Abstract
Diffusion-weighted magnetic resonance imaging (diffusion MRI) is being used to characterize morphological development of cells within developing cerebral cortical gray matter. Abnormal morphology is a shared characteristic of cerebral cortical neurons for many neurodevelopmental disorders, and therefore diffusion MRI is potentially of high value for monitoring growth-related anatomical changes of relevance to brain function. Here, the theoretical framework for analyzing diffusion MRI data is summarized. An overview of quantitative methods for validating the interpretations of diffusion MRI data using light microscopy is then presented. These theoretical modeling and validation methods have been used to precisely characterize changes in water diffusion anisotropy with development in the context of several animal model systems. Further, in diffusion MRI studies of several preclinical models of neurodevelopmental disorders, the ability is demonstrated of diffusion MRI to detect abnormal morphological neural development. These animal model studies are reviewed along with recent initial efforts to translate the findings into an approach for studies of human subjects. This body of data indicates that diffusion MRI has the requisite sensitivity to detect abnormal cellular development in the context of several models of neurodevelopmental disorders, and therefore may provide a new strategy for detecting abnormalities in early stages of brain development in humans.
Graphical abstract
Introduction
Joe Ackerman’s drive to understand the biophysical mechanisms that influence magnetic resonance signals measured in biological tissue profoundly shaped the body of data reviewed here. Joe designed many influential experiments that revealed how water diffusion, as encoded in diffusion-weighted magnetic resonance imaging (diffusion MRI) can be used to monitor brain physiology and anatomy on the cellular level. Many of these experiments were performed under his direction at the Biomedical MR Laboratory (BMRL) at Washington University. In the period from 2000-2005, I was fortunate to be a member of the BMRL and join the efforts of several researchers who were contributing to the efforts to develop a new way to non-invasively characterize anatomical development of cells within the cerebral cortex. This method utilized diffusion MRI in novel way. Rather than focusing on brain white matter structures, which at the time were known to induce anisotropy in water diffusion in ways that reflect their coherent organization as well as their pathophysiological characteristics in neurological disorders [1-3], the phenomenon being studied was water diffusion anisotropy within early developing cerebral cortical gray matter. Already by 1997, water diffusion anisotropy within the immature cerebral cortex had been reported in studies of cats [4] and pigs [5], and shortly thereafter Mori and co-workers characterized this phenomenon in the developing mouse brain [6]. Within the BMRL at this time, Jeff Neil was spearheading an effort to systematically study within neonatal humans brain development using diffusion MRI [7-9], with the objective to establish relationships between cellular-morphological development abnormalities revealed by this technique and the risk for subsequent cognitive and behavioral disorders in prematurely delivered human infants. Joe Ackerman integrated with this focused clinical objective a team of talented scientists in statistical physics, data analysis, biomechanics, cell biology neuroscience, neurophysiology, and small-animal MRI. Several of these individuals also share perspectives in this special issue of JMR.
This review summarizes contributions made in preclinical studies of the analysis of water diffusion anisotropy within developing cerebral cortical gray matter. Studies of animal model systems have facilitated the development of theoretical modeling strategies for analyzing the diffusion-attenuated MRI signal in gray matter as well as independent experimental methods for validating the interpretations of biophysical models. Further, they have enabled precise characterization of the normal trajectory of diffusion anisotropy changes with cortical maturation, and they have led to the generation of a series of animal model systems for assessing the value of diffusion MRI in identifying abnormal development. Joe Ackerman has facilitated this work through his mentorship individuals who subsequently positively affected the growth of this field and through direct contributions.
Diffusion within cerebral cortical gray matter: Dispersion in the orientation distribution of axons and dendrites.
The cerebral cortex is the 1-5 mm-thick outer-most sheet of tissue in the mammalian brain. This is where synaptic connections reside that integrate sensory input, facilitate planning of responses, and instigate motor activities. A major focus for most diffusion MRI studies is the development, pathology, or anatomical organization of brain white matter. There are fundamental differences in the biological processes that influence water diffusion anisotropy in cerebral white matter compared to other brain structures. In white matter, axon fiber bundles that are comparable in size (length as well as cross-sectional area) to an MRI voxel restrict water diffusion perpendicular to the bundle more than in the direction parallel to the bundle. Degradation of the fiber bundle structure secondary to myelin or axonal injury, or disease, can result in reduced anisotropy in water diffusion [10, 11]. Maturation of fiber bundles involves axonal organization and myelination, which are process associated with increases in water diffusion anisotropy [6, 8, 12]. Under each of these conditions, white matter exhibiting highly anisotropic water diffusion is associated with mature, healthy, often myelinated axons consisting primarily of parallel structures.
In addition to cerebral white matter, anisotropy in water diffusion can also be observed in gray matter. In particular, properties of water diffusion reflect cellular morphology in gray matter structures with laminar organization, such as the cerebral cortex and hippocampus (see [13] for a recent review). In the mature cerebral cortex, the majority of tissue volume consists of cylindrical cell processes such as axons and dendrites. Bourgeois and Rakic have estimated that approximately 60% of the cerebral cortex to be occupied by the “neuropil” at maturity [14]. Thus, on a microscopic cellular size scale, water diffusion is expected to be anisotropic. However, as a consequence of the broad distribution of cellular process orientations, water diffusion averaged over a volume of a standard diffusion MRI voxel in most gray matter stuctures exhibits very little directional dependence compared to most white matter structures. Water diffusion in the mature cerebral cortex is locally anisotropic but macroscopically nearly (but not completely [15, 16]) isotropic. The immature cerebral cortex differs from the mature cortex in this regard. Immediately following migration of pyramidal neurons from ventricular and subventricular zones to the cortical plate, such as half way through gestation in humans or non-human primates, or the first days of life in mice and rats, most synaptic connections have not yet formed, and the morphology of neurons is simple (Figure 1). Cellular processes within the cortical plate are nearly uniformly radially oriented, and correspondingly, water diffusion is as anisotropic as it is in mature white matter fiber bundles (e.g., see Figure 4). In 2002, McKinstry and co-workers posited that water diffusion anisotropy changes associated with maturation reflect morphological changes in neurons and radial glia [9]. Healthy development is associated with reductions in water diffusion anisotropy, and as has been more explicitly refined since then, water diffusion anisotropy decreases with increasing dispersion in axonal and dendritic cellular process orientations (Figure 1) [17]. It is important to precisely characterize the relationship between biological structures and cortical diffusion anisotropy because morphological development of neurons is reported to be abnormal in several neurodevelopmental disorders. If this abnormal morphology affects the trajectory of cortical FA changes with development, then the analysis of cortical FA could be of value for identifying individuals affected by neurodevelopmental disorders. This review summarizes work performed with several animal models of disease, in addition to studies of human subjects, that characterize the link between abnormal neural morphology and diffusion MRI studies focused on the developing cerebral cortex.
Figure 1.
Morphological development of cerebral cortical pyramidal neurons with development. Traces of dendritic arbors for neonatal rat neocortical pyramidal neurons were generated by Furtak and co-workers [80] and made available through the NeuroMorpho database [81] (http://www.neuromorpho.org). Neurons from rats of ages ranging from postnatal day 1 (P1) through P14 are shown. Apical dendrites are red, basal dendrites are blue, and cell bodies are represented as black spheres. The orientation distribution of dendritic processes, schematized at right, broaden with age as dendritic arbors gain complexity.
Figure 4.
