Abstract
We evaluated the multitargeted Janus kinase/TRAF family member associated NF-κβ activator (TANK)-binding kinase 1 (TBK1) inhibitor momelotinib combined with trametinib in 21 patients with Kirsten rat sarcoma viral oncogene homolog-mutated non—small-cell lung cancer. The maximum tolerated dose of momelotinib was 150 mg twice daily (insufficient to achieve significant TBK1 inhibition). No patients achieved objective response and the combination did not improve on the activity of single-agent trametinib on the basis of historic data.
Introduction:
Specific treatment options are lacking for Kirsten rat sarcoma viral oncogene homolog (KRAS)-mutated non—small-cell lung cancer (NSCLC) despite treatment advances in other mutation-drivensubgroups.
Patients and Methods:
In this study we evaluated the multitargeted Janus kinase/TANK-binding kinase 1 (TBK1) inhibitor momelotinib combined with the mitogen/extracellular signal-related kinase (MEK)1/MEK2 inhibitor trametinib in patients with platinum-treated, refractory, metastatic, KRAS-mutated NSCLC. Dose escalations (3 + 3 design) were conducted with momelotinib in combination with trametinib 1.0 mg once daily, then with trametinib inþcombination with the maximum tolerated dose (MTD) of momelotinib. MTD was determined from dose-limiting toxicity (DLT) during patients’ first 28-day cycle. Safety was the primary end point, and efficacy parameters, including disease control rate (DCR) at 8 weeks, were secondary end points.
Results:
Twenty-one patients were enrolled (median age: 68 years; 14 [66.7%] female). The MTD was momelotinib 150 mg twice daily in combination with trametinib 1.0 mg once daily. DLTs that determined the MTD were increased alanine aminotransferase and fatigue. The most common adverse events of any grade were nausea (n = 14 [66.7%]), diarrhea (n = 11 [52.4%]), and fatigue (n = 11 [52.4%]). The most common Grade ≥3 event was hypoxia (n = 3[14.3%]) No patients achieved objective response. DCR at 8 weeks was 12 patients (57.1%) (90% confidence interval [CI], 37.2%−75.5%). Median progression-free and overall survival were 3.6 months (90% CI, 2.2–5.6 months) and 7.4 months (90% CI, 4.0–15.3 months), respectively. Maximum momelotinib plasma concentrations were reached 1 to 2 hours after dosing, but were insufficient to achieve significant TBK1 inhibition.
Conclusion:
The additional use of momelotinib with trametinib does not improve on the activity of single-agent trametinib in KRAS-mutated NSCLC on the basis of historic data.
Keywords: Janus kinase, MEK inhibition, MEK pathway, Metastatic non—small-cell lung cancer, TBK1 inhibition
Introduction
Activating Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations are found in an estimated 25% of patients with lung adenocarcinomas. No molecularly targeted treatment options are available to treat KRAS-mutated non—small-cell lung cancer (NSCLC) despite treatment advances in other mutation-driven subgroups.1,2 These activating mutations drive signaling pathways such as the Janus kinase (JAK)-signal transducer and activator of transcription, TANK-binding kinase 1 (TBK1) and mitogen/extracellular signal-related kinase (MEK) pathways, which are important mitogenic and prosurvival pathways. Thus, targeting these downstream effector proteins is one potential treatment strategy.
Blocking TBK1 and MEK1 might represent an approach to targeting KRAS-mutated NSCLC. TBK1 inhibition impairs viability of KRAS-driven human cancer cells, yet also results in feedback extracellular signaleregulated kinase activation.3 Momelotinib is a selective, small molecule inhibitor of JAK1/2 and TBK1 that has been extensively evaluated in the treatmentofmyelofibrosisforitsJAKinhibitoryeffects.4–7 Momelotinib monotherapy reduced autocrine cytokine signaling, resulting in blockade of KRAS-driven lung cancer growth. Moreover, the combination of momelotinib with a MEK inhibitor induced regression of an aggressive murine lung adenocarcinoma driven by KRAS mutation and p53 loss.3 Trametinib is a potent, reversible, and selective allosteric inhibitor of MEK1 and MEK2 approved for the treatment of BRAF-mutated metastatic melanoma and metastatic NSCLC in combination with dabrafenib.8,9 Therefore, we conducted a phase IB, dose-finding study of momelotinib in combination with trametinib in patients with metastatic KRAS-mutated NSCLC.
