Abstract
Background
Studies have shown inconsistent associations of nitrite and nitrate intake with the risk of gastric cancer or its associated mortality. We performed a meta-analysis of observational studies to evaluate the correlation of nitrite and nitrate intake with the risk of gastric cancer.
Material/Methods
We searched for studies reporting effect estimates and 95% confidence intervals (CIs) of gastric cancer in PubMed, EMBASE, and the Cochrane Library through November 2018. The summary results of the included studies were pooled using a random-effects model.
Results
Eighteen case-control and 6 prospective cohort studies recruiting 800 321 participants were included in this study. The summary results indicated that the highest (odds ratio [OR], 1.27; 95%CI, 1.03–1.55; P=0.022) or moderate (OR: 1.12; 95%CI, 1.01–1.26; P=0.037) nitrite intake were associated with a higher risk of gastric cancer. However, we noted that high (OR, 0.81; 95%CI, 0.68–0.97; P=0.021) or moderate (OR, 0.86; 95%CI, 0.75–0.99; P=0.036) nitrate intakes were associated with a reduced risk of gastric cancer. These associations differed when stratified by publication year, study design, country, the percentage of male participants, assessment of exposure, adjusted model, and study quality.
Conclusions
High or moderate nitrite intake was associated with higher risk of gastric cancer, whereas high or moderate nitrate intake was correlated with lower risk of gastric cancer.
MeSH Keywords: Amyl Nitrite, Meta-Analysis, Nitrates, Stomach Neoplasms
Background
Gastric cancer (GC) is the sixth most common form of cancer and the second most common in terms of mortality worldwide [1]. The pathology of GC can be divided into 2 groups: cardia and non-cardia adenocarcinoma. Diagnostic and treatment strategies are advancing, yet the prognosis for GC patients remains poor [2]. Since there is an increasing trend of the disease burden, more research is needed on identify the risk factors for GC. Numerous studies have already demonstrated that obesity, smoking, gastroesophageal reflux disease, and Helicobacter pylori infection are significantly associated with the risk of GC [3–7]. Moreover, fruit and vegetable consumption was associated with a reduced risk of GC, irrespective of the subsite or histologic type. An explanation for this could be that the high contents of antioxidants, phytosterols, and other substances in fruits and vegetables could inhibit carcinogenesis by free-radical quenching or blocking N-nitroso compound formation [8–10].
Ingested nitrate can convert to nitrite through the bacterial flora in the mouth and digestive tract. Moreover, nitrite levels can affect the formation of N-nitroso compounds. Endogenous nitrosation accounts for an estimated 45–75% of total N-nitroso compounds exposure [11], and the acceptable daily intake values should be explored in the general population. Moreover, the potential impacts of nitrite and nitrate intake and subsequent risk of GC remain controversial. Therefore, we performed a comprehensive search of the available observational studies to assess the association between nitrite or nitrate intake and the risk of GC. We also assessed whether these relationships differed according to study or participant characteristics.
Material and Methods
Data sources, search strategy, and selection criteria
This meta-analysis was according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement, which published in 2009 [12]. The study investigated the associations of nitrite or nitrate intake with the risk of GC, and no restrictions were placed on language or status of eligible publications. The PubMed, EMBASE, and the Cochrane Library databases were systematically searched in the timeframe from their inception to November 2018 for potentially eligible publications. The core search terms of (nitrate OR nitrite OR N-nitroso compounds) AND (cancer OR neoplasm OR carcinoma OR tumor) AND (gastric OR stomach) were used. The reference lists from the retrieved studies were manually searched to identify any new eligible studies. The PICOS criteria were used to identify any potential studies.
The study selection process was conducted by 2 authors, and any disagreement was resolved by the corresponding author. The inclusion criteria of this meta-analysis were as follows: (1) study designed as case-control or prospective cohort; (2) the study reported the relationship between nitrite or nitrate intake and the risk of GC incidence or mortality; and (3) the study reporting effect estimates and 95% confidence intervals (CIs) for comparisons of various categories and the lowest nitrite or nitrate intake.
Data collection and quality assessment
Data collection and quality assessment processes were performed by 2 authors and any disagreement was settled by group discussion and by an additional author referring to the original study. The collected information included the first author’s surname, publication year, study design, country, sample size, age, the percentage of male patients, assessment of exposure, GC incidence or mortality, and adjusted factors. The Newcastle-Ottawa Scale (NOS), based on selection (4 stars), comparability (2 stars), and outcome (3 stars), was used to evaluate study quality, and the “star system” range was 0–9 for evaluating the quality of included studies [13].
Statistical analysis
The relationship between nitrite or nitrate intake and the risk of GC were examined based on the effect estimate (odds ratio [OR], relative risk [RR], or hazard ratio [HR]) and corresponding 95% CIs in each study. The multiple categories of nitrite or nitrate intake within a single study were summarized into high or moderate nitrite/nitrate intake using a fixed-effects model, while the pooled results across included studies were evaluated using a random-effects model [14,15]. Heterogeneity test was performed using the I-square and Q statistic, and significant heterogeneity was defined as P<0.010 [16,17]. Sensitivity analyses were conducted to assess the stability of the pooled results [18]. Subgroup analyses for high or moderate nitrite/nitrate intake and the risk of GC were conducted based on publication year, study design, country, the percentage of male patients, assessment of exposure, adjusted model, and study quality. The interaction tests between subgroups were also performed to compare whether these associations differed according to study or participant characteristics [19]. Publication biases for high or moderate nitrite/nitrate intake and the risk of GC were evaluated using funnel plots, Egger test [20], and Begg [21] test. The inspective levels for pooled results are 2-sided, and p values less than 0.05 were regarded as statistically significant. Stata software was employed for all statistical analyses (version 10.0; Stata Corporation, College Station, TX, USA).
