Abstract
Mitochondria have long been controversial organelles in cancer. Early discoveries in cancer metabolism placed much emphasis on cytosolic contributions. Initial debate focused on if mitochondria had a role in cancer formation and progression at all. More recently the contributions of mitochondria to cancer development and progression have become firmly established. This has led to the identification of novel targets and inhibitors being studied as new therapeutic approaches. This review will summarize the role of mitochondria in cancer and highlight several agents under development.
1. Mitochondria, passenger or key player in tumor metabolism?
Several billion years ago eukaryotic cells acquired an intracellular symbiont that allowed for much more efficient generation of cellular energy and biomolecules. These endosymbionts thought to be derived from an engulfed contemporaneous prokaryotic organism [1] allowed for the evolution of multicellular organisms and are now an essential subcellular organelle, the mitochondria. This name was first coined in 1898 by Dr. Carl Benda. The name was derived from the Greek “mito” meaning thread and “chondria” meaning granule after their appearance in spermatogenesis [2]. Mitochondria are dynamic subcellular organelles that can move within the cytoplasm, contain an independent genome, protein translational system and reproduce independently of the host cell [1]. Mitochondrial homeostasis results from a complex interplay of bidirectional signals between the nucleus and the mitochondria. Most essential mitochondrial genes are encoded in the nuclear genome requiring careful coordination of nuclear and mitochondrial gene expression. This need for careful coordination may have provided the selective pressure that resulted in the epigenetic effects of the mitochondrial derived metabolites like acetyl-CoA, α-ketoglutarate and NAD+/NADH on nuclear gene expression in one direction [3] and the effects of nuclear encoded proteins and growth factor signaling on mitochondrial function in the other [4]. Given the growth and energetic advantages provided by mitochondria it would be intuitive to assume that cancer cells would utilize them to maximize growth and survival. However, early work in cancer metabolism suggested mitochondria function was not only non-contributory but might actually be detrimental.
Initial observations by Otto Warburg revealed that most tumors contained cells that were highly glycolytic even in the presence of oxygen in contrast to normal cells. Normal cells will take up glucose and completely oxidize it to CO2 and water in the presence of oxygen and only rely on glycolytic energy generation when oxygen levels are insufficient. This behavior mimics what Pasteur described in fermenting yeasts in the 1890s. In contrast, the tumor slices that Dr. Warburg studied in vitro and in vivo took up excess glucose and converted it primarily to lactate with minimal levels of oxygen consumption regardless of the amount of oxygen present [5]. This phenomenon was so pervasive in cancer that Dr. Warburg put forth the hypothesis that mitochondrial dysfunction was a fundamental aspect of cancer development [5]. The wide differences in metabolism between normal tissues and cancer made this an attractive target to develop cancer therapeutics. Indeed, some of the earliest chemotherapies developed targeted folate metabolism [6]. However, glycolytic inhibitors like 2-deoxyglucose demonstrated unimpressive responses and issues with toxicity [7]. Recently, with the development of powerful new tools a new paradigm of mitochondrial metabolism in cancer has emerged and with it a new interest in the development of agents that can target it.
1.1. Warburg metabolism is the metabolism of proliferation
At first pass the high glucose uptake and conversion to lactate exhibited by cancer cells seems counter-intuitive. Why would highly dividing cells rely on such an inefficient means of generating ATP? The answer lays in the fact that tumor cells are not in the business of making ATP but rather the business of making the next tumor cell. Carbon in the form of glucose or glutamine must be converted to the necessary lipids, amino acids and nucleotides that will be the building blocks of the next cell. While oxidative metabolism is the most efficient means of generating high energy electrons and ATP its final products are CO2 and water, materials not sufficient to the generation of the next tumor cell. It is analogous to someone trying to construct a house but using all the lumber to build a fire. Instead, highly glycolytic tumors divert multiple glycolytic intermediates into parallel metabolic pathways like the pentose phosphate pathway to generate needed ribose and NADPH for nucleotide synthesis [8]. Those carbons that are metabolized further to trioses can be removed from the pathway and converted to glycerol for phospholipid synthesis and serine for protein or nucleotide synthesis [8]. Finally, those carbons that are fully catabolized via glycolysis and converted to pyruvate can be shunted into the mitochondria and converted to acetyl-CoA for entry into the TCA cycle [9]. TCA cycle intermediates are then either oxidized for catabolic purposes or converted to amino acids or citrate for export back to the cytoplasm [9]. When viewed as a means for re-organizing carbon skeletons into needed biomolecules the adaptive advantages of a highly glycolytic state come into focus. The conversion of pyruvate to lactate is needed to maintain an adequate NAD+/NADH ratio to allow for the continued conversion of hexoses to trioses. Additionally, the utilization of the kinetically slower isoform of pyruvate kinase (PKM2) allows for the maximal levels of glycolytic intermediates to be shunted into other pathways. This initially “wasteful” system ultimately reveals itself to be the most efficient utilization of precious nutrients once one understands the ultimate goal is cellular reproduction. Warburg’s conclusion that tumors must lack of functional mitochondria did not consider the fact that oxidative catabolism via the electron transport chain is but one of many functions carried out by the mitochondria. His experiments did not examine the conversion of TCA cycle intermediates into amino acid, lipid and nucleic acid precursors in reactions that do not consume oxygen. This metabolism of proliferation is not unique to tumor cells as normal cells like lymphocytes will adapt similar metabolic phenotypes when stimulated to proliferate [10]. The differences lie in the fact that normal cells will make these adaptations only in the presence of the appropriate growth signals and adequate nutrients. Tumor cells in contrast are driven by constant oncogenic signaling that drives these metabolic changes independent of growth factor signaling and nutrient conditions. This difference underlies a possible therapeutic window for the treatment of cancer with metabolic inhibitors.