Patients and Methods
Patients
Adult patients with KRAS-mutated metastatic NSCLC were eligible if they had disease progression after receiving ≥1 platinum-based chemotherapy regimen, or if disease progression occurred ≥ 6 months after completion of adjuvant therapy for stage I to IIIA disease including ≥ 1 platinum-based regimen; had measurable disease per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST)10; and Eastern Cooperative Oncology Group performance status of 0 or 1. Patients were excluded if they had received previous treatment or immunotherapy for NSCLC within 21 and 28 days, respectively, of study enrollment, or had been exposed previously to JAK inhibitors, MEK inhibitors, or other agents thought to have activity against the TBK1 pathway.
Study Design
The study was conducted at 4 sites in the United States from March 2015 to March 2017 in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and relevant regulatory laws. Institutional review boards approved the study protocol before patients were enrolled. All patients provided written informed consent.
This open-label, nonrandomized, dose escalation phase Ib study consisted of an initial dose-finding lead-in phase and a planned expansion phase, but was discontinued before the latter phase was initiated. Patients were screened for eligibility within 28 days of the first dose of study treatment. Study visits were held weekly during the first 28-day cycle, every 2 weeks during cycles 2 to 4, and then every 28 days of subsequent cycles. Patients could continue study treatment until disease progression, unacceptable toxicity, consent withdrawal, or their refusal of treatment.
The lead-in phase comprised two 3 + 3 dose-escalation periods (described in Table 1) to determine the maximum tolerated dose (MTD) of momelotinib in combination with trametinib. The MTD of momelotinib as a single agent was previously established in myelofibrosis patients as 200 mg once daily (q.d.). Because the hypothesized mechanism of action of momelotinib in KRAS-mutated NSCLC is different than in myelofibrosis, twice daily (b.i.d.) dosing was included to determine if continuous target coverage and/or a higher daily dose is required for efficacy. Momelotinib and trametinib tablets were self-administered orally beginning on day 1 and continued until progressive disease (PD), unacceptable toxicity, or withdrawn consent. Patients were instructed to take trametinib at least 1 hour before or 2 hours after a meal.
Table 1.
Dose Escalation Schemas for Momelotinib and Trametinib
| Momelotinib | Trametinib | ||
|---|---|---|---|
| Dose Level | Once Daily | Twice Daily | Once Daily |
| Momelotinib Dose Escalation | |||
| (If needed) −1 | 100 mg | 0.5 mg | |
| (Starting level) M1 | 100 mg | 1.0 mg | |
| M2 | (A) 200 mg | (B) 100 mg | 1.0 mg |
| M3 | 150 mg | 1.0 mg | |
| M4 | 200 mg | 1.0 mg | |
| End of Momelotinib Dose Escalation: Momelotinib MTD | |||
| Trametinib dose escalation | |||
| TI | Momelotinib MTD | 1.5 mg | |
| TII | Momelotinib MTD | 2.0 mg |
There were two 3 + 3 dose escalations. The first was conducted to determine the MTD of momelotinib in combination with trametinib, and the second dose escalation was conducted to determine the MTD of trametinib in combination with momelotinib at its MTD. A DLT within 28 days would trigger a cohort expansion. DLTs were defined as adverse events considered to be clinically significant and related to study treatment (eg, Grade 4 neutropenia or thrombocytopenia, Grade ≥ 3 neutropenia with fever; Grade ≥ 3 thrombocytopenia with bleeding; Grade ≥ 3 nonhematologic toxicity). If DLTs occurred in 0 of 3 (or <2 of 6) patients, then the next dose level was opened. The MTD was the dose level immediately below the one at which ≥2 patients had DLTs. If ≥ 2 DLTs were observed at dose level M2A but not at dose level M2B, then dose level M3 was to be opened as the last evaluated dose level for momelotinib (without precluding trametinib dose escalation). If ≥2 DLTs were observed at dose level M3, then tolerability and response would be used to decide between doses M2A and M2B as momelotinib MTD for use in the trametinib dose escalation. The trametinib dose escalation would not proceed if a trametinib-related DLT was observed during the momelotinib dose escalation. Otherwise, patients were treated with dose TI of trametinib and escalation to dose TII would move forward in the absence of DLTs. If ≥ 2 DLTs occurred at the same dose level, the MTD of trametinib would be considered to have been exceeded. Abbreviations: DLT = dose-limiting toxicity; MTD = maximum tolerated dose.
Safety
Safety data were collected from the first dose of study treatment until 30 days after the last dose. The primary end point was the incidence of dose-limiting toxicities (DLTs) experienced during patients’ first 28-day cycle.
