Abstract
Rational and Objective
Evidence from clinical trials to guide patient preparation for maintenance dialysis is limited. While anemia is associated with mortality and cardiovascular (CV) disease in individuals initiating maintenance dialysis, it is not known if treatment of anemia prior to dialysis initiation with erythropoiesis stimulating agents (ESAs) alters outcomes.
Study Design
Post-hoc analysis of a randomized controlled trial
Setting & Participants
Participants with type 2 diabetes and chronic kidney disease (CKD) who progressed to dialysis (n=590) in the Trial to Reduce cardiovascular Events with Aranesp Therapy (TREAT)
Exposure
Randomized treatment assignment (Darbepoetin versus placebo)
Outcome
All-cause mortality, CV mortality, nonfatal myocardial infarction, heart failure and stroke within the first 180 days of dialysis initiation
Analytic Approach
Proportional Hazards regression
Results
Overall, 590/4038 (14.6%) participants initiated dialysis during the trial (n=298 and 292 in the darbepoetin and placebo groups, respectively). Corresponding Hb were 11.3±1.6g/dL and 9.5±1.5g/dL (P<0.001). Death from any cause occurred in 31 participants (10.4%) assigned to darbepoetin and 28 (9.6%) assigned to placebo (HR 1.16; 95% CI 0.69–1.93), while death from CV causes occurred in 15 participants (5.0%) and 13 (4.5%) respectively (HR 1.21; 95%CI 0.58–1.93). There were no differences in the risk of non-fatal myocardial infarction or heart failure. Stroke occurred in eight participants (2.8%) assigned to darbepoetin and one (0.3%) assigned to placebo (HR 8.6; 95%CI 1.1 – 68.7).
Limitations
Post-hoc analyses of a sub-group of study participants.
Conclusion
Despite initiating dialysis with a higher Hb, prior treatment with darbepoetin was not associated with a reduction in mortality, MI or heart failure in the first 180 days, but a higher frequency of stroke was observed. In the absence of more definitive data, this may inform decisions regarding the use of ESAs to treat mild-to-moderate anemia in patients with type 2 diabetes and CKD nearing dialysis initiation.
Keywords: Anemia, Hemoglobin, ESRD, Type 2 Diabetes Mellitus, Chronic Kidney Disease
INTRODUCTION
In 2015 approximately 120,000 patients initiated maintenance dialysis in the United States.1 The transition from non-dialysis chronic kidney disease (CKD) to maintenance dialysis is a time of considerable physiologic perturbation reflected by a peak in mortality1,2 and CV events,3 particularly during the first three months. In the preparation of patients for dialysis, clinicians are faced with a variety of management issues, including the treatment of anemia, that are rarely supported by robust data from clinical trials.
Anemia, defined as hemoglobin (Hb) <12 g/dL in men and <11 g/dL in women, is a common finding in patients with progressive chronic kidney disease (CKD), largely due to inadequate erythropoiesis. The prevalence of anemia increases with declining estimated glomerular filtration rate (eGFR), such that approximately 15% of individuals with CKD stage 3 and 51% of those with CKD stage 4 or higher are anemic.4 The presence of diabetes mellitus is an additional concern, as patients with moderate to advanced CKD and diabetes mellitus are more likely to be anemic than patients without diabetes.4,5
While anemia is associated with mortality and higher rates of cardiovascular (CV) events in patients with CKD,6,7 the use of erythropoiesis stimulating agents (ESAs) to achieve normal or near normal Hb concentrations (compared with intermediate targets ~9–11 g/dL) in patients with CKD has failed to prolong survival or reduce cardiovascular event rates, while the risk of stroke may be increased.8–10 Moreover, the use of ESAs to target higher Hb concentrations in patients receiving hemodialysis (HD) did not yield beneficial effects on cardiac structure.11 An earlier trial comparing higher versus lower target hematocrit was halted prematurely due to safety concerns, with subsequent analyses reporting higher mortality, hospitalization and thrombotic events in patients randomized to the higher target hematocrit.12,13
Relatively little is known about the treatment of anemia in the period prior to initiation of maintenance dialysis. In the absence of dedicated trial data in this area, the sub-group of participants initiating dialysis from Trial to Reduce cardiovascular Events with Aranesp Therapy (TREAT)10 provided a unique opportunity to examine the association of ESA use and Hb with post-dialysis outcomes.