1.2. The mitochondria strike back
Otto Warburg was the unquestioned master of tumor metabolism of his time and reportedly wrote a one sentence grant application “I will require 10,000 marks” that was ultimately funded by the German government [11]. Given the data at the time his conclusion that mitochondrial dysfunction was needed for tumor development was understandable. This initially led the field to disregard the contributions of mitochondrial metabolism to tumor formation and growth. More recently a reemergence of interest in mitochondria in cancer has been fueled by experiments that have cast doubt on the previously held dogma. For example, experiments were done to show that cancer cells grown in ethidium bromide, which depletes mitochondrial DNA, grow slower and are less capable of making tumors in animals suggesting a mitochondrial contribution to tumor growth and aggressiveness [12,13]. As the field matured more elegant systems to show this contribution were employed. One example utilized a TFAM knockout model. TFAM is a nuclear encoded transcription factor needed for maintenance and transcription of the mitochondrial genome. In a genetically engineered mouse model of lung cancer driven by mutant Kras expression mice with floxed TFAM alleles who were exposed to an inhaled Cre recombinase developed significantly smaller and less proliferative tumors [14]. The role of mitochondrial metabolism was further established when the role IDH2 mutations in tumorigenesis was uncovered. IDH2 is a mitochondrial enzyme that catalyzes the oxidative decarboxylation of isocitrate to α-ketoglutarate. IDH2 mutations are found to occur recurrently in acute myeloid leukemia (AML), gliomas, astrocytomas and chondromas. The mutations result in a neomorphic enzyme that will catalyze the conversion of α-ketoglutarate to D-2-hydroxyglutarate (2HG). This results in an excess of 2HG that is a competitive inhibitor of the ten eleven translocation (TET) family of enzymes needed for the demethylation of cytosine nucleotides in DNA. Additionally, the Jumonji-C domain histone Nε-lysine demethylases (KDMs) responsible for histone demethylation are also inhibited by 2HG. This results in changes in DNA and histone methylation leading to aberrant transcription and ultimately to transformation [15]. The transforming potential of IDH2 mutations was confirmed in a study that showed the R140Q mutation could cooperate with FLT3 mutations in the generation of AML and importantly the loss of this mutation in an established AML resulted in severe growth inhibition validating it as a bona fide oncogene and potential therapeutic target [16]. These studies and others like have demonstrated the key role mitochondrial metabolism plays in tumorigenesis and tumor progression.
1.3. Warburg strikes back, some tumors really do require mitochondrial dysfunction
Despite the fact that the vast majority of cancers have intact mitochondrial function there are several types of rare tumors that actually do require mitochondrial dysfunction. Succinate dehydrogenase (SDH) and fumarate hydratase (FH) have been found to be recurrently mutated in hereditary paraganglioma and pheochromocytoma for the former and hereditary leiomyomatosis and renal cell cancer in the latter. In addition, mutations in genes encoding SDH subunits have also been identified in gastrointestinal stromal tumors, renal tumors and testicular seminomas among others. In the hereditary syndromes one copy of the nonfunctional allele is passed on in the germline and the tumor subsequently inactivates the remaining copy by mutation or chromosomal loss. These tumors accumulate an excess of either succinate for SDH inactivated or fumarate for FH inactivated tumors. These TCA cycle metabolites are also capable of inhibiting the TET and KDM families of demethylases much like 2HG resulting in an altered epigenome [17,18]. In addition to inhibiting these enzymes succinate and fumarate can also inhibit the prolyl hydroxylases (PHDs) resulting in elevated levels of HIF [19]. These tumors can circumvent the need for an intact TCA cycle via the reductive carboxylation of α-ketoglutarate to isocitrate by the IDH1/2 enzymes [20]. Interestingly, it is the fact that these enzymes utilize the higher energy electron carrier NADP+ that allows the reverse reaction to occur as opposed to IDH3 which utilizes NAD+ in an irreversible manner. It is the reversible nature of the IDH1/2 reactions that allows for the neomorphic activity of the mutated forms of these enzymes as discussed above. These data taken together suggest that even in tumors where mitochondrial function is impaired the metabolic flow through the mitochondria is nevertheless critical.
1.4. Mitochondria more than just a power house
In addition to generating ATP and biosynthetic precursors mitochondria are also responsible for the production of cellular reactive oxygen species (ROS). Mitochondrial derived ROS from activity of the electron transport chain can be converted to H2O2 by the action of super oxide dismutase (SOD2). H2O2 then serves as a diffusible signaling molecule that can modulate the activity of ROS sensitive proteins like the FOS–JUN transcription factors [21]. Finally, perhaps one of the most important mitochondrial functions in cancer is the initiation of the apoptotic response by the release of cytochrome C into the cytoplasm [22]. Mitochondria have the ability to undergo an outer membrane permeability transition allowing for the release of cytochrome C into the cytoplasm. The released cytochrome C initiates formation of the apoptosome that culminates in the activation of the final effector caspases 3 and 7. This transition is controlled by the relative abundance and function of the anti-apoptotic BCL-2 family members and the proapoptotic BH3 only family members [23]. Several tumor types are known to overexpress BCL-2 and its family members in order to suppress apoptosis [24,25] revealing another role of mitochondria in cancer development.
1.5. Taking the war on cancer to the mitochondria
Having derived considerable insights from the above studies as to the essential nature of mitochondrial function in cancer the question then becomes can this be targeted in a therapeutic manner? A large number of mitochondrial metabolism inhibitors are under clinical development and several have achieved FDA approval. An overview of some of these molecules is detailed below.
