to the sterile filter integrity test using pressurized air. The non-decay corrected radiochemical yield was estimated to approximately 40% at the end of the synthesis. The radiochemical purity was over 99% (Figure 2A). Fifty percent ethanol was used for the recovery of the product from the SPE cartridge and subsequently, the product was diluted with phosphate buffered saline containing 200 µL of ABY-025 solution to 7.1 ± 0.3 mL in the formulation step, so that the final ethanol concentration would be < 10%. The total content of the peptide in the final product estimated by UV-HPLC (Figure 2B) was 315 ± 15 µg in the three validation runs. The product was stable at ambient temperature for at least 2 h with radiochemical purity of 98 ± 1%. The suppression of the oxidative radiolysis was achieved by addition of ethanol (7-10%, v/v). A sample of the product was kept for subsequent determination of 68Ge content and resulted in less than 0.00002%. The product specifications and validation results are summarized in Table 1. The developed method was GMP-compliant, reliable and reproducible.
Figure 1.
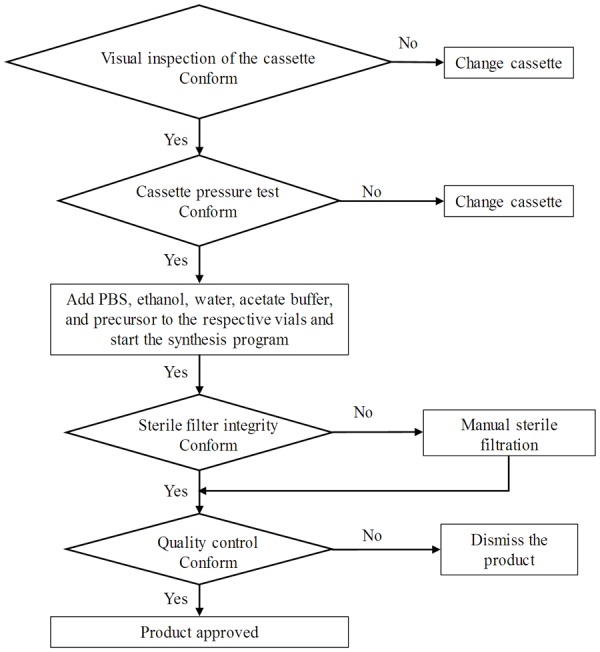
Flow chart of [68Ga]Ga-ABY-025 production on the Modular-Lab PharmTracer.
Figure 2.
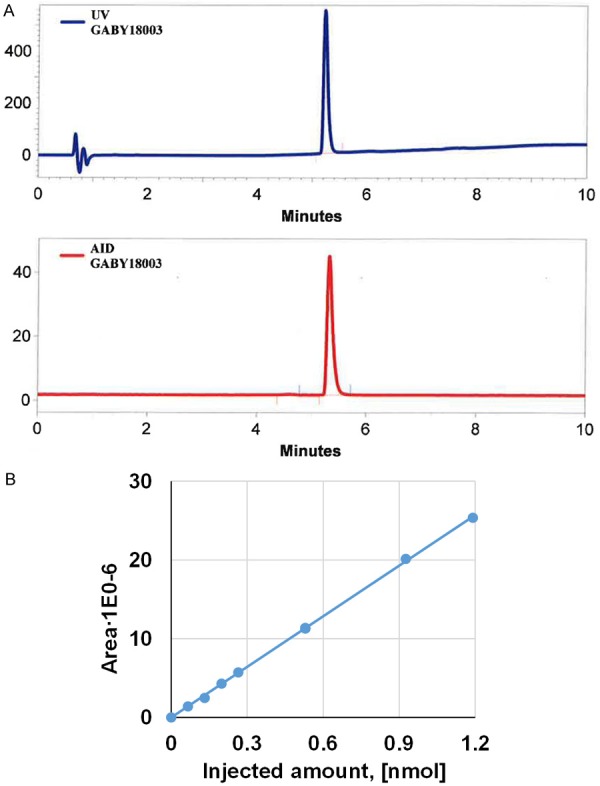
A. Typical HPLC-chromatograms of quality control with UV absorption profile (upper panel) and radioactivity signal profile (lower panel) of [68Ga]Ga-ABY-025; B. UV-HPLC signal calibration of ABY-025 (R2 = 0.9996).