METHODS
Study design and population
The design and original results of TREAT (NCT00093015) have been published.10,14 Briefly, TREAT was a prospective, double-blind, randomized controlled trial of darbepoetin alfa versus placebo for the treatment of anemia in 4,038 patients with T2DM, eGFR of 20–60 mL/min/1.73m2 according to the 4-variable MDRD (Modification of Diet in Renal Disease) Study equation, hemoglobin level <11.0 g/dL, and transferrin saturation >15%. Notable exclusion criteria included a recent (within 12 weeks) CV event, grand mal seizure, major surgery, or prior use of an ESA, uncontrolled hypertension, known human immunodeficiency virus infection, current use of intravenous antibiotics, chemotherapy or radiotherapy, malignancy (except basal cell or squamous cell carcinoma of the skin), active bleeding, hematologic diseases, pregnancy, or kidney transplantation. Local institutional review boards approved the trial and all participants provided informed consent.
Exposures and Outcomes
The primary exposure of interest in these post-hoc analyses was the randomized treatment assignment (darbepoetin versus placebo). Of note, per protocol, TREAT allowed the use of darbepoetin if the Hb fell below 9g/dL in participants randomized to the placebo arm (the median [25–75th percentile] darbepoetin dose recorded for these individuals was 0 [0–10] mcg/month).15 After the initiation of HD there was no further study protocol-driven treatment of anemia. Further management, including the use of ESA’s, was deferred to the treating physician. The primary outcome of interest was all-cause mortality; additional outcomes that were considered included CV mortality, the CV composite (death, non-fatal myocardial infarction, heart failure, stroke and hospitalization for myocardial ischemia), and the individual components of the CV composite (except for hospitalization for myocardial ischemia owing to the small number of events).10 All endpoints were adjudicated by the original Clinical Endpoints Committee, which was blinded to the treatment assignment.
Statistical Analyses
Continuous variables were examined graphically and recorded as means (± standard deviations) for normally distributed data, or medians (with 25th, 75th percentile ranges) for non-normally distributed data. Categorical variables were examined by frequency distribution and recorded as proportions. Comparisons were made using the Student t-test, Wilcoxon rank sum test or χ2 test, as appropriate. In addition, standardized differences were calculated for the baseline variables as an alternative metric of determining differences between groups.16
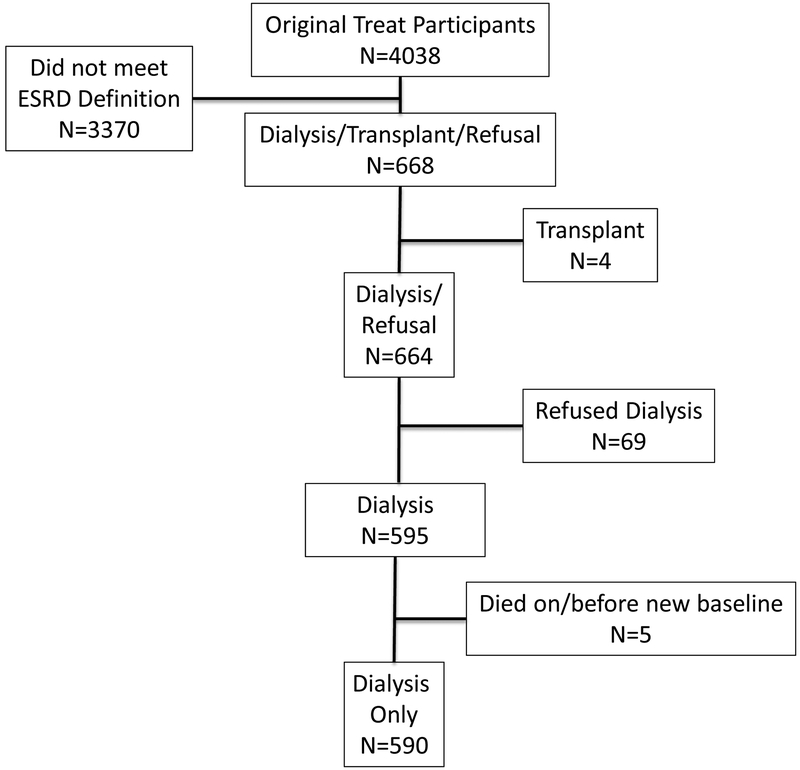
