Abstract
We have developed an integrated pipeline for countermeasure discovery that, under the auspices of the National Institutes of Health Countermeasures Against Chemical Threats network, is one of the few efforts within academia that by design spans the spectrum from discovery to phase I. The successful implementation of this approach for cyanide would enable efficient proof-of-concept studies that would lay the foundation for a generalizable strategy for parallel mechanistic studies and accelerated countermeasure development in the face of new and emerging chemical threats.
Keywords: cyanide, countermeasure, drug discovery, animal models, metabolomics
Cyanide is an archetypal chemical threat. This powerful natural toxin is encountered in many different settings, and potential exposures range from industrial accidents to major terrorist attacks. Cyanide has devastating consequences. Higher quantities lead rapidly to seizures, cardiovascular collapse, and death, while lower doses are associated with substantial long-term morbidity, including debilitating central nervous system injury. Currently available antidotes simply do not match the complexity or scale of the potential mass casualty threat scenarios, and there is an urgent need to develop novel countermeasures. To address these threats and to define an integrated approach that could be rapidly replicated for any new or emerging chemical threats, we developed a collaborative countermeasure discovery and development pipeline. In principle, the approach we describe is generalizable to virtually any therapeutic scenario that can be modeled in the zebrafish—extending beyond chemical warfare to disease models of many sorts.
The major components of the drug discovery and development process we have put in place are (1) to identify novel classes of cyanide countermeasure using unbiased approaches in a validated zebrafish model system, (2) to explore in vivo the structure–activity relationships (SARs) of these novel countermeasure classes and use medicinal chemistry to generate a series of optimized lead compounds, and (3) to validate these optimized leads in established murine and rabbit models of cyanide toxicity. Together, these individual components are focused on the overarching goal of identification of novel cyanide countermeasures poised to move directly to formal preclinical testing and phase I clinical studies.
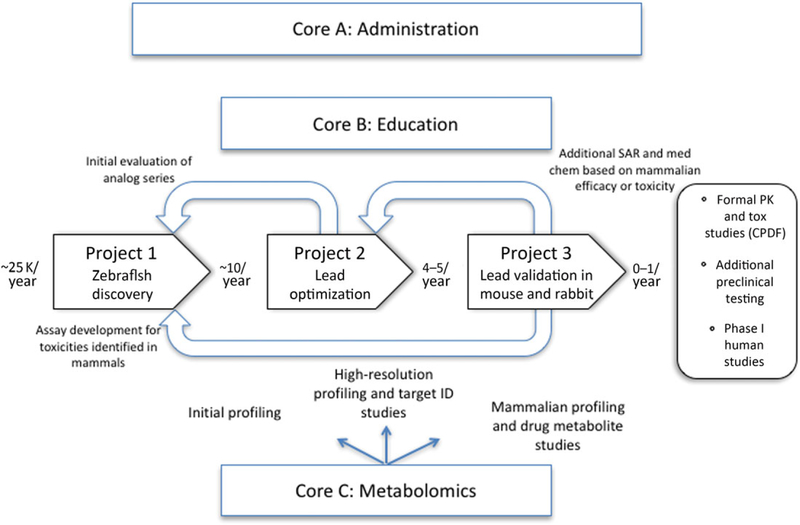
In order to accomplish this end, we integrated six discrete components: administrative, educational, and scientific cores and three research projects designed to accomplish the required steps from discovery to preclinical development. These include project 1—high-throughput in vivo discovery of cyanide antidotes; project 2—in vivo SAR and medicinal chemistry to generate optimized leads; project 3—validating leads in mouse and rabbit models of cyanide poisoning; core A—administration; core B—education; and core C—science (metabolomics).
Successful generation of validated countermeasures using this pipeline will not only lay the foundation for parallel work in preclinical development (through the Countermeasures Against Chemical Threats (CounterACT) Preclinical Development Facility (CPDF) as outlined below) and for subsequent clinical development of these compounds (in a logical extension of the current approach) but will also form a generalizable approach for the accelerated development of countermeasures to any existing or emerging chemical threat.
An outline of how each component relates and contributes to the others, including synergy between research projects and the scientific core, is given below and further elaborated in Figure 1.
Figure 1.
Schematic showing the relationship between the projects and cores. The approximate numbers of compounds moving forward at each stage are displayed in bold between each project.