2. Targeting the TCA cycle
2.1. Enasidinib
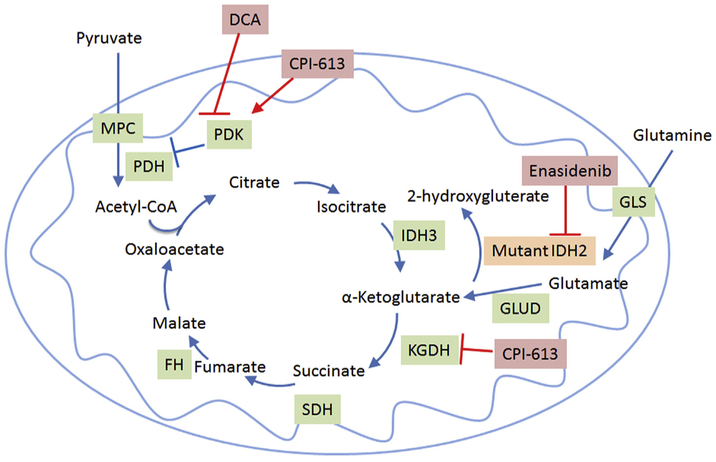
Enasidinib is a small molecule inhibitor of the mutant IDH2 protein that catalyzes the conversion of α-ketoglutarate to the oncometabolite 2HG as discussed above (Fig. 1). Enasidinib has been shown to inhibit the production of 2HG in IDH2 mutated AML in preclinical systems [26,27]. Interestingly, instead of inducing an apoptotic cell death this seems to remove the differentiation arrest imposed on the cells by the production of 2HG and leads to the maturation of the leukemia blasts into mature blood cells. In early phase clinical trials enasidinib treatment in patients with relapsed or refractory IDH2 mutated AML resulted in a 24% complete remission rate (with and without complete count recovery) and an overall response rate of 40%. Evidence of maturation of blasts was seen in samples taken from responding patients corroborating the earlier preclinical studies [28]. On the basis of the clinical efficacy the FDA granted enasidinib an accelerated approval for the treatment of IDH2 mutated AML patients with relapsed or refractory disease. Additional clinical trials are ongoing in the relapsed setting compared to conventional care regimens (ClinicalTrials.gov Identifier: NCT02577406) and in the upfront setting in combination with chemotherapy (ClinicalTrials.gov Identifier: NCT03013998).
Fig. 1.
Overview of the TCA cycle and therapeutics that target it. DCA, dichloroacetate; GLS, Glutaminase; GLUD, glutamine dehydrogenase; IDH, isocitrate dehydrogenase; KGDH, α-ketoglutarate dehydrogenase; MPC, mitochondrial pyruvate carrier complex; PDH, pyruvate dehydrogenase complex; PDK, pyruvate dehydrogenase kinase.
2.2. CPI-613
CPI-613 is a novel lipoate derivative that has shown to inhibit pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase (KGDH, Fig. 1). It inhibits PDH by activating pyruvate dehydrogenase kinase that phosphorylates and inactivates PDH [29]. CPI-613 inhibits KDGH by creating a burst of reactive oxygen species and activating a redox-sensing mechanism that results in the glutathionylation of the enzyme blocking its activity [30]. This inhibition of both PDH and KGDH effectively inhibits the points of entry for carbons derived from either glucose or glutamine into the TCA cycle. This prevents mitochondrial carbon metabolism and oxidative phosphorylation derived from the oxidation of TCA cycle intermediates. In preclinical studies CPI-613 has shown extensive anticancer effects. In a phase one study, CPI-613 has shown to be well tolerated and active in several patients with advanced myeloid malignancies [31]. In combination with chemotherapy it had encouraging activity in patients with relapsed or refractory AML especially in patients 60 years of age or older (Pardee et al. in press). CPI-613 in combination with chemotherapy roughly doubled the objective response rate for patients with metastatic pancreatic cancer and showed encouraging median survival [32]. CPI-613 is currently being evaluated in several additional clinical trials (ClinicalTrials.gov Identifiers: NCT02168140, NCT02232152, NCT02484391).
2.3. Dichloroacetate
Dichloroacetate (DCA) activates the TCA cycle by shifting cell metabolism from glycolysis to mitochondrial glucose oxidation. It does this primarily by inhibiting PDK1 (Fig. 1) which in turn leads to the activation of PDH and overall conversion of cytosolic pyruvate to mitochondrial acetyl-CoA [33]. One study showed that DCA sensitized metformin-cytotoxicity by reprogramming glucose metabolism from glycolysis to mitochondrial oxidation [34]. Another study demonstrated that DCA affects the proliferation of human colorectal cancer cells without affecting survival [35]. Additionally, DCA has been used in clinical trials including one study in glioblastoma that showed some indication of efficacy [36]. However other studies in advanced solid tumor patients demonstrated limited responses and concern for toxicity [37]. A more recent study (NCT01029925) for patients with previously treated metastatic breast or non-small cell lung cancer was terminated at the recommendation of the study safety monitoring board secondary to unexpected toxicity. Currently, there are no open cancer studies with DCA listed on clinicaltrials.gov.
3. Targeting the electron transport chain (ETC)
3.1. Metformin/Phenformin
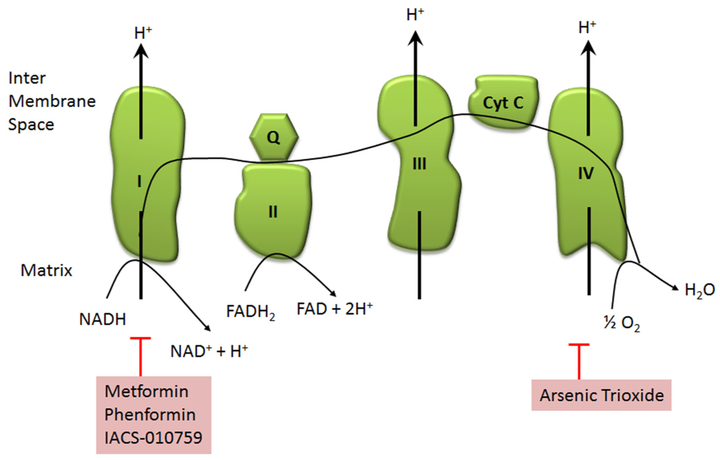
Metformin is currently used to treat diabetes however it has also shown some activity in preclinical and clinical trials settings. Metformin is a complex I inhibitor of the electron transport chain (Fig. 2). As a result it increases AMP and decreases ATP. This causes an increase in the AMP/ATP ratio leading to the activation of adenosine monophosphate activated protein kinase (AMPK) which promotes catabolism and inhibits anabolic pathways like fatty acid synthesis [38]. In pre-clinical studies it sensitized leukemia stem cells to apoptosis [39]. Metformin is currently being tested in several clinical trials. A precursor molecule Phenformin, was taken off the market for diabetes due to its increased risk of lactic acidosis but has efficacy in against multiple cancer cell lines and preclinical models [40–44]. Phenformin is not reliant on transporters for cell entry and has increased potency for the mitochondrial membrane compared to metformin [45]. It also has a 10-fold higher potency than metformin in sensitizing leukemia cells Bcl-2 inhibiton [39]. It is also being explored in clinical trials as the toxicity previously observed was in patients with diabetes where the risk benefit ratio is significantly different from those suffering with a fatal malignancy. There is currently one clinical trial looking at adding phenformin to the tyrosine kinase inhibitors dabrafenib and tremetinib for patients with metastatic BRAFV600E/K mutated melanoma (ClinicalTrials.gov Identifier: NCT03026517).