For the primary analyses, unadjusted proportional hazards regression models were fit to determine the association of randomized treatment assignment with events of interest. Participants were considered at risk from the date of dialysis initiation until the time of event or 180 days, whichever came first. Complete mortality data were available on 590 TREAT participants who initiated dialysis during the trial (Figure 1). Eighteen participants withdrew from further follow-up visits and did not have CV event data available for analysis. For all models, exposure variables and covariates were considered as the most proximal value preceding at-risk time. For the secondary analyses, analogous models were fit to determine the association of Hb with events of interest. To account for potential confounding variables, adjusted models were fit as follows: Model 1 was additionally adjusted for randomized treatment assignment; Model 2 was adjusted for randomized treatment assignment, transferrin saturation, ferritin and log-transformed urine protein/creatinine ratio; Model 3 employed a backwards selection algorithm (P for entry <0.05) that included the same variables as Model 2, in addition to age, sex, race/ethnicity (Black, Hispanic, White, Other), Quételet’s (body mass) index (BMI), systolic blood pressure, history of coronary artery, cerebrovascular, and peripheral arterial disease, history of heart failure, statin use, use of angiotensin converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), insulin, and intravenous iron, serum albumin, C-reactive protein, HbA1c, estimated glomerular filtration rate (eGFR) and duration of diabetes.
Figure 1.
Flow diagram of participant selection
The proportionality assumption for all models was assessed based on Schoenfeld residual testing. Nominal 2-sided p values of <0.05 were considered statistically significant. All analyses were performed using STATA 14.0SE (College Station, Tex., USA).
RESULTS
Baseline Characteristics
A total of 590 of the 4038 TREAT participants (14.6%) initiated dialysis during follow up, of which 541 were on hemodialysis and 49 on peritoneal dialysis. The mean follow-up time during the six-month post-dialysis initiation period was 153 days in the darbepoetin group and 162 in the placebo group. At the time of dialysis initiation, the average age was 65 ±10 years, 55% were male and 29% were black. Participants in the darbepoetin group had significantly higher Hb (11.3 versus 9.5 g/dL), transferrin saturation (28 versus 23%) and significantly lower ferritin (204 versus 209 mcg/L) compared with those in the placebo arm. Other notable differences included a lower frequency of heart disease and a higher urine protein/creatinine ratio in the darbepoetin group. Otherwise the groups were similar (Table 1 and Supplementary Table 1).
Table 1.
Participant characteristics according to randomized treatment assignment at the time of maintenance dialysis initiation
| Placebo | Darbepoetin | Pa | |
|---|---|---|---|
| n | 292 | 298 | |
| Age (years) | 65.1 ± 10.4 | 65.0 ± 10.5 | 0.85 |
| Male n, (%) | 167 (57.2%) | 158 (53.0%) | 0.31 |
