Abstract
Multiple sclerosis (MS) is a central nervous system (CNS) disease characterized by chronic neuroinflammation, demyelination, and axonal damage. Infiltration of activated lymphocytes and myeloid cells are thought to be primarily responsible for white matter damage and axonopathy. Several United States Food and Drug Administration-approved therapies exist that impede activated lymphocytes from entering the CNS thereby limiting new lesion formation in patients with relapse-remitting forms of MS. However, a significant challenge within the field of MS research is to develop effective and sustained therapies that allow for axonal protection and remyelination. In recent years, there has been increasing evidence that some kinds of stem cells and their derivatives seem to be able to mute neuroinflammation as well as promote remyelination and axonal integrity. Intracranial infection of mice with the neurotropic JHM strain of mouse hepatitis virus (JHMV) results in immune-mediated demyelination and axonopathy, making this an excellent model to interrogate the therapeutic potential of stem cell derivatives in evoking remyelination. This review provides a succinct overview of our recent findings using intraspinal injection of mouse CNS neural progenitor cells and human neural precursors into JHMV-infected mice. JHMV-infected mice receiving these cells display extensive remyelination associated with axonal sparing. In addition, we discuss possible mechanisms associated with sustained clinical recovery.
Keywords: demyelination, virus, remyelination, neural precursor cells, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory disease of the central nervous system (CNS) characterized by extensive myelin destruction (Steinman, 1996). While the cause of MS is unknown, disease onset has been attributed to multiple factors including the genetic background of the individual as well as environmental influences (Oksenberg et al., 1993; Poser, 1994). Histologic characterization of lesions reveals the presence of activated CD4+ and CD8+ T cells as well as macrophages, which are thought to act in concert with reactive microglia to release a milieu of proinflammatory factors that lead to oligodendrocyte dysregulation (Traugott et al., 1983; Lassmann et al., 2007). Multifocal demyelinating lesions eventually lead to various clinical symptoms such as impaired motor skills, cognitive decline, behavioral deficits and vision loss (Prineas and Graham, 1981; Neumann et al., 2002; Lassmann et al., 2007). Disease-modifying therapies (DMTs) for MS focus on reducing T lymphocyte infiltration into the CNS in an attempt to prevent formation of new lesions. With the exception of Ocrelizumab (anti-CD20) (Frampton, 2017), which was recently approved for progressive MS, all United States Food and Drug Administration (FDA) approved DMTs are indicated for relapsing-remitting form of MS (Weinshenker et al., 1989).
Remyelination failure in MS patients is complex and the result of a variety of factors that culminate in the inability of oligodendrocyte precursor cells (OPCs) to mature into myelin-producing oligodendrocytes. Endogenous OPCs are spread throughout the CNS and appear in high density within some subacute lesions during early stages of MS (Chang et al., 2000). Remyelination following OPC maturation leads to the formation of shadow plaques, in which patches of remyelinated white matter are composed of disproportionally thin myelin sheaths surrounding axons (Chang et al., 2000; Halfpenny et al., 2002; Lassmann, 1983; Lucchinetti et al., 1999; Prineas et al., 1989; Roy et al., 1999; Schlesinger, 1909). Therefore, understanding mechanisms associated with impaired OPC differentiation and triggering maturation of these cells into mature myelin-producing oligodendrocytes has potential for profound clinical relevance.
With this in mind, one critically important aspect related to OPC-mediated remyelination is that myelin-debris needs to be cleared by phagocytic cells, including neutrophils (Lindborg et al., 2017), inflammatory macrophages, (Healy et al., 2017; Karamita et al., 2017), and resident microglia (Zhu et al., 2016; Karamita et al., 2017; Kucharova and Stallcup, 2017). The ability to efficiently phagocytize myelin is dependent upon age in mice; macrophages from older mice have impaired ability to engulf myelin compared with macrophages derived from younger mice. Elegant studies by Franklin and colleagues (Ruckh et al., 2012) used heterochronic parabiosis to assess recovery in old mice that had undergone experimentally induced demyelination. When conjoined to younger mice, the old mice showed increased remyelination; this effect was attributed to increased clearance of myelin debris in older animals by macrophages provided from younger animals.
A recent study identified a potential mechanism associated with diminished phagocytic activity by aged macrophages. Cantuti-Castelvetri et al. (2018) demonstrated by means of transmission electron microscopy that lipids are rapidly released in response to a demyelinating injury, and this can mute OPC differentiation and remyelination. In contrast to older macrophages, young macrophages were able to efficiently engulf and process myelin lipids. Old macrophages were deficient in lipid processing, which led to formation of cholesterol crystals, phagolysosomal rupture and stimulated inflammasomes that ultimately led to an inability to resolve inflammation.
One therapeutic option to treat progressive MS would be to replenish or rejuvenate the pool of endogenous OPCs that show limited remyelination potential in the later stages of disease. Several groups have used high-throughput screening of small molecule compounds to identify potential drugs that enhance OPC maturation, with the goal of promoting remyelination in preclinical animal models of MS (Deshmukh et al., 2013; Mei et al., 2014, 2016b). Using this approach, Lairson and colleagues (Deshmukh et al., 2013) demonstrated that benztropine, an anti-muscarinic receptor compound, increased OPC maturation and remyelination in mice with experimental autoimmune encephalomyelitis (EAE), the prototypic model of MS (Deshmukh et al., 2013). More recently, clemanstine, another anti-muscarinic receptor compound, was also shown to enhance OPC maturation in EAE (Mei et al., 2016a). These results are consistent with the observation in EAE mice that ablation of the M1 muscarinic receptor in oligodendroglia resulted in accelerated remyelination, diminished axonal loss and improved clinical outcome, arguing that clemanstine may be functioning by binding to this specific receptor (Mei et al., 2016a).
Cellular replacement therapies for human neurologic diseases have also emerged as a clinically relevant area of research. NPCs possess the ability to develop into neurons, astrocytes, and oligodendrocytes (Gage, 2000). Additionally, quiescent adult NPCs have been shown to proliferate, differentiate and migrate into response to acute CNS damage in spinal cord injury, inflamma-tory demyelination and stroke (Picard-Riera et al., 2002; Yagita et al., 2001; Zhang et al., 2004). In animal models of chronic spinal cord injury, NPCs have been reported to differentiate and promote locomotor recovery (Salazar et al., 2010). Transplantation of NPCs improved cognition in a murine model of Alzheimer’s disease by increasing brain derived neurotrophic factor (Ager et al., 2015; Blurton-Jones et al., 2009). Engraftment of NPCs into murine and primate models of Huntington’s disease restore motor skills through differentiation into mature striatal neurons (Dunnett et al., 2000; Kendall et al., 1998; Palfi et al., 1998; Reidling et al., 2018).
It has also been reported that peripheral administration of hNPCs in a nonhuman primate EAE model reduces disease severity through immune regulation (Pluchino et al., 2009). A small clinical study reported that 2transplantation of human fetal-derived NPCs into the frontal lobes of children with Pelizaeus-Merbacher disease (PMD), a rare hypo-myelination disorder in children, resulted in measurable gains in motor and/or cognition associated with remyelination (Gupta et al., 2012).
