Abstract
Although progress has been made for recanalization therapies after ischemic stroke, post-treatment imaging studies show that tissue reperfusion cannot be attained despite satisfactory recanalization in a significant percentage of patients. Hence, investigation of microcirculatory changes in both surface and deep cortical levels after ischemia reperfusion is important for understanding the post-stroke blood flow dynamics. In this study, we applied optical coherence tomography (OCT) imaging of cerebral blood flow for the quantification of the microcirculatory changes. We obtained OCT microangiogram of the brain cortex in a mouse stroke model and analyzed the data to trace changes in the capillary perfusion level (CPL) before, during, and after the stroke. The CPL changes were estimated in 1 and 2 h ischemia groups as well as in a non-ischemic sham-operated group. For the estimation of CPL, a decorrelation amplitude-based algorithm was implemented and used. As a result, the CPL considerably decreased during ischemia but recovered to the baseline when recanalization was performed 1 h after ischemia; however, the CPL was significantly reduced when recanalization was delayed to 2 h after ischemia. These data demonstrate that ischemia causes microcirculation dysfunction, leading to a decreased capillary reperfusion after recanalization. Microcirculatory no-reflow warrants more rigorous assessment in clinical trials, whereas advanced optical imaging techniques may provide mechanistic insight and solutions in experimental studies.
Keywords: (110.4500) Optical coherence tomography, (170.1470) Blood or tissue constituent monitoring, (170.3880) Medical and biological imaging
1. INTRODUCTION
Ischemic stroke is one of the leading causes of mortality and morbidity around the world. Recent advances in interventional and pharmacological recanalization therapies have increased the ratio of patients with improved neurological outcome and extended the therapeutic time window on selected patients [1–5]. However, post-treatment imaging studies have also shown that in 26% of patients, reperfusion (restitution of blood flow through microcirculation) cannot be attained despite satisfactory recanalization (re-opening of occluded vessel) (see [6] for review). In most of these studies, post-treatment imaging was generally performed within 24 h; whereas a recent study has found that an incomplete recirculation is far more common (57%) when assessed within 6 h after recanalization, the most critical time window for tissue recovery [7]. In parallel with these clinical observations, a study in the mouse reported that about half of the microvessels could not be reperfused after re-opening of the middle cerebral artery (MCA) 2 h following the occlusion [8]. The latter study re-kindled interest in the no-reflow phenomenon, which was first described in 1968 and became subject of numerous studies over the years with a waxing and waning enthusiasm [9–12]. In these studies, the microcirculation was examined either histologically ex vivo or only the microvessels in the cortical surface were visualized with intravital fluorescence microscopy. However, recent advances in imaging techniques now provide an opportunity to visualize the microcirculation across the whole cortical mantle in vivo in intact animals under anesthesia. This technological advancement can bring insight to conditions predisposing to post-ischemic microcirculatory failure and its mechanisms, which may further facilitate the progress in recanalization therapies.
Surface blood flow changes can be detected with stereomicroscopes or laser speckle contrast imaging [13], but they are limited in depth-resolved volumetric imaging capability. Although two-photon microscopy offers this capability, it generally requires scanning over all three axes, which limits its speed for continuous volumetric imaging. In this regard, acquiring the axial information at once by optical coherence tomography (OCT) is advantageous for continuously repeated volumetric imaging with a higher speed [14]. For instance, Doppler OCT enables quantitative volumetric imaging of the absolute blood flow in arteries and veins [15]. Other OCT-based methods have been proposed for quantification of blood flow in capillaries [16,17]. In this study, we implemented a relatively simpler method to estimate the capillary perfusion level (CPL) from volumetric OCT angiogram data.
Using this method, we investigated the cortical capillary blood flow changes during ischemia and reperfusion in vivo in intact mice under anesthesia. We utilized the OCT to analyze perfusion changes over a large cortical depth (down to 700 μm) and showed that capillary reperfusion is incomplete if recanalization is delayed by 2 h after ischemia.