Rationale
The need for novel cyanide countermeasures
The devastating consequences of large-scale chemical exposures remain among the great vulnerabilities of modern civilian populations. Whether the result of a terrorist attack or an industrial accident, we are poorly equipped to counteract the health consequences of such exposures.1–3 New, effective countermeasures are needed. The CounterACT initiative has recognized this need for “development of new or improved therapeutic medical countermeasures against chemical threats” and prioritized efforts around several specific chemical agents.4–7 Cyanide has been identified as among the highest-priority chemical threats.8–16 Milligram quantities of cyanide can cause convulsions, seizures, cardiovascular collapse, and death in minutes, and lower doses cause a spectrum of debilitating, long-lasting pathologies, including irreversible neuronal death in select brain areas.17,18 Unlike many other chemical threats, cyanide is readily accessible in large quantities, making it feasible for terrorists to acquire. In addition, cyanide is used widely in mining and other industrial processes, so the probability of mass exposure due to accidents is much higher than for other threat agents. Cyanide exposure can also occur as a consequence of smoke inhalation, consumption of cyanide-containing foods, and infusion of certain therapeutic drugs.19–22 Therefore, development of improved cyanide countermeasures would have a positive impact on human health, even in the absence of a catastrophic mass exposure.
Existing cyanide countermeasures and their limitations
Several cyanide countermeasures are currently available in the United States: a two-component kit consisting of sodium nitrite and sodium thiosulfate4 and the single drug hydroxocobalamin. Nitrites are believed to function primarily by promoting formation of methemoglobin, which binds cyanide to form the relatively nontoxic cyanomethemoglobin.23 Sodium thiosulfate is a substrate for the cyanide-detoxifying enzyme rhodanese. The combination of nitrites and sodium thiosulfate has many limitations. Importantly, the antidote is not easy to administer. Ideally, sodium nitrite and sodium thiosulfate are infused intravenously in sequence.24 This requires significant time (sodium nitrite is typically administered over 10–20 min with continuous monitoring), relatively large infusion volumes (a typical dose of sodium thiosulfate is 12.5g in 50 mL, and hydroxocobalamin also requires substantial volume), and the involvement of experienced personnel. Thiosulfate has a distribution half-life of only 15 min after infusion20 and exhibits limited intracellular distribution, while rhodanese is predominantly located in mitochondria, so the rate of rhodanese-mediated detoxification is low.17,25–27 Fourth, the methemoglobin formed by amyl/sodium nitrite can bind only about 10 mg of cyanide—larger quantities overwhelm this detoxification mechanism20—though other unknown antidotal mechanisms allow better stoichiometry in practice. In addition, nitrites can cause dangerous decreases in blood pressure and systemic oxygen-carrying capacity.28 Thus, this drug combination can benefit only small numbers of individuals and is inadequate for mass casualty scenarios. Hydroxocobalamin, a form of vitamin B12, is a cyanide scavenger and is highly effective. It is safe at clinical doses and was licensed for use in the United States several years ago. However, like sodium nitrite and sodium thiosulfate, it requires intravenous or intraosseous infusion and so is of limited general utility as a countermeasure. Other antidotes, available outside the United States, exploit these same mechanisms, specifically methemoglobin formation (4-DMAP) or scavenging (dicobalt-EDTA).
Several cyanide antidotes are in development, including most notably cobinamide, a cobalamin analog, and sulfanegen, a substrate of the cyanide-detoxifying enzyme 3-mercaptopyruvate sulfur-transferase. Cobinamide and sulfanegen can be used singly or in combination to rescue animals from highly lethal doses of cyanide. Translation to clinical testing has been slowed by issues with solubility and stability, restriction in the total dose due to the volume required, and local toxicity with intramuscular delivery. Development of these two agents is being actively pursued, but there is room for new cyanide countermeasures that can be delivered rapidly using the intramuscular route in a mass casualty scenario. The discovery of such novel agents, enabling the neutralization of large cyanide exposures alone or in synergy with existing countermeasures, is the major focus of our countermeasure discovery platform and development pipeline.