JHMV Infection as a Model of Neuroinflammation and Demyelination
Intracranial inoculation of C56BL/6 mice with the neurotropic JHM strain of mouse hepatitis virus (JHMV) results in widespread dissemination of virus throughout the brain and spinal cord (Bergmann et al., 2006; Glass et al., 2004; Hosking and Lane, 2009). Oligodendrocytes, astrocytes and microglia are susceptible to infection while neurons are spared (Fleming et al., 1986). Type I interferons have essential roles for protecting the host against JHMV infection, as mice deficient in the interferon (IFN) −α/β receptor show elevated viral load within the CNS and higher mortality, and exogenous treatment of mice with type I interferon limits dissemination of virus (Minagawa et al., 1987; Ireland et al., 2008; Smith et al., 1987). Virus-specific CD4+ T cells function as support cells for CD8+ T cells, promoting CD8+ T cell expansion in the periphery and enhancing survival and cytolytic targeting of infected cells within the CNS (Zhou et al., 2005; Phares et al., 2012). In addition, CD4+ T cells can control viral spread through their release of IFN-γ, which serves dual roles by inhibiting viral replication within oligodendrocytes and also inducing upregulation of major histocompatibility complex (MHC) class II expression on microglia (Bergmann et al., 2003; Gonzalez et al., 2006; Parra et al., 1999; Phares et al., 2012; Ramakrishna et al., 2004).
Depletion of CD4+ T cells alters CD8+ T cell-mediated control of viral replication within the CNS, mainly a result of reduced of IFN-γ expression and elevated CD8+ T cell apoptosis (Phares et al., 2012). Virus-specific CD8+ T cells are the primary cytolytic effector cell within the CNS during JHMV infection and their peak accumulation coincides with viral clearance from glia (Lin et al., 1997; Parra et al., 1999; Ramakrishna et al., 2004). A recent study by Perlman and colleagues (Wheeler et al., 2018) used an inhibitor of colony-stimulating factor 1 receptor (CSF1R) that depletes microglia to demonstrate that microglia were required during the early days after infection to limit JHMV replication within the CNS and protect against clinical disease and death. Moreover, depletion of microglia resulted in impaired T cell responses, leading to elevated viral titers within the CNS. These results reveal nonredundant, critical roles for microglia in the early innate and virus-specific T cell responses and for subsequent host protection from viral encephalitis.
Mice that survive acute JHMV infection progress into the immune-mediated chronic demyelinating phase of the disease, with clinical symptoms manifesting as ataxia and partial-to-complete hind limb paralysis that peaks 2–3 weeks postinfection. Histologic analysis of spinal cords from mice undergoing JHMV-induced demyelination shows that oligodendrocyte dysfunction and loss of myelin integrity within white matter tracts is not due to widespread apoptosis or necrosis of mature oligodendrocytes, but instead is closely associated with the presence of both inflammatory leukocytes and presentation of viral antigen by means of MHC-I and MHC-II (Redwine et al., 2001; Stohlman and Hinton, 2001; Wu and Perlman, 1999)
Moreover, a paucity of infectious viral particles within the CNS during chronic disease suggests that productive infection of new glial cells does not amplify demyelination. More likely, viral RNA quasispecies present within the CNS of persistently infected mice promote chronic inflammation and demyelination (Adami et al., 1995; Fleming et al., 1995; Rowe et al., 1997). Luxol fast blue staining of spinal cord sections during persistent JHMV-infection reveals lesion formation primarily within the lateral funiculus and posterior funiculus (Wang et al., 1992). Additionally, there have been reports that axonal degeneration within the white matter tracts of spinal cords of JHMV-infected mice, as assessed by SMI-32 or Bielschowsky’s silver impregnation stain, occurred at the same time as demyelination, while axon damage is argued to precede oligodendrocyte dysregulation in MS (Dandekar et al., 2001; Das Sarma et al., 2009).
Several studies have reported that T cells and macrophages are the main inducers of demyelination during chronic JHMV infection, rather than viral-induced lysis of oligodendrocytes. This idea stems from results showing that JHMV-infection of RAG1−/− immunodeficient mice (lacking functional T and B lymphocytes) results in limited demyelination while there is extensive viral replication within oligodendrocytes (Pewe and Perlman, 2002; Wu and Perlman, 1999). Moreover, adoptive transfer of JHMV-sensitized splenocytes from wild-type mice into JHMV-infected RAG1−/− mice results in demyelination. Subsequent studies indicate that both CD4+ and CD8+ T cell subsets are capable of contributing to demyelination following JHMV infection (Lane et al., 2000; Pewe and Perlman, 2002). Other factors, such as epitope spreading and autoreactive T cells against host neuroantigens, are not thought to contribute to demyelination in these animals. Together, this evidence suggests that demyelination is multifaceted and numerous factors could contribute to pathology.
Effects of Mouse Neural Precursor Engraftment in JHMV-Infected Mice
As a first approach toward understanding the effects of transplanting NPCs, early studies used a syngeneic transplant protocol, in which H-2b haplotype-matched mouse striatal NPCs from postnatal day 1 (P1) C56BL/6 mice were transplanted intraspin-ally into the T8 region of C57BL/6 recipient mice undergoing JHMV-induced demyelination (Totoiu et al., 2004). Initial results demonstrated that transplanted NPCs readily proliferated and migrated up to 12 mm both rostral and caudal from the transplant site and preferentially differentiated into oligodendrocytelineage cells (Totoiu et al., 2004). Quantification of remyelinated axons resulted in up ~70% of axons remyelinated compared with 10% for nontransplanted controls, suggesting that NPCs can survive within the inflammatory niche and functionally incorporate throughout demyelinated white matter tracts following differentiation into mature oligodendrocytes (Totoiu et al., 2004).
Additional studies by Carbajal et al. (2010) demonstrating that transplanted mouse green fluorescent protein (GFP)-NPCs were shown to selectively colonize demyelinated white matter regions within the ventral and lateral funiculus regions of the spinal cord. Positional migration of NPCs was mediated, in part, by responding to the CXC chemokine ligand CXCL12 by means of the receptor CXCR4 expressed by engrafted NPCs (Carbajal et al., 2010). NPC transplantation did not alter the accumulation of T cells or macrophages within the CNS nor proinflammatory chemokine and cytokine gene expression, suggesting that the enhanced remyelination and recovery following transplantation was not a result of NPC bystander effects attenuating the inflammatory response (Hardison et al., 2006).
As an additional step to better understand the therapeutic potential of engraftment of NPCs in promoting clinical and histologic recovery, we have transplanted MHC-mismatched mouse NPCs into JHMV-infected mice with established demyelination to determine whether allogeneic NPCs are recognized as foreign and rejected by means of immunological mechanisms. Transplantation of allogeneic NPCs is clinically relevant, because transplantation of human neural stem cells into PMD patients required administration of immunosuppressive drugs to limit potential rejection (Gupta et al., 2012). Similarly, transplantation of hESC-OPCs into individuals with spinal cord injuries also was performed in conjunction with administration of immunosuppressive drugs. Studies by Palmer and colleagues (Chen et al., 2011; Phillips et al., 2013) have shown an important role for components of the innate immune response including NK cells in recognizing and rejecting MHC-mismatched NPCs following transplantation into the brains of mice.
Similarly, we have demonstrated that engraftment of allogeneic NPCs into spinal cords of JHMV-infected mice results in rejection mediated, in part, by both T lymphocytes as well as NK cells (Weinger et al., 2012, 2014). NPCs respond to both IFN-γ as well as viral infection; they react by expressing MHC class I and II that allows for T lymphocyte recognition, and retinoic acid early precursor transcript (RAE)-1 that enables NK cell recognition (Weinger et al., 2012, 2014; Plaisted et al., 2014). Collectively, these findings highlight that NPCs are recognized by cellular components of both the innate and adaptive immune system, indicating that administration of immunosuppressive drugs must be considered to promote long-term survival and function.