2. MATERIALS AND METHODS
A. Animal Preparation
Three-month-old SV129 mice (n = 9) were anesthetized with isoflurane (1.5%—2.5%, v/v), and ventilated with a mixture of air and oxygen during surgical procedures. The head was fixed in a stereotaxic frame, and the scalp was incised and retracted. Craniotomy was performed on an area of 3 mm by 3 mm over the right somatosensory cortex, corresponding to the area perfused via the MCA and subsequently exposed for imaging while keeping the dura intact. The brain surface was covered with agarose gel and a glass coverslip (8 mm in diameter), and was sealed with dental acrylic cement. After surgery, mice were placed into the OCT system for baseline imaging. For MCA occlusion (MCAO), a nylon monofilament was inserted through the internal carotid artery up to the origin of the MCA under a surgical microscope. A monofilament nylon suture (8/0) with its tip thickened with silicone resin/hardener mixture was pushed until it blocked the origin of the MCA. Immediately after MCAO, mice were placed back to the OCT system for follow-up imaging of capillary perfusion changes in the ischemic core and the penumbral area. Once the imaging was completed, recanalization was achieved 1 or 2 h after the MCAO by withdrawal of the suture and, respectively, the reperfusion imaging was performed with OCT. Tissue oxygen saturation, pulse rate, and temperature were continuously monitored with pulse oximetry and a rectal probe during the whole surgical procedure and experiment. The body temperature was maintained at 37°C and the pulse rate remained within the normal range of 250–350 pulse/ min. Blood gas tests during the baseline also resulted in the normal ranges of 7.23–7.40 pH and 25–40 pCO2 (mmHg).
All animal-based experimental procedures were reviewed and approved by the Massachusetts General Hospital Subcommittee on Research Animal Care, according to the guidelines and policies of office of laboratory animal welfare and public health service, National Institutes of Health.
B. Experiment Groups and Imaging Protocol
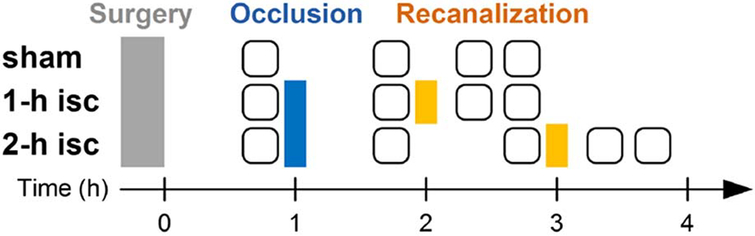
Experimental groups and surgical procedures are schematized in Fig. 1. All surgical procedures and imaging studies were performed in three animal groups: sham, 1 h ischemia followed by 1 h recanalization (“1 h isc”), and 2 h ischemia followed by 1 h recanalization (“2 h isc”) (n = 3 for each group). Preparation for imaging and MCA occlusion usually takes about 20–30 min. In all groups, baseline imaging was performed after the craniotomy and preparative surgery for MCAO. In the 1 h ischemia group, MCA was occluded and imaging was conducted ~50 min later following MCA occlusion. Furthermore, recanalization was performed 1 h after the occlusion by pulling the flament back, and two imaging sessions at 0.5 and 1 h after the recanalization were conducted. In the 2 h ischemia group, the filament was pulled out 2 h after the occlusion and another imaging session at 1 h 50 min after the occlusion was additionally performed. In the sham group, images were obtained at the same time points with the 1 h ischemia group without MCAO and recanalization.
Fig. 1.
Times of surgical manipulations and imaging sessions for each group. The rounded squares indicate sessions of OCT angiogram imaging, each of which took about 5 min.
C. Spectral-Domain Optical Coherence Tomography System
We optimized a spectral-domain OCT system (Thorlabs, Inc., Newton, NJ, USA) for imaging of the rodent cerebral cortex [14]. The 5 × objective lens was used for this study leading to a transverse resolution of 7 μm, while the axial resolution was 3.5 μm. The imaging speed was 47,000 A-scan/s.