Cyanide mechanisms
One of the major reasons why more effective mass casualty CN antidotes do not exist is the sheer complexity of the effects of cyanide. The cyanide anion has numerous toxic effects, including inhibition of the mitochondrial cytochrome c oxidase complex blocking mitochondrial respiration, inhibition of adenosine triphosphate synthesis, and inhibition of the major antioxidant enzymes catalase and superoxide dismutase.3–6 Among the earliest effects of cyanide poisoning is the release of intracellular calcium and increased nitric oxide (NO) production through the induction of NO synthase.7,8 Cyanide also leads to the generation of reactive oxygen species, including hydroxyl radicals,9,10 and may itself be converted to the potent free radical of cyanide.11 Exposure to a lethal dose of cyanide can cause death within minutes,12 but there are also long-term consequences of sublethal exposure, particularly in the central nervous system, the precise mechanisms for which remain unclear. Short-term memory loss and a Parkinson disease–like syndrome are well reported, and there are also other less well-defined neurological problems in many subjects.6
Clearly, methemoglobin formation, rhodanese-catalyzed detoxification, and cyanide scavenging are not the only potential mechanisms by which a cyanide countermeasure might function. Several other candidate mechanisms have been proposed in the literature, each with varying degrees of experimental validation. Some of the potential countermeasure mechanisms focus on detoxification or elimination of cyanide itself, while others seek to reverse the biological sequelae of cyanide. However, the range of known toxic mechanisms, the broad spectrum of possible cyanide targets, and the potential for complex interactions among these targets suggest that a distinctive approach may be necessary. In the last decade or so, many previously intractable biologic problems have yielded to phenotype-driven screens, where a range of genetic or chemical manipulations are tested in an unbiased fashion to determine those pathways or compounds that result in the desired final phenotype.29–45 As more sophisticated phenotypic assays have been developed and automated, numerous examples have emerged implicating previously unsuspected pathways in specific processes or even in specific diseases.
Targeting multiple mechanisms in parallel
For many problems, including toxic exposures, it is important to model complex systems, such as the interactions between different tissues or organs. This has led to the concept of screening in intact organisms. Whereas target-based approaches lead to discovery of compounds that modulate an in vitro system but may not be effective in vivo, phenotype-based screens in intact organisms discover compounds that are by definition effective in vivo, without regard to the specific molecular target.46,47 This phenotype-based screening approach has been referred to as “chemical genetics” because it borrows from the logic of genetics, in which phenotype-based screening is used to identify novel genes that affect a biological process of interest. The development of many drugs in use today was guided by in vivo phenotypes,7 though not in any systematic manner. For conditions that affect the complex integrated physiology of the organism (such as cyanide intoxication), phenotype-based screens are a promising approach to discover novel therapies.
While systematic in vivo screens were initially undertaken in lower organisms, such as Drosophila or nematodes, in the last few years we have pioneered the use of the zebrafish for modeling vertebrate biology in health and disease at a scale compatible with screening libraries of tens of thousands of small molecules. The zebrafish has emerged as a powerful tool for such phenotype-based screens.48–50 Its genome and body plan are similar to other vertebrates, but its optical transparency and external development make real-time observation of its internal organs simple. Numerous zebrafish disease models ranging from congenital heart defects to cancers have been developed,51–55 and the zebrafish is genetically and pharmacologically similar to humans.56,57 These attributes have already been exploited in multiple disease-suppressor screens, and the compounds identified have exhibited similar activities in mammalian models. Given the numerous potential mechanisms for reversing the effects of cyanide, we developed a large-scale, phenotype-based screen for cyanide countermeasures that we can then use to define diverse compounds with divergent mechanisms of action. Our prior work suggests that compounds that suppress chemical toxicity phenotypes in zebrafish may have direct utility as lead compounds for chemical agent countermeasures.58–75
Validation in mammalian models
The zebrafish has emerged as the dominant vertebrate for in vivo high-throughput screening, but there are clearly significant differences between fish and human physiology. In the vast majority of instances to date, compounds with activity in the zebrafish model exhibit parallel activities in mammalian or even human systems. We have explored cardiovascular physiology at each scale from single channel to the integration of multiple ion channels and found remarkable homology between the larval zebrafish and adult humans. For all of the phenotypes studied, these homologies are at least as representative or even more so than rodent physiology. Nevertheless, we anticipate some differences in the way in which zebrafish and mammals respond to toxic exposures and to specific countermeasures. These include obvious differences, such as distinctive routes of entry (e.g., inhalational, intramuscular); distinctive metabolism, distribution, and toxicity of therapeutic compounds; and specific dosing schedules. However, there are also less well-understood issues, including the partitioning of duplicated genes into discrete expression domains in the zebrafish, the effects of poikilothermia on metabolic poisons, and distinctive lipids. To ensure that the countermeasures we develop have activity in mammalian cyanide poisoning, and to understand potential differences with zebrafish, we test each of our optimized leads in validated murine and rabbit models.
Center capabilities to enhance therapeutic development
Because of the need to improve our readiness as quickly as possible, efforts that will lead directly to the advancement of candidate therapeutics through the regulatory process are of highest priority. In our U54 Center, we have created an integrated pipeline using high-throughput in vivo zebrafish screening as a discovery engine to generate promising new potential antidotes for cyanide, further exploiting the fish for in vivo SAR to guide medicinal chemistry to allow the development of distinct lead compounds for rigorous validation in two mammalian species. The overarching goal for this effort is to identify lead compounds that are suitable for formal studies of pharmacokinetics and toxicology, which would then be positioned for phase I clinical studies under the U.S. Food and Drug Administration (FDA) Animal Rule.
