Abstract
Objectives:
Effective nasal continuous positive airway pressure (CPAP) therapy reduces the cardiovascular outcomes associated with obstructive sleep apnea (OSA), but the mechanism behind this effect is unclear. We investigated if OSA patients during wakefulness showed signs of increased sympathetic activity and decreased vasoreactivity in cerebral cortical vessels as measured with near-infrared spectroscopy (NIRS), and if this may be reversed by CPAP treatment.
Subjects and methods:
23 OSA patients (mean age, 55 y) naive to CPAP were included in a prospective interventional study. The OSA patients received CPAP therapy for at least two months. Cortical low-frequency oscillation (LFO) amplitudes and vasoreactivity during a breath hold test were measured with NIRS and were compared between baseline and after CPAP treatment. Baseline values also were compared to 13 healthy controls (mean age, 52 y).
Results:
We found a decrease in LFO amplitudes after CPAP therapy (P = 0.022) in OSA patients. We found no differences in LFO amplitudes between OSA patients and healthy controls (P = 0.934). There were no differences in peak vascular response following breath hold tests in OSA patients before and after CPAP therapy (P = 0.158) or compared to healthy controls (P = 0.740).
Conclusion:
Our NIRS study revealed a decrease in LFO amplitude following two months of CPAP treatment in OSA patients, which may reflect a decrease in sympathetic activity affecting cortical vessels.
Keywords: Obstructive sleep apnea, Continuous positive airway pressure, Cortical vessels, Cerebral autoregulation, Near infrared spectroscopy, Breath-holding tests, Autonomic nervous system, Stroke
1. Introduction
In the general population the prevalence of obstructive sleep apnea (OSA) is high [1,2] and has been reported to be up to 33% [3]. OSA is more prevalent among men and increases with age [4,5]. OSA is characterized by repetitive episodes of partial or complete upper airway obstruction during sleep associated with intermittent oxygen desaturation, snoring and sleep fragmentation [6]. OSA is strongly associated with transitory ischemic attacks (TIA) [7] and cerebral infarction [7–9], independent of other risk factors. Effective nasal continuous positive airway pressure (CPAP) therapy reduces the cardiovascular outcomes associated with OSA [9], but the mechanism behind this effect is unclear.
During OSA episodes of intermittent hypoxia, recurrent arousals and intrathoracic pressure swings are suggested to induce sympathetic activation, which eventually may lead to endothelial dysfunction [10]. An increase in sympathetic activity in OSA patient may affect cortical cerebral vessels directly [11], but this theory has never been investigated before. Investigating cortical cerebral vessels in OSA patients may help to clarify the pathophysiologic mechanisms leading to ischemic vascular diseases in OSA.
Cerebral cortical vascular changes can be monitored non invasively in humans in vivo by near-infrared spectroscopy (NIRS), which can detect relative changes in oxyhemoglobin (oxyHb), deoxyhemoglobin (deoxyHb) and total hemoglobin (totalHb) [12]. So far, NIRS has been used to record oxyHb and deoxyHb changes during obstructive sleep apnea events in OSA patients [13,14]. Previous NIRS studies in awake patients and healthy subjects have investigated cortical low-frequency oscillation (LFO) oxyHb [11,15–17]. An increase in oxyHb LFO amplitude is posture dependent in healthy subjects and therefore is believed to increase during sympathetic activation [11]. NIRS also may detect cortical endothelial dysfunction by measuring vasoreactivity via a breath-hold test [18]. Therefore, NIRS may be applied to assess sympathetic activity and vasoreactivity in the cerebral cortical vasculature in awake OSA patients, but this hypothesis has not been previously investigated.
In our study, we aimed to investigate if OSA patients during wakefulness have increased LFO amplitude in cerebral cortical vessels as a sign of increased sympathetic activity by employing NIRS. We investigated changes in cortical LFO amplitude in OSA patients following two months of effective CPAP treatment and in comparison to healthy controls. Furthermore, we explored changes in vasoreactivity in response to breath-hold tests in OSA patients following CPAP treatment and in comparison to healthy controls.