Efficacy
Computed tomography scans were performed at baseline and then every 8 weeks thereafter for tumor response per RECIST criteria.10 Secondary efficacy end points included disease control rate (DCR) at week 8 (defined as complete response [CR] + partial response [PR] + stable disease [SD]), best overall response (ORR, defined as CR + PR) during the lead-in phase, progression-free survival (PFS), and overall survival (OS). Additionally, the duration of SD was determined as the time interval from the first dose of study treatment to the earlier of definitive disease progression or death.
Pharmacokinetics
Blood samples were collected on day 15 of cycle 1 before and at scheduled intervals after the momelotinib dose. Plasma concentrations of momelotinib and its major metabolite (M-21) were determined using validated bioanalytical assays. Pharmacokinetic (PK) parameters were estimated using standard noncompartmental methods using Phoenix WinNonlin software Version 6.4 (Certara, Princeton, NJ).
Statistical Analysis
Safety was evaluated in all patients who received ≥ 1 dose of study treatment. Efficacy was determined in all enrolled patients. PKs were determined in all patients who received ≥ 1 dose of study drug and had ≥ 1 post-dose plasma concentration value for the corresponding analyte.
Dose-limiting toxicities and adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities, version 19.1 and graded in terms of severity on the basis of Common Terminology Criteria for Adverse Events (version 4.03).9 The DCR and ORR were summarized for each dose level, along with corresponding 90% confidence intervals (CIs) on the basis of the Clopper—Pearson method. PFS and OS were analyzed using the Kaplan—Meier method for each dose level.
Results
Patients
Twenty-one patients were enrolled. All were included in the analyses of safety, efficacy, and PK. Patient characteristics are shown in Table 2. All patients discontinued study treatment. Momelotinib was discontinued because of PD (n = 10; 47.6%), AEs (n = 9;42.9%), and patient decision (n = 2; 9.5%). Trametinib was discontinued for the same reasons.
Table 2.
Demographic and Baseline Characteristics
| Parameter | Dose Level M1 (n = 3) | Dose Level M2A (n = 5) | Dose Level M2B (n = 4) | Dose Level M3 (n = 4) | Dose Level TI (n = 5) | Total (n = 21) |
|---|---|---|---|---|---|---|
| Age, Years | ||||||
| Mean (SD) | 63.3 (9.61) | 68.4 (7.09) | 68.3 (10.34) | 69.5 (9.33) | 69.4 (15.06) | 68.1 (9.90) |
| Median (range) | 65 (53–72) | 68 (60–76) | 70.5 (54–78) | 70 (58–80) | 70 (46–84) | 68 (46–84) |
| Sex, n (%) | ||||||
| Male | 1 (33.3) | 0(0) | 2 (50.0) | 2 (50.0) | 2 (40.0) | 7 (33.3) |
| Female | 2 (66.7) | 5 (100) | 2 (50.0) | 2 (50.0) | 3 (60.0) | 14 (66.7) |
| Race, n (%) | ||||||
| White | 3 (100) | 4 (80.0) | 2 (50.0) | 3 (75.0) | 4 (80.0) | 16 (76.2) |
| Othera | 0(0) | 1 (20.0) | 2 (50.0) | 1 (25.0) | 1 (20.0) | 5 (23.8) |
| ECOG Performance Status, n (%) | ||||||
| 0 | 1 (33.3) | 2 (40.0) | 2 (50.0) | 0(0) | 3 (60.0) | 8 (38.1) |
| 1 | 2 (66.7) | 3 (60.0) | 2 (50.0) | 4 (100) | 2 (40.0) | 13 (61.9) |
| Time Since Diagnosis, Months | ||||||
| Median (range) | 16.5 (13.3–27.4) | 16.4 (13.8–37.1) | 10.7 (3.6–32.2) | 30.6 (8.5–45.1) | 26.1 (9.1–109.5) | 16.6 (3.6–109.5) |
| Previous Lung Cancer Therapy, n (%) | ||||||
| Systemic platinum-based chemotherapy | 3 (100) | 5 (100) | 4 (100) | 4 (100) | 5 (100) | 21 (100) |
| First-line chemotherapyb | 3 (100) | 4 (80.0) | 3 (75.0) | 4 (100) | 5 (100) | 19 (90.5) |
| Second-line chemotherapy | 2 (66.7) | 3 (60.0) | 2 (50.0) | 2 (50.0) | 3 (60.0) | 12 (57.1) |
| Adjuvant chemotherapy | 0(0) | 2 (40.0) | 1 (25.0) | 0(0) | 2 (40.0) | 5 (23.8) |
| Surgery | 0(0) | 2 (40.0) | 3 (75.0) | 0(0) | 3 (60.0) | 8 (38.1) |
| Radiation therapy | 1 (33.3) | 0(0) | 3 (75.0) | 2 (50.0) | 3 (60.0) | 9 (42.9) |
Dose level M1: momelotinib 100 mg q.d. with trametinib 1.0 mg q.d.; dose level M2A: momelotinib 200 mg q.d. with trametinib 1.0 mg q.d.; dose level M2B: momelotinib 100 mg b.i.d. with trametinib 1.0 mg q.d.; dose level M3: momelotinib 150 mg b.i.d. with trametinib 1.0 mg q.d.; and dose level TI: momelotinib 150 mg b.i.d. with trametinib 1.5 mg q.d.