3. RESULTS
A. 3D OCT Angiogram
For each imaging session in Fig. 1, a 3D OCT angiogram of the cortical microvasculature was obtained and the CPL was estimated. OCT angiograms were obtained by a decorrelation- based method described elsewhere [14]. While conventional structural OCT imaging typically acquires one B-scan for each Y position, the decorrelation-based method repeats two B-scans and then analyzes differences between the two consecutive B-scan images. When a sample exhibits no movement and an OCT system has a perfect phase stability, then the difference will be zero at every voxel. However, when a sample has local motions like blood flow, such motions will result in larger differences, appearing as bright areas in the difference map. By repeating this procedure over the Y axis, we can obtain a 3D difference map, and when obtained from the animal brain cortex like in this study, it reveals blood perfusion patterns down to the finest vascular structures (i.e., capillaries).
We imaged an area of 1.8 mm by 1.8 mm with 512 B-scans at the Nyquist sampling frequency (i.e., 3.5 μm steps), where each B-scan consisted of 512 A-scans. The image resolution was 7.0 μm laterally and 3.3 μm axially. We consecutively acquired 10 volume images and averaged them to obtain a higher con- trast-to-noise ratio. The acquisition of 10 volume images took less than 5 min.
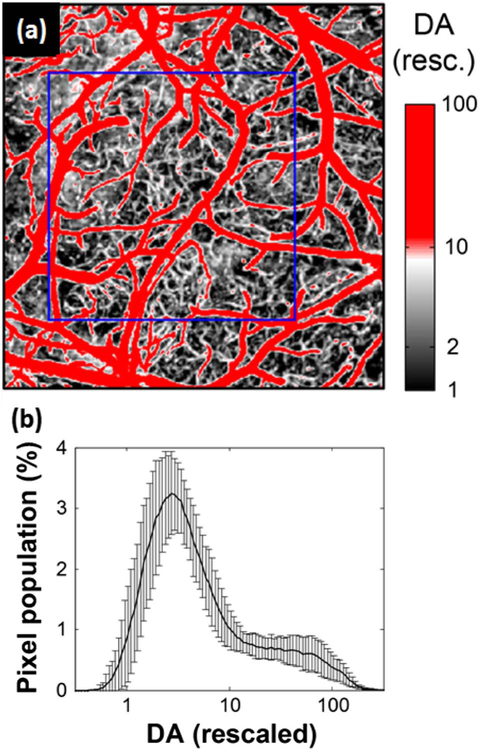
Each angiogram displayed a 3D microvascular structure over the volume of 1.8 mm by 1.8 mm by 700 μm (X by Y by Z). Figure 2(a) shows a maximum intensity projection (MIP) image of an exemplary 3D OCT angiogram.
Fig. 2.
OCT 3D angiogram and co-registration. (a) A maximum intensity projection (MIP) image of 3D OCT angiogram. The decorrelation amplitude (DA) is presented in log scale (arbitrary unit). Scale bar = 0.5 mm. (b) Co-registration across imaging sessions. The white image displays the MIP angiogram at the baseline and the overlapped red image shows the angiogram at the occlusion, which was co-registered to the baseline imaging. The white box indicates a common FOV across the stages.
B. Co-Registration Across Imaging Sessions
Since the animal had to be moved between the stages to occlude and re-canalize the MCA, the field of view (FOV) was slightly different across the imaging sessions. Therefore, we performed simple co-registration of angiograms, where the optimum translation and rotation of a given angiogram MIP was found relative to the baseline angiogram MIP by maximizing the cross correlation between the two images [Fig. 2(b), for example]. Then, we identified a common FOV across the set of images and cropped all angiogram volumes to this common FOV. This co-registration and cropping led to slightly smaller FOVs than the acquired 1.8 mm by 1.8 mm but made it much simpler and robust to choose the region of interest (ROI) and analyze how the CPL changed in time over the same area.