Fig. 2.
Overview of the electron transport chain and therapies that target these complexes. Complex I – NADH dehydrogenase; Complex II-succinate dehydrogenase; Complex III-cytochrome bc1 complex; complex IV-cytochrome c oxidase; ETC – electron transport chain, Cyt C – cytochrome C; Q – quinone.
3.2. IACS-010759
IACS-010759 works in a similar manner as metformin (Fig. 2). It is a complex I inhibitor of the electron transport chain [46]. In preclinical studies it has promising activity against AML cells [47]. This is currently being tested in patients with advanced solid tumors (ClinicalTrials.gov Identifier: NCT03291938) as well as in acute myeloid leukemia (ClinicalTrials.gov Identifier: NCT02882321) in phase I trials.
3.3. Arsenic trioxide
Arsenic trioxide has been widely used in Chinese medicine. Arsenic trioxide is a complex IV inhibitor (Fig. 2); it ultimately suppresses mitochondrial function by inhibiting cytochrome c oxidase, a main component of complex IV [48]. This inhibition leads to subsequent electron leakage which ultimately leads to the formation of superoxide. With the formation of reactive oxygen species this drug is able to effectively decrease the mitochondrial membrane potential thus ultimately leading to apoptosis [49,50]. It is currently FDA approved for the treatment of acute promyelocytic leukemia (APL) in the upfront and relapsed settings. In APL it stimulates the degradation of the fusion protein PML-RARα resulting in the terminal differentiation of the malignant cells. It is unclear if this mechanism involves the ETC. It has been tested in the clinic in a wide range of solid tumors and hematologic malignancies at various stages of clinical trials. To date its role outside of the treatment of APL has not been firmly established.
4. Targeting mitochondrial translation and fission
4.1. Tigecycline
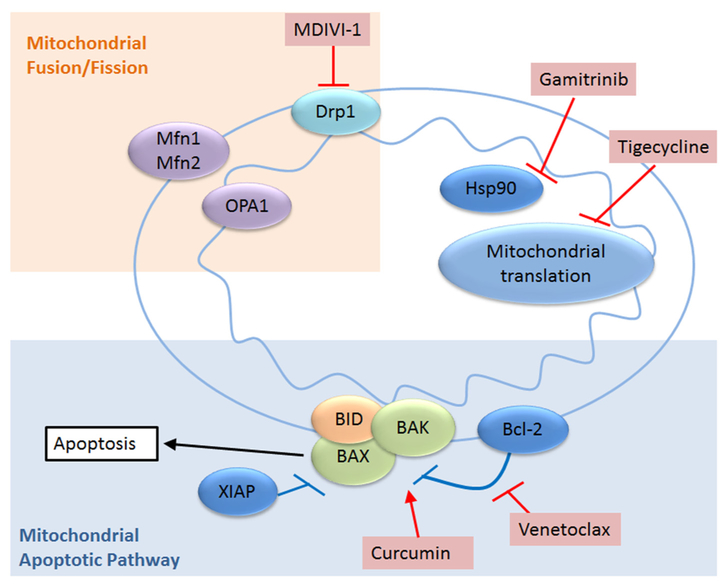
Tigecycline inhibits mitochondrial translation (Fig. 3). It binds to the 30S ribosomal subunit and blocks the interaction of aminoacyl-tRNA with the A site of the ribosome [51]. Currently it is used to treat a number of bacterial infections. Preclinically, tigecycline has shown to inhibit mitochondrial translation in AML and was more efficacious than other small molecules that were tested [52]. Tigecycline in combination with imatinib decreased the CML stem cell population dramatically in vitro and in vivo [53]. Since the toxicology and pharmacology is already known in humans this can lead to rapid advancement into clinical trials. A phase I trial in relapsed or refractory acute myeloid leukemia was conducted and the MTD in this population was established [54]. Unfortunately, no clinical responses were observed in this trial. A recent preclinical study showed promising activity of tigecycline in combination with venetoclax (discussed below) against double hit lymphomas indicating that a combination treatments may be more effective [55].
Fig. 3.
Overview of other specific mitochondrial functions that are targeted by cancer therapies. Apaf-1/BAD/BAK/BID, pro-apoptotic proteins; XIAP, anti-apoptotic protein; Mfn1/Mfn2, mitofusin; OPA1, mitochondrial dynamin like 120 kDa protein; Drp1, dynamin related protein 1; Hsp90, heat shock protein 90.
4.2. MDIVI-1
Mitochondria are mobile and dynamic organelles that can fuse with (fusion) or break off from (fission) other mitochondria [56]. MDIVI-1 is a mitochondrial fission inhibitor. It is suggested that it works in mammalian cells by inhibiting Drp1 activity [57] resulting in hyperfused mitochondria and a decrease in mitophagy. There are currently no clinical trials that use this drug but it is showing promise pre-clinically. It was shown to increase the response of ovarian cancer cells to death receptor mediated apoptosis while sparing normal cells [58]. In combination with cisplatin, MDIVI-1 induces mitochondrial uncoupling and swelling and promotes apoptosis in drug resistant breast cancer cells [59]. Interestingly, MDIVI-1 has shown to attenuate the cardiotoxicity of doxorubicin treatment by inhibiting the fragmentation of the mitochondria that is proposed to play a major role in increasing heart failure [60]. The role of mitochondrial fission in cancer cell growth and survival is an area of intense research interest.
5. Targeting mitochondrial apoptotic pathway
5.1. Venetoclax
Ventoclax is a small molecule BH3 only mimetic that binds and inhibits BCL-2 allowing the oligomerization of BAX and BAK resulting in the formation of the mitochondrial outer membrane pore complex and the cytosolic release of cytochrome C triggering apoptosis (Fig. 3). It is active in multiple cancers including lymphomas, chronic lymphocytic leukemia, small cell lung cancer and AML among others [61–63]. In clinical trials it has shown activity in relapsed and refractory chronic lymphocytic leukemia [64]. Interestingly it also shows excellent activity in CLL patients with p53 deletions [65] suggesting that loss of p53 function does not protect from venetoclax in this setting. These trial results have led to the approval of venetoclax for the treatment of CLL. More recent studies have shown activity of this agent in AML [63] and intriguingly there was a suggestion that patients harboring an IDH mutation might be particularly responsive. In a more recent study presented as an abstract venetoclax was combined with the hypomethylating agent azacytidine for elderly patients with AML. This small study revealed very encouraging response rates with an 85% remission rate [66]. The final results of this study are pending at this time.