Table 1.
Summary of the product specifications and validation results for the three consecutive validation productions of [68Ga]Ga-ABY-025
| Test | Acceptance criteria | GABY18002 | GABY18003 | GABY18004 |
|---|---|---|---|---|
| Radiochemical purity | ≥ 90%; no unknown impurity corresponds to > 5% | 99 | 99 | 98 |
| Chemical purity | Absence of unknown UV signals | > 99 | > 99 | > 99 |
| pH | 4.0-8.0 | 7.4 | 7.4 | 7.4 |
| Radioactivity concentration | 5-100 MBq/mL | 35 | 40 | 49 |
| Radioactivity | 50-500 MBq | 240.8 | 290 | 350 |
| Volume | 5-10 mL | 6.79 | 7.28 | 7.18 |
| Radionuclidic purity | > 99.9% | > 99.9999 | > 99.9999 | > 99.9999 |
| 68Ge breakthrough | < 0.001% | 0.000005 | 0.000006 | 0.000004 |
| Sterility test | Sterile | Sterile | Sterile | Sterile |
| Endotoxins test | < 3 EU/mL | < 0.25 | < 0.25 | < 0.25 |
| Stability | RCP ≥ 90% within 120 min | 99 | 98 | 97 |
| Ethanol* | < 10% | 3.2 | 3.5 | 3.3 |
| Sterile filter integrity | ≥ 1 bar | Passed | Passed | Passed |
Determined by gas chromatography.
Quality control
The chemical purity, radiochemical purity and amount of the peptide were determined by UV-Radio-HPLC. The HPLC method for the determination of [68Ga]Ga-ABY-025 radiochemical purity and concentration by UV, and radio-detection was validated previously [13] with respect to specificity, linearity, repeatability, radioactivity recovery from the HPLC column as well as stability of the product at room temperature. It was re-qualified with respect to specificity, linearity and precision. The calibration of the UV-signal was conducted to enable accurate determination of the peptide content in the final product, and it resulted in high Pearson correlation coefficient (R2) of 0.9996 (Figure 2B). The range of the peptide concentration for the calibration corresponded to that expected for the product concentration. The HPLC method was developed to assure a baseline separation between the oxidized and non-oxidized products and demonstrated high specificity. The investigation of the detection limit demonstrated broad marginal for the accurate detection of both UV- and radio-signals.
Clinical examination
The biodistribution of [68Ga]Ga-ABY-025 was in agreement with the previously conducted phase I study [8,14]. Physiological uptake was observed in salivary glands, liver, gastric wall, and intestines (Figure 3A-C). The patient had a known locally advanced breast cancer in the right breast, from which a previous biopsy indicated a borderline expression of HER2 (IHC score: 1-2+). The whole-body scan visualized the primary cancer with SUVmax values of 7.2 (2 h) and 8.3 (3 h), and unexpectedly showed intense uptake in a 7 mm large lytic bone metastasis with SUVmax of 13.8 (2 h) and 16.0 (3 h). [18F]FDG/PET-CT detected the primary tumor (SUVmax: 6.1) and metastasis (SUVmax: 6.1). The false positive was detected in the sentinel lymph node indicating post-surgical inflammation after biopsy wherein sentinel node was found tumor- and HER2-negative (Figure 3D-F).
Figure 3.