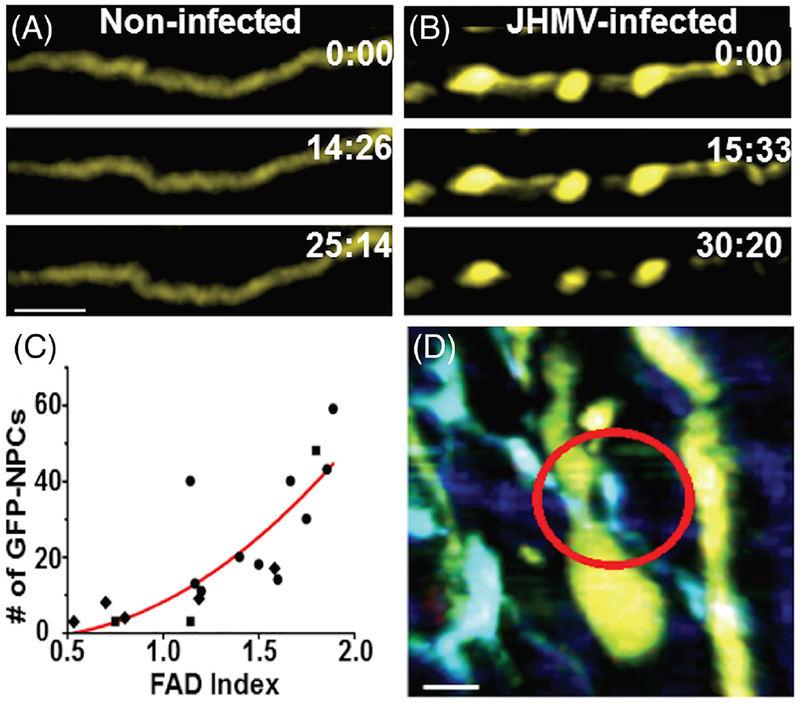
We have recently used two-photon microscopy to assess intercellular interactions of transplanted mouse NPCs ex vivo (Greenberg et al., 2014). JHMV-infected Thy1-yellow fluorescent protein (YFP) mice, which express YFP from medium-to-large caliber axons within the spinal cord, received subventricular zone-derived NPCs that express GFP following their differentiation into oligodendrocytes (proteolipid protein-GFP). Several important observations were derived from this study, including the finding that JHMV-infected Thy1-YFP mice displayed extensive axonal damage earlier than expected during JHMV-induced disease, suggesting that appearance of axonopathy precedes robust immune-mediated demyelination. This argues that axonal damage may be important in contributing to white matter damage and myelin loss. It is not yet clear whether viral infection of neurons and/or transport of viral proteins along axons is important in this process (Das Sarma et al., 2009). In addition, two-photon imaging showed that engrafted NPCs interacted with damaged axons and this resulted in improved axonal integrity and remyelination as determined by YFP expression (Fig. 1A–D) (Greenberg et al., 2014; Kerschen-steiner et al., 2005).
Fig. 1.
Axonal damage in JHMV-infected mice is reversed following NPC engraftment. A: Time-lapse images (times marked in min:s) depicting absence of focal axonal degeneration (FAD) in a noninfected Thy1-YFP spinal cord. B: Time-lapse images showing progression of FAD in a Thy1-YFP spinal cord 7 days following JHMV infection. Scale bar = 20 μm. C: GFP-NPC localization correlates with the FAD severity of lesions in the JHMV infected Thy1-YFP spinal cord 8 days posttransfer. Number of transferred GFP-NPCs found in lesions is plotted vs. FAD severity of the lesions for each 10−5 cm3 imaging volume. D: Time-lapse images showing GFP-NPCs initiating intercellular interactions with “Stage 1 FAD” axons in the JHMV infected Thy1-YFP spinal cord 8 days posttransfer. Circle indicates a GFP-NPC actively extending a process toward the axon. Scale bars = 10 μm. Figures derived from Greenberg et al. 2014.
We have also examined the effect of S1P receptor antagonism on the biology of mouse NPCs following transplantation into JHMV-infected mice. Earlier studies from our laboratory showed that treatment of JHMV-infected mice with FTY720 (fingolimod), the first oral drug approved by the FDA for treatment of patients with the relapsing-remitting form of MS, mutes effective anti-viral immune responses by affecting migration and accumulation of virus-specific T cells within the CNS during acute viral-induced encephalomyelitis (Blanc et al., 2014). FTY720 treatment reduced the severity of neuroinflammation-mediated demyelination by restricting the access of disease-causing lymphocytes into the CNS, but this did not result in viral recrudescence.
As a result of this work, we were interested if the therapeutic benefit of mouse NPC transplantation into JHMV-infected mice would be augmented if FTY720 was also administered, since previously published studies showed a beneficial effect of FTY720 in combination with benztropine in reducing clinical disease and increasing remyelination in the mouse EAE model of MS (Deshmukh et al., 2013). We found that cultured NPCs expressed transcripts for S1P receptors S1P1, S1P2, S1P3, S1P4, and S1P5. Administration of FTY720 to JHMV-infected mice resulted in enhanced migration and increased proliferation of transplanted NPCs following spinal cord engraftment. FTY720 treatment did not improve clinical disease, diminish neuroinflammation or the severity of demyelination and did not increase remyelination (Blanc et al., 2015).
Glial-committed neural precursor cells have been previously suggested as a potential treatment for autoimmune demyelinating diseases such as MS, as they are sources for generation of mature remyelinating oligodendrocytes (Ben-Hur et al., 1998; Brustle et al., 1999). Glial progenitors derived from NPCs can remyelinate axons following transplantation into regions of experimentally induced demyelination (Keirstead et al., 1999). Transplantation of these cells into rodent autoimmune models of demyelination resulted in improved clinical outcomes as a result of migration of cells into the inflamed white matter tracts (Ben-Hur et al., 2003). Glial precursor cells have been suggested to act either as modulators of the immune system or by replacement of the damaged or lost endogenous neural precursors in animal models of MS (Pluchino et al., 2003,2009; Aharonowiz et al., 2008).
Most of these studies used models of demyelination caused by injury or infiltration of myelin-reactive T cells to demonstrate the effect of implanting myelin-competent NPCs in promoting remyelination. But viral infections have also been considered as potential triggers of MS in genetically susceptible individuals (Giovannoni et al., 2006), and a clinically relevant question is whether glial-committed stem cells can ameliorate demyelination caused by persistent neurotropic viruses. To address this question, we have shown that engraftment of glial-committed progenitors in JHMV infected mice with established neurological disease resulted in remyelination and axonal sparing (Totoiu et al., 2004). This result raises another relevant question, whether glial cells derived from NPCs are susceptible to viral infection. There are several known neurotropic viruses that have been shown to infect and replicate in NPCs and cells derived from NPCs.