The fundamental innovation underlying our pipeline is the integration of in vivo phenotype-driven screening in the zebrafish with robust mammalian models in what may be the only integrated discovery and development platform funded by the National Institutes of Health (NIH). Exploiting the comprehensive native context of the larval zebrafish and automated or semiautomated assays scaled for 96-well plates, it is possible to directly screen large chemical libraries for compounds that rescue end points relevant to human cyanide poisoning in this representative vertebrate. In addition, first-pass in vivo toxicology is also undertaken in the zebrafish allowing parallel evaluation for potential adverse effects during the screening phase. These capabilities for parallel counterscreening are further exploited through an in vivo approach to SAR that enables specific phenotypes to be correlated with structural features of the small molecule. When combined iteratively with streamlined efficacy and follow-up toxicity studies in mammalian models, these techniques offer a unique strategy for lead optimization. Notably, the murine models themselves incorporate features of the most likely threat scenarios, and the continuous optical and physiologic assessment available in the rabbit model allows the pathophysiology of cyanide toxicity and its rescue to be explored at a resolution that was not previously feasible. Along the pipeline, we have positioned metabolomics at each stage to link mechanisms in the different models and different tasks. This cutting-edge technology is very relevant to cyanide toxicity (and other chemical threats) and opens the possibility for novel insights into the mechanisms of action of cyanide and different countermeasures. Notably, we have previously validated the overall strategy with several agents in diverse therapeutic areas that are now in preclinical and clinical testing.
Implications for human health
Cyanide poisoning can result from accidental spills, ingestion, smoke inhalation, or as an iatrogenic outcome, such as from clinical sodium nitroprusside infusion.4 Novel, more effective cyanide countermeasures would provide measurable, life-saving benefits to the victims of these small-scale occurrences. However, safe and effective cyanide countermeasures would offer an even more dramatic impact in the face of a large-scale exposure, as might occur during an industrial accident or a terrorist attack, where no feasible options currently exist. In such an event, ready access to cyanide antidotes could transform a potentially devastating situation into a manageable one. Existing cyanide antidotes require administration by qualified personnel and have several associated toxicities that limit their utility.4,24,28,76 More importantly, they are limited in their mode of action. Existing antidotes focus on clearing cyanide from the system, typically by sequestration or conversion to a less toxic form. As such, they are not used prophylactically and do not mitigate the chronic effects of cyanide but are of utility when administered during the acute phase of cyanide exposure. The efforts in this proposal will lead to discovery of novel, potent, efficacious cyanide countermeasures. By identifying safe and effective cyanide countermeasures and expanding the range of mechanisms by which they function, our approach is designed to significantly enhance our ability to respond to chemical threats and protect human lives.
The integrated approach that we have designed screens ~25,000 novel compounds, optimizes four to five lead compounds, and validates one to two of these leads each year. Moreover, our approach has developed the infrastructure, scientific interaction, and mechanistic understanding necessary to generalize this novel approach to countermeasure and drug development for other chemical threats and biomedical problems.
Specific components and their interactions
Phase I: high-throughput in vivo discovery of cyanide antidotes
This effort is focused on the discovery of novel cyanide countermeasures in phenotype-based chemical screens in the zebrafish.17,18 In prior work funded through the NIH CounterACT Program, we developed a zebrafish model of cyanide poisoning with stereotypical toxicities, including bradycardia, neuronal necrosis, and death. We have adapted this model for high-throughput screening and performed proof-of-concept studies in a screen for compounds that reproducibly rescue zebrafish from lethal cyanide exposure. In this screen (>100,000 compounds), we identified 26 small molecules that rescue cyanide toxicity at concentrations ≤1 uM. We performed initial SAR with commercially available derivatives and identified two compounds with EC50s against cyanide of approximately 100 nM. These discovery efforts continue in several different ways. We are able to continue to prime the pipeline with discrete agents that emerge from new chemical libraries. We are able to screen in parallel zebrafish models of uniquely vulnerable or resistant populations, specifically early development, adolescence, and the aged. We are also prioritizing screen hits for development as optimized leads through the development of mode-of-activity profile assays for neurologic, cardiac, and systemic effects in the context of cyanide exposure and the testing of candidates in all potential pairwise combinations with all existing countermeasures and cobinamide and sulfanegen.