Abbreviations: b.i.d. = twice daily; ECOG = Eastern Cooperative Oncology Group; q.d. = once daily.
Includes 1 Asian patient (dose level 2B), 1 patient whose race was not permitted to be reported (dose level 2A), and 3 patients whose race was classified as other (ie, not classified as black/African American, American Indian/Alaska Native, or Native Hawaiian/Pacific Islander).
Most commonly carboplatin and pemetrexed with or without bevacizumab (n = 16, 76.2%).
Exposure
Median duration of exposure to momelotinib and trametinib was 8.1 weeks (range, 1.0–42.3 weeks). Of the 18 patients (85.7%) evaluable for DLTs, 4 patients (19.0%) completed ≥6 cycles of treatment.
Safety
Two of 5 patients (40.0%) at dose level M2A (200 mg momelotinib q.d.) had DLTs, and there were no DLTs at dose levels 2B and 3, so the study progressed to the dose escalation of trametinib. Two of 3 patients (66.7%) at dose level TI (momelotinib 150 mg b.i.d.) had DLTs. All DLTs were Grade 3 in severity and considered by the investigator to be related to momelotinib, with first onset generally occurring during the second or third week of therapy. The DLTs included increased amylase level associated with mild nausea but not abdominal pain, in 1 patient (dose level M2A, momelotinib dose reduced/trametinib interrupted), increases in aspartate aminotransferase (AST), alkaline phosphatase, and gamma-glutamyltransferase levels in 1 patient (dose level M2A, momelotinib and trametinib interrupted), increased AST level in 1 patient (dose level TI, momelotinib and trametinib interrupted), and fatigue in 1 patient (dose level TI, momelotinib and trametinib dose reduced). None of these DLTs were reported as serious. On the basis of these findings, the MTD was determined to be momelotinib 150 mg b.i.d. in combination with trametinib 1.0 mg q.d. (ie, dose level M3).
All 21 patients reported AEs (Table 3). Most cases of nausea, diarrhea, and fatigue, as well as AEs of decreased appetite, dizziness, and increased AST were considered by the investigator to be related to momelotinib and trametinib. Cases of dry skin were considered related to trametinib. Fifteen patients (71.4%) had AEs of Grade 3 or higher, most commonly hypoxia (n = 3; 14.3%) followed by increased amylase level, increased AST level, fatigue, and pneumonia (n = 2 each; 9.5%). AEs of interest were not prespecified in the study protocol; however, on the basis of previous studies of momelotinib in patients with myelofibrosis, AEs of interest include peripheral neuropathy, cataracts, and first-dose effect (defined as dizziness, flushing, hot flush, headache, hypotension, and/or nausea occurring on the first dosing day and resolving by the following day). Two patients had Grade 1 peripheral sensory neuropathy, and no patients had cataracts or first-dose effect. Overall, AEs leading to dose interruptions or dose modifications of momelotinib and trametinib occurred in 10 patients (47.6%) and 11 patients (52.4%), respectively. Increased amylase and AST levels, and fatigue led to dose interruptions or modifications in 2 patients each. All other AEs responsible for dose adjustments occurred in 1 patient each.
Table 3.