For example, a neonatal neurotropic virus called Coxsackievirus B3 (CVB3) persists in the CNS and preferentially infects proliferating neural stem cells and infiltrating myeloid cells (Tabor-Godwin et al., 2010). CVB3 persists within the murine neurogenic region and infects neural stem cells, causing cell death, decrease in brain size, and eventually developmental defects (Ruller et al., 2012). This suggests that persistent viral infections in the CNS can have long-term neurological sequelae (Ruller et al., 2012). Borna disease virus, a human pathogen associated with behavioral disorders, is capable of severely impairing neurogenesis by infecting human neural progenitors (Brnic et al., 2012).
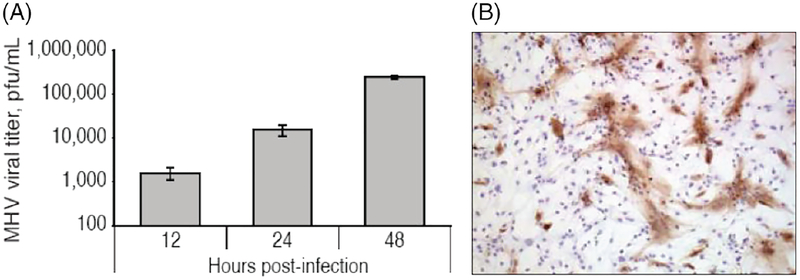
Another human neurotropic virus, herpes simplex virus type 1 (HSV-1) that causes herpes simplex encephalitis, was shown to infect and deplete mouse NPCs in the subventricular zone, causing a loss of neuroblasts (Chucair-Elliott et al., 2014). Furthermore, NPCs are depleted by viral-induced lysis due to their susceptibility to infection by Enterovirus 71 (Huang et al., 2014). In addition, human ESC-derived oligodendrocyte progenitors are highly susceptible to infection by JC virus, the causative pathogen of progressive multifocal leukoencephalopathy (Schaumburg et al., 2008). We have shown that glial cells derived from murine NPCs are susceptible to JHMV infection and these cells can actively replicate JHMV, as evidenced by increasing viral titers and extensive distribution of viral antigen throughout the infected monolayer (Fig. 2A,B) (Whitman et al., 2009).
Fig. 2.
JHMV replicates in glial cells derived from mouse NPCs. A: Differentiated progenitor cultures were infected with JHMV (multiplicity of infection = 0.1) and viral titers in supernatants determined at 12, 24, and 48 hr postinfection (p.i.) by plaque assay. B: Immunocytochemical staining for viral antigen at 24 hr p.i. revealed wide-spread distribution of virus throughout the cell culture (100 × magnification). Figures derived from Whitman et al. 2008.
IFN-γ plays an important role in controlling JHMV infection of persistently infected mice (Parra et al., 1999). Treatment of JHMV-infected cells with IFN-γ led to inhibition of viral replication in a dose-dependent manner (Whitman et al., 2009). IFN-γ treatment also limited the cytopathic effects of JHMV infection, demonstrating the importance of this cytokine in host defense following JHMV infection (Whitman et al., 2009). JHMV is capable of infecting and replicating in primary OPC cultures, indicating that these cells are susceptible to infection in vivo. Remyelination is relatively slow in JHMV-infected mice, yet OPCs can be found in the vicinity of on-going demyelination. Overall, these findings suggest that susceptibility of NPCs and their derivatives to viral infection should be considered in plans to use these cells for cell replacement therapy for neurological disorders.
Immunosuppression used to prevent rejection of allogeneic cells may cause reemergence of persistent neurotropic viruses. These reactivated viruses could infect and diminish the transplanted cells, impeding therapeutic benefits. Problems associated with immunosuppression could be mitigated by using patient-specific induced pluripotent stem cells (iPSCs) to produce immune-matched cells for transplantation. Interestingly, we recently learned that mouse iPSC-derived NPCs expressed low levels of the JHMV receptor CEACAM1a, which made them resistant to infection and viral induced cell death in vitro (Mangale et al., 2017). This suggests that iPSC-derived cells may be a good option for cell replacement therapy, because they would avoid both rejection and viral-mediated cell death. An overview of our results with transplantation of moues NPCs into JHMV-infected mice is provided in Table 1.
TABLE 1.
Overview of Mouse and Human Stem Cell Engraftment Into JHMV-Infected Mice
| Cell type | Antigenicity | Cell survival & Migration | Clinical improvement | Spinal cord demyelination | Spinal cord remyelination | Immuno-modulation | Reference | |
|---|---|---|---|---|---|---|---|---|
| Mouse | NPCs | Syngeneic | Yes | Yes | Yes | Yes | No |
Totoiu et al., 2004 Carbajal et al., 2010 Greenberg et al., 2014 Blanc et al., 2015 |
| NPCs | Allogeneic | No | No | Yes | Not determined | No |
Weinger et al., 2012 Weinger et al., 2014 |
|
| Human | ESC-OPCs | Xenogeneic | No | No | Yes | Focal at site of transplant | Not determined | Hatch et al., 2009 |
| ESC-NCLCs | Xenogeneic | No | Yes | Reduced | Yes | Yes | Chen et al., 2014 | |
| iPSC-NPCs | Xenogeneic | No | No | Reduced | Focal at site of transplant | Yes | Plaisted et al., 2016 |
NPCs: neural progenitor cells; ESC-OPCs: embryonic stem cell-derived oligodendrocyte progenitor cells; ESC-NCLCs: embryonic stem cell-derived neural crest like cells; iPSC-NPCs: inducible pluripotent cell-derived neural progenitor cells.
Effects of Transplantation of Human Pluripotent Stem Cell-Derived Cells in Virally Induced Models of Neuroinflammation and Demyelination
The long-term goal of studying MS model mice is to guide the development of effective treatments for the human disease. In our early work, we saw very limited clinical recovery after transplantation of predifferentiated human OPCs in mice undergoing JHMV-induced demyelination (Hatch et al., 2009). Engrafted cells were rejected within 2 weeks after transplantation, even in the presence of immunosuppressive drugs targeting activated T lymphocytes. There was only a slight increase in remyelination near the transplant site compared with mice receiving a saline control (Hatch et al., 2009). This in contrast to earlier studies using human embryonic stem cell (hESC)-derived early stage OPCs in a model of spinal cord injury in rat, in which enhanced remyelination and improved motor function were observed following transplantation (Keirstead et al., 2005). Less mature human neural lineage cells have previously been shown to exert neuroprotective effects in mouse and nonhuman primate models of EAE, suggesting that they possess broader functionality in vivo (Aharonowiz et al., 2008; Pluchino et al., 2009).
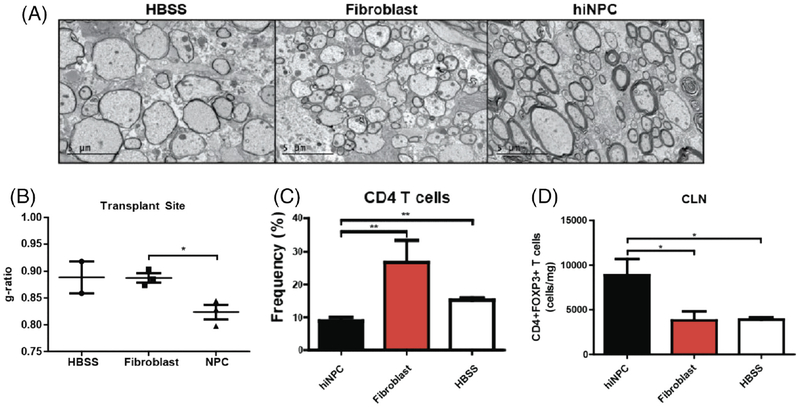
When we transplanted NPCs derived from human iPSCs into the spinal cords of JHMV-infected mice, the cells were rejected, but there was focal remyelination at the site of transplantation (Fig. 3A,B) (Plaisted et al., 2016). There was also reduced recruitment of CD4+ T cells into the CNS, and a transient increase in CD4+FoxP3 + Tregs was observed (Fig. 3C,D). Importantly, ablation of Tregs by means of PC61.5 treatment abrogated histopathological recovery. These findings support an immunomodulatory role for Tregs, where they may suppress neuroinflammation or promote tissue repair mechanisms. The cells used for this study were generated by an embryoid-body-based technique; they were characterized by gene expression analysis and found to be positive for the transcription factor PAX6, a classical marker of CNS neural precursor cells.