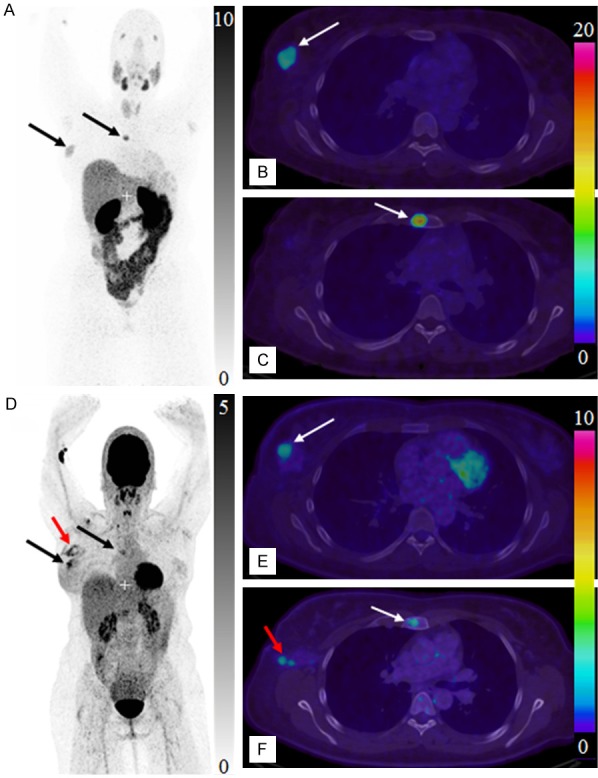
Maximum intensity projection PET images of whole body scan with [68Ga]Ga-ABY-025 (A, 2 h) and [18F]FDG (D). Transaxial PET-CT fused images of the primary tumor (B, E, respectively for [68Ga]Ga-ABY-025 (2 h) and [18F]FDG) and metastasis (C, F, respectively for [68Ga]Ga-ABY-025 (2 h) and [18F]FDG). The black and white arrows indicate known tumor deposits. The red arrow (D, F) indicate post-surgical inflammation after biopsy wherein sentinel lymph node was tumor- and HER2-negative.
Discussion
Affibody molecules are ligands that meet the criteria for molecular imaging in terms of high specificity binding to the target receptor and fast pharmacokinetics, blood and non-target tissue clearance. The second generation anti-HER2 Affibody molecules with amino acid variation in both binding and non-binding surfaces resulted in an analogue, ABY-025, presented with picomolar affinity to HER2 [17-22]. ABY-025 has a cysteine at C-terminus which is used for site-specific conjugation of a DOTA chelator moiety. The small three α-helical structure of the polypeptide is heat stable and refolds rapidly to its native structure after heating to high temperatures (e.g. 80°C) required for 68Ga-labelling.
68Ga has proved its potential to facilitate development of clinically practical PET and worldwide promote PET technique for earlier better diagnostics and individualized medicine. The introduction of [68Ga]Ga-ABY-025/PET-CT into clinical routine would provide HER2 targeted imaging for tumor-type specific, non-invasive, whole-body diagnosis with precise localization of tumors and metastases, most importantly it would allow for pre-therapeutic quantification of receptor status, uptake kinetics and dosimetry that would enable accurate staging, therapy selection and planning as well as monitoring response to the therapy resulting in personalized medicine.
The development and validation of GMP compliant manual production of [68Ga]Ga-ABY-025 was previously accomplished for two peptide doses demonstrating 100% success rate [13]. The clinical phase I study was conducted using manually produced [68Ga]Ga-ABY-025 [8,13]. However, the transition to the subsequent study involving larger cohort of patients require automation of the manufacturing procedure in order to minimize radiation dose to the chemist, assure robustness of the production and minimize human mistake factor as well as provide on-line documentation of the manufacturing process for GMP compliance which has outmost importance in the clinical environment involving patient examinations. Moreover, the automated production of the radiopharmaceutical allows easy transfer of the process to other laboratories enabling standardization and harmonization of multicenter clinical trials that in turn may accelerate regulatory approvals for routine clinical use [15]. The availability of an automated commercial production mode might accelerate the worldwide dissemination of [68Ga]Ga-ABY-025/PET-CT diagnostic technology for theranostic application.