2. Subjects and methods
2.1. Subjects
Patients with moderate to severe OSA who had never received CPAP therapy were recruited from April 2011 to November 2011 at the Danish Center for Sleep Medicine, Glostrup Hospital, Denmark. OSA was diagnosed according to the International Classification of Sleep Disorders by the American Academy of Sleep Medicine [19] by sleep disorder specialists. OSA patients had an AHI (apnea-hypopnea index) ⩾15 evaluated with either polysomnography (PSG) or cardiorespiratory monitory (CRM).
Healthy controls were recruited via internet advertising from the general population matching OSA for gender, age, body mass index (BMI), and time of recording. The healthy controls underwent a clinical interview and screening questions for snoring, witnessed apneas, and shirt size. All healthy controls were examined one night with CRM to exclude the presence of OSA or periodic nocturnal leg movements. PSG and CRM data were assessed by trained staff at the Danish Center for Sleep Medicine following the guidelines of the American Academy of Sleep Medicine [20].
Exclusion criteria for OSA patients and healthy controls were previous history of stroke, myocardial infarction, known stenosis of the carotid arteries (>50%), unstable chronic lung disease, anemia, present cardiac arrhythmia, central sleep apnea, rapid eye movement sleep behavior disorder, narcolepsy, medication with effects on the central nervous system, weekly alcohol use (more than 21 drinks for men or 14 drinks for women; 12 g of alcohol per drink), current use of illegal drugs, pregnancy, or breast feeding.
All subjects were examined with NIRS during daytime hours and OSA patients at a mean time of day of 12:15 pm (±0.42) and healthy controls at 12:16 pm (±0.56). OSA patients were reexamined with NIRS at the same time of the day within one hour following at least two months of CPAP treatment (IN551S, REMstar Auto A-flex w/SD CARD, Respironics Inc., Murrysville, USA). CPAP compliance was defined as CPAP used more than 50% of days at least four hours at night and was controlled by recording scripts from the patients’ CPAP systems.
2.2. Near-infrared spectroscopy acquisition
Measurement of oxyHb, deoxyHb, and totalHb LFO was performed using continuous-wave NIRS (NIRS2; TechEn Inc, Milford, MA, USA). All recordings were performed by B.E.J. The NIRS optodes were placed bilaterally on the forehead with one source (two wavelengths, 690 nm and 830 nm) and two detectors on each side avoiding the frontal sinus. The distance between sources and detectors were three cm with the detectors lateral to the source. Thus, the detectors were measured at the frontal cortex supplied by the anterior cerebral artery or possibly the anterior cerebral artery or middle cerebral artery watershed areas.
LFO measurement was performed with the subjects in a comfortable supine position in a quiet room with a constant temperature (23 °C). The subjects were instructed to lie still and relax with their eyes open for 10 minutes. If subjects accidently fell asleep, they were gently awakened with a touch.
Breath hold was measured after a normal exhalation and subjects were instructed to hold their breath for up to 30-second tests, if possible. A maximum of four breath holds for each breath subject were recorded with two minute intervals. Breath holds lasting less than 15-seconds were excluded. To control that breath holds were properly performed subjects wore an open mask that caused no respiratory resistance to record PETCO2 (ProPaq Encore; Welch Allyn Protocol, Beaverton, OR, USA). If any changes in PETCO2 that indicated breathing were observed, the breath hold was excluded.
2.3. Data analysis
Postprocessing of NIRS data was performed by an expert who was blinded to the OSA and healthy control status of the subjects. Data processing was performed in MATLAB (The MathWorks Inc., Natick, Massachusetts, USA).