The hits generated are progressed for subsequent optimization through SAR and medicinal chemistry in project 2. Each of these hits must meet criteria for potency and satisfy initial screens for toxicity and mode-of-action profiling. Analog series generated in project 2 are also fed back to project 1 for efficacy and toxicity screens. Similarly, specific biological questions on the therapeutic or toxic effects of drug metabolites identified in project 3, as well as combination screens with existing agents, are undertaken in project 1. Project 1 uses the metabolomics platform for initial mode-of-action profiling and for characterization of the distinctive metabolic responses to cyanide across different age groups. These approaches exploit the compressed developmental timeline exhibited in the zebrafish and allow ongoing assessment of the interaction between cyanide and various countermeasures in both acute and chronic contexts.
Phase 2: optimizing novel cyanide countermeasures
This project builds on the hits with activity in our three cyanide toxicity–screening assays and uses SAR and medicinal chemistry to optimize the potency, toxicity, safety, and solubility of these novel classes to generate genuine leads that are then fed into project 3 for mammalian validation. Efforts furnish a series of optimized leads with potency, as well as absorption, distribution, metabolism, excretion, and toxicology characteristics suitable for further validation in mammalian models.
Interactions.
This project handles ~10 compounds per year, which undergo systematic SAR studies. Iterative rounds of compound design, compound acquisition (by purchase and synthesis), and compound testing deliver optimized compounds with high potency and efficacy. These optimized compounds are then profiled for physical and pharmacological properties, including pharmacokinetic stability and toxicology. Potent molecules with acceptable profiles are moved forward for further testing in mammalian models. Project 2 receives hits from project 1 and also returns optimized compounds to project 1 for high-resolution cardiotoxicity assessment. Project 2 passes optimized leads on to project 3 and also receives mouse and rabbit serum samples back from project 3 to assess compound pharmacokinetics. Project 2 exploits core C for absorption, distribution, metabolism, and excretion (ADME) studies of the optimized leads and for high-resolution mechanism of action studies.
Project 3: validating promising drug candidates in mammalian models of cyanide poisoning
Optimized lead compounds from project 2 are tested in two established mammalian models of cyanide poisoning, both singly and in limited combinations. Inaddition, range-finding toxicity studies are performed on the drugs in mice. Of the approximately 25 drug hits identified annually in project 1, we are able to optimize four to five per year for testing in mammals in project 3.
The mouse model is a cyanide gas inhalational model, which complements well with a potassium cyanide intravenous infusion model in rabbits. The drugs are administered by intramuscular injection to the mice and by intramuscular injection and inhalation in the rabbits, since the CounterACT program is primarily interested in finding a cyanide antidote that could be given rapidly in the field. The leads discovered in project 2 are also evaluated in a unique rabbit CN toxicity model monitored with noninvasive broadband optical technologies, including diffuse optical spectroscopy and continuous-wave infrared spectroscopy. This component enables the assessment of rate and extent of pathophysiologic events of CN poisoning as well as the therapeutic responses. It is also feasible to assess the effects of cyanide treatment agents in combined CN and smoke inhalation injury. All drugs are assessed for toxicity in the mice.
Interactions.
Project 3 tests each of the optimized leads emerging from project 2 for their efficacy and safety in mammals. This project uses core C for efficacy profiling as well as initial ADME studies in mammals. Drug metabolites identified in mammalian studies will be tested in project 1 and will also be used to inform additional rounds of medicinal chemistry in project 2. In addition, focused questions on mechanism of action or undetected drug toxicity will be explored further in projects 1 and 2. Ultimately, understanding the difference among the models employed in this proposal will inform future efforts to exploit this pipeline in the development of other countermeasures or other drugs and will contribute systems level information for the Animal Rule pathway.
The center is supported by three cores: an administrative core, an education core, and a metabolomics core. The administrative core is responsible for the fiduciary aspects of the proposal. The education core focuses on the educational component of the U54 mission, executing it through the interdisciplinary training of a new generation of countermeasure scientists focusing on fostering interactions between countermeasure scientists and a range of emerging biologic disciplines. The metabolomics core offers scientific integration across the other disciplines withintheprogram.75–86
The metabolomics core
Recent advances in metabolic profiling technologies have significantly enhanced the feasibility of high throughput, phenotypic “snapshots” of a whole organism’s response to a given perturbation, though to date such technologies have principally been applied to studies in model organisms. The profiling of low-molecular-weight biochemicals, including lipids, sugars, and amino acids, that serve as substrates and products in metabolic pathways is particularly relevant to identifying diagnostic biomarkers that indicate exposure to toxic agents. In addition to serving as biomarkers, circulating metabolites may themselves participate as regulatory signals, such as in the control of blood pressure.