Automation of labelling synthesis
The commercial synthesis platform (Modular-Lab PharmTracer platform, Eckert & Ziegler Eurotope GmbH, Berlin, Germany) used in this study utilizes disposable cassettes that exclude the risk of cross-contamination, allow high frequency of the synthesis, and improve reproducibility. It allows for the most common labelling methods involving either fractionation or pre-concentration of the generator eluate. The synthesis procedure was based on the respective manual method [13] and did not require further optimization. Since [68Ga]Ga-ABY-025 of lower specific radioactivity demonstrated advantages in terms of higher detection rate and image contrast, favorable organ distribution and lower effective dose [8], the simpler fractionation method [23,24] requiring larger amount of the precursor ABY-025 was selected even for the automated process. The translation of the manual preparation procedure was straightforward even though the automated process is continuous and cannot be interrupted for the adjustments of the conditions, e.g. amount of the radioactivity or pH. Such key parameters as reaction time, temperature, acidity of the reaction mixture, precursor peptide concentration, performance of solid phase extraction cartridge, radiochemical yield, radiochemical purity and reproducibility of the process correlated well with those of the manual preparation.
The liquid transport on the automated synthesis system requires larger volumes and the losses of the peptide can be larger due to the liquid transport through the tubing and manifold network. The total peptide loss on the plastic surfaces of vials, syringes and cassette resulted in lower peptide amount in the final product (315 ± 15 µg, n = 3 production validation) as compared to the manual preparation (427 ± 19 µg, n = 23). The accurate determination of the final peptide concentration in the product was crucial to confirm sufficiently high peptide content. The robustness of the production was assured by the three times higher amount of the precursor (300 µg) used in the automated production as compared to that of manual preparation. The high concentration of ABY-025 compensating for metal cation impurities provided high radioactivity incorporation and reproducibility of the labelling reaction. The desired high peptide amount allowed low specific radioactivity of the imaging agent, wherein effective specific radioactivity (SRA) was determined as a ratio of the radioactivity in MBq and amount of the peptide in the final product associated with the measured radioactivity. The high amount of the peptide (300 µg, 40 nmoles) in the reaction mixture resulted in high radiochemical yield, and together with the rest of the peptide (200 µg, 26 nmoles) added to the purified product prior to the sterile filtration provided reliable reproducibility of the peptide concentration in the final product. The variation in the radioactivity associated with the final product depended on the generator pre-elution time delay.
To avoid the direct exposure of the peptide to the acidic generator eluate [13], the latter was first added to the reaction buffer and then the buffered solution was transferred to the reactor containing the precursor, ABY-025. The peptide contains such amino acid residues as methionine and tryptophan that are sensitive to radiolytic oxidation and it is important to suppress the latter to provide required radiochemical purity of the labelled product. It was sufficient to add 7-10% (v/v) of ethanol to the reaction mixture to suppress the formation of the oxidation by-product to less than 1%, similar to the case of the manual preparation. Three times higher amount of ABY-025 used in the automated procedure (i.e. 300 µg vs. 100 µg in the manual synthesis) and lower amount of the radioactivity also contributed to the suppression of the radiolysis. No other radical scavenger such as ascorbic acid or dihydroxybenzoic acid [24-26] were needed. [68Ga]Ga-ABY-025 was stable for at least 2 h at ambient temperature without formation of detectable radioactive by-products. Most probably, the content of 3.3 ± 0.2% of ethanol in the final formulation was sufficient to exclude radiolysis and assure the product stability. The formulation of the product allowed adequate dilution to reduce the EtOH concentration to less than 10% and to provide accurate UV-HPLC determination of the peptide content in the product and patient administered peptide dose crucial for the adequate interpretation of imaging results [8,13].
The concentration of the acetate buffer optimized during the manual procedure (0.1 M) [13] was slightly higher in the automated production (0.15 M). However, despite the possibility of the excess buffer to compete with the DOTA comprising precursor [23], the radiochemical yield was similar most probably due to the 3-fold higher concentration of the precursor ABY-025. The product recovery from the Oasis HLB solid phase extraction cartridge during automated synthesis was high although approximately 10% lower compared to the manual preparation since the fine tuning of the liquid flow rate and thus desorption from SPE cartridges was limited. The heating temperature was set to 80°C slightly higher compared to the manual procedure (75°C), considering the possible difference in heat transfer between the glass and plastic material of the reaction vial requiring slightly higher temperature when using the plastic vial. The fast labelling synthesis (35 min) and high radioactivity incorporation provided high radiochemical yield. The method presented high repeatability with three production validation runs meeting the pre-determined specifications (Table 1).