Adverse Events Reported in ≥20% of Patients
| Adverse Event, n (%) | Dose Level M1 (n = 3) | Dose Level M2A (n = 5) | Dose Level M2B (n = 4) | Dose Level M3 (n = 4) | Dose Level TI (n = 5) | Total (n = 21) | |
|---|---|---|---|---|---|---|---|
| Any Grade | Grade ≥3 | ||||||
| Any Adverse Event | 3 (100) | 5 (100) | 4 (100) | 4 (100) | 5 (100) | 21 (100) | 15 (71.4) |
| Nausea | 1 (33.3) | 4 (80.0) | 3 (75.0) | 3 (75.0) | 3 (60.0) | 14 (66.7) | - |
| Diarrhea | 1 (33.3) | 2 (40.0) | 3 (75.0) | 1 (25.0) | 4 (80.0) | 11 (52.4) | - |
| Fatigue | 2 (66.7) | 4 (80.0) | 2 (50.0) | 1 (25.0) | 2 (40.0) | 11 (52.4) | 2 (9.5) |
| Decreased Appetite | 0(0) | 2 (40.0) | 2 (50.0) | 1 (25.0) | 2 (40.0) | 7 (33.3) | - |
| Dizziness | 2 (66.7) | 1 (20.0) | 0(0) | 1 (25.0) | 2 (40.0) | 6 (28.6) | 6 (28.6) |
| Vomiting | 0(0) | 1 (20.0) | 1 (25.0) | 1 (25.0) | 2 (40.0) | 5 (23.8) | - |
| Pyrexia | 0(0) | 3 (60.0) | 2 (50.0) | 0(0) | 0(0) | 5 (23.8) | - |
| Cough | 0(0) | 1 (20.0) | 1 (25.0) | 2 (50.0) | 1 (20.0) | 5 (23.8) | - |
| Dyspnea | 0(0) | 2 (40.0) | 1 (25.0) | 0(0) | 2 (40.0) | 5 (23.8) | - |
| Dry Skin | 0(0) | 2 (40.0) | 2 (50.0) | 1 (25.0) | 0(0) | 5 (23.8) | - |
| Musculoskeletal Chest Pain | 0(0) | 2 (40.0) | 2 (50.0) | 0(0) | 1 (20.0) | 5 (23.8) | - |
| Amylase Increased | 0(0) | 2 (40.0) | 2 (50.0) | 0(0) | 1 (20.0) | 5 (23.8) | 2 (9.5) |
| AST Increased | 0(0) | 3 (60.0) | 0(0) | 0(0) | 2 (40.0) | 5 (23.8) | - |
| Anemia | 0(0) | 3 (60.0) | 1 (25.0) | 0(0) | 1 (20.0) | 5 (23.8) | - |
Data are presented as n (%). Most cases of nausea, diarrhea, and fatigue, as well as adverse events of decreased appetite, dizziness, and increased AST, were considered by the investigator to be related to momelotinib and trametinib; cases of dry skin were considered related to trametinib. Dose level M1: momelotinib 100 mg q.d. with trametinib 1.0 mg q.d.; dose level M2A: momelotinib 200 mg q.d. with trametinib 1.0 mg q.d.; dose level M2B: momelotinib 100 mg b.i.d. with trametinib 1.0 mg q.d.; dose level M3: momelotinib 150 mg b.i.d. with trametinib 1.0 mg q.d.; dose level TI: momelotinib 150 mg b.i.d. with trametinib 1.5 mg q.d.
Abbreviations: AST = aspartate aminotransferase; b.i.d. = twice daily; q.d. = once daily.
A total of 14 deaths occurred during the study, with ≥ 2 deaths reported at each dose level. Most deaths were caused by disease progression, and typically occurred ≥ 30 days after the last dose of study treatment.
Efficacy
The best ORR during the study was 0, with no patient achieving a CR or PR at any time and 13 (61.9%) achieving SD (Table 4). At week 8, 12 patients (≥1 patient at each dose level) achieved SD, 1 patient had PD, and 8 patients were nonevaluable for response. Therefore, the DCR at week 8 was 12 patients (57.1%) (90% CI, 37.2%−75.5%). The duration of SD ranged from 1.6 to 12.7 months, with 7 patients censored (5 because of initiation of new anticancer therapy and 2 because of study discontinuation without a progressive event). Median PFS was 3.6 months (90% CI, 2.2–5.6 months), and median OS was 7.4 months (90% CI, 4.0–15.3 months).
Table 4.