Results
Literature search and study characteristics
The initial searches of the electronic databases produced 831 articles; of these, 769 were discarded due to duplication or irrelevance. The remaining 62 studies underwent full-text evaluations; 38 of these were excluded for not assigning nitrite or nitrate as exposure markers (n=21), for reporting the sample population (n=12), or for being a systematic review (n=5). Ultimately, 18 case-control studies and 6 cohort studies were included in the final quantitative meta-analysis [22–45]. No additional eligible studies were found in the manual search of the references of retrieved studies. Details of the study selection process are presented in Figure 1, while the baseline characteristics of the patients included in the examined studies are shown in Table 1.
Figure 1.
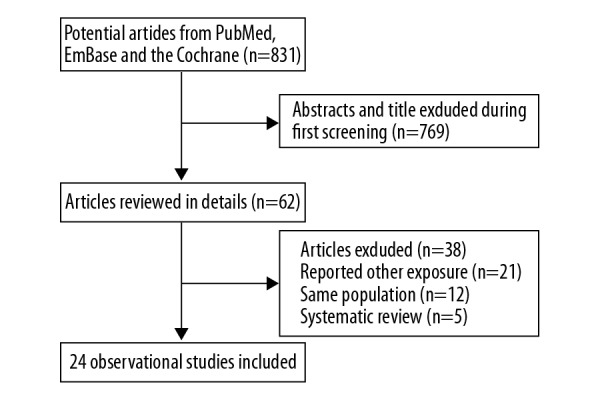
Flow diagram of the literature search and studies selection process.
Table 1.
Baseline characteristic of studies included in the systematic review and meta-analysis.
| Study | Publication year | Study design | Country | Sample size | Age (years) | Percentage male (%) | Assessment of exposure | Reported outcomes | Adjusted factors | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Risch [22] | 1985 | Case-control | Canada | 492 | 35–79 | 66.3 | FFQ | Nitrite and nitrate | Age, sex, and area of residence | 7 |
| Buiatti [23] | 1990 | Case-control | Italy | 1,782 | <75 | NA | FFQ | Nitrite and nitrate | Non-dietary variables and kilocalorie | 7 |
| Boeing [24] | 1991 | Case-control | Germany | 722 | 32–80 | 20.0–30.0 | IAQ | Nitrate | Age, sex and hospital | 6 |
| Hansson [25] | 1994 | Case-control | Sweden | 1,017 | 40–79 | 64.4 | FFQ | Nitrate | Age, gender, ascorbic acid, β-carotene, α-tocopherol | 7 |
| La Vecchia [26] | 1994 | Case-control | Italy | 2,747 | 19–74 | 59.4 | FFQ | Nitrite and nitrate | age, sex, education, family history of gastric cancer, BMI, TEI | 8 |
| Pobel [27] | 1995 | Case-control | France | 220 | 66.5 | 69.5 | FFQ | Nitrite and nitrate | Age, sex, occupation and total calorie intake | 6 |
| La Vecchia [28] | 1997 | Case-control | Italy | 2,799 | 19–74 | 59.4 | FFQ | Nitrite | Sex, age, and education | 7 |
| van Loon [29] | 1998 | Prospective cohort | The Netherlands | 120,852 | 55–69 | 48.2 | FFQ | Nitrite and nitrate | Age, sex, smoking, education, coffee consumption, intake of vitamin C and beta-carotene, family history of stomach cancer, prevalence of stomach disorders, use of refrigerator and use of freezer | 8 |
| Galanis [30] | 1998 | Prospective cohort | USA | 11 907 | >18.0 | 47.1 | FFQ | Nitrate | Age, education, Japanese place of birth, and gender. Analyses among men were also adjusted for cigarette and alcohol | 7 |
| De Stefani [31] | 1998 | Case-control | France | 1038 | 25–84 | 65.8 | FFQ | Nitrite | Age, sex, residence, urban/rural status, smoking duration, alcohol consumption, and „mate” consumption | 6 |
| Knekt [32] | 1999 | Prospective cohort | Finland | 9985 | 15–99 | 52.8 | FFQ | Nitrite and nitrate | Sex, age, municipality, smoking and TEI | 7 |
| Palli [33] | 2001 | Case-control | Italy | 943 | All stages | 60.1 | FFQ | Nitrite and nitrate | Age, sex, social class, family history of gastric cancer, area of rural residence, BMI, total energy and each nutrient of interest | 7 |
| Mayne [34] | 2001 | Case-control | USA | 1294 | 30–79 | 78.1 | IAQ | Nitrite | Sex, site, age; race, proxy status, income, education, BMI, cigarettes/day, years of consuming beer, wine, and liquor, and TEI | 7 |
| De Stefani [35] | 2001 | Case-control | France | 405 | 30–89 | 65.2 | FFQ | Nitrite and nitrate | Age, gender, residence, urban/rural status, and education | 6 |
| Engel [36] | 2003 | Case-control | USA | 1324 | 30–79 | 77.9 | IAQ | Nitrite | Geographic center, age, sex, race, income, respondent type, TEI | 7 |
| Lopez-Carrillo [37] | 2004 | Case-control | Mexico | 665 | >20 | 56.7 | FFQ | Nitrite | Age, gender, residence, TEI, education, Hp/CagA status and ascorbic acid | 8 |
| Kim [38] | 2007 | Case-control | Korea | 272 | 57.2 | 68.4 | FFQ | Nitrate | Age, sex, socioeconomic status, refrigerator use, H. pylori infection, and foods | 7 |
| Ward [39] | 2008 | Case-control | USA | 400 | >21 | NA | IAQ | Nitrite and nitrate | Year of birth, gender, education, smoking, alcohol, TEI, vitamin C, fiber, carbohydrate | 6 |
| Hernández-Ramírez [40] | 2009 | Case-control | Mexico | 735 | >20 | 54.0 | FFQ | Nitrite and nitrate | Energy, age, gender, H. pylori CagA status, schooling and consumptions of salt, chili and alcohol | 7 |
| Loh [41] | 2011 | Prospective cohort | UK | 23 363 | 40–79 | 46.2 | FFQ | Nitrite | Age, sex, BMI, cigarette smoking status, alcohol intake, TEI, PI, educational, and menopausal status | 8 |
| Cross [42] | 2011 | Prospective cohort | France | 494 979 | 50–71 | 59.7 | FFQ | Nitrite and nitrate | Age, sex, BMI, education, ethnicity, tobacco smoking, alcohol drinking, PI, vigorous physical activity, and the daily intake of fruit, vegetables, saturated fat, and TEI | 8 |
| Navarro Silvera [43] | 2011 | Case-control | USA | 1294 | 30–79 | NA | IAQ | Nitrite | Gender, age, site, race, income, education, proxy status, TEI, and mutual adjustment for other principle components | 7 |
| Keszei [44] | 2013 | Prospective cohort | The Netherlands | 120 852 | 55–69 | 48.2 | FFQ | Nitrite and nitrate | Age, smoking status, TEI, BMI, alcoholic intake, vegetable intake, fruit intake, education, and PI | 8 |
| Taneja [45] | 2017 | Case-control | India | 234 | All stages | 67.1 | IAQ | Nitrate | Age, gender, and tobacco consumption | 7 |
BMI – body mass index; FFQ – food-frequency questionnaire; IAQ – interviewer-administered questionnaire; PI – physical activity; TEI – total energy intake.