The core platform technology couples liquid chromatography with tandem mass spectrometry. A total of over 400 parent/daughter ion pairs (i.e., metabolites) are monitored through three selective reaction-monitoring experiments on each sample and quantitated based on internal standards. The core presently has the capability to analyze thousands of samples from humans or model organisms each year. The activities of the metabolomics core will directly benefit all three proposed projects. Metabolic profiling in zebrafish and mammalian models will contribute to the center in three significant ways: (1) it will lead to the discovery of novel diagnostic biomarkers of vulnerability to cyanide exposure, (2) it will help to establish similarities and differences between the various models of cyanide exposure, and (3) it will aid in the evaluation of countermeasure efficacy in each of the systems. The metabolomic platform is working actively with all three scientific projects at present, and we have tested the logistics of sample transfer between all the sites within the U54.
Long-term strategy
The work undertaken in this program will lead to the preclinical development of novel cyanide countermeasures, which will require formal phase I testing in humans under the Animal Rule pathway at the FDA, as cyanide cannot ethically be given to humans. This mechanism allows drugs to be approved through rigorous testing in animals in the absence of phase II and III clinical trials. If this platform proves as successful as we propose, we anticipate that three to four drugs will make it through all three projects, and we would plan in future projects to undertake rigorous preclinical testing via the unique mechanism of the CPDF at SRI. This resource within the CounterACT network facilitates the progression of bona fide lead compounds through formal preclinical testing toward phase I clinical testing. These studies bridge the gap between the initial efficacy and toxicity studies within our pipeline and the first-in-humans phase I studies that will be required for ultimate deployment as countermeasures. The specific studies required to progress toward phase I testing will vary with each compound but will likely include good manufacturing practices–compliant studies of stability, manufacturing, and formulation in addition to formal ADME and toxicity studies.87–91
Summary
The integrated pipeline for countermeasure discovery that we have developed under the auspices of the NIH CounterACT Network is one of the few efforts within academia that by design spans the spectrum from discovery to phase I. The successful implementation of this approach for cyanide would enable efficient proof-of-concept studies laying the foundation for a generalizable strategy to undertake parallel mechanistic studies and accelerated countermeasure development in the face of new and emerging chemical threats.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ciraulo DL, Frykberg ER, Feliciano DV, et al. 2004. A survey assessment of the level of preparedness for domestic terrorism and mass casualty incidents among Eastern Association for the Surgery of Trauma members. J. Trauma 56: 1033–1039; [DOI] [PubMed] [Google Scholar]
- 2.Keim ME, Pesik N & Twum-Danso NA 2003. Lack of hospital preparedness for chemical terrorism in a major US city: 1996–2000. Prehosp. Disaster Med 18: 193–199. [DOI] [PubMed] [Google Scholar]
- 3.Burda AM & Sigg T 2001. Pharmacy preparedness for incidents involving weapons of mass destruction. Am. J. Health Syst. Pharm 58: 2274–2284. [DOI] [PubMed] [Google Scholar]
- 4.Gracia R & Shepherd G 2004. Cyanide poisoning and its treatment. Pharmacotherapy 24: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 5.Isom GE & Borowitz JL 1995. Modification of cyanide toxicodynamics: mechanistic based antidote development. Toxicol. Lett 82–83: [DOI] [PubMed] [Google Scholar]
- 6.Peterson RT & Fishman MC 2004. Discovery and use of small molecules for probing biological processes in zebrafish. Methods Cell Biol 76: 569–591. [DOI] [PubMed] [Google Scholar]
- 7.Zon LI & Peterson RT 2005. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov 4: 35–44. [DOI] [PubMed] [Google Scholar]