For the oscillation analysis, power spectra were estimated by computing the Fourier transform of the NIRS signal time series. NIRS intensity time series were first divided into windows of 100 seconds in duration with 50 seconds of overlap. Within each 100 s window, intensity variations at 690 and 830 nm were converted into relative changes in oxyHb and totalHb (ie, oxy-Hb + deoxyHb) using the modified Beer-Lambert Law [21] with a differential pathlength factor of six at both wavelengths. No correction for partial volume effect was employed because the NIRS measured oscillations are not expected to be very much localized. The oxyHb and deoxyHb signals were then Fourier transformed to obtain their power spectra (MATLAB function pwelch). The LFO frequency was defined as the frequency within the 0.05-Hz to 0.15-Hz range. To assess sympathetic activity the LFO amplitudes of oxyHb and totalHb were extracted as the value of the oxyHb spectrum at the LFO frequency. The values of oxyHb and totalHb amplitude were then averaged over all 100s windows and over the four NIRS channels.
For the breath-hold tests intensity variations at 690 and 830 nm also were converted into relative changes in oxyHb and totalHb as described above. Data collection was down sampled to 25 Hz and automatic motion detection was applied. The data also were low pass filtered at 0.5 Hz (to remove cardiac and high-frequency noise) and high-pass filter at 0.01 Hz (to remove slow drifts). The cortical cerebrovascular reactivity was assessed as the maximal increase in oxyHb and totalHb relative to baseline within the first 30 seconds of breath hold. The mean of four breath holds was used for analysis. Baseline was defined as the 10 seconds before breath hold instruction and was normalized to one.
2.4. Statistics
Data are presented as mean value ± standard error of the mean (±SEM). Sample size could not be properly determined before the study, as there is no previously known variation coefficient for the NIRS measurements performed in our study. However, other studies investigating cerebrovascular reactivity to hypercapnia included between eight and 20 subjects [22,23] and similar studies investigating LFO and cerebral oscillatory hemodynamics included between 10 and 38 subjects [11,24]. Gender match between OSA group and controls was analyzed with the Pearson X2 test. Age, BMI, time of recording, and time between somnography and NIRS recordings between OSA patients and healthy controls were analyzed with the Mann–Whitney U test. Time of recording, changes after CPAP treatment in LFO oscillations, breath-hold data, and breath-hold duration within OSA patients were tested using the Wilcoxon signed rank test. Differences in oscillation, breath-hold data, and breath-hold duration between OSA patients and healthy controls were tested using the Mann–Whitney U test. All analyses were performed with IBM SPSS 19.0 for Windows (Chicago, IL, USA). Five percent (P = 0.05) was chosen as the level of significance.
3. Results
Overall, 23 OSA patients (mean, 55 ± 1.5, range, 41–67) and 13 healthy controls (mean, 52 ± 2.8, range, 39–68) fulfilled the study criteria and were enrolled for NIRS sampling and analysis. 19 OSA patients were diagnosed based on CRM and four on PSG. All healthy subjects were investigated with CRM. SaO2 data was missing in one OSA patient investigated with CRM. Three healthy controls had an AHI >five (6.2, 9.7 and 12.0) but were asymptomatic and therefore were included in the analysis. There were no differences in gender (P = 0.841), age (P =0.322), or BMI (P =0.126). There were no differences in time of day for NIRS recordings between CPAP days (P = 0.863) or OSA patients and healthy controls (P = 0.934). There were no differences in vital parameters between CPAP days or healthy controls and OSA patients. As expected there was a significant difference in AHI, oxygen desaturation index, and time with saturation <90% between healthy controls and OSA patients (all P < 0.001). Time from PSG or CRM to NIRS recordings were significantly longer in OSA patients compared to healthy controls (P = 0.002).
The influence of CPAP on LFO amplitude was examined in 14 OSA patients as nine subjects were excluded (six due to failure to meet for reexamination and three subjects due to CPAP non compliance) (Table 1). The influence of CPAP on maximal increase in oxyHb and totalHb during breath hold were examined in 13 OSA patients, as 10 were excluded (6 due to failure to meet for reexamination, three patients due to CPAP non compliance and one patient unable to perform breath holding on day two).
Table 1.