A total of 24 studies that recruited a total of 800 321 individuals were included in this study; the publication dates ranged from 1985 to 2017. The sample sizes ranged from 220 to 494 979, and the percentage of male patients ranged from 20.0% to 78.1%. Thirteen studies were conducted in Europe, while the rest were conducted in Canada, the USA, Mexico, Korea, and India. Eighteen studies used a food-frequency questionnaire (FFQ) to assess exposure, while the remaining 6 studies used an interviewer-administered questionnaire (IAQ) to evaluate exposure. The association between nitrite intake and the risk of GC was reported in 19 studies, and the impact of nitrate intake was reported in 17 studies. NOS was used for evaluation of study quality and is shown in Table 1. Six studies had 8 stars, 13 studies had 7 stars, and the remaining 5 studies had 6 stars.
Nitrite intake and gastric cancer
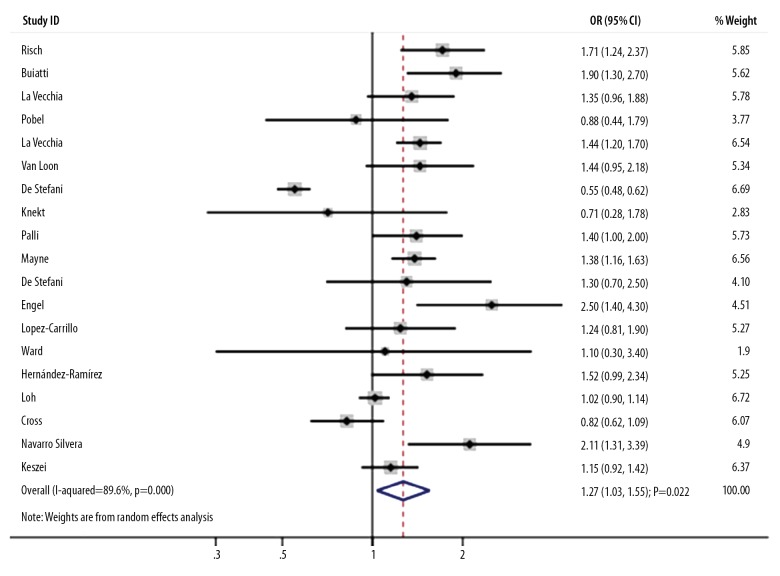
The association between high nitrite intake and the risk of GC was reported in 19 studies. A high nitrite intake was associated with an increased risk of GC (OR, 1.27; 95%CI, 1.03–1.55; P=0.022; Figure 2), and significant heterogeneity across included studies (I-square, 89.6%; P<0.001). A sensitivity analysis indicated that the conclusion was not altered by excluding any particular study (data not shown). Subgroup analyses suggested the significantly increased GC risk mainly included pooled studies published in or after 2000 (OR, 1.28; 95%CI, 1.09–1.51; P=0.003), studies designed as case-control (OR, 1.38; 95%CI, 1.02–1.85; P=0.034), studies conducted in other countries (OR, 1.56; 95%CI, 1.32–1.84; P<0.001), studies with a percentage of male patients <60.0% (OR, 1.18; 95%CI, 1.01–1.37; P=0.032), studies using an IAQ to assess exposure (OR, 1.73; 95%CI, 1.22–2.44; P=0.002), studies with partial adjustment (OR, 1.47; 95%CI, 1.20–1.79; P<0.001), and studies of high quality (OR, 1.36; 95%CI, 1.18–1.56; P<0.001). Moreover, we assessed whether publication year, country, the percentage of male patients, assessment of exposure, adjusted extent, and study quality could bias the correlation between high nitrite intake and the risk of GC (Table 2). We found no significant publication bias for high nitrite intake and GC risk (P for Egger=0.061; P for Begg=0.576).
Figure 2.
Association between high nitrite intake and the risk of gastric cancer.
Table 2.