5.2. Curcumin
Curcumin is derived from the turmeric spice. It is believed that curcumin is able to induce apoptosis by specifically targeting the mitochondria [38]. One study suggested that it works by inducing Apaf-1 dependent caspase activation [67]. Cancer cells that were treated with curcumin were able to activate caspase 3 which is further enhanced by Apaf-1 silencing. Additionally, cytochrome c release and caspase activation were inhibited with the absence of p21. The upregulation of Bax, Bak, Bid, and Bim was observed. Another study looked at a combination treatment between curcumin and tamoxifen. Tamoxifen is an estrogen receptor inhibitor. Both curcumin and tamoxifen worked synergistically and induced apoptosis in chemoresistant melanoma A375 cells and G361 cells [68]. This combination treatment also had an increase in reactive oxygen species formation and shift in mitochondrial membrane potential. Currently, curcumin is being tested in combination with standard chemotherapeutics in a variety of solid tumors, specifically cancers of the colon, breast, pancreas, prostate, and rectum, as well as chronic lymphocytic leukemia.
6. Targeting mitochondrial HSP90
6.1. Gamitrinib
Gamitrinib is a highly selective small molecule that is able to inhibit mitochondrial HSP90 [69]. HSP90 in the mitochondrial matrix serves as a chaperone protein and is increased in cancer. Inhibition of mitochondrial HSP90 causes mitochondrial collapse and selective tumor cell death [70]. It synergized with doxorubicin in preclinical xenograft models of breast and prostate cancer with no increase in cardiotoxicity [71]. When used in combination with bromodomain inhibitors gamitrinib caused synergistic cell death in glioblastoma models [72]. Gamitrinib also enhanced the activity of MAPK inhibition in BRAF mutated melanoma cell lines [73].
7. Conclusions/future directions
The mitochondria have gone from being considered superfluous to cancer metabolism to becoming one of the main centers of focus of the field. Several promising new agents are currently in the clinic and several have already changed the way cancer patients are treated. One emerging theme is the power of mitochondrial targeted attacks to sensitize cancer cells to chemotherapy, kinase inhibitors or even other metabolic inhibitors. Most agents examined clinically to date have limited single agent activity pointing to metabolic flexibility of cancer cells that allows for resistance. This should not be surprising or discouraging and lack of single agent activity should not determine the fate of a promising agent. As the understanding of the mechanisms of metabolic flexibility utilized by tumors is increasingly understood new targets and strategies will be developed and a completely metabolically targeted approach to cancer treatment will be possible. The metabolic requirements of the immune system and its manipulation in the tumor microenvironment open up additional future avenues of attack. Specifically impairing the tumor’s ability to utilize immunosuppressive metabolic pathways will allow increased opportunities to harness the power of the immune system to help more patients. Collaboration between metabolic inhibitors, chemotherapy, tyrosine kinase inhibitors and immune therapies is the future of cancer care. If the pharmaceutical, clinical and scientific communities are to develop these strategies, close collaboration will be needed there as well.
Funding
TSP, LPG and RGA are supported by 1R01CA197991–01A1.
Footnotes
Conflicts of interest
TSP is the Chief Medical Officer of Rafael Pharmaceuticals and has received research funding from them. Rafael Pharmaceuticals owns the licensing rights to CPI-613 and is currently developing it for use in oncology patients. Rafael Pharmaceuticals had no input over the content of this manuscript.
References
- [1].Margulis L, Origin of Eukaryotic Cells; Evidence and Research Implications for a Theory of the Origin and Evolution of Microbial, Plant, and Animal Cells on the Precambrian Earth, Yale University Press, 1970. Place Published. [Google Scholar]
- [2].C B, Uber die Spermatogenese Arch, Anal. Physiol (1898) 393–398. [Google Scholar]
- [3].Kinnaird A, Zhao S, Wellen KE, Michelakis ED, Metabolic control of epigenetics in cancer, Nat. Rev. Cancer 16 (2016) 694–707. [DOI] [PubMed] [Google Scholar]
- [4].Fan J, Shan C, Kang HB, Elf S, Xie J, Tucker M, Gu TL, Aguiar M, Lonning S, Chen H, Mohammadi M, Britton LM, Garcia BA, Aleckovic M, Kang Y, Kaluz S, Devi N, Van Meir EG, Hitosugi T, Seo JH, Lonial S, Gaddh M, Arellano M, Khoury HJ, Khuri FR, Boggon TJ, Kang S, Chen J, Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex, Mol. Cell 53 (2014) 534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Warburg O, On the origin of cancer cells, Science 123 (1956) 309–314. [DOI] [PubMed] [Google Scholar]
- [6].Papac RJ, Origins of cancer therapy, Yale J. Biol. Med 74 (2001) 391–398. [PMC free article] [PubMed] [Google Scholar]
- [7].Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK, Kroll S, Jung DT, Kurtoglu M, Rosenblatt J, Lampidis TJ, A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors, Cancer Chemother. Pharmacol 71 (2013) 523–530. [DOI] [PubMed] [Google Scholar]
- [8].Wallace DC, Mitochondria and cancer, Nat. Rev. Cancer 12 (2012) 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weinberg SE, Chandel NS, Targeting mitochondria metabolism for cancer therapy, Nat. Chem. Biol 11 (2014) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chi Van D, Jung-whan K, Convergence of cancer metabolism and immunity: an overview, Biomol. Ther 26 (2018) 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koppenol WH, Bounds PL, Dang CV, Otto Warburg's contributions to current concepts of cancer metabolism, Nat. Rev. Cancer 11 (2011) 325. [DOI] [PubMed] [Google Scholar]