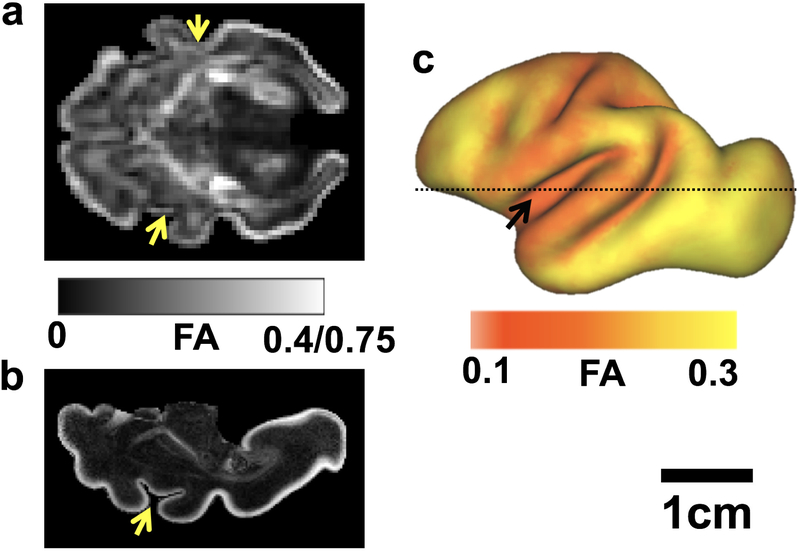
Regional patterns of cerebral cortical diffusion anisotropy in the fetal rhesus macaque brain. (a-b) Axial views of FA maps are shown for G110 brains (a) in utero and (b) post mortem. The FA scale ranges from 0 to 0.4 for the in utero parameter map, and from 0 to 0.75 in the post mortem parameter map. Yellow arrowheads indicate the location of the insular cortex, near the source of the transverse neurogenic gradient, which is characterized by relatively low diffusion anisotropy, for orientation relative to the surface map in (c). FA values were projected onto models of the G110 cerebral cortical surface from multiple brains, and averaged onto a common surface [41]. (c) Lateral view of the G110 average surface. Black arrowhead indicates the location of the insula, and the dashed line is the approximate location of the axial planes shown in (a-b).
Biophysical Modeling of Water Diffusion in the Developing Cerebral Cortex
A common approach has been adopted for quantitative modeling of the influence of dispersion in the orientations of axon, dendrites, and other cylindrical cellular processes within a voxel [18-21]. This framework relies on three fundamental assumptions:
Water molecules reside within one of two environments. The “cylindrical” environment consists of cellular processes such as axons and dendrites and is characterized by the volume fraction v. The “extra-cylindrical” environment consists of non-cylindrical structures such as cell bodies, and occupies the remaining 1- v volume fraction of the voxel.
Water exchange between the two environments during the diffusion inter-pulse delay, Δ, is negligible.
The Gaussian phase approximation is made within each environment.
The consequences of the first two assumptions are that the dependence of the signal intensity S(b) on diffusion weighting b, relative to the reference intensity S(0) can be written as the sum of two terms
| (1) |
in which Sc(b) and Se(b) correspond to the signal intensity components arising from the cylindrical and extracylindrical compartments, respectively, and b is the standard diffusion weighting term defined in diffusion measurements using pulsed-field gradient magnetic resonance techniques [22]. If water diffusion is assumed to be isotropic within the extracylindrical compartment, diffusion is characterized by the extracylindrical apparent diffusion coefficient (ADCe) according to the expression
| (2) |
To account for anisotropic diffusion within the extracylindrical environment, the scalar-valued ADCe can be replaced with a diffusion tensor D [23, 24], as in [25].
Mathematical expressions for the cylindrical term Sc(b) as a function of diffusion weighting oriented along an arbitrary diffusion sensitization direction, are first provided for the two limiting cases of extreme anisotropy of cellular process orientation, and perfectly isotropic orientation distribution of cellular processes. Under conditions of extreme anisotropy, all cylindrical cellular process are oriented parallel to each other, and α is the angle between this common orientation and the direction of the applied diffusion-sensitizing magnetic field gradients. Local diffusion anisotropy within the cylindrical structures is represented as a difference between the (larger) diffusion coefficient characterizing displacements parallel to the cellular process axis (Dax) and the (smaller) diffusion coefficient characterizing displacements perpendicular to the cellular process axis, oriented radially in the local frame (Drad). As a consequence of the Gaussian phase approximation
| (3) |
In order to account for heterogeneity in the orientations of different axon, dendrite, or other cellular processes, the contributions of all cylinder orientations are integrated over the orientation distribution. For the extreme case of a uniform cylinder orientation distribution, in which all cylinder orientations are equally probable, the distribution of angles α, f(α), equals sin(α)/2. As detailed in [26, 27],
| (4) |
in which Φ(χ) is the error function of χ. In a diffusion-weighted magnetic resonance spectroscopy study of the adult rat brain, expression [4] was used to analyze diffusion of N-acetylaspartate (NAA) [28]. This endogenous molecule is present in high concentrations in neurons, and therefore provides an opportunity to examine a situation in which v ≈ 1, and under these conditions, remarkable agreement between Eq. [4] was observed with the experimental data.
To more generally account for cylinder orientation distributions that span the range from extreme anisotropy to isotropic, it is useful to consider the orientation distribution function (ODF) expressed as a function of the polar angles θ and ϕ, f(θ, ϕ). The expression analogous to Eq. [4], but for a general ODF is [29]
| (5) |
Thus, the analysis of dispersion in axon and dendrite orientations involves characterizing the function f(θ, ϕ). One approach is to approximate f(θ, ϕ) as a truncated spherical harmonic expansion [20, 25, 29]. A challenge encountered with numerical estimation of f(θ, ϕ) using a spherical harmonic expansion is that a high-order expansion is needed to precisely approximate a narrow distribution of orientations, such as in white matter fibers, or the early-developing cerebral cortex. As a result, a large number of adjustable parameters are needed, which has the disadvantage of introducing instability in model parameter estimation. To overcome this issue, one approach has been to model f(θ, ϕ) as a Watson [21, 30] (or Bingham [31]) distribution, a strategy adopted in the popular neurite orientation dispersion and density imaging (NODDI) method of data analysis. In these cases, the width of the fiber orientation distribution is reflected as model distribution parameters, such as the Watson distribution concentration parameter κ [32] which is high for a narrow distribution of fibers (e.g. immature cerebral cortex) and low for a broad distribution (mature cortex). Drawbacks to this approach, however, are that the minimum number of diffusion encodings necessary for its use have not been characterized, and nonlinear optimization, or other specialized computational methods, are necessary for its implementation. In the context of the model assumptions, the issue of parameter estimation has specific challenges associated with it [33].
Another approach that facilitates interpretation of water diffusion anisotropy in terms of the axon and dendrite ODF, that avoids the difficulties posed by analytical modeling and closely parallels the standard diffusion tensor analysis [23, 24], results from expanding Eq. [5] as a Taylor series with respect to b [34]. The term that is linear in b contains the diffusion tensor, D, and the second moment of f(θ, ϕ), which is the scatter matrix T. The standard way to quantify anisotropy in D is to express variation in its eigenvalues through the fractional anisotropy (FA) [35], which ranges from 0 (isotropic diffusion) to 1 (extreme anisotropy). In a similar manner, it is possible to compute eigenvalues of T and to express anisotropy in the ODF’s scatter matrix as FAT. This analysis leads to a monotonic relationship between FA and FAT, which is a result that facilitates an interpretation of FA in terms of anatomical properties of the cerebral cortex; namely, the neuropil volume fraction, the local diffusion coefficient magnitudes, and anisotropy in the axon/dendrite orientation distribution. In the following sections, it is assumed that v, Dax, and Drad do not change with age, and that changes in cortical FA with development are caused by changes in FAT. Experimental support of this interpretation can be gleaned from the data to be reviewed, however further direct experimental investigation of these assumptions would clearly be of value.