Response Assessments for the Combination of Momelotinib With Trametinib
| Parameter | Dose Level M1 (n = 3) | Dose Level M2A (n = 5) | Dose Level M2B (n = 4) | Dose Level M3 (n = 4) | Dose Level TI (n = 5) | Total (n = 21) |
|---|---|---|---|---|---|---|
| Best Overall Response, n (%) | ||||||
| SD | 3 (100) | 4 (80.0) | 2 (50.0) | 1 (25.0) | 3 (60.0) | 13 (61.9) |
| PD | 0(0) | 0(0) | 2 (50.0) | 2 (50.0) | 1 (20.0) | 5 (23.8) |
| Nonevaluable | 0(0) | 1 (20.0) | 0(0) | 1 (25.0) | 1 (20.0) | 3 (14.3) |
| Median PFS, Months (90% CI) | NR (5.4-NR) | 5.6 (2.2-NR) | 2.8 (1.8–9.7) | 1.7 (1.6–2.2) | 3.9 (0.7–3.9) | 3.6 (2.2–5.6) |
| Median OS, Months (90% CI) | 9.3 (7.4–11.2) | NR (2.2-NR) | 9.9 (4.0–16.2) | 5.1 (2.2-NR) | 3.9 (1.6-NR) | 7.4 (4.0–15.3) |
Dose level M1: momelotinib 100 mg q.d. with trametinib 1.0 mg q.d.; dose level M2A: momelotinib 200 mg q.d. with trametinib 1.0 mg q.d.; dose level M2B: momelotinib 100 mg b.i.d. with trametinib 1.0 mg q.d.; dose level M3: momelotinib 150 mg b.i.d. with trametinib 1.0 mg q.d.; and dose level TI: momelotinib 150 mg b.i.d. with trametinib 1.5 mg q.d.
Abbreviations: b.i.d. = twice daily; NR = not reached; OS = overall survival; PFS = progression-free survival; q.d. = once daily.
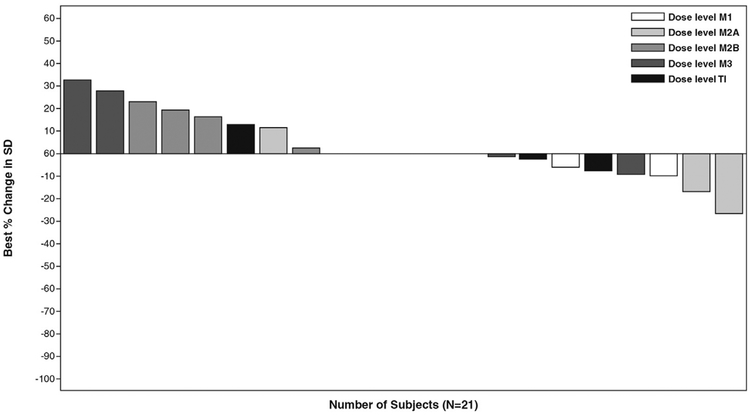
Study treatment reduced tumor size in some patients on the basis of analysis of the sum of diameters (longest for non-nodal lesion, short axis for nodal lesion) of target lesions. The baseline mean sum of the diameters of target lesions was 82.5 mm (standard deviation, 55.6 mm) and the mean best percentage change in the sum of diameters from baseline was 3.8% (standard deviation, 15.7%; ranging from a 26.7% reduction to a 32.3% increase; Figure 1).
Figure 1.
Best Percentage Change From Baseline in Sum of Diameters (SD, Longest for Non-Nodal Lesion, Short Axis for Nodal Lesion) of Target Lesions. Dose Level M1: Momelotinib 100 mg Once Daily (q.d.) With Trametinib 1.0 mg q.d.; Dose Level M2A: Momelotinib 200 mg q.d. With Trametinib 1.0 mg q.d.; Dose Level M2B: Momelotinib 100 mg Twice Daily (b.i.d.) With Trametinib 1.0 mg q.d.; Dose Level M3: Momelotinib 150 mg b.i.d. With Trametinib 1.0 mg q.d.; and Dose Level TI: Momelotinib 150 mg b.i.d. With Trametinib 1.5 mg q.d.
Pharmacokinetics
Peak plasma concentration (Cmax) and exposure (area under the plasma concentration-time curve uring the dosing interval [AUCtau]) for momelotinib and its primary metabolite M-21 on day 15 of cycle 1 showed more than dose-proportional increases between dose level 1 (100 mg q.d.) and dose level 2A (200 mg q.d.; Table 5). However, with b.i.d. dosing, the Cmax and AUCtau values for momelotinib and M-21 were similar across 100 mg and 150 mg dose levels (dose levels 2B, 3, and I).
Table 5.