| Race n, (%) | 0.17 | ||
| Black | 91 (31.2%) | 79 (26.5%) | |
| Hispanic | 37 (12.7%) | 51 (17.1%) | |
| Other | 6 (2.1%) | 12 (4.0%) | |
| White | 158 (54.1%) | 156 (52.3%) | |
| BMI (kg/m2) | 31.1 ± 6.7 | 31.1 ± 7.7 | 0.98 |
| SBP (mmHg) | 141 ± 24 | 140 ± 22 | 0.61 |
| CAD n, (%) | 130 (44.5%) | 106 (35.6%) | 0.03 |
| CVD n, (%) | 48 (16.4%) | 57 (19.1%) | 0.39 |
| PAD n, (%) | 64 (21.9%) | 60 (20.1%) | 0.59 |
| HF n, (%) | 135 (46.2%) | 130 (43.6%) | 0.52 |
| Statin n, (%) | 136 (46.6%) | 130 (43.6%) | 0.47 |
| ACEi n, (%) | 93 (31.8%) | 104 (34.9%) | 0.43 |
| ARB n, (%) | 84 (28.8%) | 93 (31.2%) | 0.52 |
| Insulin use n, (%) | 149 (51.0%) | 127 (42.6%) | 0.04 |
| Iron use n, (%) | 159 (54.5%) | 144 (48.3%) | 0.14 |
| Hemoglobin (g/dL) | 9.5 ± 1.4 | 11.3 ± 1.6 | <0.001 |
| Albumin (g/dL) | 3.6 ± 0.5 | 3.6 ± 0.5 | 0.08 |
| Transferrin Saturation (%) | 23 [18, 30] | 28 [20, 37] | <0.001 |
| Ferritin (mcg/L) | 210 [114, 404] | 204 [101, 312] | 0.04 |
| CRP (mg/L) | 4.5 [3.0, 11.9] | 4.1 [3.0, 10.8] | 0.6 |
| HbA1c (%) | 6.7 [6.1, 7.6] | 6.7 [6.0, 7.9] | 0.88 |
| Creatinine (mg/dL) | 3.7 [2.8, 4.9] | 4.0 [3.0, 5.1] | 0.16 |
| eGFR (mL/min/1.73m2) | 16 [13, 22] | 15 [11, 21] | 0.07 |
| Urine Pr/Cr ratio (g/g) | 3.2 [1.3, 6.4] | 4.7 [1.6, 7.9] | 0.01 |
| Duration of T2DM (years) | 17.9 [11.5, 24.5] | 17.9 [11.7, 23.9] | 0.88 |
| Hemodialysisn, (%) | 271 (92.8%) | 270 (90.6%) | 0.33 |
| FACT score | 31 [20, 39] | 31 [21, 40] | 0.35 |
| EQ5D score | 60 [45, 70] | 60 [50, 71] | 0.27 |
Values for continuous variables are given as mean ±standard deviation or median (25th – 75th percentile).
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; CAD, coronary artery disease; CVD, cerebrovascular disease; PAD, peripheral arterial disease; HF, heart failure; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; T2DM, type 2 diabetes mellitus; FACT, functional assessment of cancer therapy fatigue score; EQ5D, EuroQol 5 dimension visual analogue score
P values refer to a test for difference according to randomized treatment assignment
Randomized Treatment Assignment - All-cause and CV mortality
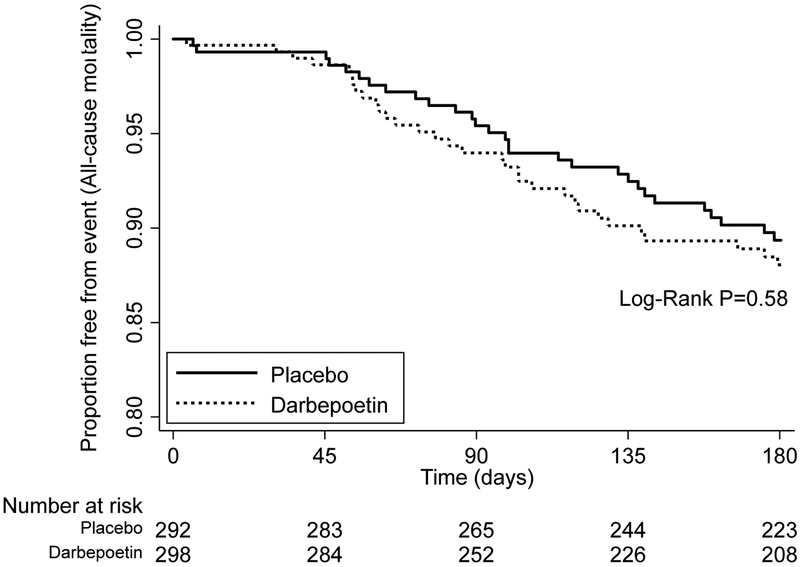
Death from any cause after HD initiation occurred in 31 of 298 (10.4%) participants in the darbepoetin group and 28 of 292 (9.6%) participants from the placebo group (unadjusted HR for darbepoetin vs. placebo 1.16; 95%CI 0.69 – 1.93; P=0.58) (Table 2 and Figure 2). Death attributable to a cardiovascular cause occurred in 15 (5.0%) participants from the darbepoetin group and 13 (4.5%) from the placebo group (HR for darbepoetin vs. placebo 1.21; 95%CI 0.58 – 2.55; P=0.61) (Table 2). Results were similar when only patients initiating hemodialysis were considered (Supplementary Table 2). There was no association of Hb at the time of dialysis initiation with all-cause or CV mortality in unadjusted or adjusted analyses (Supplementary Tables 3 and 4).