Subgroup analyses for nitrite and nitrate intake and the risk of gastric cancer.
| Outcomes | Factor | Groups | Number of studies | OR and 95% CI | P value | Heterogeneity (%) | P value for heterogeneity | P value between subgroups |
|---|---|---|---|---|---|---|---|---|
| High versus low nitrite intake | Publication year | 2000 or after | 11 | 1.28 (1.09–1.51) | 0.003 | 67.2 | 0.001 | <0.001 |
| Before 2000 | 8 | 1.18 (0.76–1.83) | 0.462 | 94.5 | <0.001 | |||
| Study design | Case-control | 14 | 1.38 (1.02–1.85) | 0.034 | 92.2 | <0.001 | 0.588 | |
| Cohort | 5 | 1.04 (0.89–1.21) | 0.633 | 39.8 | 0.156 | |||
| Country | Europe | 12 | 1.11 (0.87–1.43) | 0.402 | 91.2 | <0.001 | <0.001 | |
| Other | 7 | 1.56 (1.32–1.84) | <0.001 | 22.4 | 0.259 | |||
| Percent male | ≥60.0 | 7 | 1.25 (0.78–2.02) | 0.353 | 94.8 | <0.001 | <0.001 | |
| <60.0 | 9 | 1.18 (1.01–1.37) | 0.032 | 61.2 | 0.008 | |||
| Assessment of exposure | FFQ | 15 | 1.18 (0.94–1.48) | 0.150 | 90.2 | <0.001 | <0.001 | |
| IAQ | 4 | 1.73 (1.22–2.44) | 0.002 | 52.7 | 0.096 | |||
| Adjusted extent | Fully | 13 | 1.23 (0.97–1.57) | 0.091 | 91.1 | <0.001 | <0.001 | |
| Partial | 6 | 1.47 (1.20–1.79) | <0.001 | 31.9 | 0.196 | |||
| Study quality | High | 15 | 1.36 (1.18–1.56) | <0.001 | 70.1 | <0.001 | <0.001 | |
| Low | 4 | 0.83 (0.50–1.38) | 0.466 | 67.6 | 0.026 | |||
| Moderate versus low nitrite intake | Publication year | 2000 or after | 10 | 1.14 (0.97–1.34) | 0.104 | 74.1 | <0.001 | 0.265 |
| Before 2000 | 5 | 1.11 (0.99–1.23) | 0.070 | 0.0 | 0.593 | |||
| Study design | Case-control | 10 | 1.23 (1.07–1.43) | 0.005 | 46.9 | 0.049 | <0.001 | |
| Cohort | 5 | 0.98 (0.88–1.10) | 0.744 | 42.7 | 0.137 | |||
| Country | Europe | 10 | 1.04 (0.95–1.14) | 0.375 | 43.5 | 0.068 | <0.001 | |
| Other | 5 | 1.30 (1.01–1.67) | 0.045 | 53.5 | 0.072 | |||
| Percent male | ≥60.0 | 4 | 1.43 (1.16–1.77) | 0.001 | 0.0 | 0.407 | <0.001 | |
| <60.0 | 8 | 0.99 (0.92–1.05) | 0.672 | 7.1 | 0.376 | |||
| Assessment of exposure | FFQ | 12 | 1.03 (0.95–1.12) | 0.414 | 31.6 | 0.139 | <0.001 | |
| IAQ | 3 | 1.56 (1.27–1.93) | <0.001 | 14.6 | 0.310 | |||
| Adjusted extent | Fully | 11 | 1.11 (0.97–1.26) | 0.127 | 69.9 | <0.001 | 0.078 | |
| Partial | 4 | 1.20 (1.02–1.40) | 0.025 | 0.0 | 0.479 | |||
| Study quality | High | 12 | 1.13 (1.00–1.27) | 0.044 | 70.1 | <0.001 | 0.759 | |
| Low | 3 | 1.11 (0.76–1.61) | 0.583 | 0.0 | 0.375 | |||
| High versus low nitrate intake | Publication year | 2000 or after | 8 | 0.89 (0.73–1.09) | 0.268 | 61.3 | 0.011 | <0.001 |
| Before 2000 | 9 | 0.75 (0.60–0.93) | 0.010 | 59.1 | 0.012 | |||
| Study design | Case-control | 12 | 0.79 (0.63–1.01) | 0.058 | 83.1 | <0.001 | 0.599 | |
| Cohort | 5 | 0.92 (0.77–1.09) | 0.321 | 0.0 | 0.745 | |||
| Country | Europe | 11 | 0.78 (0.64–0.95) | 0.015 | 59.3 | 0.006 | <0.001 | |
| Other | 6 | 0.88 (0.65–1.21) | 0.444 | 82.2 | <0.001 | |||
| Percent male | ≥60.0 | 7 | 0.81 (0.61–1.08) | 0.148 | 80.3 | <0.001 | 0.002 | |
| <60.0 | 8 | 0.77 (0.58–1.01) | 0.055 | 68.6 | 0.002 | |||
| Assessment of exposure | FFQ | 14 | 0.75 (0.64–0.88) | <0.001 | 51.3 | 0.014 | <0.001 | |
| IAQ | 3 | 1.10 (1.03–1.19) | 0.008 | 0.0 | 0.633 | |||
| Adjusted extent | Fully | 9 | 0.78 (0.61–1.02) | 0.065 | 68.0 | 0.002 | <0.001 | |
| Partial | 8 | 0.85 (0.68–1.08) | 0.179 | 74.6 | <0.001 | |||
| Study quality | High | 12 | 0.75 (0.63–0.89) | 0.001 | 58.7 | 0.005 | <0.001 | |
| Low | 5 | 1.07 (0.94–1.23) | 0.304 | 6.2 | 0.371 | |||
| Moderate versus low nitrate intake | Publication year | 2000 or after | 7 | 0.94 (0.84–1.05) | 0.294 | 0.0 | 0.486 | 0.003 |
| Before 2000 | 8 | 0.80 (0.65–0.98) | 0.032 | 75.8 | <0.001 | |||
| Study design | Case-control | 10 | 0.80 (0.66–0.96) | 0.018 | 66.8 | 0.001 | <0.001 | |
| Cohort | 5 | 0.96 (0.86–1.08) | 0.512 | 0.0 | 0.517 | |||
| Country | Europe | 11 | 0.81 (0.70–0.94) | 0.006 | 71.2 | <0.001 | 0.008 | |
| Other | 4 | 1.13 (0.89–1.45) | 0.324 | 0.0 | 0.671 | |||
| Percent male | ≥60.0 | 5 | 0.82 (0.67–1.02) | 0.069 | 10.8 | 0.344 | 0.679 | |
| <60.0 | 8 | 0.86 (0.70–1.07) | 0.170 | 80.2 | <0.001 | |||
| Assessment of exposure | FFQ | 13 | 0.85 (0.74–0.99) | 0.037 | 69.8 | <0.001 | 0.587 | |
| IAQ | 2 | 0.95 (0.54–1.67) | 0.853 | 69.2 | 0.072 | |||
| Adjusted extent | Fully | 9 | 0.91 (0.73–1.13) | 0.387 | 79.9 | <0.001 | 0.883 | |
| Partial | 6 | 0.83 (0.74–0.93) | 0.002 | 0.0 | 0.632 | |||
| Study quality | High | 11 | 0.86 (0.74–1.01) | 0.065 | 73.1 | <0.001 | 0.786 | |
| Low | 4 | 0.85 (0.59–1.23) | 0.389 | 49.5 | 0.115 |
CI – confidence interval; FFQ – food-frequency questionnaire; IAQ – interviewer-administered questionnaire; OR – odds ratio.