C. Estimation of Capillary Perfusion Level (CPL)
The CPL was estimated from each data of the co-registered angiogram. Our data processing procedures for the estimation consists of three steps. First, we minimized slow drift effects, if any, by rescaling the amplitude of each angiogram. As our experiments compare angiograms acquired over several hours from the same animal, there is a possibility that the light source intensity varied over hours and it affects quantitative estimation of the CPL. To remove any effect of this variation in light source intensity, we rescaled the decorrelation amplitude (DA) of each angiogram to a range of 1–100 such that the values 1 and 100 corresponded to the means of a minimum of 5% and a maximum of 5% of the DAs in the MIP, respectively. The value 1 corresponded to baseline noise from static tissue and the value 100 corresponded to large blood perfusion in arteries and veins. The rescale size was chosen to be 100 since it was similar to the actual DA contrast-to-noise ratios; (mean of max5%)/(mean of min5%) =80 ± 33 (n = 9). The minimization of the slow drift effect was confirmed by similar histograms of the rescaled DA between different animals [Fig. 3(b)].
Fig. 3.
Estimation of the capillary perfusion level (CPL) from the rescaled OCT angiogram. (a) DA-based segmentation of capillaries in the rescaled MIP image. The blue box indicated the ROI for estimation of CPL. (b) Histogram of the rescaled DA in log scale (averaged over n = 9 animals). Data are presented as mean ± SD.
Second, we segmented capillaries based on differences in the rescaled DA between capillaries and large vessels. We found that in the rescaled angiogram, arteries and veins exhibited DAs between 10 and 100 while capillaries exhibited DAs between 2 and 10 [Fig. 3(a)]. This factor of 10 may correspond to the fact that the blood flow speed in arteries and veins (1–10 mm/s; [5]) is approximately 10 times larger than that in capillaries (0.1–1 mm/s; [18–20]), although the DA does not map one-to-one to the blood flow speed due to its dependence on flow direction and hematocrit [17]. Here, we segmented capillaries based on the approximate relation of the DA, meaning the identification of pixels whose DA lies between 2 and 10 as capillaries [white color in Fig. 3(a)].
Finally, the CPL was estimated by the sum of the DA of the segmented capillaries within an ROI from each MIP angiogram, and changes in the CPL were traced during ischemia and reperfusion for each animal. The CPL was estimated from the MIP angiogram to include all capillaries over the depths we could observe with our OCT (down to 700 μm). The ROI was chosen such that it included a clearly seen capillary bed in the sham group [e.g., the blue box in Fig. 3(a)] or a capillary bed affected by the MCAO in the other groups. To minimize a ROI selection bias, the same criteria were used across groups: we opened and imaged similar locations, the area about 6 mm lateral to the midline, which generally corresponds to the ischemic core when the MCA is occluded; all ROIs were chosen such that they should be 0.8 mm by 0.4 mm at least; and they showed similar baseline histograms of the DA over all animals [Fig. 3(b)].