Table 2.
Association of randomized treatment assignment with death, cardiovascular death, heart failure, myocardial infarction and stroke during the first six months after maintenance dialysis initiation (n=590)
| Placebo (N=292) | Darbepoetin (N=298) | Hazard Ratio (95% CI) | P | |
|---|---|---|---|---|
|
End Point (Events/Total) |
Risk at 6 mths (Events/total) |
Risk at 6 mths (Events/total) | ||
| Death (59/590) |
10.2 % (28/292) |
11.5 % (31/298) |
1.16 (0.69 – 1.93) | 0.58 |
| CV Death (28/590) |
5.0 % (13/292) |
5.8 % (15/298) |
1.21 (0.58 – 2.55) | 0.61 |
| CV Composite (66/586) |
10.8 % (29/292) |
13.8 % (37/294) |
1.35 (0.83 – 2.20) | 0.23 |
| Heart Failure (19/572) |
3.9 % (10/286) |
3.5 % (9/286) |
0.95 (0.38 – 2.33) | 0.90 |
| Myocardial Infarction (17/572) |
3.5 % (9/286) |
3.2 % (8/286) |
0.95 (0.37 – 2.45) | 0.91 |
| Stroke (9/572) |
0.4 % (1/286) |
3.4 % (8/286) |
8.59 (1.07 – 68.7) | 0.04 |
Figure 2.
Kaplan-Meier plot examining effect of randomized treatment assignment on all-cause mortality from time of maintenance dialysis initiation to 6 months
Randomized Treatment Assignment - Heart Failure, Myocardial Infarction and Stroke
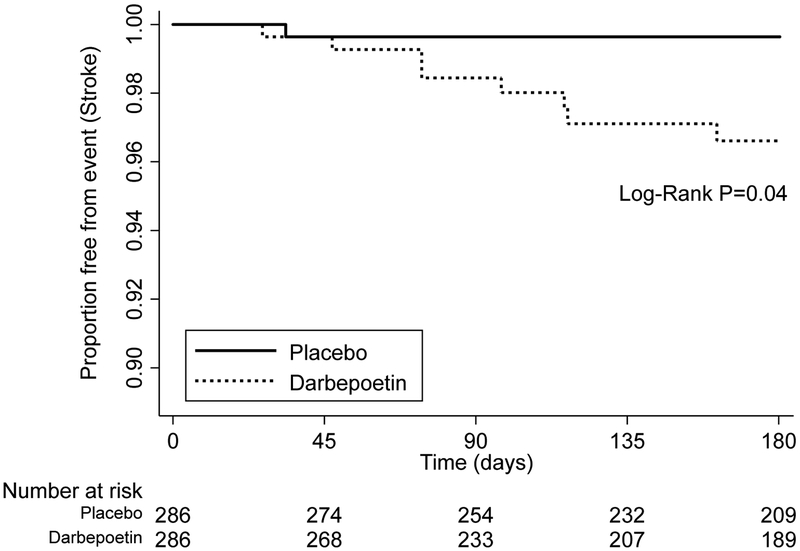
Among the 590 participants who initiated dialysis during the trial, there were no significant between-group differences in the time from dialysis initiation to occurrence of heart failure or myocardial infarction, according to the randomized treatment assignment (Table 2). However, the development of fatal or non-fatal stroke appeared to be more likely to occur in patients who had been randomized to darbepoetin (HR 8.59; 95%CI 1.07 – 68.7; P=0.04), although the number of events was small (8 (2.8%) versus 1 (0.3%) respectively; Table 2 and Figure 3). Results were similar when only patients initiating hemodialysis were considered (Supplementary Table 2). There was no association of Hb at the time of dialysis initiation with heart failure, myocardial infarction or stroke in unadjusted or adjusted analyses (Supplementary Tables 3 and 4).
Figure 3.