Mean (±SEM) characteristics of continuous positive airway pressure treatment in 14 compliant obstructive sleep apnea patients with continuous positive airway pressure treatment for at least two months.
| CPAP titration (cm H20) | 8(±1) |
|---|---|
| Days with CPAP ⩾4 h | 82 (±4) |
| Days of usage | 67 (±1) |
CPAP, continuous positive airway pressure.
For breath hold analysis 20 OSA patients were compared to 13 healthy controls, as three OSA patients were excluded (two due to movement artifacts and 1 due to technical concerns). One OSA subject could only perform acceptable breath holds three times and another OSA subject one time, but they were still included in the analysis.
3.1. Low frequency oscillation amplitudes
There was a decrease in LFO amplitudes between OSA patients before and after CPAP therapy for oxyHb (P = 0.022) and a trend toward decrease for totalHb (P = 0.056) values (Fig. 1).
Fig. 1.
Low frequency oscillation amplitudes of oxygenated hemoglobin (oxyHb) and total hemoglobin (totalHb) for 14 obstructive sleep apnea (OSA) patients before and after continuous positive airway pressure (CPAP) treatment for at least two months 23 OSA patients before CPAP treatment were also compared to 13 healthy controls, right sided figure. Error bars are standard error of the mean. μM = micromolar oxyHb or totalHb concentration. Black boxes are OSA patients before CPAP treatment, striped grey boxes are OSA patients after CPAP treatment, and grey boxes are healthy subjects. *Indicates a P value = 0.022.
We found no differences in LFO amplitudes between OSA patients and healthy controls during rest for oxyHb (P = 0.934) and totalHb (P = 0.681) values (Fig. 1).
3.2. Breath hold analysis
We found no differences in mean breath-hold durations between CPAP days for OSA patients (P = 0.423) or between healthy controls and OSA patients (P = 0.377) (Table 2).
Table 2.
Basic characteristics and vital parameters in healthy controls and obstructive sleep apnea patients (±SEM).
| Healthy controls | OSA patients | P value | |
|---|---|---|---|
| Gender (M/W) | 10/3 | 17/6 | 0.841 |
| Age | 52.0 (±2.8) | 55.0 (±1.5) | 0.322 |
| BMI | 28.5 (±0.9) | 32.1(±1.3) | 0.126 |
| MAP | 95.1 (±4.1) | 90.9 (±2.6) | 0.242 |
| HR | 59.9 (±2.2) | 64.9 (±1.9) | 0.106 |
| SAT | 97.4 (±0.4) | 97.3 (±0.3) | 0.880 |
| PETCO2 | 35.2 (±0.9) | 35.0 (±0.5) | 0.842 |
| Respiration frequency | 10.8 (±1.3) | 10.2 (±0.8) | 0.758 |
| AHI | 3.3 (±1.1) | 34.6 (±3.6) | <0.001 |
| ODI | 1.0 (±0.4) | 20.3 (±3.7) | <0.001 |
| Time (%) with SaO2 <90% | 0.4 (±0.2) | 15.7 (±3.7) | <0.001 |
| Breath-hold duration | 27.5 (±1.2) | 26.9 (±1.1) | 0.377 |
| Days somnography to NIRS | 13 (±6) | 23 (±8) | 0.002 |
Mean (±SEM) characteristics in healthy controls and OSA patients. M, man; W, woman; BMI, body mass index; MAP, mean arterial blood pressure; HR, heart rate; SAT, peripheral saturation; PETCO2, partial pressure of end tidal CO2 in mmHg; AHI, apnea-hypopnea index; ODI, oxygen desaturation index.
There were no differences between OSA patients before and after CPAP therapy for maximum increase in oxyHb (P = 0.152) or totalHb (P = 0.173).