The specifications of the pharmaceutical grade 68Ge/68Ga generator classified as a medicinal product (GalliaPharm®) meet the requirements defined in the Ph. Eur. monograph [27] and thus no additional generator validation is required. Nevertheless, the breakthrough of 68Ge was monitored for the three process validation productions. The elution profile was also investigated to optimize the selection of the top fraction with the highest 68Ga concentration, and the synthesis program was adjusted respectively to assure the highest possible radiochemical yield. The generator was regularly eluted at < 24 h prior to the synthesis to reduce the metal cation impurity content [23,24] and assure high process reproducibility.
Peptide and radioactivity dose adjustment
Our previous study, wherein the patients (n = 10) received bolus intravenous administration of the low (ABYA, 78 ± 8 µg) and high (ABYB; 427 ± 19 µg) peptide content radiopharmaceuticals on two occasions one week apart [8], demonstrated superiority of the high peptide content radiopharmaceutical in terms of biodistribution, detection rate, image contrast, and dosimetry. The combination of high peptide content and whole-body imaging at 2 hours after injection appeared to be optimal for routine clinical use. Thus, it was crucial to assure administration of maximum amount of the produced radiopharmaceutical (GABY, Table 1), and consequently the total volume of the final product had to be administered.
The manual synthesis allows flexibility and it was easy to adjust the amount of radioactivity taken for the synthesis by pre-buffering the eluate prior to the addition to the reaction mixture, measuring the total radioactivity amount and using as much as it was needed to provide desired product radioactivity content independent on the age of the generator. However, this approach cannot be applied to the automated production since each change in the continuous process, e.g. change of the generator eluate volume would require respective validation which is impossible in practice.
In the case of automated production, the strategy must be different to assure the amount of the administered radioactivity adjusted to 50-200 MBq. The amount of the radioactivity associated with the radiopharmaceutical at the end of the production can be regulated using such amount of the eluted radioactivity from the generator that would correspond to none-decay corrected radiochemical yield (RCY) of approximately 40%. The amount of the radioactivity entering the production process can be modulated by the pre-elution of the generator. The time for the pre-elution delay depends of the age of the generator and respective expected maximum amount of the eluted 68Ga. The pre-elution delay time can be calculated using equation describing the ingrowth of 68Ga (Equation 1) in secular equilibrium with 68Ge decay (Equation 2).
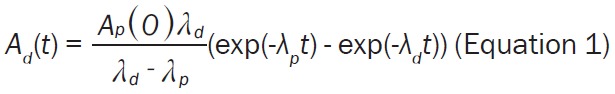 |
The Equation 1 can be re-arranged into the Equation 3, wherein the substitution of the desired 68Ga radioactivity at time point t (Ad(t); MBq) and amount of 68Ge (Ap(0); MBq) assessed from the Equation 2 allows estimation of the delay time (t in min if λd is in min-1) between the generator pre-elution and the start of the synthesis (SOS).
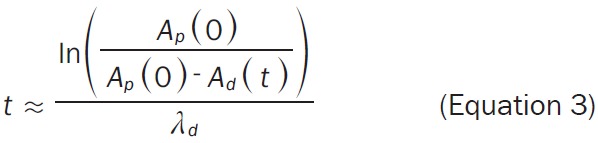 |
The amount of 68Ge radioactivity (Ap(0), MBq) must include correction for the generator elution efficiency (e.g. 75%) and fractionation (e.g. 85%) [24]. The amount of 68Ga radioactivity (Ad(t), MBq) entering the labelling synthesis should be corrected for the RCY at the end of the synthesis (EOS, e.g. 40%). For example, the amount of 68Ga radioactivity entering the labelling synthesis assuming 200 MBq at the EOS would be 500 MBq including the correction for the RCY. Assuming a 6-month-old 50 mCi generator, 68Ge content is theoretically expected to be 1155 MBq which would translate into 68Ga radioactivity of 736 MBq given the elution efficiency of 75% and fractionation of 85%. The post production quality control, radiopharmaceutical release and delivery documentation may require additional 20 minutes and reduce the radioactivity amount to the desirable administration dose of 150-160 MBq.