- 8.Okumura T, Ninomiya N & Ohta M 2003. The chemical disaster response system in Japan. Prehosp. Disaster Med 18: 189–192. [DOI] [PubMed] [Google Scholar]
- 9.Schecter WP & Fry DE 2005. The surgeon and acts of civilian terrorism: chemical agents. J. Am. Coll. Surg 200: 128–135. [DOI] [PubMed] [Google Scholar]
- 10.Eckstein M 2004. Cyanide as a chemical terrorism weapon. JEMS 29(Suppl.): 22–31. [PubMed] [Google Scholar]
- 11.Bismuth C, Borron SW, Baud FJ & Barriot P 2004. Chemical weapons: documented use and compounds on the horizon. Toxicol. Lett 149: 11–18. [DOI] [PubMed] [Google Scholar]
- 12.Rotenberg JS 2003. Cyanide as a weapon of terror. Pediatr. Ann 32: 236–240. [DOI] [PubMed] [Google Scholar]
- 13.Greenfield RA, Brown BR, Hutchins JB, et al. 2002. Microbiological, biological, and chemical weapons of warfare and terrorism. Am. J. Med. Sci 323: 326–340. [DOI] [PubMed] [Google Scholar]
- 14.Goozner B, Lutwick LI & Bourke E 2002. Chemical terrorism: a primer for 2002. J. Assoc. Acad. Minor. Phys 13: 14–18. [PubMed] [Google Scholar]
- 15.Noeller TP 2001. Biological and chemical terrorism: recognition and management. Cleve. Clin. J. Med 68: 1001–1002, [DOI] [PubMed] [Google Scholar]
- 16.Wong SH 2000. Challenges of toxicology for the millennium. Ther. Drug Monit 22: 52–57. [DOI] [PubMed] [Google Scholar]
- 17.Way JL, Sylvester D, Morgan RL, et al. 1984. Recent perspectives on the toxicodynamic basis of cyanide antagonism. Fundam. Appl. Toxicol 4: S231–S239. [DOI] [PubMed] [Google Scholar]
- 18.Kanthasamy AG, Borowitz JL, Pavlakovic G & Isom GE 1994. Dopaminergic neurotoxicity of cyanide: neurochemical, histological, and behavioral characterization. Toxicol. Appl. Pharmacol 126: 156–163. [DOI] [PubMed] [Google Scholar]
- 19.Aregheore EM & Agunbiade OO 1991. The toxic effects of cassava (Manihot esculenta Grantz) diets on humans: a review. Vet. Hum. Toxicol 33: 274–275. [PubMed] [Google Scholar]
- 20.Schulz V 1984. Clinical pharmacokinetics of nitroprusside, cyanide, thiosulphate and thiocyanate. Clin. Pharmacokinet 9: 239–251. [DOI] [PubMed] [Google Scholar]
- 21.Alcorta R 2004. Smoke inhalation & acute cyanide poisoning. Hydrogen cyanide poisoning proves increasingly common in smoke-inhalation victims. JEMS 29(Suppl.): 6–15, [PubMed] [Google Scholar]
- 22.Alarie Y 2002. Toxicity of fire smoke. Crit. Rev. Toxicol 32: 259–289. [DOI] [PubMed] [Google Scholar]
- 23.Cummings TF 2004. The treatment of cyanide poisoning. Occup. Med. (Lond.) 54: 82–85. [DOI] [PubMed] [Google Scholar]
- 24.Baskin SI, Horowitz AM & Nealley EW 1992. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J. Clin. Pharmacol 32: 368–375. [DOI] [PubMed] [Google Scholar]
- 25.Sylvester DM, Hayton WL, Morgan RL & Way JL 1983. Effects of thiosulfate on cyanide pharmacokinetics in dogs. Toxicol. Appl. Pharmacol 69: 265–271. [DOI] [PubMed] [Google Scholar]
- 26.Westley J 1981. Thiosulfate: cyanide sulfurtransferase (rhodanese). Methods Enzymol. 77: 285–291. [DOI] [PubMed] [Google Scholar]
- 27.Baskin SI, Porter DW, Rockwood GA, et al. 1999In vitro and in vivo comparison of sulfur donors as antidotes to acute cyanide intoxication. J. Appl. Toxicol 19: 173–183. [DOI] [PubMed] [Google Scholar]
- 28.Hall AH, Kulig KW & Rumack BH 1989. Suspected cyanide poisoning in smoke inhalation: complications of sodium nitrite therapy. J. Toxicol. Clin. Exp 9: 3–9. [PubMed] [Google Scholar]
- 29.Sauer SW & Keim ME 2001. Hydroxocobalamin: improved public health readiness for cyanide disasters. Ann. Emerg. Med 37: 635–641. [DOI] [PubMed] [Google Scholar]
- 30.Hall AH & Rumack BH 1987. Hydroxycobalamin/sodium thiosulfate as a cyanide antidote. J. Emerg. Med 5: 115–121. [DOI] [PubMed] [Google Scholar]
- 31.Marrs TC, Swanston DW & Bright JE 1985. 4-Dimethylaminophenol and dicobalt edetate (kelocyanor) in the treatment of experimental cyanide poisoning. Hum. Toxicol 4: 591–600. [DOI] [PubMed] [Google Scholar]
- 32.Nagahara N, Li Q & Sawada N 2003. Do antidotes for acute cyanide poisoning act on mercaptopyruvate sulfur-transferase to facilitate detoxification? Curr. Drug Targets Immune Endocr. Metabol. Disord 3: 198–204. [DOI] [PubMed] [Google Scholar]