D. Changes in CPL During Ischemia and After Reperfusion
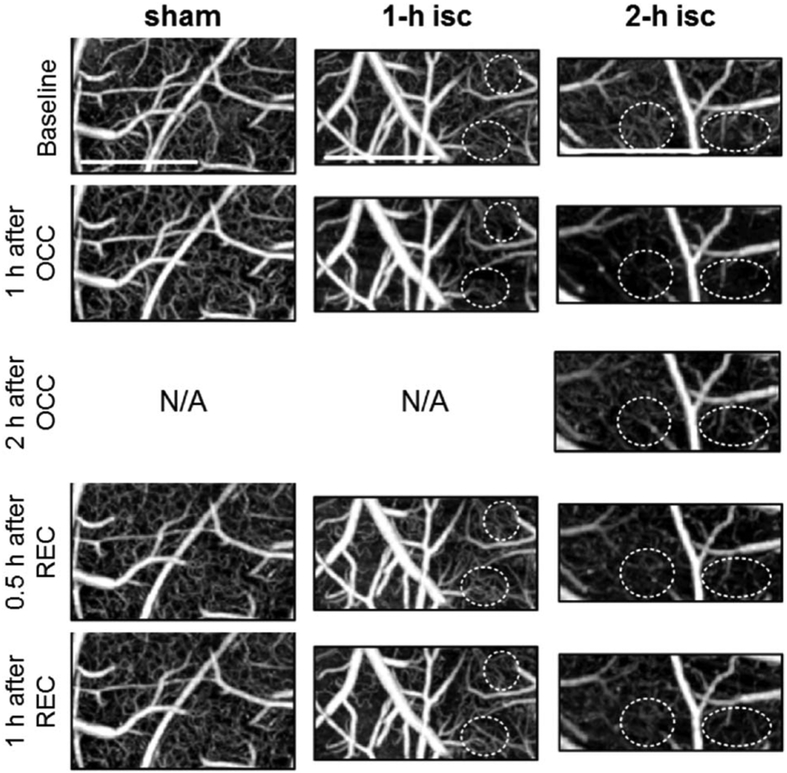
1 h after the MCA occlusion, the ischemic groups (1 h isc and 2 h isc) exhibited significant reductions in the CPL from their baselines (62 ± 7% and 56 ± 11%; lower than the sham group with p = 0.002 and 0.006, respectively). The relative CPL at this stage was not significantly different between the 1 h ischemia and 2 h ischemia groups (p = 0.6). However, after the recanalization was established by withdrawing the filament, the 1 h ischemia group showed recovery of CPL (93 ± 11%; not different from the sham group with p = 0.4); on the other hand, the 2 h ischemia group exhibited significantly reduced recovery (55 + ∕ 13%; lower than the sham and 1 h ischemia groups with p = 0.0001 and 0.0006, respectively). This tendency could be seen in the MIPs as well (see the dashed circles in Fig. 4). These data are summarized in Fig. 4, which displays representative MIPs at each stage and for each group. Note that significant reduction in MIPs during ischemia was observed as designated by dashed circles.
Fig. 4.
MIPs of OCT angiogram from a representative animal at every stage for each group. The dashed white circles indicate areas where the representative changes in the CPL can be seen clearly. All MIPs are presented over selected ROIs and displayed in log scale over the same range of the rescaled DA from 1 to 100. Scale bar = 0.5 mm.
Figure 5 shows how the CPL changed relative to its baseline for all animals. A significant CPL reduction following ischemia (nearly half of the baseline) and partial restoration following 2h ischemia as well as the different responses of each animal together with nearly total restoration and homogenous response of each mouse after 1 h of ischemia can be clearly seen in Fig. 5(a). Figure 5(b) shows statistical analysis of the CPL data.
Fig. 5.
CPL data of all animals. (a) Relative changes in the CPL (rCPL). The bold line of rCPL indicates data from the animal whose MIPs are presented in Fig. 4. (b) Statistical analysis of rCPL. Stars indicate that its rCPL is statistically significantly different from the sham group (p < 0.01).
4. DISCUSSION
A. Reduced Capillary Reperfusion after Prolonged Ischemic Stroke
In this study, we have clearly demonstrated that effective capillary reperfusion could be obtained if the ischemic period is less than 1 h but not longer than 2 h in line with the previous observations made on superficial cortical microcirculation in mice [8]. This finding is in accordance with clinical stroke studies reporting that the early administration of rt-PA within the first hour of stroke has the highest improvement rate and the time to needle is an important parameter predicting a good outcome within 3.5 h [4]. Close correlation between the reappearance of capillaries in post-treatment angiographies (capillary blushing) and good clinical outcome after interventional re-opening of the occluded MCA also conforms to experimental findings [8,21,22]. The emergence of impaired capillary reperfusion after ischemia lasting for as short as 2 h underscores the neglected potential importance of no-reflow in clinical practice [6].