If the delay of the patient examination should occur, the radiopharmaceutical with radioactivity amount corresponding to 200 MBq at the EOS can still be used within 2 h and produce high quality images and accurate uptake quantification. It is important to stress that it is not the specific radioactivity per se that is critical for the biodistribution pattern but the amount of the administered peptide. The respective amount of the radioactivity should provide sufficiently high counts for the uptake registration. The variation in specific radioactivity depends not only on the age of the generator and labelling synthesis reproducibility but also occasional delays between the product delivery and radiopharmaceutical administration to the patient due to logistical reasons. However, as mentioned the most important parameter that should present high reproducibility is the amount of the administered peptide.
After the age of 10 months the highest achievable 68Ga radioactivity from the generator should be used implying that the shortest pre-elution delay time corresponds to 4 hours. The product radioactivity will gradually decrease below 200 MBq (EOS). However, given the advantages of modern PET scanners equipped with highly sensitive detectors, administration of rather low radioactivity amount still results in high quality images and accurate quantification. Thus, a 50 mCi generator can be used for 12 months.
Clinical aspects
As mentioned above the total amount of the administered peptide is a critical factor. We previously demonstrated the influence of the injected peptide mass on the organ distribution. The high dose of the peptide resulted in lower background uptake in the liver and higher lesion detection rate at early time points, and higher image contrast. To use [68Ga]Ga-ABY-025/PET for receptor quantification in a routine setting, it is important to control the amount of the administered peptide. During synthesis, the loss of the peptide takes place on various surfaces during transfer due to hydrophobic or static adsorption. This should especially be taken into consideration during the automated synthesis wherein the peptide transfer occurs multiple times and on larger total surface as compared to the manual procedure. The concentration in the final formulation was determined using HPLC calibration plot as a part of quality control (Figure 2B). The system suitability test must be conducted, and in addition the results can be used for one-point calibration to confirm the validity of the calibration plot created earlier during the process validation. Thereafter, the SRA (MBq/µg) can be calculated. The total volume of the formulation was administered, and the radioactivity of the syringe was measured before and after administration for the accurate determination of the injected radioactivity amount (MBq). The linear correlation between the radioactivity amount and the respective amount of the peptide allows for the estimation of the administered amount of the peptide (Equation 4). The radioactivity values must be decay corrected to the same time point, e.g. injection time.
 |
Our previous phase I clinical study demonstrated advantages of using radiopharmaceutical with high peptide content (427 ± 19 µg, n = 23) combined with a 2 h post administration PET examination to achieve an optimal examination protocol [8,13,14]. The automated production reported herein provided [68Ga]Ga-ABY-025 of approximately 25% lower peptide content, nevertheless the radiopharmaceutical presented high contrast images (Figure 3) comparable to that with the high content radiopharmaceutical in the phase I study, even though the healthy liver uptake was somewhat higher. This case report is a typical example and supports the strong influence of the total peptide amount on the healthy tissue uptake and image contrast.
Prior immunohistochemical analysis of the primary tumor biopsy specimen showed a borderline expression of HER2 and consequently the treatment with Trastuzumab was not considered. However, subsequent [68Ga]Ga-ABY-025/PET-CT examination demonstrated uptake in a 7 mm bone metastasis with SUVmax of 16.0, which was considerably higher than that in the 25 mm primary tumor (SUVmax: 8.3). Increased HER2-expression in the metastasis was confirmed by immunohistochemistry. The [68Ga]Ga-ABY-025/PET-CT findings lead to the significant changes in the treatment regimen for the patient. The false positive finding by [18F]FDG/PET-CT (Figure 3D, 3E) of post-surgical inflammation in the axilla also stresses the importance of the targeting specificity of [68Ga]Ga-ABY-025/PET-CT.