Fig. 3.
Intraspinal transplantation of iPSC-derived NPCs into JHMV-infected mice. A: Focal remyelination in animals transplanted with hiNPCs. Representative electron micrographs of coronal spinal cord sections from HBSS, fibroblast, and hiNPC injected mice. B: Analysis of the ratio of the axon diameter vs. total fiber diameter (g-ratio) confirmed enhanced remyelination. C: Quantification of the percent of CD4+ T cells demonstrated a significant (P < 0.05) decrease in the CLNs of hiNPC transplanted mice compared with controls at 5 days posttransplant (p.t.) D: Quantification of the number of CD4+FoxP3 + Tregs demonstrated a significant (P < 0.05) increase in the CLNs of hiNPC transplanted mice compared with controls at 5 days p.t. Figures derived from Plaisted et al. 2016.
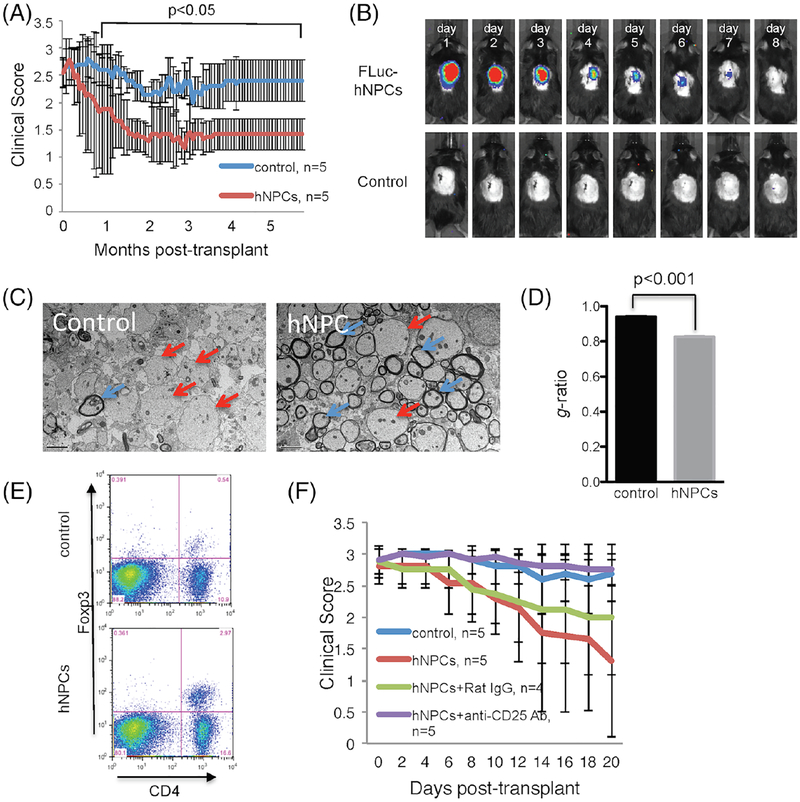
However, the results differed when we transplanted a population of PAX6-negative hPSC-derived cells that we referred to as “neural precursor-like cells” (NPLCs) into JHMV-infected mice. The NPLC transplantation resulted in clinical and histological improvement out to 6 months posttransplant, despite the rejection of transplanted cells within 8 days (Fig. 4A,B) (Chen et al., 2014). Strikingly, while the transplanted cells did not migrate from the site of implantation, the remyelinated axons were distributed both rostrally and caudally, rather than localized to the region of cell delivery (Fig. 4C,D). The remyelination was not likely to be the result of acute inflammatory-mediated rejection, as the spinal cords had reduced infiltration of CD4+ and CD8+ effector T cells compared with controls, and the total number of CD4+CD25+FoxP3 regulatory T cells (Tregs) within the spinal cords was elevated (Fig. 4D) (Chen et al., 2014). Depletion of Tregs in NPLC-transplanted mice by means of anti-CD25 (PC61.5) treatment abolished the therapeutic benefits, highlighting the likely importance of Tregs in this more extensive recovery (Fig. 4E).
Fig. 4.
Intraspinal transplantation of hNPCs into JHMV-infected mice. (A) Improved (p < 0.05) clinical recovery in hNPC-transplanted JHMV-infected mice was sustained out to 168 days post-transplantation (p.t.) when compared to infected mice treated with vehicle alone. (B) Daily IVIS® imaging of luciferase-labeled hNPCs revealed that following intraspinal transplantation, cells are reduced to below the level of detection by day 8 post-transplantation; representative mice are shown. IVIS® imaging was performed on vehicle-transplanted mice as a control. (C) Representative EM images (1200×) showing increased numbers of remyelinated axons (red arrows) compared to demyelinated axons (blue arrows) in hNPC-transplanted mice compared to control mice. (D) Calculation of g-ratio, as a measurement of structural and functional axonal remyelination, revealed a significantly (p < 0.001) lower g-ratio (indicative of remyelination) in hNPC-treated mice compared to control mice at 3 weeks pt. (E) Quantification of Treg numbers in spinal cords of mice indicated a significant (p < 0.05) increase in numbers of Tregs in hNPC-transplanted mice versus controls between 8–10 days post-transplantation. (F) hNPC-transplanted mice receiving anti-CD25 antibody (purple line) did not display recovery in motor skills as compared to either hNPC-treated mice (red line), hNPC-treated mice receiving isotype-matched control antibody (green line), or vehicle control mice (blue line). Figures derived from Chen et al., 2014.
The PAX6-negative NPLCs were not classic neural precursor cells; they were produced by a method that enhanced the differentiation of peripheral neural lineage cells rather than CNS neural lineage derivatives. The differences were confirmed by gene expression studies, which showed that the NPLCs had an expression profile that considerably differed from the CNS-NPCs as well as ineffective fibroblasts and undifferentiated hESCs and iPSCs (Plaisted et al., 2016). The gene expression signature gave clues to the characteristics that may underlie the disease-modifying activity of NPLCs; for example, these cells produced higher levels of TGF-ß2 than NPCs, fibroblasts, and undifferentiated hESC cells that did not elicit clinical recovery (Chen et al., 2014).
Previous work has shown that this anti-inflammatory cytokine promotes FoxP3 expression in the peripheral Treg compartment, influencing the frequency and suppressive activity of Tregs (Marie et al., 2005). Tregs have been shown to have an important role during both acute and chronic JHMV-infection (Anghelina et al., 2009). IL-10-expressing virus-specific Tregs dampen proliferation of virus-specific effector CD4+ T cells, and depletion of Tregs increases mortality, suggesting that during acute JHMV infection, Tregs limit immunopathological disease without negatively impacting viral clearance. In addition, studies from Trandem et al. (2010) have shown that adoptive transfer of Tregs into JHMV-infected mice attenuates clinical disease severity by dampening neuroinflammation and subsequent demyelination. An overview of our results with transplantation of human progenitor cells into JHMV-infected mice is provided in Table 1.