B. Potential of OCT for Stroke Research
The OCT method, which we have adapted to study the microcirculation across the cortical mantle, offers several options for providing answers to some clinically significant questions to prevent no-reflow after recanalization therapies. For example, combination of the OCT with tissue perfusion studies may disclose the critical residual cerebral blood flow (CBF) values required to preclude no-reflow. These threshold CBF values may be used for selection of stroke patients for late recanalization treatments. Combination of the OCT with multi-photon imaging may provide mechanistic insight to examine the potential role of ischemia-induced pericyte contractions in capillary flow disturbance by using transgenic mice expressing fluorescent pericyte marker proteins (e.g., NG2-DS red mice) [8,22], and simultaneously monitoring pericyte activation and capillary flow with multi-photon microscopy and OCT, respectively. Similarly, the potential role of leukocytes, fibrin, and platelets as well as distal microembolism from the thrombolyzed clot could be investigated using fluorescently labeled markers [6,10]. Understanding the mechanisms of microcirculatory obstructions are essential for the development of treatments that can be combined with recanalization therapies to improve the success rate and extend the therapeutic window.
C. Technical Limitations
Potential limitations of the study were the necessity of moving mice in and out of the OCT system for each step of surgery and imaging as well as the relatively long period of imaging follow-up. To overcome these obstacles, we rescaled the DA for each imaging session to suppress the effect of any variation in the light source intensity over hours, under a reasonable assumption that the saturated DA of high blood flow and the DA noise from static tissue will not significantly change during imaging sessions. There can be a concern on this assumption because the saturated DA can vary when some high blood flow within the ROI is decreased by MCAO, although the ROIs we used generally included not only MCA branches but also other arteries and veins that are expected not to decrease during MCAO. However, this effect of MCAO, even if it exists, would not jeopardize the statistical result in Fig. 5(b); in fact, the existence of such an effect and its compensation would enforce the statistical significance by the following logic: (1) the effect of MCAO would decrease the saturated DA (mean of the top 5%) in the occluded stage; (2) the rescaling based on this lower saturated DA would result in a CPL data larger than the true value; (3) if we compensated for this error, the CPL data for the occluded state would become lower than the current data, enlarging the difference from the sham data in Fig. 5(b), top.
5. CONCLUSION
In conclusion, our data demonstrate that capillaries distributed over a large depth encompassing almost the entire mouse cortex are affected during ischemia reperfusion, causing a decreased capillary reperfusion. This complication emerges after ischemia lasting as short as 2 h and may negatively impact the recovery after re-canalization of the occluded artery. This finding warrants more rigorous assessment in clinical trials, whereas further experimental studies in animals may provide mechanistic insight as well as solutions with translational potential by combining the OCT with recently developed multimodal imaging techniques.
Acknowledgment.
Travel expenses of Yasemin Gursoy-Ozdemir to Massachusetts General Hospital, Harvard University, Boston, were financed by a grant from Scientific Research Projects Coordination Unit of Hacettepe University.
Funding. National Institute of Biomedical Imaging and Bioengineering (NIBIB) (R00-EB014879, R01-EB000790); National Institute of Neurological Disorders and Stroke (NINDS) (P01-NS055104); Air Force Office of Scientific Research (AFOSR) (MFEL FA9550-07-1-0101).