The automated production procedure is currently used in the ongoing phase II/III study, aiming to validate the use of [68Ga]Ga-ABY-025/PET-CT for non-invasive assessment of HER2-status in breast cancers in a multicenter setting (ClinicalTrials.gov: NCT03655353).
Conclusions
Fully automated GMP/GRPP-compliant and reproducible production of [68Ga]Ga-ABY-025 was developed for quantitative HER2-imaging in patients with metastatic breast cancer. The process was fully validated meeting the acceptance criteria on the radiochemical purity, radionuclidic purity, stability, sterility, endotoxin content, residual solvent content, and sterile filter integrity. The content of the peptide in the final product was sufficiently high to assure low background uptake. The administration of the total product volume was possible due to the pre-eluting of the generator and thus modulating the amount of 68Ga(III) used for the production. The clinical examination presented high contrast PET images adequate for the quantification of HER2 expression.
Acknowledgements
E&Z Eurotope GmbH and WIIK Pharma are acknowledged for the supply of disposable cassettes for the production implementation and validation process. The work was sponsored by The Swedish Cancer Foundation (Cancerfonden) and The Swedish Breast Cancer Foundation (BRO). Affibody AB donated ABY-025 peptide.
Disclosure of conflict of interest
Fredrik Frejd and Joachim Feldwisch are employees of Affibody AB. Philip Schweighöfer and Johanna Seemann are employees of Eckert & Ziegler.
References
- 1.Cortes J, Fumoleau P, Bianchi GV, Petrella TM, Gelmon K, Pivot X, Verma S, Albanell J, Conte P, Lluch A, Salvagni S, Servent V, Gianni L, Scaltriti M, Ross GA, Dixon J, Szado T, Baselga J. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2012;30:1594–1600. doi: 10.1200/JCO.2011.37.4207. [DOI] [PubMed] [Google Scholar]
- 2.Houssami N, Macaskill P, Balleine RL, Bilous M, Pegram MD. HER2 discordance between primary breast cancer and its paired metastasis: tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res Treat. 2011;129:659–674. doi: 10.1007/s10549-011-1632-x. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K, Group ES. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26:3637–3643. doi: 10.1038/sj.onc.1210379. [DOI] [PubMed] [Google Scholar]
- 6.Molina R, Barak V, van Dalen A, Duffy MJ, Einarsson R, Gion M, Goike H, Lamerz R, Nap M, Soletormos G, Stieber P. Tumor markers in breast cancer-European group on tumor markers recommendations. Tumour Biol. 2005;26:281–293. doi: 10.1159/000089260. [DOI] [PubMed] [Google Scholar]
- 7.Viani GA, Afonso SL, Stefano EJ, De Fendi LI, Soares FV. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer. 2007;7:153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sörensen J, Velikyan I, Sandberg D, Wennborg A, Feldwisch J, Tolmachev V, Orlova A, Sandström M, Lubberink M, Olofsson H, Carlsson J, Lindman H. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 affibody PET/CT. Theranostics. 2016;6:262–271. doi: 10.7150/thno.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsson E, Sandelin K, Appelgren J, Zhou W, Jirstrom K, Bergh J, Warnberg F. Clonal alteration of breast cancer receptors between primary ductal carcinoma in situ (DCIS) and corresponding local events. Eur J Cancer. 2014;50:517–524. doi: 10.1016/j.ejca.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Lindstrom LS, Karlsson E, Wilking UM, Johansson U, Hartman J, Lidbrink EK, Hatschek T, Skoog L, Bergh J. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J. Clin. Oncol. 2012;30:2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 11.Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93:552–556. doi: 10.1038/sj.bjc.6602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandstrom M, Lindskog K, Velikyan I, Wennborg A, Feldwisch J, Sandberg D, Tolmachev V, Orlova A, Sorensen J, Carlsson J, Lindman H, Lubberink M. Biodistribution and radiation dosimetry of the anti-HER2 affibody molecule 68Ga-ABY-025 in breast cancer patients. J Nucl Med. 2016;57:867–871. doi: 10.2967/jnumed.115.169342. [DOI] [PubMed] [Google Scholar]