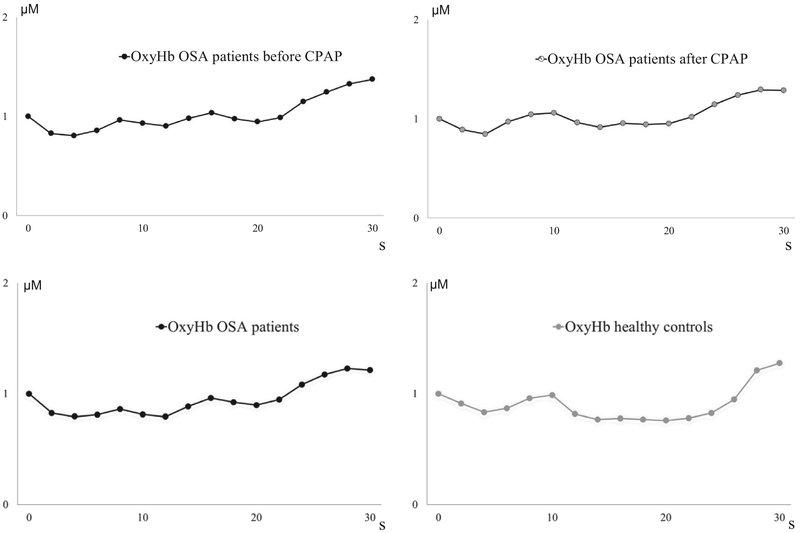
There were no differences between OSA patients and healthy controls for maximum increase in oxyHb (P = 0.740) or totalHb (P = 0.606) (Fig. 2–4).
Fig. 2.
Increase in oxygenated hemoglobin (oxyHb) and total hemoglobin (totalHb) following breath hold in 13 obstructive sleep apnea (OSA) patients before and after continuous positive airway pressure (CPAP) treatment for at least two months. 20 OSA patients before CPAP treatment were also compared to 13 healthy controls, right sided figure. Error bars are standard error of the mean. μM = micromolar oxyHb or totalHb concentration. Black boxes are OSA patients before CPAP treatment, striped grey boxes are OSA patients after CPAP treatment, and grey boxes are healthy subjects.
Fig. 4.
Mean changes in total hemoglobin (totalHb) following 30-second breath-hold tests in 13 obstructive sleep apnea (OSA) patients before and after continuous positive airway pressure (CPAP) treatment for at least two months. The two bottom figures show 20 OSA patients and 13 healthy controls at baseline. μM = micromolar oxyHb or totalHb concentration.
4. Discussion
The major finding of our study was a decrease in LFO amplitude following two months of effective CPAP treatment in OSA patients, which may reflect a modulation in sympathetic activity. We found no difference in cortical vasoreactivity to the breath hold test following CPAP treatment and showed no differences on cortical LFO amplitude or cortical vasoreactivity between OSA patients and healthy controls. Our study is the first to show a modulating effect of CPAP therapy on cortical vessels in OSA patients, which thereby contributes to the current knowledge on the beneficial effect of CPAP therapy in OSA [9].
OSA is associated with increased sympathetic activity, which has been shown in animal and human models [10]. In rats hypoxia for seven hours daily for 30 days leads to increased blood pressure, which can be blocked by chemical denervation of the peripheral sympathetic nervous system [25]. Mice exposed to hypoxia for eight hours daily leads to increased levels of urine catecholamine after 14 days [26]. In OSA patients urine catecholamine levels are elevated throughout the day compared to controls [27], and awake OSA patients show elevated muscle sympathetic nerve activity and plasma norepinephrine compared to controls [28]. Increased sympathetic activity leads to an increase in cortical vascular LFO amplitude. Thus, Tachtsidis et al. [11] measured increased oxyHb LFO amplitude with NIRS on posture-dependent activation of the autonomic reflex. Experimental animal models have also shown increased cortical LFO amplitude following sympathetic alterations [29,30]. A decrease in LFO amplitude, found in our study indicates a decrease in sympathetic activity affecting cortical vessels following CPAP treatment in OSA patients. This finding is in agreement with studies demonstrating that CPAP treatment in OSA patients reduces daytime plasma norepinephrine urinary levels [31] and daytime muscle sympathetic nerve activity [32]. However, our study is the first to indicate that changes in sympathetic activity might induce changes in cortical vessels.