Concluding Remarks
Research using a mouse model of virally induced demyelination has provided support for the potential of cell transplantation therapy for human disease. Experiments indicate that transplantation of certain types of cells can promote sustained recovery both through promoting remyelination and limiting ongoing demyelination by muting neuroinflammation. These reports also highlight the importance of comparing differing cell types transplanted to the same model of human disease. In designing cell therapies for human disease, it is important to standardize criteria for defining cell types to be used for transplantation. Our analysis of gene expression profiles of a variety of human precursors and stem cells revealed that they are very diverse; for example, while pluripotent stem cells were very similar to each other, cells that had been designated as neural stem cells were clustered into multiple subgroups (Muller et al., 2008). Similarly, mesenchymal stem cells are very divergent in their behavior and capabilities depending on fundamental factors, including organ or tissue of origin, age of donor, preparation methods, degree and means of expansion, and assays used to assess their differentiation capabilities (Robey, 2017).
The mechanisms by which different transplanted cells elicit clinical improvements appear to be different, but the experimental evidence converges on common themes. The transplanted cells all appear to mute the effects of inflammatory immune cells and involve signaling by Tregs, which are anti-inflammatory. Some of the cell types either function as OPCs or to stimulate remyelination by endogenous OPCs. In order for cell therapies to advance to clinical relevance, the properties of each cell type should be examined by multiple methods to determine what characteristics are responsible for clinical recovery in mouse models of demyelinating disease. This approach could lead to identification of the best cell type for transplantation therapy, or perhaps more promising, identification of the key ameliorative factors that can be translated into therapy without the need for cells.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01 NS041249 and R01 NS074987 (TEL) as well as National Multiple Sclerosis Society Collaborative Research Center grant RG 4925 (TEL) and funding from the Ray & Tye Noorda Foundation (TEL); NIH grant R01 AI121945 (CMW); California Institute for Regenerative Medicine (CIRM) grants RM1–01717 and CL1–00502 (JFL) and TR3–05603 (CMW and JFL); and training grant NIH 5T32NS082174 (LLM).
References
- Adami C, Pooley J, Glomb J, Stecker E, Fazal F, Fleming JO, Baker SC. 1995. Evolution of mouse hepatitis virus (MHV) during chronic infection: quasispecies nature of the persisting MHV RNA. Virology 209:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ager RR, Davis JL, Agazaryan A, Benavente F, Poon WW, Laferla FM, Blurton-Jones M. 2015. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 25:813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben-Hur T. 2008. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS One 3:E3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anghelina D, Zhao J, Trandem K, Perlman S. 2009. Role of regulatory T cells in coronavirus-induced acute encephalitis. Virology 385:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T, Einstein O, Mizrachi-Kol R, Ben-Menachem O, Reinhartz E, Karussis D, Abramsky O. 2003. Transplanted multi-potential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia 41:73–80. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Rogister B, Murray K, Rougon G, Dubois-Dalcq M. 1998. Growth and fate of PSA-NCAM + precursors of the postnatal brain. J Neurosci 18:5777–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Lane TE, Stohlman SA. 2006. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol 4:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Parra B, Hinton DR, Chandran R, Morrison M, Stohlman SA. 2003. Perforin-mediated effector function within the central nervous system requires IFN-gamma-mediated MHC up-regulation. J Immunol 170:3204–3213. [DOI] [PubMed] [Google Scholar]
- Blanc CA, Grist JJ, Rosen H, Sears-Kraxberger I, Steward O, Lane TE. 2015. Sphingosine-1-phosphate receptor antagonism enhances proliferation and migration of engrafted neural progenitor cells in a model of viral-induced demyelination. Am J Pathol 185:2819–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc CA, Rosen H, Lane TE. 2014. Fty720 (fingolimod) modulates the severity of viral-induced encephalomyelitis and demyelination. J Neuroinflammation 11:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, Laferla FM. 2009. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A 106:13594–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brnic D, Stevanovic V, Cochet M, Agier C, Richardson J, Montero-Menei CN, Milhavet O, Eloit M, Coulpier M. 2012. Borna disease virus infects human neural progenitor cells and impairs neurogenesis. J Virol 86:2512–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, Mckay RD. 1999. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science 285:754–756. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil MT, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lutjohann D, Mobius W, Simons M. 2018. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359v684–v688. [DOI] [PubMed] [Google Scholar]
- Carbajal KS, Schaumburg C, Strieter R, Kane J, Lane TE. 2010. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A 107:11068–11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. 2000. Ng2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 20:6404–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Coleman R, Leang R, Tran H, Kopf A, Walsh Craig M, Sears-Kraxberger I, Steward O, Macklin WB, Loring JF, Lane TE. 2014. Human neural precursor cells promote neurologic recovery in a viral model of multiple sclerosis. Stem Cell Reports 2: 825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Phillips LK, Gould E, Campisi J, Lee SW, Ormerod BK, Zwierzchoniewska M, Martinez OM, Palmer TD. 2011. MHC mismatch inhibits neurogenesis and neuron maturation in stem cell allografts. PLoS One 6:E14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chucair-Elliott AJ, Conrady C, Zheng M, Kroll CM, Lane TE, Carr DJ. 2014. Microglia-induced IL-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells. Glia 62: 1418–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar AA, Wu GF, Pewe L, Perlman S. 2001. Axonal damage is T cell mediated and occurs concomitantly with demyelination in mice infected with a neurotropic coronavirus. J Virol 75: 6115–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sarma J, Kenyon LC, Hingley ST, Shindler KS. 2009. Mechanisms of primary axonal damage in a viral model of multiple sclerosis. J Neurosci 29:10272–10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, Kim HJ, Padmanabhan K, Swoboda JG, Ahmad I, Kondo T, Gage FH, Theofilopoulos AN, Lawson BR, Schultz PG, Lairson LL. 2013. A regenerative approach to the treatment of multiple sclerosis. Nature 502:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Nathwani F, Bjorklund A. 2000. The integration and function of striatal grafts. Prog Brain Res 127:345–380. [DOI] [PubMed] [Google Scholar]
- Fleming JO, Adami C, Pooley J, Glomb J, Stecker E, Fazal F, Baker SC. 1995. Mutations associated with viral sequences isolated from mice persistently infected with MHV-JHM. Adv Exp Med Biol 380:591–595. [DOI] [PubMed] [Google Scholar]
- Fleming JO, Trousdale MD, Elzaatari FAK, Stohlman SA, Weiner LP. 1986. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal-antibodies. J Virol 58:869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton JE. 2017. Ocrelizumab: first global approval. Drugs 77: 1035–1041. [DOI] [PubMed] [Google Scholar]
- Gage fH. 2000. Mammalian neural stem cells. Science 287:1433–1438. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, Cutter GR, Lunemann J, Martin R, Munz C, Sriram S, Steiner I, Hammerschlag MR, Gaydos CA. 2006. Infectious causes of multiple sclerosis. Lancet Neurol 5:887–894. [DOI] [PubMed] [Google Scholar]