Validation of the Interpretation of FA Changes in Terms of Neuron Morphological Development Using Independent Experimental Methods
In order to validate interpretations of cortical FA based on the intricate biophysical modeling reviewed in the previous section, quantitative comparisons to results from independent experimental methods are needed. A typical voxel volume in a diffusion MRI experiment conducted on a human brain is on the order of 8 microliters (2×2×2 mm3). Therefore, MRI measurement of water diffusion anisotropy provides a quantitative aggregate measure of cellular complexity that reflects the structural characteristics of thousands of neurons. Morphological complexity of large populations of cells can be difficult to quantify using traditional light microscopy, because standard methods involve manually tracing neuronal processes, which presents challenges for use as a practical validation strategy. Microscopy-based validation studies have thus incorporated novel methods for tracing, as well as statistical methods for analyzing images that circumvent the need to perform extensive tracing operations.
Explicit orientation distribution determinations using cell tracing data
One early study in which the orientation distribution of myelinated axons was directly measured in gray matter of the superior colliculus, as well as the white matter of the corpus callosum, was performed by Leergaard and co-workers [36]. Diffusion MRI measurements were performed on post mortem rat brain tissue, and subsequently, using the same tissue, a stereological approach was taken to estimate the orientation distribution of myelinated axons. Within regions that matched the in-plane resolution of the MRI data set, myelinated axons were manually traced, and the orientation distribution of fibers was compared to the fiber orientation distribution calculated from the diffusion MRI data. The magnitude and direction of anisotropy in the orientation distribution closely matched the orientation distribution predicted from the diffusion MRI data. An automated approach for analyzing cell tracings in terms of cell process orientation distribution functions is illustrated in Figure 2a-c. Each segment of 10 μm in length (the distance that approximately matches the root-mean-squared displacement in a diffusion MRI experiment) is fitted to a line, and the orientation distribution of resulting lines can be estimated. Such an analysis has been performed for neuron traces that have been deposited in the NeuroMorpho database [37].
Figure 2.
Light microscopy methods for validating cellular process orientation distributions inferred through diffusion MRI measurements of diffusion anisotropy. (a-c) Cell tracing methods enable explicit determination of cell process orientation distributions. (a) Tracings of a P4 (upper) and P14 (lower) rat pyramidal neuron are illustrated as in Figure 1. (b) Each dendrite branch is separated into 25 μm sections, and approximated as a cylinder. Cylinders are translated to a common origin in (c) to illustrate the difference in dendrite orientation distribution width between the two different neurons. (d) 3D structure tensor calculations reveal anisotropy in cell process orientation along three orthogonal axes. (e) A 3D montage of hippocampal gray matter (CA1) and neighboring white matter (alveus, inferior longitudinal fasciculus (ILF)), stained with DiI, is shown in the lower panel, as described in [39]. The primary orientations of cell processes are indicated with arrows that are color-coded according to direction following the color scale used for (d). In (d), 3D structure tensors are overlaid on the 3D histological images, indicating the direction and magnitude of diffusion anisotopy expected for each of the tissue sub-regions.
A subsequent post mortem diffusion MRI and light microscopy study extended the tracing approach to a 3D strategy that focused on early developing cerebral cortical gray matter [34]. Following diffusion MRI procedures, cerebral cortical tissue from postnatal day 13 (P13) and P31 ferret brains was stained using the rapid Golgi method, and examined with confocal microscopy. The through-plane resolution of the confocal images was sufficiently high to generate 3D traces of the Golgi-stained neurons. An automated tracing method was used to analyze 3D image stacks, and obtain traces of multiple stained neurons throughout tissue volumes comparable to a diffusion MRI voxel size. Similarity in fractional anisotropy of the axon/dendrite orientation distribution scatter matrix (FAT) and water diffusion FA confirmed the theoretical predictions relating these two parameters.
Other image analysis methods to corroborate diffusion MRI findings
Although cell tracing-based methods facilitate explicit estimations of the axon/dendrite orientation distribution, there are disadvantages associated with relying on their calculation for validating interpretations of diffusion MRI data. First, it is difficult to trace neural processes accurately, but with sufficiently high throughput to provide an estimate of the average morphological characteristics that affect water diffusion. Second, many histological procedures are not appropriate for cell tracing. For example, some procedures stain tissue too densely to trace individual cells or cell processes. Image analysis procedures that to not require tracing can provide straightforward strategies for quantifying cell morphology, and can be applied to a broader set of staining techniques.
In 2012, Budde and Frank [38] introduced using structure tensor analyses for validating diffusion MRI data. The structure tensor of an image quantifies the direction dependence of image variation in intensity. For most staining methods, image intensity is homogeneous within a given cell process, and therefore in directions parallel to the principal axis if a diffusion tensor, image intensity variation is expected to be minimal. In directions perpendicular to the primary orientation of cell processes, image intensity varies. Thus, the orientation, and magnitude of anisotropy within a structure tensor is expected to correspond to the orientation and magnitude of anisotropy of the diffusion tensor for the same region of tissue. The first implementation of structure tensor calculations for guiding the interpretation of diffusion MRI [38], demonstrated striking correlations between diffusion tensor and structure tensor orientation and magnitude of anisotropy. These authors similarly demonstrated that structure tensor analysis is a simple yet robust analysis that can be applied to tissue stained using a variety of immunohistochemical, as well as other staining methods. Subsequent work [39, 40] generalized the structure tensor analysis to 3D (Figure 2d-e), which is beneficial for studying complex structures that are oblique to any given sectioning plane, such as is commonly encountered in studies of the convoluted cerebral cortex of gyrencephalic species. Recent comparisons between 3D structure tensors and diffusion MRI results in the fetal rhesus macaque brain have shown that the morphology of dendritic processes closely corresponds to anisotropy of water diffusion in the developing cerebral cortex [41], which supports the role of development of dendritic arbors in maturation-related changes in cerebral cortical diffusion anisotropy.
Although structure tensor analysis has the advantage of being highly flexible for performing validation studies of diffusion MRI data, a drawback is that the relationship between the structure tensor and the axon/dendrite orientation distribution is in most cases not well understood. Thus, although quantitative, structure tensor analyses are of limited value for more detailed biophysical modeling. Other statistical analyses of histological images have also been proposed. As one example, Novikov and co-workers have proposed a classification scheme based on the diffusion time dependence of the diffusion MRI signal, which has implications in terms of the spatial autocorrelation function of the underlying tissue [42]. This approach has yet to be incorporated into a validation analysis of developing cerebral cortical tissue.
Empirical Model of the Loss of Water Diffusion Anisotropy with Maturation of the Cerebral Cortical Gray Matter
Precise knowledge of the developmental trajectory of cerebral cortical diffusion anisotropy in the normally-developing brain is important for optimizing the sensitivity with which diffusion MRI can be used to detect abnormal development. Cortical FA values have been determined as a function of age in several species across multiple laboratories. Remarkable consistency in the dependence of FA on age, laminar position, and cortical region has been observed in comparisons between these studies.