- [12].Morais R, Zinkewich-Peotti K, Parent M, Wang H, Babai F, Zollinger M, Tumor-forming ability in athymic nude mice of human cell lines devoid of mitochondrial DNA, Cancer Res. 54 (1994) 3889–3896. [PubMed] [Google Scholar]
- [13].Cavalli LR, Varella-Garcia M, Liang BC, Diminished tumorigenic phenotype after depletion of mitochondrial DNA, Cell Growth Differ. 8 (1997) 1189–1198. [PubMed] [Google Scholar]
- [14].Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GRS, Chandel NS, Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity, Proc. Natl. Acad. Sci 107 (2010) 8788–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, Tallman MS, Sun Z, Wolniak K, Peeters JK, Liu W, Choe SE, Fantin VR, Paietta E, Löwenberg B, Licht JD, Godley LA, Delwel R, Valk PJM, Thompson CB, Levine RL, Melnick A, Leukemic IDH1 and IDH2 mutations result in a Hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation, Cancer Cell 18 (2010) 553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kats Lev M., Reschke M, Taulli R, Pozdnyakova O, Burgess K, Bhargava P, Straley K, Karnik R, Meissner A, Small D, Su Shinsan M, Yen K, Zhang J, Pandolfi Pier P., Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance, Cell Stem Cell 14 (2014) 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y, Zhao S, Ye D, Xiong Y, Guan KL, Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors, Genes Dev. 26 (2012) 1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, Quezado M, Smith WI Jr., Jahromi MS, Xekouki P, Szarek E, Walker RL, Lasota J, Raffeld M, Klotzle B, Wang Z, Jones L, Zhu Y, Wang Y, Waterfall JJ, O’Sullivan MJ, Bibikova M, Pacak K, Stratakis C, Janeway KA, Schiffman JD, Fan JB, Helman L, Meltzer PS, Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor, Cancer Discovery 3 (2013) 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E, Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase, Cancer Cell 7 (2005) 77–85. [DOI] [PubMed] [Google Scholar]
- [20].Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ, Reductive carboxylation supports growth in tumour cells with defective mitochondria, Nature 481 (2011) 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abate C, Patel L, Rauscher FJ 3rd, Curran T, Redox regulation of fos and Jun DNA-binding activity in vitro, Science 249 (1990) 1157–1161. [DOI] [PubMed] [Google Scholar]
- [22].Liu X, Kim CN, Yang J, Jemmerson R, Wang X, Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c, Cell 86 (1996) 147–157. [DOI] [PubMed] [Google Scholar]
- [23].Czabotar PE, Lessene G, Strasser A, Adams JM, Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy, Nat. Rev. Mol. Cell Biol 15 (2013) 49. [DOI] [PubMed] [Google Scholar]
- [24].Davids MS, Letai A, Targeting the B-cell lymphoma/leukemia 2 family in Cancer, J. Clin. Oncol 30 (2012) 3127–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Adams JM, Cory S, The Bcl-2 apoptotic switch in cancer development and therapy, Oncogene 26 (2007) 1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang F, Travins J, DeLaBarre B, Penard-Lacronique V, Schalm S, Hansen E, Straley K, Kernytsky A, Liu W, Gliser C, Yang H, Gross S, Artin E, Saada V, Mylonas E, Quivoron C, Popovici-Muller J, Saunders JO, Salituro FG, Yan S, Murray S, Wei W, Gao Y, Dang L, Dorsch M, Agresta S, Schenkein DP, Biller SA, Su M, de Botton S, Yen KE, Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation, Science 340 (2013) 622–626. [DOI] [PubMed] [Google Scholar]
- [27].Yen K, Travins J, Wang F, David MD, Artin E, Straley K, Padyana A, Gross S, DeLaBarre B, Tobin E, Chen Y, Nagaraja R, Choe S, Jin L, Konteatis Z, Cianchetta G, Saunders JO, Salituro FG, Quivoron C, Opolon P, Bawa O, Saada V, Paci A, Broutin S, Bernard OA, de Botton S, Marteyn BS, Pilichowska M, Xu Y, Fang C, Jiang F, Wei W, Jin S, Silverman L, Liu W, Yang H, Dang L, Dorsch M, Penard-Lacronique V, Biller SA, Su M, AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations, Cancer Discov. 7 (2017) 478–493. [DOI] [PubMed] [Google Scholar]
- [28].Amatangelo MD, Quek L, Shih A, Stein EM, Roshal M, David MD, Marteyn B, Farnoud NR, de Botton S, Bernard OA, Wu B, Yen KE, Tallman MS, Papaemmanuil E, Penard-Lacronique V, Thakurta A, Vyas P, Levine RL, Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response, Blood 130 (2017) 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zachar Z, Marecek J, Maturo C, Gupta S, Stuart SD, Howell K, Schauble A, Lem J, Piramzadian A, Karnik S, Lee K, Rodriguez R, Shorr R, Bingham PM, Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo, J. Mol. Med 89 (2011) 1137–1148. [DOI] [PubMed] [Google Scholar]
- [30].Stuart SD, Schauble A, Gupta S, Kennedy AD, Keppler BR, Bingham PM, Zachar Z, A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process, Cancer Metab. 2 (2014) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pardee TS, Lee K, Luddy J, Maturo C, Rodriguez R, Isom S, Miller LD, Stadelman KM, Levitan D, Hurd D, Ellis LR, Harrelson R, Manuel M, Dralle S, Lyerly S, Powell BL, A phase I study of the first-in-class antimitochondrial metabolism agent, CPI-613, in patients with advanced hematologic malignancies, Clin. Cancer Res. 20 (2014) 5255–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Alistar A, Morris BB, Desnoyer R, Klepin HD, Hosseinzadeh K, Clark C, Cameron A, Leyendecker J, D’Agostino R, Topaloglu U, Boteju LW, Boteju AR, Shorr R, Zachar Z, Bingham PM, Ahmed T, Crane S, Shah R, Migliano JJ, Pardee TS, Miller L, Hawkins G, Jin G, Zhang W, Pasche B, Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-Centre, open-label, dose-escalation, phase 1 trial, Lancet Oncol. 18 (2017) 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ruggieri V, Agriesti F, Scrima R, Laurenzana I, Perrone D, Tataranni T, Mazzoccoli C, Lo Muzio L, Capitanio N, Piccoli C, Dichloroacetate, a selective mitochondria-targeting drug for oral squamous cell carcinoma: a metabolic perspective of treatment, Oncotarget 6 (2015) 1217–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Choi YW, Lim IK, Sensitization of metformin-cytotoxicity by dichloroacetate via reprogramming glucose metabolism in cancer cells, Cancer Lett. 346 (2014) 300–308. [DOI] [PubMed] [Google Scholar]