In our study, we found no difference between LFO amplitude in OSA patients and healthy controls, which may be due to sympathetic activity being less pronounced during wakefulness than during sleep with obstructive apnea episodes. This finding has previously been shown measuring muscle sympathetic nerve activity in OSA patients [33]. The lack of statistical difference in vascular LFO amplitude between OSA patients and healthy controls also could be attributed to endothelial dysfunction [34], which might attenuate the amplitude response to the increased sympathetic activity in OSA patients. Furthermore using NIRS Schroeter et al. [16] have shown how cortical vascular LFO amplitude are lower in a group of healthy seniors (mean age, 65) compared to young healthy subjects (mean age, 24). Thus, endothelial dysfunction and attenuation of LFO amplitude with age may cause alterations, which cancels out the possible effect of sympathetic activity on increasing cortical vascular LFO amplitude.
Our study is the first study to investigate cortical vasoreactivity to a breath-hold test with NIRS in OSA patients during wakefulness. Previously Diomedi et al. [35] and Placidi et al. [22] measured the blood flow velocity in the middle cerebral artery via transcranial Doppler. Vasoreactivity was estimated after an inspiration and then quantified using a breath-holding index (BHI). BHI was calculated by dividing the percent increase in maximum flow volume occurring during breath holding by the length of time (in seconds) and therefore is not directly comparable to our method. Both BHI studies reported a significant lower BHI at 8:00 am and 5:30 pm in OSA patients compared to healthy controls [35,22]. In addition, an increase in afternoon BHI compared to morning BHI was reported to be significant in OSA patients [22]. The authors proposed that the morning compromised cerebrovascular reactivity may be interpreted as a consequence of down regulation of pH receptors due to the high partial pressure of CO2 (pCO2) levels raised during the night. In our study, vasoreactivity was measured primarily between 11 pm and 12 am, and failed to show a difference between healthy controls. This discrepancy may be caused by cortical vessels not being affected in OSA, differences in the methodology and vascular compartments investigated or that cortical vasoreactivity normalizes during wakefulness in OSA. Of interest, Hsieh et al. [36] prospectively studied 71 patients with mild to moderate ischemic stroke during hospitalization and classified patients into wake-up stroke and non wake-up stroke. The degree of AHI was then measured between three and 14 days after the stroke onset. The study showed that patients with wake-up stroke had larger AHI than non wake-up stroke patients [36]. Thus, temporary nocturnal decrease in cerebral vasoreactivity in OSA may increase the risk for ischemic event. However, this proposed mechanism needs to be prospectively investigated in OSA patients without previous stroke with an otherwise matched control group.
Some limitations to our study need to be acknowledged. The differential pathlength factor and the partial volume error may vary between subjects due to different head anatomy, which can introduce errors and inter subject variation in the LFO amplitudes we reported, NIRS recordings may be contaminated by changes in extracranial tissue [37], which can be due to alterations in the skin microcirculation [38]. Thus, in our study we have not characterized the contamination by skin vasculature, which can be a confounding effect in the results. A breath-hold test may be limited for quantitative purposes as PETCO2 may rise at different rates during breath holding in different subjects. It would have been desirable if the time from PSG or CRM to NIRS recordings were the same between healthy controls and OSA patients, but there are no reasons to assume that the subjects changed between recordings. Furthermore, it is important to note that OSA patients did not start CPAP treatment until after the NIRS recordings. We cannot rule out that some of the present results may be due to a type 2 error, as it turned out that the variation groups were relatively large (SEM up to 1.7 with LFO amplitude of about 3, see Fig. 1). Hence, there might be relative small alterations between sessions and groups, which the study was not powered to detect with the current limited sample sizes.
In conclusion, our study using NIRS in OSA patients showed a decrease in LFO amplitude following two months of CPAP treatment in OSA patients, which may reflect a decrease in sympathetic activity affecting cortical vessels. NIRS may be a non invasive tool to assess sympathetic induced changes in OSA in the cortical vasculature but needs to be further investigated.
Fig. 3.