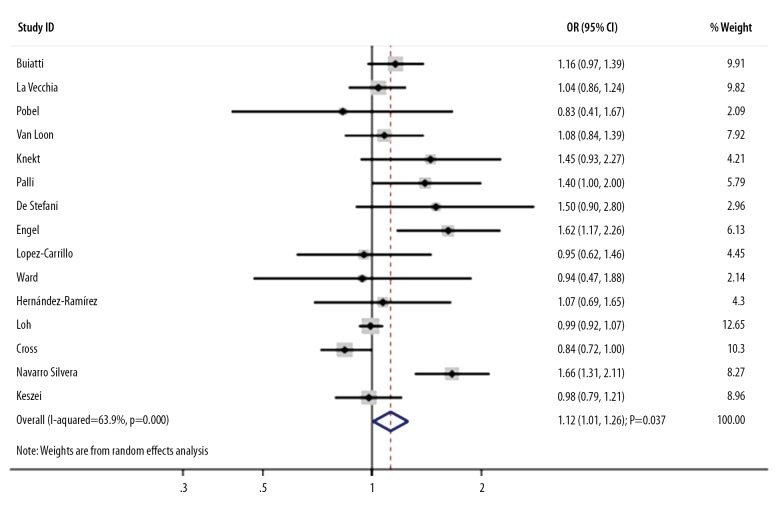
An association between moderate nitrite intake and the risk of GC was reported in 15 studies. The pooled OR indicated that moderate nitrite intake produced additional risk for GC risk by 12% (OR, 1.12; 95%CI, 1.01–1.26; P=0.037; Figure 3), and significant heterogeneity was detected (I-square, 63.9%; P<0.001). Sensitivity analyses indicated that the pooled results changed after the exclusion of several studies due to marginal 95%CI (data not shown). The subgroup analysis upon pooling of the case-control studies (OR, 1.23; 95%CI, 1.07–1.43; P=0.005), the studies conducted in other countries (OR, 1.30; 95%CI, 1.01–1.67; P=0.045), studies with a percentage of male patients ≥60.0% (OR, 1.43; 95%CI, 1.16–1.77; P=0.001), studies using IAQ to assess exposure (OR, 1.56; 95%CI, 1.27–1.93; P<0.001), studies with partial adjustment (OR, 1.20; 95%CI, 1.02–1.40; P=0.025), and studies of high quality (OR, 1.13; 95%CI, 1.00–1.27; P=0.044) indicated that moderate nitrite intake increases the risk of GC. Study design, country, the percentage of male patients, and assessment of exposure played an important role in the correlation between moderate nitrite intake and GC risk (Table 2). No significant publication bias was observed (P for Egger, 0.115; P for Begg, 0.692).
Figure 3.
Association between moderate nitrite intake and the risk of gastric cancer.
Nitrate intake and gastric cancer
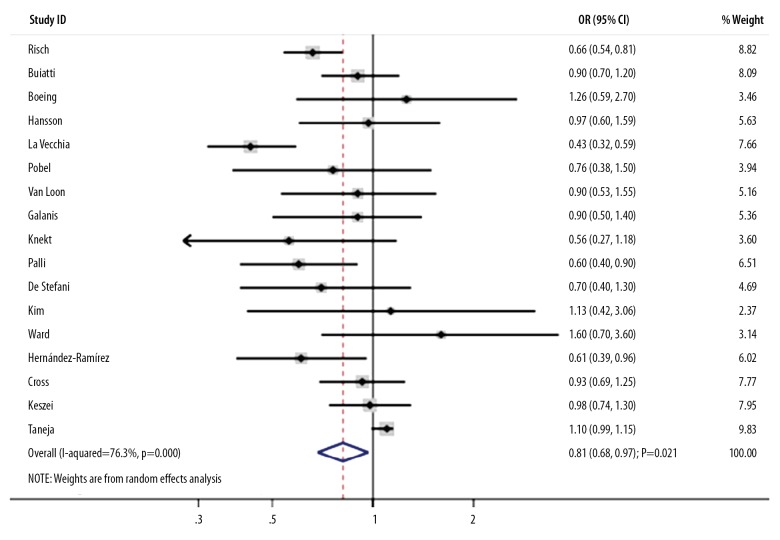
The association between high nitrate intake and the risk of GC was reported in 17 studies. The summary OR indicated that a high nitrate intake plays a protective role on the progression of GC (OR, 0.81; 95%CI, 0.68–0.97; P=0.021; Figure 4), and showed significant heterogeneity across the included studies (I-square, 76.3%; P<0.001). A sensitivity analysis found the conclusion was stable and not changed by excluding individual studies (data not shown). Subgroup analyses indicated that high nitrate intake was associated with decreased risk of GC in studies published before 2000 (OR, 0.75; 95%CI, 0.60–0.93; P=0.010), those conducted in Europe (OR, 0.78; 95%CI, 0.64–0.95; P=0.015), those that used FFQ to assess exposure (OR, 0.75; 95%CI, 0.64–0.88; P<0.001), and those of high quality (OR, 0.75; 95%CI, 0.63–0.89; P=0.001). Moreover, publication year, country, the percentage of male patients, assessment of exposure, adjustment extent, and study quality could bias the correlation between high nitrate intake and GC (Table 2). There was no publication bias among included studies (P for Egger, 0.054; P for Begg, 0.343).