Pharmacokinetic Parameters for Momelotinib and its major metabolite M-21
| Parametera | Dose Level M1 (n = 3) | Dose Level M2A (n = 5) | Dose Level M2B (n = 4) | Dose Level M3 (n = 4) | Dose Level TI (n = 5) |
|---|---|---|---|---|---|
| Momelotinib | |||||
| Cmax, ng/mL | 136.3 (49.4) | 571.8 (32.9) | 318.5 (72.6) | 289.7 (48.0) | 359.0 (31.3) |
| AUCtau, ng/mL/hb | 774.3 (47.1) | 3901.9 (48.2) | 2568.3 (53.0) | 2214.1 (71.5) | 1871.1 (8.2) |
| Tmax, h | 1.9 (0.5, 2.1) | 2.0 (1.0, 2.0) | 0.9 (0.7, 4.5) | 1.1 (0.9, 6.2) | 1.0 (0.5, 1.3) |
| t1/2, h | 6.9 (2.1, 9.2) | 8.7 (8.3, 12.6) | 8.3 (3.7, 12.9) | 6.1 (3.9, 8.4) | 5.4 (4.2, 8.3) |
| M-21 | |||||
| Cmax, ng/mL | 268.0 (23.5) | 645.0 (33.2) | 441.5 (39.0) | 368.3 (34.0) | 386.3 (29.0) |
| AUCtau, ng/mL/hb | 1992.1 (44.7) | 5606.4 (25.8) | 4564.5c | 2531.0 (36.5) | 2604.5 (14.6) |
| Tmax, hours | 1.9 (1.0, 2.1) | 2.0 (2.0, 2.0) | 1.5 (1.0, 2.0) | 1.1 (0.9, 8.0) | 1.3 (1.0, 2.0) |
| t1/2, hours | 8.1 (2.5, 15.3) | 12.4 (8.4, 20.6) | 5.5 (5.5, 5.5) | 6.8 (5.7, 7.9) | 15.3 (11.3, 19.3) |
| M-21/Momelotinib Ratio | |||||
| AUCtau | 2.6 (44.6) | 1.7 (52.8) | 2.7c | 1.3 (40.3) | 1.4 (20.6) |
| Cmax | 2.2 (52.0) | 1.2 (47.2) | 1.7 (38.3) | 1.3 (25.8) | 1.1 (13.7) |
Dose level M1: momelotinib 100 mg q.d. with trametinib 1.0 mg q.d.; dose level M2A: momelotinib 200 mg q.d. with trametinib 1.0 mg q.d.; dose level M2B: momelotinib 100 mg b.i.d. with trametinib 1.0 mg q.d.; dose level M3: momelotinib 150 mg b.i.d. with trametinib 1.0 mg q.d.; and dose level TI: momelotinib 150 mg b.i.d. with trametinib 1.5 mg q.d.
Abbreviations: AUCtau = area under the plasma concentration-time curve during the dosing interval; b.i.d. = twice daily; Cmax = peak plasma concentration; PK = pharmacokinetics; q.d. = once daily; Q1/Q3 = first quarter/third quarter; t1/2 = half-life; tmax = amount of time that a drug is present at the maximum concentration in serum.
Data for Cmax and AUCtau are presented as the mean (percent coefficient of variation); data for Tmax and t1/2 are presented as median (Q1, Q3), and M-21/momelotinib ratios are presented as the mean (percent coefficient of variation).
Number of evaluable patients for the lambda Z dependent parameters (AUCtau and half-life) were: dose level 1, n = 3; dose level 2A, n = 5; dose level 2B, n = 2; dose level 3, n = 2; dose level I, n = 3.
Standard deviation was not calculated because n = 1 for this parameter at the given dose level.
Discussion
In this study we evaluated the combination of momelotinib, a JAK1/JAK2 and TBK1 inhibitor, with the MEK1/MEK2 inhibitor trametinib in treating KRAS-mutated metastatic NSCLC. On the basis of the dose escalations conducted in this study, we concluded that the MTD for the combination was momelotinib 150 mg b.i.d. and trametinib 1 mg q.d. (ie, dose level 3). Two of the 4 patients treated with the MTD achieved SD as their best response. Across dose levels, the DCR at 8 weeks was 12 patients (57.1%), and the SD rate overall was 13 patients (61.9%). No patients achieved CR or PR. The safety profile of momelotinib and trametinib at the doses tested were generally consistent with the known safety profile of each agent. The PK analysis was generally comparable with previous evaluations11 and showed that maximum momelotinib plasma concentrations were approximately 300 ng/mL (equal to 0.72 μmol/L), which is below levels required to inhibit TBK1 kinase activity.3
In a previous randomized phase II study, single-agent trametinib 2.0 mg q.d. produced a PR in 10 of 86 patients (11.6%) with previously treated KRAS-mutated advanced NSCLC, with an additional 38 patients (44.2%) having SD.12 Two of 30 patients (7%) with KRAS-mutated NSCLC in a phase I dose escalation study of trametinib also had PR and 16 (53%) had SD at doses of ≥ 2 mg trametinib.13 In our study, the MTD of trametinib in combination with momelotinib was only 1 mg and the activity observed with the momelotinib 150 mg b.i.d.-trametinib 1 mg q.d. combination did not improve on the historic data for single-agent trametinib and accordingly did not support proceeding to the planned expansion phase for further evaluation of the combination at the MTD. Thus, the study was discontinued.