- Glass WG, Hickey MJ, Hardison JL, Liu MT, Manning JE, Lane TE. 2004. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J Immunol 172:4018–4025. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Bergmann CC, Ramakrishna C, Hinton DR, Atkinson R, Hoskin J, Macklin WB, Stohlman SA. 2006. Inhibition of interferon-gamma signaling in oligodendroglia delays coronavirus clearance without altering demyelination. Am J Pathol 168:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ML, Weinger JG, Matheu MP, Carbajal KS, Parker I, Macklin WB, Lane TE, Cahalan MD. 2014. Two-photon imaging of remyelination of spinal cord axons by engrafted neural precursor cells in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A 111:E2349–E2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, Perry R, Farrell J, Jeremy RJ, Ulman M, Huhn SL, Barkovich AJ, Rowitch DH. 2012. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med 4:155ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfpenny C, Benn T, Scolding N. 2002. Cell transplantation, myelin repair, and multiple sclerosis. Lancet Neurol 1:31–40. [DOI] [PubMed] [Google Scholar]
- Hardison JL, Nistor G, Gonzalez R, Keirstead HS, Lane TE. 2006. Transplantation of glial-committed progenitor cells into a viral model of multiple sclerosis induces remyelination in the absence of an attenuated inflammatory response. Exp Neurol 197:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MN, Schaumburg CS, Lane TE, Keirstead HS. 2009. Endogenous remyelination is induced by transplant rejection in a viral model of multiple sclerosis. J Neuroimmunol 212:74–81. [DOI] [PubMed] [Google Scholar]
- Healy LM, Jang JH, Won SY, Lin YH, Touil H, Aljarallah S, Bar-Or A, Antel JP. 2017. Mertk-mediated regulation of myelin phagocytosis by macrophages generated from patients with ms. Neurol Neuroimmunol Neuroinflamm 4:E402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking MP, Lane TE. 2009. The biology of persistent infection: inflammation and demyelination following murine coronavirus infection of the central nervous system. Curr Immunol Rev 5: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HI, Lin JY, Chen HH, Yeh SB, Kuo RL, Weng KF, Shih SR. 2014. Enterovirus 71 infects brain-derived neural progenitor cells. Virology 468–470, 592–600. [DOI] [PubMed] [Google Scholar]
- Ireland DD, Stohlman SA, Hinton DR, Atkinson R, Bergmann CC. 2008. Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells. J Virol 82:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamita M, Barnum C, Mobius W, Tansey MG, Szymkowski DE, Lassmann H, Probert L. 2017. Therapeutic inhibition of soluble brain TNF promotes remyelination by increasing myelin phagocytosis by microglia. JCI Insight 2:pii: 87455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Ben-Hur T, Rogister B, O’Leary MT, Dubois-Dalcq M, Blakemore WF. 1999. Polysialylated neural cell adhesion molecule-positive CNS precursors generate both oligodendrocytes and Schwann cells to remyelinate the CNS after transplantation. J Neurosci 19:7529–7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. 2005. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 25:4694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall AL, Rayment FD, Torres EM, Baker HF, Ridley RM, Dunnett SB. 1998. Functional integration of striatal allografts in a primate model of Huntington’s disease. Nat Med 4:727–729. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. 2005. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med 11:572–577. [DOI] [PubMed] [Google Scholar]
- Kucharova K, Stallcup WB. 2017. Distinct NG2 proteoglycan-dependent roles of resident microglia and bone marrow-derived macrophages during myelin damage and repair. PLoS One 12: E0187530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Liu MT, Chen BP, Asensio VC, Samawi RM, Paoletti AD, Campbell IL, Kunkel SL, Fox HS, Buchmeier MJ. 2000. A central role for CD4(+) T cells and rantes in virus-induced central nervous system inflammation and demyelination. J Virol 74: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H 1983. Comparative neuropathology of chronic experimental allergic encephalomyelitis and multiple sclerosis. Schriftenr Neurol 25:1–135. [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti CF. 2007. The immunopathology of multiple sclerosis: an overview. Brain Pathol 17:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Stohlman SA, Hinton DR. 1997. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J Virol 71:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindborg JA, Mack M, Zigmond RE. 2017. Neutrophils are critical for myelin removal in a peripheral nerve injury model of wallerian degeneration. J Neurosci 37:10258–10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. 1999. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain 122(Pt 12): 2279–2295. [DOI] [PubMed] [Google Scholar]
- Mangale V, Marro BS, Plaisted WC, Walsh CM, Lane TE. 2017. Neural precursor cells derived from induced pluripotent stem cells exhibit reduced susceptibility to infection with a neurotropic coronavirus. Virology 511:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M, Rudensky AY. 2005. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4 +CD25 + regulatory T cells. J Exp Med 201:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Fancy SPJ, Shen YA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, Redmond SA, Etxeberria A, Xiao L, Franklin RJM, Green A, Hauser SL, Chan JR. 2014. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat Med 20:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Lehmann-Horn K, Shen YA, Rankin KA, Stebbins KJ, Lorrain DS, Pekarek K, A Sagan S, Xiao L, Teuscher C, Von Budingen HC, Wess J, Lawrence JJ, Green AJ, Fancy SP, Zamvil SS, Chan JR. 2016a. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife 5:pii. e18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Mayoral SR, Nobuta H, Wang F, Desponts C, Lorrain DS, Xiao L, Green AJ, Rowitch D, Whistler J, Chan JR. 2016b. Identification of the kappa-opioid receptor as a therapeutic target for oligodendrocyte remyelination. J Neurosci 36:7925–7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa H, Takenaka A, Mohri S, Mori R. 1987. Protective effect of recombinant murine interferon beta against mouse hepatitis virus infection. Antiviral Res 8:85–95. [DOI] [PubMed] [Google Scholar]
- Muller FJ, Laurent LC, Kostka D, Ulitsky I, Williams R, Lu C, Park IH, Rao MS, Shamir R, Schwartz PH, Schmidt NO, Loring JF. 2008. Regulatory networks define phenotypic classes of human stem cell lines. Nature 455:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Medana IM, Bauer J, Lassmann H. 2002. Cytotoxic t lymphocytes in autoimmune and degenerative cns diseases. Trends Neurosci 25:313–319. [DOI] [PubMed] [Google Scholar]
- Oksenberg JR, Begovich AB, Erlich HA, Steinman L. 1993. Genetic factors in multiple sclerosis. JAMA 270:2362–2369. [PubMed] [Google Scholar]
- Palfi S, Conde F, Riche D, Brouillet E, Dautry C, Mittoux V, Chibois A, Peschanski M, Hantraye P. 1998. Fetal striatal allo-grafts reverse cognitive deficits in a primate model of Huntington disease. Nat Med 4:963–936. [DOI] [PubMed] [Google Scholar]
- Parra B, Hinton DR, Marten NW, Bergmann CC, Lin MT, Yang CS, Stohlman SA. 1999. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J Immunol 162: 1641–1647. [PubMed] [Google Scholar]
- Pewe L, Perlman S. 2002. Cutting edge: CD8 T cell-mediated demyelination is IFN-gamma dependent in mice infected with a neurotropic coronavirus. J Immunol 168:1547–1551. [DOI] [PubMed] [Google Scholar]