Figure 4.
Association between high nitrate intake and the risk of gastric cancer.
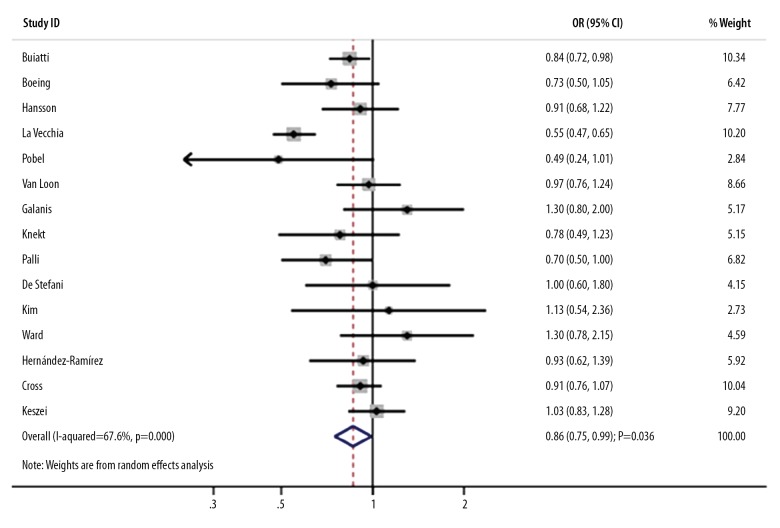
The association between moderate nitrate intake and the risk of GC was reported in 15 studies. We noted that a moderate nitrate intake was associated with a decreased risk of GC (OR, 0.86; 95%CI, 0.75–0.99; P=0.036; Figure 5), and significant heterogeneity was noted (I-square, 67.6%; P<0.001). The pooled results were variable after the sequential exclusion of individual studies due to marginal 95%CI (data not shown). The subgroup analyses indicated that the risk of GC was decreased in the moderate versus the lowest nitrate intake when studies were conducted before 2000 (OR, 0.80; 95%CI, 0.65–0.98; P=0.032), studies had a case-control design (OR, 0.80; 95%CI, 0.66–0.96; P=0.018), studies conducted in Europe (OR, 0.81; 95%CI, 0.70–0.94; P=0.006), studies that used FFQ to assess exposure (OR, 0.85; 95%CI, 0.74–0.99; P=0.037), and studies with partial adjustment (OR, 0.83; 95%CI, 0.74–0.93; P=0.002). Moreover, publication year, study design, and country played an important role in the correlation between moderate nitrate intake and the risk of GC (Table 2). No evidence of publication bias was observed by using Egger and Begg tests (P for Egger, 0.323; P for Begg, 0.921).
Figure 5.
Association between moderate nitrate intake and the risk of gastric cancer.
Discussion
This comprehensive quantitative meta-analysis aimed to assess any potential associations between nitrite or nitrate intake and subsequent GC risk based on all available observational studies. The current study included 800 321 individuals from 18 case-control and 6 cohort studies with a wide range of participant characteristics. Overall, a high or moderate nitrite intake was associated with an increased risk of GC. Conversely, a high or moderate nitrate intake provides a protective effect against GC. The summary results for moderate nitrite or nitrate intake on the risk of GC were variable and therefore require further large-scale studies for verification. Moreover, these associations differed when stratified by publication year, study design, country, the percentage of male patients, assessment of exposure, adjusted model, and study quality.
The study conducted by Xie et al. contained 62 observational studies and found dietary nitrate intake was inversely associated with the risk of GC, whereas dietary nitrite intake did not yield a significant association with the risk of GC [46]. However, the study compared only the highest versus lowest dietary nitrate or nitrite intake, while the effect estimates at various medium exposures were not included; this may result in the oversight of several important datapoints for these associations. Moreover, several additional studies should be updated in this study. Therefore, the current study aimed to systematically evaluate the potential role of nitrite and nitrate intake in the risk of GC.
The summary results indicated that a high or moderate nitrite intake was associated with an increased risk of GC. Most of the included studies reported a positive trend between high or moderate nitrite intake and the risk of GC, while several studies reported reverse trend. Pobel et al. indicated that nitrite and nitrate intakes were not correlated with the risk of GC, and speculated that this could be due to the contribution of vegetables and fruits to dietary nitrite [27]. De Stefani et al. found that high nitrite intake produced a protective effect on GC risk, which could be due to exogenous N-nitroso compounds contributing to a similar role in the risk of GC [31]. This study indicated that a high or moderate nitrite intake was correlated with a higher risk of GC; the reason for this was correlated with the sources of nitrite intake, and nitrite from animal products play an important role on endogenous nitrosation [47].