Conclusion
The additional use of momelotinib with trametinib at the doses evaluated in our study did not improve on the activity of single-agent trametinib in KRAS-mutated NSCLC. However, because the PK analyses indicated momelotinib concentrations were below levels required to inhibit TBK1 kinase activity, the present findings did not invalidate the concept of combining a TBK1 inhibitor with a MEK1/MEK2 inhibitor, or for that matter, another agent targeting a key KRAS-signaling pathway. Indeed, novel TBK1 inhibitors with significantly improved potency and specificity are being developed and will enable more robust testing of this and other clinical hypotheses.14
Clinical Practice Points.
Specific options are lacking to treat patients with KRAS-mutated NSCLC.
Direct inhibition of KRAS has proven clinically challenging, and therefore one approach to target KRAS-mutated NSCLC has focused on downstream effector proteins.
In this study we evaluated the combination of momelotinib, a JAK1/JAK2 and TBK1 inhibitor, with the MEK1/MEK2 inhibitor trametinib in treating this cancer.
Although the activity observed with the momelotinib-trametinib combination at the doses evaluated did not improve on the historic data for single-agent trametinib, momelotinib concentrations were too low to inhibit TBK1; thus, the present findings did not invalidate the concept of combining a TBK1 inhibitor with a MEK1/MEK2 inhibitor, or for that matter, another agent targeting a key KRAS signaling pathway.
Acknowledgments
Writing and editorial assistance for this article was provided by Impact Communication Partners and funded by Gilead Sciences, Inc. This study was funded by Gilead Sciences, Inc.
Footnotes
This study is registered at ClinicalTrials.gov: NCT02258607
Disclosure
D.A.B. is a consultant for N of One; R.H., J.K., and S.K. are Gilead Sciences employees; M.K. is part of the speaker’s bureau for AstraZeneca; A.S. and K.K. have stated that they have no conflicts of interest.
References
- 1.Rieley GJ, Marks J, Pao W. KRAS mutations in nonesmall-cell lung cancer. Proc Am Thorac Soc 2009; 6:201–5. [DOI] [PubMed] [Google Scholar]
- 2.Matikas A, Mistriotis D, Georgoulias V, Kotsakis A. Targeting KRAS mutated nonesmall-cell lung cancer: a history of failures and a future of hope for a diverse entity. Crit Rev Oncol Hematol 2017; 110:1–12. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Z, Aref AR, Cohoon TJ, et al. Inhibition of KRAS-driven tumorigenicity by interruption of an autocrine cytokine circuit. Cancer Discov 2014; 4:452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyner JW, Bumm TG, Deininger J, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood 2010; 115:5232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and pre-clinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia 2009; 23:1441–5. [DOI] [PubMed] [Google Scholar]
- 6.Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol 2017; 35:3844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison CN, Vannucchi AM, Platzbecker U, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol 2018; 5: e73–81. [DOI] [PubMed] [Google Scholar]
- 8.Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol 2012; 13:782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mekinist [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2018. [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 11.Xin Y, Kawashima J, Weng W, Kwan E, Tarnowski T, Silverman JA. Pharmacokinetics and safety of momelotinib in subjects with hepatic or renal impairment. J Clin Pharmacol 2018; 58:522–32. [DOI] [PubMed] [Google Scholar]
- 12.Blumenschein JG, Smit EF, Planchard D, et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced nonesmall-cell lung cancer (NSCLC). Ann Oncol 2015; 26:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmaco-dynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol 2012; 13:773–81. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins RW, Aref AR, Lizotte PH, et al. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov 2018; 8:196–215. [DOI] [PMC free article] [PubMed] [Google Scholar]