Mean changes in oxygenated hemoglobin (oxyHb) following 30-second breath-hold tests in 13 obstructive sleep apnea (OSA) patients before and after continuous positive airway pressure (CPAP) treatment for at least two months. The two bottom figures show 20 OSA patients and 13 healthy controls at baseline. μM = micromolar oxyHb or totalHb concentration.
Acknowledgments
The authors wish to thank lab technicians, Winnie Grønning Nielsen and Lene Elkjaer, for their excellent and dedicated assistance. The study was supported by the Lundbeck Foundation via the Lundbeck Foundation Center for Neurovascular Signaling (LUCENS), the Gangsted Foundation, the Frimodt-Heineke Foundation, the Toyota Foundation and the Augustinus Foundation.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: http://dx.doi.org/10.1016/j.sleep.2012.12.009.
References
- [1].Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–5. [DOI] [PubMed] [Google Scholar]
- [2].Hrubos-Strom H, Randby A, Namtvedt SK, Kristiansen HA, Einvik G, Benth J, et al. A Norwegian population-based study on the risk and prevalence of obstructive sleep apnea. The Akershus Sleep Apnea Project (ASAP). J Sleep Res 2011;20:162–70. [DOI] [PubMed] [Google Scholar]
- [3].Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo epidemiologic sleep study. Sleep Med 2010;11:441–6. [DOI] [PubMed] [Google Scholar]
- [4].Davies RJ, Stradling JR. The epidemiology of sleep apnoea. Thorax 1996;51(Suppl. 2):S65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jennum P, Sjol A. Snoring, sleep apnoea and cardiovascular risk factors: the MONICA II study. Int J Epidemiol 1993;22:439–44. [DOI] [PubMed] [Google Scholar]
- [6].Sleep-related breathing disorders in adults. Recommendations for syndrome definition and measurement techniques in clinical research.The report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667–89. [PubMed] [Google Scholar]
- [7].Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–41. [DOI] [PubMed] [Google Scholar]
- [8].Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–53. [DOI] [PubMed] [Google Scholar]
- [10].Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 2010;7:677–85. [DOI] [PubMed] [Google Scholar]
- [11].Tachtsidis I, Elwell CE, Leung TS, Lee CW, Smith M, Delpy DT. Investigation of cerebral haemodynamics by near-infrared spectroscopy in young healthy volunteers reveals posture-dependent spontaneous oscillations. Physiol Meas 2004;25:437–45. [DOI] [PubMed] [Google Scholar]
- [12].Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhaupl K, et al. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage 2000;12:623–39. [DOI] [PubMed] [Google Scholar]
- [13].Matsuo A, Inoue Y, Namba K, Chiba H. Changes in cerebral hemoglobin indices in obstructive sleep apnea syndrome with nasal continuous positive airway pressure treatment. Sleep Breath 2011;15:487–92. [DOI] [PubMed] [Google Scholar]
- [14].Pizza F, Biallas M, Wolf M, Werth E, Bassetti CL. Nocturnal cerebral hemodynamics in snorers and in patients with obstructive sleep apnea: a near-infrared spectroscopy study. Sleep 2010;33:205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Phillip D, Schytz HW, Selb J, Payne S, Iversen HK, Skovgaard LT, et al. Low frequency oscillations in cephalic vessels assessed by near infrared spectroscopy. Eur J Clin Invest 2012;42:1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schroeter ML, Schmiedel O, von Cramon DY. Spontaneous low-frequency oscillations decline in the aging brain. J Cereb Blood Flow Metab 2004;24:1183–91. [DOI] [PubMed] [Google Scholar]
- [17].Tachtsidis I, Elwell CE, Lee CW, Leung TS, Smith M, Delpy DT. Spectral characteristics of spontaneous oscillations in cerebral haemodynamics are posture dependent. Adv Exp Med Biol 2003;540:31–6. [DOI] [PubMed] [Google Scholar]