We noted that high or moderate nitrate intakes are correlated with a reduced risk of GC than the lowest nitrate intake. Taneja et al. indicated that the intake of >45 mg/L nitrate via drinking water produced an additional GC risk [45]. The potential reason for this could be the source of nitrate. In this study, the nitrate source in most of the included studies was vegetables that contain nutrients that inhibit the N-nitrosation in food, and the beneficial effects of nitrate intake could be affected by vitamin C and other antioxidants [48]. Vitamins C and E could inhibit endogenous nitrosation and hinder the formation of nitrosation compounds [49].
Subgroup analyses indicated that publication year, study design, country, the percentage of male patients, assessment of exposure, adjusted model, and study quality could bias the correlation between nitrite or nitrate intake and the risk of GC. First, the diagnosing strategy and timing were developed through the study publication year. Second, the current study included case-control and cohort studies, which associated with the evidence level. Third, the percentage of male patients contributed to the heterogeneity of the associations, possibly due to the different prevalence of GC between men and women. Fourth, the assessment of exposure was correlated with the accuracy of data collection. Fifth, the extent of adjustment could affect the intrinsic association of nitrite or nitrate intake with the risk of GC. Sixth, the quality of the included studies reflects the reliability of the conclusions made therein.
We were aware of several limitations of this meta-analysis. First, most of the included studies were retrospective case-control studies, which might have introduced potential selection and recall biases. Second, the cutoff values of nitrite and nitrate intakes differed among the included studies, which could have affected the comparability between exposure and control. Third, the significant heterogeneity could not be fully interpreted in the sensitivity and subgroup analyses. Fourth, the adjusted factors differed among the included studies, and these factors may have played an important role in the progression of GC. Fifth, publication bias is inevitable since this study was based on published articles.
Conclusions
This study concluded that a high or moderate nitrite intake increases the risk of GC, whereas a high or moderate nitrate intake was associated with a decreased risk of GC. These associations are variable according to several characteristics of study or patients. Further large-scale prospective cohort studies are required to evaluate the correlation between nitrite or nitrate intake from various sources and the risk of GC.
Footnotes
Source of support: Funding was provided by the National Natural Science Foundation of China (81460370)
Conflicts of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Otsuji E, Fujiyama J, Takagi T, et al. Results of total gastrectomy with extended lymphadenectomy for gastric cancer in elderly patients. J Surg Oncol. 2005;91:232–36. doi: 10.1002/jso.20330. [DOI] [PubMed] [Google Scholar]
- 3.Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–55. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 4.Vaughan TL, Davis S, Kristal A, et al. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: Adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomark Prev. 1995;4:85–92. [PubMed] [Google Scholar]
- 5.Chow WH, Swanson CA, Lissowska J, et al. Risk of stomach cancer in relation to consumption of cigarettes, alcohol, tea and coffee in Warsaw, Poland. Int J Cancer. 1999;81:871–76. doi: 10.1002/(sici)1097-0215(19990611)81:6<871::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Farrow DC, Vaughan TL, Sweeney C, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231–38. doi: 10.1023/a:1008913828105. [DOI] [PubMed] [Google Scholar]
- 7.Kamangar F, Dawsey SM, Blaser MJ, et al. Opposing risks of gastric cardia and noncardiac gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445–52. doi: 10.1093/jnci/djj393. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund. Food, physical activity and the prevention of cancer: A global perspective. American Institute for Cancer Research; Washington DC: 2007. [Google Scholar]
- 9.Mirvish SS, Wallcave L, Eagen M, et al. Ascorbate-nitrite reaction: Possible means of blocking the formation of carcinogenic N-nitroso compounds. Science. 1972;177:65–68. doi: 10.1126/science.177.4043.65. [DOI] [PubMed] [Google Scholar]
- 10.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control. 1991;2:427–42. doi: 10.1007/BF00054304. [DOI] [PubMed] [Google Scholar]
- 11.Tricker AR. N-nitroso compounds and man: Sources of exposure, endogenous formation and occurrence in body fluids. Eur J Cancer Prev. 1997;6:226–68. [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Plos Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute; 2009. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Ades AE, Lu G, Higgins JP. The interpretation of random-effects metaanalysis in decision models. Med Decis Making. 2005;25:646–54. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 16.Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. In: Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions 5.0.1. chapter 9 Oxford, UK: The Cochrane Collaboration; 2008. [Google Scholar]
- 17.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. 1999;47:15–17. [Google Scholar]
- 19.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: Metaanalysis in context. 2nd ed. London: BMJ Books; 2011. pp. 285–312. [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 22.Risch HA, Jain M, Choi NW, et al. Dietary factors and the incidence of cancer of the stomach. Am J Epidemiology. 1985;122:947–59. doi: 10.1093/oxfordjournals.aje.a114199. [DOI] [PubMed] [Google Scholar]