- [35].Delaney LM, Ho N, Morrison J, Farias NR, Mosser DD, Coomber BL, Dichloroacetate affects proliferation but not survival of human colorectal cancer cells, Apoptosis 20 (2015) 63–74. [DOI] [PubMed] [Google Scholar]
- [36].Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC, Metabolic modulation of glioblastoma with dichloroacetate, Sci. Transl. Med 2 (2010) 31ra34. [DOI] [PubMed] [Google Scholar]
- [37].Chu QS, Sangha R, Spratlin J, Vos LJ, Mackey JR, McEwan AJ, Venner P, Michelakis ED, A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors, Investig. New Drugs 33 (2015) 603–610. [DOI] [PubMed] [Google Scholar]
- [38].Wen S, Zhu D, Huang P, Targeting cancer cell mitochondria as a therapeutic approach, Future Med. Chem 5 (2013) 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Velez J, Pan R, Lee JT, Enciso L, Suarez M, Duque JE, Jaramillo D, Lopez C, Morales L, Bornmann W, Konopleva M, Krystal G, Andreeff M, Samudio I, Biguanides sensitize leukemia cells to ABT-737-induced apoptosis by inhibiting mitochondrial electron transport, Oncotarget 7 (2016) 51435–51449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jackson AL, Sun W, Kilgore J, Guo H, Fang Z, Yin Y, Jones HM, Gilliam TP, Zhou C, Bae-Jump VL, Phenformin has anti-tumorigenic effects in human ovarian cancer cells and in an orthotopic mouse model of serous ovarian cancer, Oncotarget 8 (2017) 100113–100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Guo Z, Zhao M, Howard EW, Zhao Q, Parris AB, Ma Z, Yang X, Phenformin inhibits growth and epithelial-mesenchymal transition of ErbB2-overexpressing breast cancer cells through targeting the IGF1R pathway, Oncotarget 8 (2017) 60342–60357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rajeshkumar NV, Yabuuchi S, Pai SG, De Oliveira E, Kamphorst JJ, Rabinowitz JD, Tejero H, Al-Shahrour F, Hidalgo M, Maitra A, Dang CV, Treatment of pancreatic cancer patient-derived xenograft panel with metabolic inhibitors reveals efficacy of phenformin, Clin. Cancer Res. 23 (2017) 5639–5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Petrachi T, Romagnani A, Albini A, Longo C, Argenziano G, Grisendi G, Dominici M, Ciarrocchi A, Dallaglio K, Therapeutic potential of the metabolic modulator phenformin in targeting the stem cell compartment in melanoma, Oncotarget 8 (2017) 6914–6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kang JH, Lee SH, Lee JS, Nam B, Seong TW, Son J, Jang H, Hong KM, Lee C, Kim SY, Aldehyde dehydrogenase inhibition combined with phenformin treatment reversed NSCLC through ATP depletion, Oncotarget 7 (2016) 49397–49410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Iversen AB, Horsman MR, Jakobsen S, Jensen JB, Garm C, Jessen N, Breining P, Frøkiaer J, Busk M, Results from 11C-metformin-PET scans, tissue analysis and cellular drug-sensitivity assays questions the view that biguanides affects tumor respiration directly, Sci. Rep 7 (2017) 9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bosc C, Selak MA, Sarry JE, Resistance is futile: targeting mitochondrial energetics and metabolism to overcome drug resistance in Cancer treatment, Cell Metab. 26 (2017) 705–707. [DOI] [PubMed] [Google Scholar]
- [47].Molina JR, Protopopova M, Bandi M, Bardenhagen J, Bristow C, Alimova M, Carroll C, Chang E, Feng N, Gay J, Geck Do M, Greer J, Huang S, Jiang Y, Konopleva M, Matre P, Kang Z, Liu G, McAfoos T, Morlacchi P, Smith M, Sonal S, Theroff J, Xu Q, Draetta G, Jones P, Toniatti C, Di Francesco ME, Marszalek JR, Abstract LB-A15: IACS-010759 is a novel inhibitor of oxidative phosphorylation that selectively targets AML cells by inducing a metabolic catastrophe, Mol. Cancer Ther 14 (2015) (LB-A15–LB-A15). [Google Scholar]
- [48].Sun RC, Board PG, Blackburn AC, Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells, Mol. Cancer 10 (2011) 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen YC, Lin-Shiau SY, Lin JK, Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis, J. Cell. Physiol 177 (1998) 324–333. [DOI] [PubMed] [Google Scholar]
- [50].Wang TS, Kuo CF, Jan KY, Huang H, Arsenite induces apoptosis in Chinese hamster ovary cells by generation of reactive oxygen species, J. Cell. Physiol 169 (1996) 256–268. [DOI] [PubMed] [Google Scholar]
- [51].Aleksandrov A, Simonson T, Molecular dynamics simulations of the 30S ribosomal subunit reveal a preferred tetracycline binding site, J. Am. Chem. Soc 130 (2008) 1114–1115. [DOI] [PubMed] [Google Scholar]
- [52].Skrtić M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z, Hurren R, Jitkova Y, Gronda M, Maclean N, Lai CK, Eberhard Y, Bartoszko J, Spagnuolo P, Rutledge AC, Datti A, Ketela T, Moffat J, Robinson BH, Cameron JH, Wrana J, Eaves CJ, Minden MD, Wang JC, Dick JE, Humphries K, Nislow C, Giaever G, Schimmer AD, Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia, Cancer Cell 20 (2011) 674–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kuntz EM, Baquero P, Michie AM, Dunn K, Tardito S, Holyoake TL, Helgason GV, Gottlieb E, Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells, Nat. Med 23 (2017) 1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Reed GA, Schiller GJ, Kambhampati S, Tallman MS, Douer D, Minden MD, Yee KW, Gupta V, Brandwein J, Jitkova Y, Gronda M, Hurren R, Shamas-Din A, Schuh AC, Schimmer AD, A phase 1 study of intravenous infusions of tigecycline in patients with acute myeloid leukemia, Cancer Med. 5 (2016) 3031–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ravà M, D’Andrea A, Nicoli P, Gritti I, Donati G, Doni M, Giorgio M, Olivero D, first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 a preclinical model of < em > MYC < /em > / < em > BCL2 < /em > double-hit B cell lymphoma, Sci. Transl. Med (2018) 10. [DOI] [PubMed] [Google Scholar]