Kaplan-Meier plot examining effect of randomized treatment assignment on stroke from time of maintenance dialysis initiation to 6 months
DISCUSSION
In a post-hoc analysis of TREAT participants initiating maintenance dialysis, those randomly assigned to the darbepoetin arm began dialysis with a higher Hb than those in the placebo group, but did not have an associated reduction in the risk of all-cause or CV mortality, heart failure or myocardial infarction at 180 days. Although the number of events was limited, there was no suggestion of benefit, and there appeared to be a higher risk of stroke for patients initiating dialysis who had been randomized to darbepoetin. As it is unlikely that adequately powered trials of ESAs to treat anemia will be performed around the time of maintenance dialysis initiation, our analyses can help to inform clinicians caring for patients approaching ESRD. Overall, our results are consistent with the primary results of TREAT,10 showing no benefit (and potential harm) from ESA treatment of mild to moderate anemia to a target of ~13g/dL in patients with T2DM and CKD.
Anemia has been associated with a plethora of adverse consequences in ESRD, including left ventricular hypertrophy, heart failure,17 risk of hospitalization18,19 and mortality.20,21 While some observational studies report evidence for a U-shaped association of Hb with mortality,22,23 and others suggest that the association differs according to ESA dose,24 concerns remain that these reports are subject to the inherent biases of observational research that may obscure causal effects of lower or higher Hb concentrations on clinical outcomes. In a randomized trial of patients receiving hemodialysis, Parfrey et al. examined the effect treatment with epoetin alfa to higher (13.4 to 14.5 g/dL) versus lower (9.5 to 11.5 g/dL) target Hb on changes in left ventricular volume index (LVVI). This study recruited 596 adult patients with LVVI <100 mL/m2, free from symptomatic ischemic heart disease and heart failure at baseline, who were receiving HD therapy for the preceding 3 to 18 months. At the end of the 96-week study there were no differences between the higher and lower target Hb groups in terms of the percentage change in LVVI (7.6% vs. 8.3%) or left ventricular mass index (16.8% vs. 14.2%). As in our analysis, Parfrey et al. also reported a significantly higher risk of stroke in patients randomized to the higher target Hb arm (12 versus 4 participants; P=0.045).11 The Normal Hematocrit Study randomized 1233 participants on maintenance HD, with a history of heart failure or ischemic heart disease, to higher (42%) vs. lower target (30%) hematocrit using epoetin alfa. The study was halted after the third interim analysis. No benefits were observed and again there was a tendency towards harm (higher mortality, hospitalization and thrombotic events) with the higher target Hb arm.12,13
The focus of our current study was in the period of transition from CKD to maintenance dialysis. Anemia is common at the time of dialysis initiation, with a mean Hb for patients starting dialysis in the US of 9.4 g/dL in 2015, with around 13% reporting the pre-dialysis use of ESAs.1 We recently reported that the trajectory of Hb declines precipitously in the months prior to the development of ESRD in participants of TREAT, despite increasing doses of darbepoetin.15 Similar to other observational findings, a report from Canada reported that lower Hb was independently associated with mortality in a subset of patients (n=924) with eGFR <15mL/min/1.73m2.25 The period following initiation of maintenance dialysis is particularly turbulent and high risk, marked by a peak in mortality and CV events during the first three months, with a one-year mortality rate around 22%.1–3 The reasons for excess mortality and event rates during this period remain incompletely understood and strategies to protect patients from this increased risk have not been defined. Our current approach, investigating treatment of anemia prior to dialysis initiation, was made possible by the fact that the intervention (randomized assignment to darbepoetin versus placebo) in TREAT had no effect on the progression of CKD to ESRD. Therefore, approximately equal numbers of participants in the randomized arms progressed to require maintenance dialysis, with a higher mean Hb in those assigned to the darbepoetin arm. However, the provision of darbepoetin did not prolong survival or reduce the likelihood of MI or heart failure; moreover, darbepoetin treatment appeared to be associated with a higher risk of stroke.