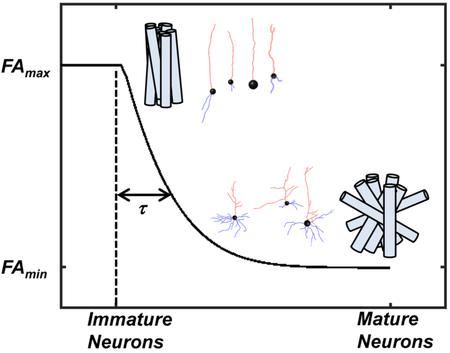
One way to compare results reported by different researchers is through reference to an analytical model of FA changes with development (Figure 3a). Cortical FA ranges between extreme cases of a maximal FA value observed in the immature cortex, FAmax, and a minimal (but non-zero [43]) value at maturity, FAmin. At the conclusion of neural migration to the cortical plate, the addition of new morphologically simple neurons to the cortical plate ceases, neurons initiate morphological differentiation, and cortical FA begins to decrease from FAmax toward FAmin. The time in which the reduction in FA is initiated is termed tinit. Reduction in FA with development has been empirically approximated as an exponential decay, characterized by the species-dependent decay time constant τ [17] (Figure 3a). The resulting expression for cortical changes in FA as a function of time, t, is
| (6) |
Figure 3.
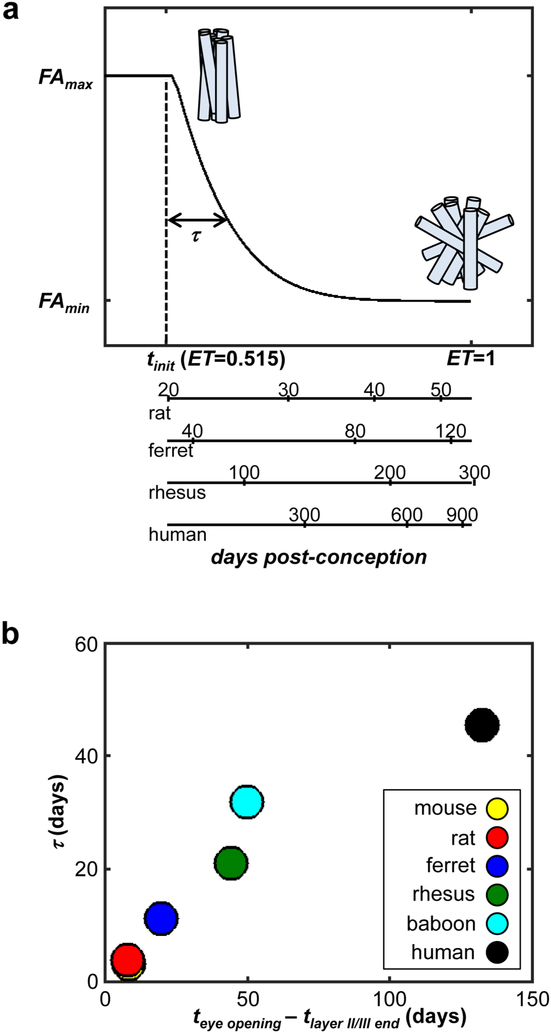
Comparison of the rate of age-dependent FA reduction between species. (a) For a given species, beginning at the time tinit, FA decreases exponentially with a decay time constant τ. Over this period, neural morphological development occurs, as represented by the cylinders with increasing orientation dispersion, as shown in Figure 1. To compare changes in FA between species, the post-conception age can be converted to a species-independent event time (ET). The FA changes for rhesus macaques is plotted as a function of ET, and the approximate value of τ is illustrated on the plot. For reference, corresponding ages for rats, ferrets, and humans are shown alongside the rhesus time line. (b) Comparisons between τ and the interval length between the conclusion of layer II/III neurogenesis and eye opening are shown for six species, following procedures described in [56]. The approximately linear relationship indicates consistency in the rates of development measured by FA changes and by the meta-analysis performed by Workman and colleagues [57].
Thus, the time constant τ reflects the pace of cerebral cortical development for a given species. For example, τ is expected to be longer in humans than in more rapidly developing species such as mice, because human cortical development occurs over a more protracted period of time. Within this framework, it is noted that the cerebral cortical thickness is comparable to, or even smaller than the voxel dimensions typically used in diffusion MRI studies. The observed FA value is a volume-fraction-weighted average of FA within the cortex, as well as within neighboring cerebrospinal fluid (CSF) and subplate tissue [44]. Water diffusion is isotropic in the CSF and subplate, and therefore the parameters FAmax and FAmin, as well as FA(t) in general, will be reduced by partial volume averaging of the cortex with neighboring tissue or CSF. The extent of this reduction will depend on the image resolution. However to a first approximation, the relative sizes of FAmax, FAmin, and FA(t) are expected to be consistent for a given image resolution. Due to the fact that parameters tinit and τ are sensitive to relative values of FA at varying values of t rather than absolute values, tinit and τ are expected to be (approximately) comparable between studies, in spite of different degrees of partial volume averaging between studies.
Changes in cortical FA with development have also been expressed using other analytical models. In studies of human subjects, changes in FA have been modeled as a linear reduction with time [45-47]. One drawback to such an approximation is that it is inaccurate for limiting cases. Physically impossible FA values larger than unity, or smaller than 0 are predicted for young or old ages, respectively. Further, inter-study comparisons are only possible if subjects are analyzed over similar developmental age ranges, because changes in FA per unit time decrease as the value at maturity, FAmin, is approached. There have also been studies focused on very early stages of cerebral cortical development that have documented FA increases with age [48-50]. In these cases, the thickness of the cortical plate changes dramatically compared to the image resolution, and this leads to an increase in FA with age. Such observations reflect changes in partial volume averaging between cell types, rather than changes in cellular morphology. Under such conditions, Eq. [6] is not appropriate for analyzing FA(t) due to its dependence on partial volume averaging noted above. The objective of this review is to focus on subsequent stages of development, in which cortical thickness does not change as dramatically.
Published cortical FA values for mouse [51], rat [52, 53], ferret [17, 54], baboon [55], and human [9, 50], have been analyzed using Eq. [6], and the fitted values of τ are comparable to independently estimated species-dependent rates of central nervous system development (Figure 3b) [56]. The independent estimate of the rate of CNS development is provided by Workman and co-workers [57], (http://www.translatingtime.net) who have compiled developmental data published for multiple species, and built a regression model that can convert the age of an individual, in terms of days post-conception, into a species-independent event time (ET) which ranges between values of 0 and 1. It was found that the exponential time constant for cortical FA changes was proportional to the predicted time elapsed between two well-conserved developmental events (the conclusion of neurogenesis for cortical layers II/III, and eye opening, Figure 3b) [56]. This proportionality implies that the rate of change of cortical FA follows that predicted by the rate of CNS development for a given species. Therefore, FA changes observed in an animal model of a developmental process or a neurodevelopmental disorder are expected to relate to FA changes observed in humans in a comparable way. Indeed, the onset of the reduction in FA, tinit, is now included in the translating time regression model (http://www.translatingtime.net), and the units for the abscissa in Figures 3a and 3b are species-independent ET values defined by Workman and colleagues [57].