- Phares TW, Stohlman SA, Hwang M, Min B, Hinton DR, Bergmann CC. 2012. CD4 T cells promote CD8 tT cell immunity at the priming and effector site during viral encephalitis. J Virol 86: 2416–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LK, Gould EA, Babu H, Krams SM, Palmer TD, Martinez OM. 2013. Natural killer cell-activating receptor NKG2D mediates innate immune targeting of allogeneic neural progenitor cell grafts. Stem Cells 31:1829–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Baron-Van Evercooren A. 2002. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A 99:13211–13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisted WC, Weinger JG, Walsh CM, Lane TE. 2014. T cell mediated suppression of neurotropic coronavirus replication in neural precursor cells. Virology 449:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisted WC, Zavala A, Hingco E, Tran H, Coleman R, Lane TE, Loring JF, Walsh CM. 2016. Remyelination is correlated with regulatory T cell induction following human embryoid body-derived neural precursor cell transplantation in a viral model of multiple sclerosis. PLoS One 11:E0157620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Gritti A, Blezer E, Amadio S, Brambilla E, Borsellino G, Cossetti C, Del Carro U, Comi G, ‘t Hart B, Vescovi A, Martino G. 2009. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann Neurol 66:343–354. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. 2003. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 422:688–94. [DOI] [PubMed] [Google Scholar]
- Poser CM. 1994. The epidemiology of multiple sclerosis: a general overview. Ann Neurol 36(suppl 2):S180–S193. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Graham JS. 1981. Multiple sclerosis: capping of surface immunoglobulin g on macrophages engaged in myelin breakdown. Ann Neurol 10:149–158. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Goldenberg PZ, Ilyas AA, Quarles RH, Benjamins JA, Sprinkle TJ. 1989. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest 61:489–503. [PubMed] [Google Scholar]
- Ramakrishna C, Stohlman SA, Atkinson RA, Hinton DR, Bergmann CC. 2004. Differential regulation of primary and secondary CD8(+) T cells in the central nervous system. J Immunol 173:6265–6273. [DOI] [PubMed] [Google Scholar]
- Redwine JM, Buchmeier MJ, Evans CF. 2001. In vivo expression of major histocompatibility complex molecules on oligodendrocytes and neurons during viral infection. Am J Pathol 159:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidling JC, Relano-Gines A, Holley SM, Ochaba J, Moore C, Fury B, Lau A, Tran AH, Yeung S, Salamati D, Zhu C, Hatami A, Cepeda C, Barry JA, Kamdjou T, King A, Coleal-Bergum D, Franich NR, Laferla FM, Steffan JS, Blurton-Jones M, Meshul CK, Bauer G, Levine MS, Chesselet MF, Thompson LM. 2018. Human neural stem cell transplantation rescues functional deficits in R6/2 and Q140 Huntington’s disease mice. Stem Cell Reports 10:58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey PG. 2017. “Mesenchymal stem cells”: fact or fiction, and implications in their therapeutic use. F1000Res 6:pii: F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CL, Baker SC, Nathan MJ, Fleming JO. 1997. Evolution of mouse hepatitis virus: detection and characterization of spike deletion variants during persistent infection. J Virol 71:2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, Braun PE, Goldman SA. 1999. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci 19:9986–9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckh JM, Zhao JW, Shadrach JL, Van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. 2012. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruller CM, Tabor-Godwin JM, Van Deren DA Jr, Robinson SM, Maciejewski S, Gluhm S, Gilbert PE, An N, Gude NA, Sussman MA, Whitton JL, Feuer R. 2012. Neural stem cell depletion and CNS developmental defects after enteroviral infection. Am J Pathol 180:1107–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar DL, Uchida N, Hamers FP, Cummings BJ, Anderson AJ. 2010. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury nod-scid mouse model. PLoS One 5:E12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumburg C, O’Hara BA, Lane TE, Atwood WJ. 2008. Human embryonic stem cell-derived oligodendrocyte progenitor cells express the serotonin receptor and are susceptible to JC virus infection. J Virol 82:8896–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger H 1909. Zur Frage Der Akuten Multiplen Sklerose Und Der encephalomyelitis disseminata im kindesalter. Arb Neurol Inst [Wien] 17:410–432. [Google Scholar]
- Smith AL, Barthold SW, Beck DS. 1987. Intranasally administered alpha/-beta interferon prevents extension of mouse hepatitis virus, strain JHM, into the brains of BALB/cByJ mice. Antiviral Res 8:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L 1996. Multiple Sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85:299–302. [DOI] [PubMed] [Google Scholar]
- Stohlman SA, Hinton DR. 2001. Viral Induced Demyelination. Brain Pathol 11:92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor-Godwin JM, Ruller CM, Bagalso N, An N, Pagarigan RR, Harkins S, Gilbert PE, Kiosses WB, Gude NA, Cornell CT, Doran KS, Sussman MA, Whitton JL, Feuer R. 2010. A novel population of myeloid cells responding to coxsackievirus infection assists in the dissemination of virus within the neonatal CNS. J Neurosci 30:8676–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totoiu MO, Nistor GI, Lane TE, Keirstead HS. 2004. Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis. Exp Neurol 187:254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trandem K, Anghelina D, Zhao J, Perlman S. 2010. Regulatory T cells inhibit T cell proliferation and decrease demyelination in mice chronically infected with a coronavirus. J Immunol 184: 4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traugott U, Reinherz EL, Raine CS. 1983. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science 219:308–310. [DOI] [PubMed] [Google Scholar]
- Wang FI, Hinton DR, Gilmore W, Trousdale MD, Fleming JO. 1992. Sequential infection of glial cells by the murine hepatitis virus jhm strain (MHV-4) leads to a characteristic distribution of demyelination. Lab Invest 66:744–754. [PubMed] [Google Scholar]
- Weinger JG, Plaisted WC, Maciejewski SM, Lanier LL, Walsh CM, Lane TE. 2014. Activating receptor NKG2D targets RAE-1-expressing allogeneic neural precursor cells in a viral model of multiple sclerosis. Stem Cells 32:2690–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger JG, Weist BM, Plaisted WC, Klaus SM, Walsh CM, Lane TE. 2012. MHC mismatch results in neural progenitor cell rejection following spinal cord transplantation in a model of viral-induced demyelination. Stem Cells 30:2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, Ebers GC. 1989. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 112(Pt 1):133–146. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Sariol A, Meyerholz DK, Perlman S. 2018. Microglia are required for protection against lethal coronavirus encephalitis in mice. J Clin Invest 128:931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman L, Zhou H, Perlman S, Lane TE. 2009. IFN-gamma-mediated suppression of coronavirus replication in glial-committed progenitor cells. Virology 384:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GF, Perlman S. 1999. Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. J Virol 73: 8771–8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K, Miyata T, Okano H, Hori M, Matsumoto M. 2001. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke 32: 1890–1896. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. 2004. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab 24:441–448. [DOI] [PubMed] [Google Scholar]
- Zhou J, Hinton DR, Stohlman SA, Liu CP, Zhong L, Marten NW. 2005. Maintenance of CD8+ T cells during acute viral infection of the central nervous system requires CD4+ T cells but not interleukin-2. Viral Immunol 18:162–169. [DOI] [PubMed] [Google Scholar]
- Zhu K, Sun J, Kang Z, Zou Z, Wu G, Wang J. 2016. Electroacupuncture promotes remyelination after cuprizone treatment by enhancing myelin debris clearance. Front Neurosci 10:613. [DOI] [PMC free article] [PubMed] [Google Scholar]