The major strength of our study is the ability to analyze, in the setting of a randomized placebo-controlled trial, the effect of higher vs. lower target Hb (achieved by darbepoetin versus placebo) at the time of dialysis initiation in a relatively large number of well-characterized participants of TREAT. It is unlikely that prospective trials of Hb targets will be performed in the future, making our findings highly relevant to the medical community providing care to patients with progressive CKD. Moreover, since the timing of initiation of dialysis is not uniform or necessarily predictable, a trial comparing higher versus lower hemoglobin targets at the time of dialysis initiation would not be feasible. There are some limitations of our analyses that warrant discussion. TREAT was not designed to test the effect of higher versus lower Hb targets at the start of dialysis, while the provision for darbepoetin therapy if the Hb fell below 9 g/dL in the placebo arm precluded analyses involving lower Hb concentrations. However, we took advantage of the fact that similar numbers of patients reached ESRD according to the original randomized treatment assignment, and were reassured that the groups were balanced on most characteristics at the time of dialysis initiation, except Hb and use of darbepoetin. Despite these reassurances, we cannot exclude the possibility that the groups were imbalanced on other factors. After dialysis was initiated there was no further protocol-driven management of Hb, and the use of ESA was at the treating physician’s discretion. It is likely that most participants commenced ESA therapy in their dialysis centers, which would attenuate the differences in Hb observed at the initiation of dialysis. Moreover, follow-up Hb data (and data related to dialysis access and subsequent treatments) were not available following dialysis initiation. For this reason, and to capture adequate numbers of clinical events, we limited our research question to examine the effect of higher versus lower Hb at the initiation of dialysis (both hemodialysis and peritoneal dialysis) on outcomes, and limited the follow-up period to six months post-initiation. As a result of the relatively small numbers of events, the confidence intervals for most outcomes were wide and cannot confidently rule out a small benefit (or harm) of prior use of darbepoetin in the months to years preceding initiation of dialysis. However, there was an increased number of strokes in the 180 days following dialysis initiation in TREAT participants who had been randomized to darbepoetin. Although consistent with prior data from the original TREAT findings,10,26 as well post-hoc findings of TREAT-like participants from RED-HF (a placebo-controlled trial of darbepoetin in patients with anemia and heart failure),27,28 the effect estimates for the risk of stroke were underpowered and should be considered as hypothesis generating. Longer-term cardiovascular, and short- and longer-term non-cardiovascular outcomes, including metrics related to transfusion, sensitization, transplant listing and transplantation would help to inform the “ESA or no ESA” decision in patients with progressive CKD and mild to moderate anemia preparing for dialysis initiation. Finally, TREAT was performed in participants with CKD, anemia and T2DM; thus, it is unclear if the results are generalizable to patients without this triad of comorbid disease.
In summary, despite the above limitations, our results suggest that in participants of TREAT who required initiation of dialysis there is no apparent benefit from starting dialysis with a higher Hb in terms of six-month risk of death, MI or heart failure, and there may be a greater risk of stroke. In the absence of more definitive data, our results suggest that treating clinicians and patients should carefully consider the decision to use ESA-based strategies for mild-moderate anemia management for patients preparing for the initiation of dialysis.
Supplementary Material
Acknowledgements and Disclosures
Support: Dr. Mc Causland is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK102511. At the time of TREAT Dr. Pfeffer received grant support from (research grant to Brigham and Women’s Hospital) and served as a consultant to Amgen. No payments in the last 24 months. In addition, MAP has received research support from Novartis and served as a consultant for AstraZeneca, Bayer, Boehringer Ingelheim, DalCor, Genzyme, Gilead, GlaxoSmithKline, Janssen, Lilly, Novartis, Novo Nordisk, Relypsa, Sanofi, Teva, Thracos. He holds stock options for DalCor and a patent awarded to Brigham and Women’s Hospital regarding the use of inhibitors of the renin-angiotensin system in MI. Licensed by Novartis, Dr. Pfeffer’s share irrevocably assigned to charity.
Financial Disclosure: The TREAT Study was funded by Amgen. This analysis was conducted independently by the authors and used the data set held at the Brigham & Women’s Hospital; the authors designed and conducted all analyses described herein and were solely responsible for the drafting and editing of this manuscript.
REFERENCES
- 1.USRDS 2016 Annual Data Report.