Data collected from in vivo, as well as aldehyde-fixed post mortem tissue are included in the Figure 3b plot. Several studies have directly investigated the comparability of diffusion measurements performed before and after death and fixation in brain tissue, and while the directionally-averaged water diffusivity is markedly reduced in post mortem tissue compared to in vivo conditions, the extent of diffusion anisotropy is preserved [58]. Figure 4a shows an FA parameter map measured from a G110 rhesus macaque in utero, compared to an FA map derived from a G110 hemisphere from a different animal, determined post mortem in Figure 4b. As discussed above, cortical FA is higher in the post mortem data as a consequence of higher image resolution and reduced partial volume averaging. However, the relative variation in FA values, apparent in the regional patterns of FA, are preserved between the two tissue conditions. Systematic differences between living and ex vivo tissue are not observed the data used to produce Figure 3b, though the differences in physiological state of the tissue for these measurements should be recognized, as discussed in greater detail in [56], and references cited therein.
The developmental reduction in FA with time, averaged over the entire cerebral cortex, suggests a relationship between water diffusion anisotropy and the age of cortical neurons within the tissue being studied. The association between reductions in diffusion anisotropy with neuron age is also apparent on the finer scales of cerebral cortex anatomy such as the laminar and regional position. There is a gradient in neuron age across the layers of the cerebral cortex due to its “inside-out” development. Neurons that form the inner cortical layers (layers V/VI) are born at an earlier age than the neurons present in the outer layers (layers II/III), and as a consequence, neurons of inner cortical layers exhibit more mature and complex morphology than neurons of the outer layers at early stages of development [59, 60]. Expressed in terms of concepts relevant to water diffusion anisotropy, the orientation distributions of axons and dendrites of inner cortical layers at a given stage of development are broader than those of the outer layers due to the difference in neuron age. Therefore, water diffusion anisotropy is expected to be lower within the deeper layers. This layer dependence has been explicitly demonstrated in baboons [55, 61], rhesus macaques [44], and ferrets [17], and is apparent in images of other diffusion MRI studies that generated high-resolution parametric maps of diffusion anisotropy (for example, [48, 53]). In addition to the laminar gradient in neuron age, a regional gradient termed the translational neurogenic gradient (TNG), also exists [62, 63]. The TNG is structured such that neurons of a given lamina are oldest near the insular cortex, which is located on the ventral and lateral cortex bordering the frontal lobe, and neurons are progressively younger for regions more distant to the insular TNG source. A regional gradient in FA has been quantified in ferrets [17], rhesus macaques [41], and baboons [55], that is parallel to that expected from the TNG (Figure 4c). Specifically, FA is lowest near the insula and it increases as a function of distance from the presumed TNG source. Efforts to quantify the TNG magnitude (e.g. the difference in age of neurons at the TNG source vs. the frontal and occipital poles) indicate quantitative agreement with values estimated based on histological and cell birthdating studies [17]. The close associations observed between cerebral cortical diffusion anisotropy and neuronal age strongly support that morphological development of neurons critically influence FA changes in the developing cerebral cortex.
A final consideration is whether cytoarchitectural differences lead to distinct developmental trajectories for different areas of the cerebral cortex. Cortical areas involved in different functions have different cytoarchitectural characteristics. Recent advances in neuroimaging approaches for studying the mature human brain have created the possibility of generating fine-scale parcellations of the cerebral cortex within individuals [64], which would be an exceptionally valuable capability for neuroscience and neurological research. Further, although cortical diffusion anisotropy is not currently used for cytoarchitectural parcellation of the cerebral cortex, high-resolution diffusion MRI studies of post mortem human brain sections have demonstrated rich differences in cortical diffusion anisotropy between areas with different cytoarchitecture [16]. The need for high spatial resolution is well-recognized, to avoid confounds associated with neighboring white matter structures [65]. Nevertheless, an intriguing finding is that it has been possible to detect differences in the orientation of the least restricted direction of diffusion, relative to the radial orientation of pyramidal neuron apical dendrites, in high-resolution diffusion MRI studies of adult human subjects [15]. It is therefore of high interest to determine whether diffusion MRI can be used to monitor the transition of the developing cerebral cortex from the cortical plate to a cytoarchitecturally mature, functionally distinct region of cortex. Two reproducible patterns observed in multiple species are a highly conspicuous transition from high to low FA that marks the boundary between isocortex (high FA) and allocortex (low FA), and the observation that primary motor and sensory cortical areas experience an earlier tinit than their neighboring cortical areas, resulting in relatively low FA [17, 55]. Based on the findings reported in studies of mature brains, it is anticipated that improvements in the precision of cytoarchitectural development will be facilitated as diffusion MRI measurements with increasing image resolution become feasible.
Abnormal Developmental Trajectories of FA Changes Observed in Preclinical and Clinical Studies
Abnormal morphology of dendritic arbors of neurons in the cerebral cortex is consistently reported in animal models of several neurodevelopmental disorders. The influence of environmental factors on cerebral cortical neuron morphology was famously demonstrated in studies showing that environmental enrichment can cause increased dendritic arbor complexity [66]. Inversely, poorly enriched environments, such as social isolation results in the appearance of abnormally simplified cortical dendritic arbors [67]. Linkages between more specific sensory input have been observed for corresponding regions of the cortex. As an example, perturbed visual experience [68] has been shown to result in disturbances in dendritic arbor complexity in visual cortex. Thus, it is perhaps unsurprising that environmental exposures known to cause neurodevelopmental disorders, such as fetal alcohol exposure [69] or perinatal hypoxia/ischemia [70], are also associated with markedly reduced cerebral cortical dendritic arbor complexity. In addition to environmental influences, neurodevelopmental disorders of genetic origin, such as Rett syndrome [71], are additional example cases in which abnormal dendritic morphology is implicated disease. The common finding of abnormal neural morphological development in neurodevelopmental disorders with diverse etiologies suggest this effect is central to their pathophysiology, and therefore a non-invasive method to detect abnormal anatomical development could be of value for early detection, or prediction of risk for future functional impairment.
Mounting evidence from preclinical studies of animal model systems indicates that abnormalities in dendritic arbor complexity secondary to insults associated with neurodevelopmental disorders in humans can lead to increased anisotropy in cortical water diffusion compared to age-matched controls. In a study of fetal exposure to alcohol, diffusion MRI measurements performed on post mortem neonatal rat brain tissue revealed increased cortical FA in offspring to rats that were fed alcohol throughout pregnancy compared to animals fed an isocaloric control solution [72]. Two dimensional structure tensor calculations performed on the same tissue following DiI staining [38] served to validate that abnormal dendritic arborization was associated with the observed abnormally high FA. In a post mortem diffusion MRI study of perinatal sheep following experimentally induced hypoxia/ischemia, cortical FA was observed to be higher than in control animals, and corroborative analyses of the same tissue following Golgi staining indicated basal dendrites exhibited simpler morphology in affected animal brains [70]. Visual deprivation has similarly been shown to produce abnormally high FA in the visual cortex of early postnatal ferrets, and these findings were supported by 2D dendrite orientation distribution analyses [73]. Lastly, in utero diffusion anisotropy measurements have been shown to result in increased cortical FA in the context of a rhesus macaque fetus as a result of a functional deficit in the placenta [74]. These results are summarized with respect to overall changes in cortical FA in Figure 5. Although it is currently not possible to quantitatively interpret differences in FA of control vs. experimentally-perturbed cases in terms of specific anatomical differences, together, these findings indicate in a qualitative sense that diffusion MRI directed at the developing cerebral cortex potentially has the sensitivity needed to detect abnormal morphological development of neurons in the context of neurodevelopmental disorders. It should also be noted that not all perinatal insults lead to increased cortical FA. For example, in rat pups subjected to hypoxic/ischemic injury on the third day of life, cerebral cortical FA was reduced, compared to controls, when assessed three days later [75]. Subsequent histological examination of the brains of these animals indicated that disrupted organization of nestin-positive radial glial cells was the cause of the relatively low FA. Given this expected heterogeneity in biological processes associated with abnormal development, continued use of animal models will be of value to validate the histological underpinnings of diffusion MRI measurements.