- [18].Vasdekis SN, Tsivgoulis G, Athanasiadis D, Andrikopoulou A, Voumvourakis K, Lazaris AM, et al. Cerebrovascular reactivity assessment in patients with carotid artery disease: a combined TCD and NIRS study. J Neuroimaging 2011. [DOI] [PubMed] [Google Scholar]
- [19].American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester (IL): American Academy of Sleep Medicine; 2005. [Google Scholar]
- [20].Iber C, Ancoli-Israel S, CAQS. For the American Academy of Sleep Medicine The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester (IL): American Academy of Sleep Medicine; 2007. [Google Scholar]
- [21].Cope M, Delpy DT, Reynolds EO, Wray S, Wyatt J, van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Adv Exp Med Biol 1988;222:183–9. [DOI] [PubMed] [Google Scholar]
- [22].Placidi F, Diomedi M, Cupini LM, Bernardi G, Silvestrini M. Impairment of daytime cerebrovascular reactivity in patients with obstructive sleep apnoea syndrome. J Sleep Res 1998;7:288–92. [DOI] [PubMed] [Google Scholar]
- [23].Reichmuth KJ, Dopp JM, Barczi SR, Skatrud JB, Wojdyla P, Hayes D Jr, et al. Impaired vascular regulation in patients with obstructive sleep apnea: effects of continuous positive airway pressure treatment. Am J Respir Crit Care Med 2009;180:1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reinhard M, Wehrle-Wieland E, Grabiak D, Roth M, Guschlbauer B, Timmer J, et al. Oscillatory cerebral hemodynamics-the macro- vs. microvascular level. J Neurol Sci 2006;250:103–9. [DOI] [PubMed] [Google Scholar]
- [25].Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia-influence of chemoreceptors and sympathetic nervous system. J Hypertens 1997;15:1593–603. [DOI] [PubMed] [Google Scholar]
- [26].Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, et al. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med 2008;177:227–35. [DOI] [PubMed] [Google Scholar]
- [27].Fletcher EC, Miller J, Schaaf JW, Fletcher JG. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep 1987;10:35–44. [DOI] [PubMed] [Google Scholar]
- [28].Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 1993;103:1763–8. [DOI] [PubMed] [Google Scholar]
- [29].Japundzic N, Grichois ML, Zitoun P, Laude D, Elghozi JL. Spectral analysis of blood pressure and heart rate in conscious rats: effects of autonomic blockers. J Auton Nerv Syst 1990;30:91–100. [DOI] [PubMed] [Google Scholar]
- [30].Cerutti C, Barres C, Paultre C. Baroreflex modulation of blood pressure and heart rate variabilities in rats: assessment by spectral analysis. Am J Physiol 1994;266:1993–2000. [DOI] [PubMed] [Google Scholar]
- [31].Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest 2001;120:887–93. [DOI] [PubMed] [Google Scholar]
- [32].Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 1999;100:2332–5. [DOI] [PubMed] [Google Scholar]
- [33].Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96:1897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lurie A Endothelial dysfunction in adults with obstructive sleep apnea. Adv Cardiol 2011;46:139–70. [DOI] [PubMed] [Google Scholar]
- [35].Diomedi M, Placidi F, Cupini LM, Bernardi G, Silvestrini M. Cerebral hemodynamic changes in sleep apnea syndrome and effect of continuous positive airway pressure treatment. Neurology 1998;51:1051–6. [DOI] [PubMed] [Google Scholar]
- [36].Hsieh SW, Lai CL, Liu CK, Hsieh CF, Hsu CY. Obstructive sleep apnea linked to wake-up strokes. J Neurol 2012;259:1433–9. [DOI] [PubMed] [Google Scholar]
- [37].Canova D, Roatta S, Bosone D, Micieli G. Inconsistent detection of changes in cerebral blood volume by near infrared spectroscopy in standard clinical tests. J Appl Physiol 2011;110:1646–55. [DOI] [PubMed] [Google Scholar]
- [38].Smielewski P, Czosnyka M, Pickard JD, Kirkpatrick P. Clinical evaluation of near-infrared spectroscopy for testing cerebrovascular reactivity in patients with carotid artery disease. Stroke 1997;28:331–8. [DOI] [PubMed] [Google Scholar]