- 2.Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney International. 2014;85(1):158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckardt K-U, Gillespie IA, Kronenberg F, et al. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney International. 2015;88(5):1117–1125. doi: 10.1038/ki.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Achkar TM, Ohmit SE, McCullough PA, et al. Higher prevalence of anemia with diabetes mellitus in moderate kidney insufficiency: The Kidney Early Evaluation Program. Kidney Int. 2005;67(4):1483–1488. doi: 10.1111/j.1523-1755.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- 5.Thomas MC, MacIsaac RJ, Tsalamandris C, Power D, Jerums G. Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care. 2003;26(4):1164–1169. [DOI] [PubMed] [Google Scholar]
- 6.Vlagopoulos PT, Tighiouart H, Weiner DE, et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. JASN. 2005;16(11):3403–3410. doi: 10.1681/ASN.2005030226. [DOI] [PubMed] [Google Scholar]
- 7.Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD. Anemia and end-stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int. 2004;66(3):1131–1138. doi: 10.1111/j.1523-1755.2004.00863.x. [DOI] [PubMed] [Google Scholar]
- 8.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–2098. [DOI] [PubMed] [Google Scholar]
- 9.Drüeke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer MA, Burdmann EA, Chen C-Y, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 11.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. JASN. 2005;16(7):2180–2189. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- 12.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 13.Coyne DW. The health-related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney International. 2012;82(2):235–241. doi: 10.1038/ki.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeffer MA, Burdmann EA, Chen C-Y, et al. Baseline characteristics in the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT). Am J Kidney Dis. 2009;54(1):59–69. doi: 10.1053/j.ajkd.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Mc Causland FR, Claggett B, Pfeffer MA, et al. Change in Hemoglobin Trajectory and Darbepoetin Dose Approaching End-Stage Renal Disease: Data from the Trial to Reduce Cardiovascular Events with Aranesp Therapy Trial. Am J Nephrol. 2017;46(6):488–497. doi: 10.1159/000485326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in Medicine. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. American Journal of Kidney Diseases. 1996;28(1):53–61. [DOI] [PubMed] [Google Scholar]
- 18.Xia H, Ebben J, Ma JZ, Collins AJ. Hematocrit levels and hospitalization risks in hemodialysis patients. JASN. 1999;10(6):1309–1316. [DOI] [PubMed] [Google Scholar]
- 19.Locatelli F, Pisoni RL, Combe C, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2004;19(1):121–132. [DOI] [PubMed] [Google Scholar]
- 20.Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. JASN. 1999;10(3):610–619. [DOI] [PubMed] [Google Scholar]
- 21.Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int. 2003;63(5):1908–1914. doi: 10.1046/j.1523-1755.2003.00937.x. [DOI] [PubMed] [Google Scholar]
- 22.Messana JM, Chuang C-C, Turenne M, et al. Association of quarterly average achieved hematocrit with mortality in dialysis patients: a time-dependent comorbidity-adjusted model. Am J Kidney Dis. 2009;53(3):503–512. doi: 10.1053/j.ajkd.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Regidor DL, Kopple JD, Kovesdy CP, et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. JASN. 2006;17(4):1181–1191. doi: 10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- 24.Brookhart MA, Schneeweiss S, Avorn J, Bradbury BD, Liu J, Winkelmayer WC. Comparative mortality risk of anemia management practices in incident hemodialysis patients. JAMA. 2010;303(9):857–864. doi: 10.1001/jama.2010.206. [DOI] [PubMed] [Google Scholar]
- 25.Levin A, Djurdjev O, Duncan J, Rosenbaum D, Werb R. Haemoglobin at time of referral prior to dialysis predicts survival: an association of haemoglobin with long-term outcomes. Nephrol Dial Transplant. 2006;21(2):370–377. doi: 10.1093/ndt/gfi209. [DOI] [PubMed] [Google Scholar]
- 26.Skali H, Parving H-H, Parfrey PS, et al. Stroke in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia treated with Darbepoetin Alfa: the trial to reduce cardiovascular events with Aranesp therapy (TREAT) experience. Circulation. 2011;124(25):2903–2908. doi: 10.1161/CIRCULATIONAHA.111.030411. [DOI] [PubMed] [Google Scholar]
- 27.Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368(13):1210–1219. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 28.Bello NA, Lewis EF, Desai AS, et al. Increased risk of stroke with darbepoetin alfa in anaemic heart failure patients with diabetes and chronic kidney disease. Eur J Heart Fail. 2015;17(11):1201–1207. doi: 10.1002/ejhf.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.