Figure 5.
Experimental models of neurodevelopmental disorders that have been demonstrated to produce an increase in cerebral cortical FA relative to age-matched controls. The species-averaged FA value for control animals (black trace) was determined by converting curves for each species (such as shown for rhesus macaques in Figure 4a) to species independent ET, and averaging the result. The ages of animals for individual experimental perturbations were translated to the ET scale using species-dependent parameters given in [57].
Recent human subject studies support the interpretations of cortical diffusion MRI based on preclinical work. Comparisons of cortical FA between prematurely born and termborn infants show that early exposure to the extra-uterine environment hampers the loss of diffusion anisotropy [47]. Further, in prematurely-delivered human infants, the extent of reduction in cerebral cortical FA has been shown to correlate with measures of infant growth [76]. Regional patterns in the loss of cortical FA in human infants have been reported [77], and comparisons to measures of white matter development indicate that cortical maturation, as reflected in the reduction in diffusion anisotropy, is coupled to white matter development [46]. This integration with other aspects of brain development suggest that abnormalities in cortical diffusion anisotropy may be of functional relevance. A critical next step for this imaging modality will be to establish linkages between diffusion MRI findings during the perinatal period and subsequent cognitive and behavioral outcomes.
Conclusion
Diffusion MRI provides a unique opportunity to non-invasively characterize cellular morphological development of neurons in the early developing cerebral cortex. This aspect of brain development is critical to support the formation of synaptic connections and hence functional circuits. Invasive studies using microscopy have identified that abnormal morphology of developing cortical neurons is associated with various neurodevelopmental disorders. Preclinical, as well as initial human subject studies indicate that measurements of cerebral cortical diffusion anisotropy possess the requisite sensitivity to detect abnormal development of neurons. However, due to the lack of specificity of the characteristics of water diffusion averaged over an MRI voxel, it has been necessary to design specialized analyses of microscopy data to quantitatively validate the interpretations of diffusion MRI findings. Yet from another perspective, the averaging of water diffusion over thousands of cellular processes, that is intrinsic to diffusion MRI measurements, presents an advantage of efficiently measuring the average structural characteristics of large ensembles of cells. Such averaging enables the detection of subtle structural effects, provided they are sufficiently widespread to affect a large number of cells. This could be considered a valuable capability, relative to other experimental approaches, for research focused on neurodevelopmental disorders.
The developmental period associated with the loss of cerebral cortical water diffusion anisotropy is a critical time for CNS development, but is difficult to access using other techniques. Ideally, non-invasive modalities would be of use for detecting abnormalities at early stages of development, prior to the emergence of cognitive and behavioral deficits. Diffusion MRI directed at the immature brain meets this need. In addition, the endogenous image contrast obtained in diffusion MRI is possible to use in the study of vulnerable populations, such as prematurely delivered human infants (see Neil and Smyser, this issue). Improvements in retrospective motion correction techniques will further bolster the value of diffusion MRI by extending the ability to apply this approach to the study of developing fetuses [78, 79]. In summary, it is hoped that this versatile technique for characterizing cerebral cortical maturation throughout the entire brain will provide new strategies for early detection of anatomical abnormalities associated with neurodevelopmental disorders, and as a potential way to monitor responses to proposed therapeutic interventions.
Acknowledgements
The author would like to acknowledge discussions with Sune Jespersen (Aarhus University) for several conversations, beginning in the Ackerman laboratory, that have made much of this work possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Beaulieu C, The basis of anisotropic water diffusion in the nervous system - a technical review, NMR Biomed, 15 (2002) 435–455. [DOI] [PubMed] [Google Scholar]
- [2].Le Bihan D, Looking into the functional architecture of the brain with diffusion MRI, Nat.Rev. Neurosci, 4 (2003) 469–480. [DOI] [PubMed] [Google Scholar]
- [3].Mori S, Zhang J, Principles of diffusion tensor imaging and its applications to basic neuroscience research, Neuron, 51 (2006) 527–539. [DOI] [PubMed] [Google Scholar]
- [4].Baratti C, Barnett A, Pierpaoli C, Comparative MRI Study of Brain Maturation Using T1, T2, and the Diffusion Tensor, in: Proceedings of the ISMRM, Vancouver, CA, 1997. [Google Scholar]
- [5].Thornton JS, Ordidge RJ, Penrice J, Cady EB, Amess PN, Punwani S, Clemence M, Wyatt JS, Anisotropic water diffusion in white and gray matter of the neonatal piglet brain before and after transient hypoxia-ischaemia, Magn Reson Imaging, 15 (1997) 433–440. [DOI] [PubMed] [Google Scholar]
- [6].Mori S, Itoh R, Zhang J, Kaufmann WE, van Zijl PC, Solaiyappan M, Yarowsky P, Diffusion tensor imaging of the developing mouse brain, Magn Reson Med, 46 (2001) 18–23. [DOI] [PubMed] [Google Scholar]
- [7].Neil J, Miller J, Mukherjee P, Huppi PS, Diffusion tensor imaging of normal and injured developing human brain - a technical review, NMR Biomed, 15 (2002) 543–552. [DOI] [PubMed] [Google Scholar]
- [8].Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, Akbudak E, Aronovitz JA, Miller JP, Lee BC, Conturo TE, Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging, Radiology, 209 (1998) 57–66. [DOI] [PubMed] [Google Scholar]
- [9].McKinstry RC, Mathur A, Miller JP, Ozcan AO, Snyder AZ, Schefft GL, Almli CR, Shiran SI, Conturo TE, Neil JJ, Radial organization of developing human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI, Cereb Cortex, 12 (2002) 1237–1243. [DOI] [PubMed] [Google Scholar]
- [10].Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK, Toward accurate diagnosis of white matter pathology using diffusion tensor imaging, Magn Reson Med, 57 (2007) 688–695. [DOI] [PubMed] [Google Scholar]
- [11].Zilles K, Palomero-Gallagher N, Amunts K, Development of cortical folding during evolution and ontogeny, Trends Neurosci, 36 (2013) 275–284. [DOI] [PubMed] [Google Scholar]
- [12].Zanin E, Ranjeva JP, Confort-Gouny S, Guye M, Denis D, Cozzone PJ, Girard N, White matter maturation of normal human fetal brain. An in vivo diffusion tensor tractography study, Brain Behav, 1 (2011) 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Assaf Y, Imaging laminar structures in the gray matter with diffusion MRI, NeuroImage, (2018) In press. [DOI] [PubMed] [Google Scholar]