- 13.Velikyan I, Wennborg A, Feldwisch J, Lindman H, Carlsson J, Sorensen J. Good manufacturing practice production of [(68)Ga]Ga-ABY-025 for HER2 specific breast cancer imaging. Am J Nucl Med Mol Imaging. 2016;6:135–153. [PMC free article] [PubMed] [Google Scholar]
- 14.Sandberg D, Tolmachev V, Velikyan I, Olofsson H, Wennborg A, Feldwisch J, Carlsson J, Lindman H, Sorensen J. Intra-image referencing for simplified assessment of HER2-expression in breast cancer metastases using the Affibody molecule ABY-025 with PET and SPECT. Eur J Nucl Med Mol Imaging. 2017;44:1337–1346. doi: 10.1007/s00259-017-3650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velikyan I. Prospective of 68Ga-Radiopharmaceutical development. Theranostics. 2014;4:47–80. doi: 10.7150/thno.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallium (68Ga) edotreotide injection. European Pharmacopoeia. 2014:1062. [Google Scholar]
- 17.Nilsson B, Moks T, Jansson B, Abrahmsen L, Elmblad A, Holmgren E, Henrichson C, Jones TA, Uhlen M. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1987;1:107–113. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]
- 18.Nord K, Nilsson J, Nilsson B, Uhlen M, Nygren PA. A combinatorial library of an alpha-helical bacterial receptor domain. Protein Eng. 1995;8:601–608. doi: 10.1093/protein/8.6.601. [DOI] [PubMed] [Google Scholar]
- 19.Nord K, Gunneriusson E, Ringdahl J, Stahl S, Uhlen M, Nygren PA. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat Biotechnol. 1997;15:772–777. doi: 10.1038/nbt0897-772. [DOI] [PubMed] [Google Scholar]
- 20.Feldwisch J, Tolmachev V, Lendel C, Herne N, Sjoberg A, Larsson B, Rosik D, Lindqvist E, Fant G, Hoiden-Guthenberg I, Galli J, Jonasson P, Abrahmsen L. Design of an optimized scaffold for affibody molecules. J Mol Biol. 2010;398:232–247. doi: 10.1016/j.jmb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Ahlgren S, Orlova A, Wallberg H, Hansson M, Sandstrom M, Lewsley R, Wennborg A, Abrahmsen L, Tolmachev V, Feldwisch J. Targeting of HER2-expressing tumors using 111In-ABY-025, a second-generation affibody molecule with a fundamentally reengineered scaffold. J Nucl Med. 2010;51:1131–1138. doi: 10.2967/jnumed.109.073346. [DOI] [PubMed] [Google Scholar]
- 22.Eigenbrot C, Ultsch M, Dubnovitsky A, Abrahmsen L, Hard T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc Natl Acad Sci U S A. 2010;107:15039–15044. doi: 10.1073/pnas.1005025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velikyan I, Beyer GJ, Langstrom B. Microwave-supported preparation of 68Ga-bioconjugates with high specific radioactivity. Bioconjugate Chem. 2004;15:554–560. doi: 10.1021/bc030078f. [DOI] [PubMed] [Google Scholar]
- 24.Velikyan I. 68Ga-based radiopharmaceuticals: production and application relationship. Molecules. 2015;20:12913–12943. doi: 10.3390/molecules200712913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jodal A, Lankat-Buttgereit B, Brom M, Schibli R, Behe M. A comparison of three (67/68)Ga-labelled exendin-4 derivatives for beta-cell imaging on the GLP-1 receptor: the influence of the conjugation site of NODAGA as chelator. EJNMMI Res. 2014;4:31. doi: 10.1186/s13550-014-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velikyan I, Rosenstrom U, Eriksson O. Fully automated GMP production of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 for clinical use. Am J Nucl Med Mol Imaging. 2017;7:111–125. [PMC free article] [PubMed] [Google Scholar]
- 27.Gallium (68Ga) chloride solution for radiolabelling. Ph Eur. 2014:1060. [Google Scholar]