- 23.Buiatti E, Palli D, Decarli A, et al. A case-control study of gastric cancer and diet in Italy. Int J Cancer. 1990;45:896–901. doi: 10.1002/ijc.2910450520. [DOI] [PubMed] [Google Scholar]
- 24.Boeing H, Frentzel-Beyme R, Berger M, et al. Case-control study on stomach cancer in Germany. Int J Cancer. 2010;47:858–64. doi: 10.1002/ijc.2910470612. [DOI] [PubMed] [Google Scholar]
- 25.Hansson LE, Nykén Olof, Beagstrom R, et al. Nutrients and gastric cancer risk. A population-based case-control study in Sweden. Int J Cancer. 1994;57:638–44. doi: 10.1002/ijc.2910570505. [DOI] [PubMed] [Google Scholar]
- 26.Vecchia CL, Ferraroni M, D’Avanzo B, et al. Selected micronutrient intake and the risk of gastric cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:393–98. [PubMed] [Google Scholar]
- 27.Pobel D, Riboli E, Cornée J, et al. Nitrosamine, nitrate and nitrite in relation to gastric cancer: A case-control study in Marseille, France. Eur J Epidemiology. 1995;11:67–73. doi: 10.1007/BF01719947. [DOI] [PubMed] [Google Scholar]
- 28.La Vecchia C, Negri E, Franceschi S, et al. Case-control study on influence of methionine, nitrite, and salt on gastric carcinogenesis in northern Italy. Nutr Cancer. 1997;27:65–68. doi: 10.1080/01635589709514503. [DOI] [PubMed] [Google Scholar]
- 29.Van Loon A, Botterweck A, Goldbohm R, et al. Intake of nitrate and nitrite and the risk of gastric cancer: a prospective cohort study. Brit J Cancer. 1998;78:129–35. doi: 10.1038/bjc.1998.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galanis DJ, Kolonel LN, Lee J, et al. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: A prospective study. Int J Epidemiology. 1998;27:173–80. doi: 10.1093/ije/27.2.173. [DOI] [PubMed] [Google Scholar]
- 31.De Stefani E, Boffetta P, Mendilaharsu M, et al. Dietary nitrosamines, heterocyclic amines, and risk of gastric cancer: A case-control study in Uruguay. Nutr Cancer. 1998;30:158–62. doi: 10.1080/01635589809514656. [DOI] [PubMed] [Google Scholar]
- 32.Knekt P, Jarvinen R, Dich J, et al. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: A follow-up study. Int J Cancer. 1999;80:852–56. doi: 10.1002/(sici)1097-0215(19990315)80:6<852::aid-ijc9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 33.Palli D, Russo A, Decarli A. Dietary patterns, nutrient intake and gastric cancer in a high-risk area of Italy. Cancer Causes Control. 2001;12:163–72. doi: 10.1023/a:1008970310963. [DOI] [PubMed] [Google Scholar]
- 34.Mayne ST, Risch HA, Dubrow R, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055–62. [PubMed] [Google Scholar]
- 35.De Stefani E, Ronco A, Brennan P, et al. Meat consumption and risk of stomach cancer in Uruguay: A case-control study. Nutr Cancer. 2001;40:103–7. doi: 10.1207/S15327914NC402_5. [DOI] [PubMed] [Google Scholar]
- 36.Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404–13. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Carrillo L, Torres-Lopez J, Galvan-Portillo M, et al. Helicobacter pylori-CagA seropositivity and nitrite and ascorbic acid food intake as predictors for Gastric Cancer. Eur J Cancer. 2004;40:1752–59. doi: 10.1016/j.ejca.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Kim HJ, Lee SS, Choi BY, et al. Nitrate intake relative to antioxidant vitamin intake affects gastric cancer risk: A case-control study in Korea. Nutr Cancer. 2007;59:185–91. doi: 10.1080/01635580701460554. [DOI] [PubMed] [Google Scholar]
- 39.Ward MH, Heineman EF, Markin RS, et al. Adenocarcinoma of the stomach and esophagus and drinking water and dietary sources of nitrate and nitrite. Int J Occup Environ Health. 2008;14:193–97. doi: 10.1179/oeh.2008.14.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernández-Ramírez RU, Galván-Portillo MV, Ward MH, et al. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int J Cancer. 2009;125:1424–30. doi: 10.1002/ijc.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loh YH, Jakszyn P, Luben RN, et al. N-nitroso compounds and cancer incidence: the European Prospective Investigation into Cancer and Nutrition (EPIC) – Norfolk Study. Am J Clin Nutr. 2011;93:1053–61. doi: 10.3945/ajcn.111.012377. [DOI] [PubMed] [Google Scholar]
- 42.Cross AJ, Freedman ND, Ren J, et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol. 2011;106:432–42. doi: 10.1038/ajg.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Navarro Silvera SA, Mayne ST, Risch HA, et al. Principal component analysis of dietary and lifestyle patterns in relation to risk of subtypes of esophageal and gastric cancer. Ann Epidemiol. 2011;21:543–50. doi: 10.1016/j.annepidem.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keszei AP, Goldbohm RA, Schouten LJ, et al. Dietary N-nitroso compounds, endogenous nitrosation, and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Am J Clin Nutr. 2013;97:135–46. doi: 10.3945/ajcn.112.043885. [DOI] [PubMed] [Google Scholar]
- 45.Taneja P, Labhasetwar P, Nagarnaik P, et al. The risk of cancer as a result of elevated levels of nitrate in drinking water and vegetables in Central India. J Water Health. 2017;15:602–14. doi: 10.2166/wh.2017.283. [DOI] [PubMed] [Google Scholar]
- 46.Xie L, Mo M, Jia HX, et al. Association between dietary nitrate and nitrite intake and site-specific cancer risk: Evidence from observational studies. Oncotarget. 2016;7:56915–32. doi: 10.18632/oncotarget.10917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grosse Y, Baan R, Straif K, et al. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol. 2006;7:628–29. doi: 10.1016/s1470-2045(06)70789-6. [DOI] [PubMed] [Google Scholar]
- 48.McKnight GM, Duncan CW, Leifert C, et al. Dietary nitrate in man: friend or foe? Brit J Nutr. 1999;81:349–58. doi: 10.1017/s000711459900063x. [DOI] [PubMed] [Google Scholar]
- 49.Zeegers MP, Selen RFM, Kleinjans JCS, et al. Nitrate intake does not influence bladder cancer risk: The Netherlands cohort study. Environ Health Perspect. 2006;114:1527–31. doi: 10.1289/ehp.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]