- [14].Bourgeois JP, Rakic P, Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage, J Neurosci, 13 (1993) 2801–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McNab JA, Polimeni JR, Wang R, Augustinack JC, Fujimoto K, Stevens A, Triantafyllou C, Janssens T, Farivar R, Folkerth RD, Vanduffel W, Wald LL, Surface based analysis of diffusion orientation for identifying architectonic domains in the in vivo human cortex, Neuroimage, 69 (2013) 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aggarwal M, Nauen DW, Troncoso JC, Mori S, Probing region-specific microstructure of human cortical areas using high angular and spatial resolution diffusion MRI, Neuroimage, 105 (2015) 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kroenke CD, Taber EN, Leigland LA, Knutsen AK, Bayly PV, Regional patterns of cerebral cortical differentiation determined by diffusion tensor MRI, Cereb Cortex, 19 (2009) 2916–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Assaf Y, Basser PJ, Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain, Neuroimage, 27 (2005) 48–58. [DOI] [PubMed] [Google Scholar]
- [19].Barazany D, Basser PJ, Assaf Y, In vivo measurement of axon diameter distribution in the corpus callosum of rat brain, Brain, 132 (2009) 1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jespersen SN, Kroenke CD, Ostergaard L, Ackerman JJ, Yablonskiy DA, Modeling dendrite density from magnetic resonance diffusion measurements, Neuroimage, 34 (2007) 1473–1486. [DOI] [PubMed] [Google Scholar]
- [21].Zhang H, Hubbard PL, Parker GJ, Alexander DC, Axon diameter mapping in the presence of orientation dispersion with diffusion MRI, Neuroimage, 56 (2011) 1301–1315. [DOI] [PubMed] [Google Scholar]
- [22].Callaghan PT, Translational dynamics and magnetic resonance, Oxford, New York, 2011. [Google Scholar]
- [23].Basser PJ, Mattiello J, LeBihan D, Estimation of the effective self-diffusion tensor from the NMR spin echo, Journal of magnetic resonance, 103 (1994) 247–254. [DOI] [PubMed] [Google Scholar]
- [24].Basser PJ, Mattiello J, LeBihan D, MR diffusion tensor spectroscopy and imaging, Biophysical journal, 66 (1994) 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jespersen SN, Bjarkam CR, Nyengaard JR, Chakravarty MM, Hansen B, Vosegaard T, Ostergaard L, Yablonskiy D, Nielsen NC, Vestergaard-Poulsen P, Neurite density from magnetic resonance diffusion measurements at ultrahigh field: comparison with light microscopy and electron microscopy, Neuroimage, 49 (2010) 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yablonskiy DA, Sukstanskii AL, Leawoods JC, Gierada DS, Bretthorst GL, Lefrak SS, Cooper JD, Conradi MS, Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized 3He diffusion MRI, Proc Natl Acad Sci U S A, 99 (2002) 3111–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Callaghan PT, Jolley KW, Lelievre J, Diffusion of water in the endosperm tissue of wheat grains as studied by pulsed field gradient nuclear magnetic resonance, Biophysical journal, 28 (1979) 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kroenke CD, Ackerman JJ, Yablonskiy DA, On the nature of the NAA diffusion attenuated MR signal in the central nervous system, Magn Reson Med, 52 (2004) 1052–1059. [DOI] [PubMed] [Google Scholar]
- [29].Anderson AW, Measurement of fiber orientation distributions using high angular resolution diffusion imaging, Magn Reson Med, 54 (2005) 1194–1206. [DOI] [PubMed] [Google Scholar]
- [30].Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC, NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain, Neuroimage, 61 (2012) 1000–1016. [DOI] [PubMed] [Google Scholar]
- [31].Tariq M, Schneider T, Alexander DC, Gandini Wheeler-Kingshott CA, Zhang H, Bingham-NODDI: Mapping anisotropic orientation dispersion of neurites using diffusion MRI, Neuroimage, 133 (2016) 207–223. [DOI] [PubMed] [Google Scholar]
- [32].Fisher NI, Lewis T, Embleton BJJ, Statistical analysis of spherical data, Cambridge University Press, Cambridge, UK, 1987. [Google Scholar]
- [33].Jelescu IO, Veraart J, Fieremans E, Novikov DS, Degeneracy in model parameter estimation for multi-compartmental diffusion in neuronal tissue, NMR Biomed, 29 (2016) 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jespersen SN, Leigland LA, Cornea A, Kroenke CD, Determination of axonal and dendritic orientation distributions within the developing cerebral cortex by diffusion tensor imaging, IEEE Trans Med Imaging, 31 (2012) 16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Basser PJ, Pierpaoli C, Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI, J. Mag. Reson., Ser. B, 111 (1996) 209–219. [DOI] [PubMed] [Google Scholar]
- [36].Leergaard TB, White NS, de Crespigny A, Bolstad I, D'Arceuil H, Bjaalie JG, Dale AM, Quantitative histological validation of diffusion MRI fiber orientation distributions in the rat brain, PLoS One, 5 (2010) e8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hansen MB, Jespersen SN, Leigland LA, Kroenke CD, Using diffusion anisotropy to characterize neuronal morphology in gray matter: the orientation distribution of axons and dendrites in the NeuroMorpho.org database, Front Integr Neurosci, 7 (2013) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Budde MD, Frank JA, Examining brain microstructure using structure tensor analysis of histological sections, Neuroimage, 63 (2012) 1–10. [DOI] [PubMed] [Google Scholar]
- [39].Khan AR, Cornea A, Leigland LA, Kohama SG, Jespersen SN, Kroenke CD, 3D structure tensor analysis of light microscopy data for validating diffusion MRI, Neuroimage, 111 (2015) 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schilling K, Janve V, Gao Y, Stepniewska I, Landman BA, Anderson AW, Comparison of 3D orientation distribution functions measured with confocal microscopy and diffusion MRI, Neuroimage, 129 (2016) 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang X, Studholme C, Grigsby PL, Frias AE, Cuzon Carlson VC, Kroenke CD, Folding, But Not Surface Area Expansion, Is Associated with Cellular Morphological Maturation in the Fetal Cerebral Cortex, J Neurosci, 37 (2017) 1971–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Novikov DS, Jensen JH, Helpern JA, Fieremans E, Revealing mesoscopic structural universality with diffusion, Proc Natl Acad Sci U S A, 111 (2014) 5088–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, Glasser MF, Hernandez M, Sapiro G, Jenkinson M, Feinberg DA, Yacoub E, Lenglet C, Van Essen DC, Ugurbil K, Behrens TE, W.U.-M.H. Consortium, Advances in diffusion MRI acquisition and processing in the Human Connectome Project, Neuroimage, 80 (2013) 125–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang X, Pettersson DR, Studholme C, Kroenke CD, Characterization of Laminar Zones in the Mid-Gestation Primate Brain with Magnetic Resonance Imaging and Histological Methods, Front Neuroanat, 9 (2015) 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Deipolyi AR, Mukherjee P, Gill K, Henry RG, Partridge SC, Veeraraghavan S, Jin H, Lu Y, Miller SP, Ferriero DM, Vigneron DB, Barkovich AJ, Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration, Neuroimage, 27 (2005) 579–586. [DOI] [PubMed] [Google Scholar]
- [46].Smyser TA, Smyser CD, Rogers CE, Gillespie SK, Inder TE, Neil JJ, Cortical Gray and Adjacent White Matter Demonstrate Synchronous Maturation in Very Preterm Infants, Cereb Cortex, 26 (2016) 3370–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ball G, Srinivasan L, Aljabar P, Counsell SJ, Durighel G, Hajnal JV, Rutherford MA, Edwards AD, Development of cortical microstructure in the preterm human brain, Proc Natl Acad Sci U S A, 110 (2013) 9541–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Aggarwal M, Gobius I, Richards LJ, Mori S, Diffusion MR Microscopy of Cortical Development in the Mouse Embryo, Cereb Cortex, 25 (2015) 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gupta RK, Hasan KM, Trivedi R, Pradhan M, Das V, Parikh NA, Narayana PA, Diffusion tensor imaging of the developing human cerebrum, Journal of neuroscience research, 81 (2005) 172–178. [DOI] [PubMed] [Google Scholar]