REFERENCES
- 1.Wheeler HM, Mlynash M, Inoue M, Tipirnini A, Liggins J, Bammer R, Lansberg MG, Kemp S, Zaharchuk G, Straka M, and Albers GW, “The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion,” Int. J. Stroke 10, 723–729 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lansberg MG, Cereda CW, Mlynash M, Mishra NK, Inoue M, Kemp S, Christensen S, Straka M, Zaharchuk G, Marks MP, Bammer R, and Albers GW, “Response to endovascular reperfusion is not time-dependent in patients with salvageable tissue,” Neurology 85, 708–714 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, Campbell BC, Bammer R, Olivot J-M, Desmond P, Davis SM, Donnan GA, and Albers GW, “RAPID automated patient selection for reperfusion therapy: a pooled analysis of the echoplanar imaging thrombolytic evaluation trial (EPITHET) and the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study,” Stroke 42, 1608–1614 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W, Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, Butcher KS, Parsons MW, Barber PA, Levi C, Bladin C, and Byrnes G, “Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials,” Lancet 375, 1695–1703 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Donnan GA, Davis SM, Parsons MW, Ma H, Dewey HM, and Howells DW, “How to make better use of thrombolytic therapy in acute ischemic stroke,” Nat. Rev. Neurol 7, 400–409 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Dalkaraand T and Arsava EM, “Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis?” J. Cereb. Blood Flow Metab 32, 2091–2099 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horsch AD, Dankbaar JW, Niesten JM, van Seeters T, van der Schaaf IC, van der Graaf Y, Mali WPTM, and Velthuis BK, “Predictors of reperfusion in patients with acute ischemic stroke,” Am. J. Neuroradiol 36, 1056–1062 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, and Dalkara T, “Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery,” Nat. Med 15, 1031–1037 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Ames A, Wright RL, Kowada M, Thurston JM, and Majno G, “Cerebral ischemia. II. The no-reflow phenomenon,” Am. J. Pathol 52, 437–453 (1968). [PMC free article] [PubMed] [Google Scholar]
- 10.del Zoppo GJ, “Microvascular changes during cerebral ischemia and reperfusion,” Cerebrovasc. Brain Metab. Rev 6, 47–96 (1994). [PubMed] [Google Scholar]
- 11.del Zoppo GJ and Mabuchi T, “Cerebral microvessel responses to focal ischemia,” J. Cereb. Blood Flow Metab 23, 879–894 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Garcia JH, Liu KF, Yoshida Y, Chen S, and Lian J, “Brain microvessels: factors altering their patency after the occlusion of a middle cerebral artery (Wistar rat),” Am. J. Pathol 145, 728–740 (1994). [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn AK, Bolay H, Moskowitz MA, and Boas DA, “Dynamic imaging of cerebral blood flow using laser speckle,” J. Cereb. Blood Flow Metab 21, 195–201 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan VJ, Jiang JY, Yaseen MA, Radhakrishnan H, Wu W, Barry S, Cable AE, and Boas DA, “Rapid volumetric angiography of cortical microvasculature with optical coherence tomography,” Opt. Lett 35, 43–45 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan VJ, Atochin DN, Radhakrishnan H, Jiang JY, Ruvinskaya S, Wu W, Barry S, Cable AE, Ayata C, Huang PL, and Boas DA, “Optical coherence tomography for the quantitative study of cerebrovascular physiology,” J. Cereb. Blood Flow Metab 31, 1339–1345 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Radhakrishnan H, Wu W, Daneshmand A, Climov M, Ayata C, and Boas DA, “Quantitative imaging of cerebral blood flow velocity and intracellular motility using dynamic light scattering-optical coherence tomography,” J. Cereb. Blood Flow Metab 33, 819–825 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Jiang JY, Wu W, Lesage F, and Boas DA, “Statistical intensity variation analysis for rapid volumetric imaging of capillary network flux,” Biomed. Opt. Express 5, 1160–1172 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinfeld D, Mitra PP, Helmchen F, and Denk W, “Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex,” Proc. Natl. Acad. Sci. USA 95, 15741–15746 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulte ML, Wood JD, and Hudetz AG, “Cortical electrical stimulation alters erythrocyte perfusion pattern in the cerebral capillary network of the rat,” Brain Res. 963, 81–92 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Stefanovic B, Hutchinson E, Yakovleva V, Schram V, Russell JT, Belluscio L, Koretsky AP, and Silva AC, “Functional reactivity of cerebral capillaries,” J. Cereb. Blood Flow Metab 28, 961–972 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Ali F, Jefferson A, Barrow T, Cree T, Louis S, Luke K, Major K, Nemeth D, Smoker S, and Walker S, “The capillary index score: rethinking the acute ischemic stroke treatment algorithm. Results from the Borgess medical center acute ischemic stroke registry,” J. Neurointerv. Surg 5, 139–143 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, and Attwell D, “Capillary pericytes regulate cerebral blood flow in health and disease,” Nature 508, 55–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]