- [56].Archer SL, Mitochondrial dynamics — mitochondrial fission and fusion in human diseases, N. Engl. J. Med 369 (2013) 2236–2251. [DOI] [PubMed] [Google Scholar]
- [57].Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J, Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization, Dev. Cell 14 (2008) 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang J, Hansen K, Edwards R, Van Houten B, Qian W, Mitochondrial division inhibitor 1 (mdivi-1) enhances death receptor-mediated apoptosis in human ovarian cancer cells, Biochem. Biophys. Res. Commun 456 (2015) 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Qian W, Wang J, Roginskaya V, McDermott LA, Edwards RP, Stolz DB, Llambi F, Green DR, Van Houten B, Novel combination of mitochondrial division inhibitor 1 (mdivi-1) and platinum agents produces synergistic pro-apoptotic effect in drug resistant tumor cells, Oncotarget 5 (2014) 4180–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gharanei M, Hussain A, Janneh O, Maddock H, Attenuation of doxorubicin-induced cardiotoxicity by mdivi-1: a mitochondrial division/mitophagy inhibitor, PLoS One 8 (2013) e77713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jiang H, Lwin T, Zhao X, Ren Y, Li G, Moscinski L, Shah B, Tao J, Venetoclax as a single agent and in combination with PI3K-MTOR1/2 kinase inhibitors against ibrutinib sensitive and resistant mantle cell lymphoma, Br. J. Haematol (January 2018), 10.1111/bjh.15079 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lochmann TL, Floros KV, Naseri M, Powell KM, Cook W, March RJ, Stein GT, Greninger P, Maves YK, Saunders LR, Dylla SJ, Costa C, Boikos SA, Leverson JD, Souers AJ, Krystal GW, Harada H, Benes CH, Faber AC, Venetoclax is effective in small-cell lung cancers with high BCL-2 expression, Clin. Cancer Res 24 (2018) 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, Blum W, DiNardo CD, Kadia T, Dunbar M, Kirby R, Falotico N, Leverson J, Humerickhouse R, Mabry M, Stone R, Kantarjian H, Letai A, Efficacy and biological correlates of response in a phase II study of Venetoclax monotherapy in patients with acute myelogenous leukemia, Cancer Discov. 6 (2016) 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, Wong S, Dunbar M, Zhu M, Desai MB, Cerri E, Heitner Enschede S, Humerickhouse RA, Wierda WG, Seymour JF, Targeting BCL2 with Venetoclax in relapsed chronic lymphocytic leukemia, N. Engl. J. Med 374 (2016) 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, Puvvada SD, Wendtner CM, Roberts AW, Jurczak W, Mulligan SP, Bottcher S, Mobasher M, Zhu M, Desai M, Chyla B, Verdugo M, Enschede SH, Cerri E, Humerickhouse R, Gordon G, Hallek M, Wierda WG, Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study, Lancet Oncol. 17 (2016) 768–778. [DOI] [PubMed] [Google Scholar]
- [66].Pollyea DA, Stevens BM, Winters A, Minhajuddin M, Gutman JA, Purev E, Smith C, Abbott D, Jordan CT, Venetoclax (Ven) with Azacitidine (Aza) for untreated elderly acute myeloid leukemia (AML) patients (pts) unfit for induction chemotherapy: single center clinical experience and mechanistic insights from correlative studies, Blood 130 (2017) 181.28515093 [Google Scholar]
- [67].Gogada R, Amadori M, Zhang H, Jones A, Verone A, Pitarresi J, Jandhyam S, Prabhu V, Black JD, Chandra D, Curcumin induces Apaf-1-dependent, p21-mediated caspase activation and apoptosis, Cell Cycle 10 (2011) 4128–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chatterjee SJ, Pandey S, Chemo-resistant melanoma sensitized by tamoxifen to low dose curcumin treatment through induction of apoptosis and autophagy, Cancer Biol. Ther 11 (2011) 216–228. [DOI] [PubMed] [Google Scholar]
- [69].Kang BH, Tavecchio M, Goel HL, Hsieh CC, Garlick DS, Raskett CM, Lian JB, Stein GS, Languino LR, Altieri DC, Targeted inhibition of mitochondrial Hsp90 suppresses localised and metastatic prostate cancer growth in a genetic mouse model of disease, Br. J. Cancer 104 (2011) 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC, Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network, Cell 131 (2007) 257–270. [DOI] [PubMed] [Google Scholar]
- [71].Park HK, Lee JE, Lim J, Jo DE, Park SA, Suh PG, Kang BH, Combination treatment with doxorubicin and gamitrinib synergistically augments anticancer activity through enhanced activation of Bim, BMC Cancer 14 (2014) 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ishida CT, Shu C, Halatsch ME, Westhoff MA, Altieri DC, Karpel-Massler G, Siegelin MD, Mitochondrial matrix chaperone and c-myc inhibition causes enhanced lethality in glioblastoma, Oncotarget 8 (2017) 37140–37153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhang G, Frederick DT, Wu L, Wei Z, Krepler C, Srinivasan S, Chae YC, Xu X, Choi H, Dimwamwa E, Ope O, Shannan B, Basu D, Zhang D, Guha M, Xiao M, Randell S, Sproesser K, Xu W, Liu J, Karakousis GC, Schuchter LM, Gangadhar TC, Amaravadi RK, Gu M, Xu C, Ghosh A, Xu W, Tian T, Zhang J, Zha S, Liu Q, Brafford P, Weeraratna A, Davies MA, Wargo JA, Avadhani NG, Lu Y, Mills GB, Altieri DC, Flaherty KT, Herlyn M, Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors, J. Clin. Invest 126 (2016) 1834–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]