- [50].Trivedi R, Gupta RK, Husain N, Rathore RK, Saksena S, Srivastava S, Malik GK, Das V, Pradhan M, Sarma MK, Pandey CM, Narayana PA, Region-specific maturation of cerebral cortex in human fetal brain: diffusion tensor imaging and histology, Neuroradiology, 51 (2009) 567–576. [DOI] [PubMed] [Google Scholar]
- [51].Baloch S, Verma R, Huang H, Khurd P, Clark S, Yarowsky P, Abel T, Mori S, Davatzikos C, Quantification of brain maturation and growth patterns in C57BL/6J mice via computational neuroanatomy of diffusion tensor images, Cereb Cortex, 19 (2009) 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bockhorst KH, Narayana PA, Liu R, Ahobila-Vijjula P, Ramu J, Kamel M, Wosik J, Bockhorst T, Hahn K, Hasan KM, Perez-Polo JR, Early postnatal development of rat brain: in vivo diffusion tensor imaging, Journal of neuroscience research, 86 (2008) 1520–1528. [DOI] [PubMed] [Google Scholar]
- [53].Huang H, Yamamoto A, Hossain MA, Younes L, Mori S, Quantitative cortical mapping of fractional anisotropy in developing rat brains, J Neurosci, 28 (2008) 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Barnette AR, Neil JJ, Kroenke CD, Griffith JL, Epstein AA, Bayly PV, Knutsen AK, Inder TE, Characterization of brain development in the ferret via MRI, Pediatric research, 66 (2009) 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kroenke CD, Van Essen DC, Inder TE, Rees S, Bretthorst GL, Neil JJ, Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy, J Neurosci, 27 (2007) 12506–12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Leigland LA, Kroenke CD, A comparative analysis of cellular morphological differentiation within the cerebral cortex using diffusion tensor imaging, Neuromethods, 50 (2011) 329–351. [Google Scholar]
- [57].Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL, Modeling transformations of neurodevelopmental sequences across mammalian species, J Neurosci, 33 (2013) 7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sun SW, Neil JJ, Song SK, Relative indices of water diffusion anisotropy are equivalent in live and formalin-fixed mouse brains, Magn Reson Med, 50 (2003) 743–748. [DOI] [PubMed] [Google Scholar]
- [59].Marin-Padilla M, Prenatal and early postnatal ontogenesis of the human motor cortex: a golgi study. I. The sequential development of the cortical layers, Brain Res, 23 (1970) 167–183. [DOI] [PubMed] [Google Scholar]
- [60].Sidman RL, Rakic P, Development of the human central nervous system, in: Haymaker W, Adams RD (Eds.) Histology and histopathology of the nervous system, Charles C. Thomas, Springfield, IL, 1982, pp. 3–145. [Google Scholar]
- [61].Kroenke CD, Bretthorst GL, Inder TE, Neil JJ, Diffusion MR imaging characteristics of the developing primate brain, Neuroimage, 25 (2005) 1205–1213. [DOI] [PubMed] [Google Scholar]
- [62].Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS Jr., Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall, J Neurosci, 19 (1999) 10357–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bayer SA, Altman J, Neocortical Development, Raven Press, New York, 1991. [Google Scholar]
- [64].Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC, A multi-modal parcellation of human cerebral cortex, Nature, 536 (2016) 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, Leopold DA, Ye FQ, Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography, Proc Natl Acad Sci U S A, 112 (2015) E2820–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Volkmar FR, Greenough WT, Rearing complexity affects branching of dendrites in the visual cortex of the rat, Science, 176 (1972) 1445–1447. [DOI] [PubMed] [Google Scholar]
- [67].Struble RG, Riesen AH, Changes in cortical dendritic branching subsequent to partial social isolation in stumptailed monkeys, Dev Psychobiol, 11 (1978) 479–486. [DOI] [PubMed] [Google Scholar]
- [68].Tieman SB, Hirsch HV, Exposure to lines of only one orientation modifies dendritic morphology of cells in the visual cortex of the cat, The Journal of comparative neurology, 211 (1982) 353–362. [DOI] [PubMed] [Google Scholar]
- [69].Granato A, Van Pelt J, Effects of early ethanol exposure on dendrite growth of cortical pyramidal neurons: inferences from a computational model, Brain research, 142 (2003) 223–227. [DOI] [PubMed] [Google Scholar]
- [70].Dean JM, McClendon E, Hansen K, Azimi-Zonooz A, Chen K, Riddle A, Gong X, Sharifnia E, Hagen M, Ahmad T, Leigland LA, Hohimer AR, Kroenke CD, Back SA, Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization, Sci Transl Med, 5 (2013) 168ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Armstrong D, Dunn JK, Antalffy B, Trivedi R, Selective dendritic alterations in the cortex of Rett syndrome, Journal of neuropathology and experimental neurology, 54 (1995) 195–201. [DOI] [PubMed] [Google Scholar]
- [72].Leigland LA, Budde MD, Cornea A, Kroenke CD, Diffusion MRI of the developing cerebral cortical gray matter can be used to detect abnormalities in tissue microstructure associated with fetal ethanol exposure, Neuroimage, 83 (2013) 1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bock AS, Olavarria JF, Leigland LA, Taber EN, Jespersen SN, Kroenke CD, Diffusion tensor imaging detects early cerebral cortex abnormalities in neuronal architecture induced by bilateral neonatal enucleation: an experimental model in the ferret, Front Syst Neurosci, 4 (2010) 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lo JO, Roberts VHJ, Schabel MC, Wang X, Morgan TK, Liu Z, Studholme C, Kroenke CD, Frias AE, Novel Detection of Placental Insufficiency by Magnetic Resonance Imaging in the Nonhuman Primate, Reprod Sci, 25 (2018) 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sizonenko SV, Camm EJ, Garbow JR, Maier SE, Inder TE, Williams CE, Neil JJ, Huppi PS, Developmental changes and injury induced disruption of the radial organization of the cortex in the immature rat brain revealed by in vivo diffusion tensor MRI, Cereb Cortex, 17 (2007) 2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Vinall J, Grunau RE, Brant R, Chau V, Poskitt KJ, Synnes AR, Miller SP, Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns, Sci Transl Med, 5 (2013) 168ra168. [DOI] [PubMed] [Google Scholar]
- [77].Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, Miller MI, Mori S, Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging, J Neurosci, 29 (2009) 4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Studholme C, Mapping fetal brain development in utero using magnetic resonance imaging: the Big Bang of brain mapping, Annu Rev Biomed Eng, 13 (2011) 345–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jiang S, Xue H, Counsell S, Anjari M, Allsop J, Rutherford M, Rueckert D, Hajnal JV, In-utero three dimension high resolution fetal brain diffusion tensor imaging, Med Image Comput Comput Assist Interv, 10 (2007) 18–26. [DOI] [PubMed] [Google Scholar]
- [80].Furtak SC, Moyer JR Jr., Brown TH, Morphology and ontogeny of rat perirhinal cortical neurons, The Journal of comparative neurology, 505 (2007) 493–510. [DOI] [PubMed] [Google Scholar]
- [81].Ascoli GA, Donohue DE, Halavi M, NeuroMorpho.Org: a central resource for neuronal morphologies, J. Neurosci, 27 (2007) 9247–9251. [DOI] [PMC free article] [PubMed] [Google Scholar]