- 33.Pearce LL, Bominaar EL, Hill BC & Peterson J 2003. Reversal of cyanide inhibition of cytochrome c oxidase by the auxiliary substrate nitric oxide: an endogenous antidote to cyanide poisoning? J. Biol. Chem 278: 52139–52145. [DOI] [PubMed] [Google Scholar]
- 34.Muller U & Krieglstein J 1995. Inhibitors of lipid peroxidation protect cultured neurons against cyanide-induced injury. Brain Res. 678: 265–268. [DOI] [PubMed] [Google Scholar]
- 35.Ardelt BK, Borowitz JL & Isom GE 1989. Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology 56: 147–154. [DOI] [PubMed] [Google Scholar]
- 36.Lee QP, Park HW, Thayer J, et al. 1996. Apoptosis induced in cultured rat embryos by intra-amniotically microinjected sodium nitroprusside. Teratology 53: 21–30. [DOI] [PubMed] [Google Scholar]
- 37.Yamada M, Momose K & Richelson E 1996. Sodium nitroprusside-induced apoptotic cellular death via production of hydrogen peroxide in murine neuroblastoma N1E115 cells. J. Pharmacol. Toxicol. Methods 35: 11–17. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu S, Eguchi Y, Kamiike W, et al. 1996. Retardation of chemicalhypoxia-induced necrotic cell death by Bcl-2 and ICE inhibitors: possible involvement of common mediators in apoptotic and necrotic signal transductions. Oncogene 12: 2045–2050. [PubMed] [Google Scholar]
- 39.Mills EM, Gunasekar PG, Pavlakovic G & Isom GE 1996. Cyanide-induced apoptosis and oxidative stress in differentiated PC12 cells. J. Neurochem 67: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 40.Biswas G, Adebanjo OA, Freedman BD, et al. 1999. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J 18: 522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suh SH, Droogmans G & Nilius B 2000. Effects of cyanide and deoxyglucose on Ca2+ signalling in macrovascular endothelial cells. Endothelium 7: 155–168. [DOI] [PubMed] [Google Scholar]
- 42.Kulik A, Brockhaus J, Pedarzani P & Ballanyi K 2002. Chemical anoxia activates ATP-sensitive and blocks Ca2+-dependent K+ channels in rat dorsal vagal neurons in situ. Neuroscience 110: 541–554. [DOI] [PubMed] [Google Scholar]
- 43.Kiang JG & Smallridge RC 1994. Sodium cyanide increases cytosolic free calcium: evidence for activation of the reversed mode of the Na+/Ca2+ exchanger and Ca2+ mobilization from inositol trisphosphate-insensitive pools. Toxicol. Appl. Pharmacol 127: 173–181. [DOI] [PubMed] [Google Scholar]
- 44.Bleicher KH, Bohm HJ, Muller K & Alanine AI 2003. Hit and lead generation: beyond high-throughput screening. Nat. Rev. Drug Discov 2: 369–378. [DOI] [PubMed] [Google Scholar]
- 45.Armer RE & Morris ID 2004. Trends in early drug safety. Drug News Perspect 17: 143–148. [PubMed] [Google Scholar]
- 46.Yeh JR & Crews CM 2003. Chemical genetics: adding to the developmental biology toolbox. Dev. Cell 5: 11–19. [DOI] [PubMed] [Google Scholar]
- 47.Stockwell BR 2000. Chemical genetics: ligand-based discovery of gene function. Nat. Rev. Genet 1: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson KV & Ingham PW 2003. The transformation of the model organism: a decade of developmental genetics. Nat. Genet 33(Suppl.): 285–293. [DOI] [PubMed] [Google Scholar]
- 49.Grunwald DJ & Eisen JS 2002. Headwaters of the zebrafish—emergence of a new model vertebrate. Nat. Rev. Genet 3: 717–724. [DOI] [PubMed] [Google Scholar]
- 50.Patton EE & Zon LI 2001. The art and design of genetic screens: zebrafish. Nat. Rev. Genet 2: 956–966. [DOI] [PubMed] [Google Scholar]
- 51.Gajewski M, Sieger D, Alt B, et al. 2003. Anterior and posterior waves of cyclic her1 gene expression are differentially regulated in the presomitic mesoderm of zebrafish. Development 130: 4269–4278. [DOI] [PubMed] [Google Scholar]
- 52.Liu NA, Huang H, Yang Z, et al. 2003. Pituitary corticotroph ontogeny and regulation in transgenic zebrafish. Mol. Endocrinol 17: 959–966. [DOI] [PubMed] [Google Scholar]
- 53.Penberthy WT, Shafizadeh E & Lin S 2002. The zebrafish as a model for human disease. Front. Biosci 7: d1439–d1453. [DOI] [PubMed] [Google Scholar]
- 54.Amatruda JF, Shepard JL, Stern HM & Zon LI 2002. Zebrafish as a cancer model system. Cancer Cell 1: 229–231. [DOI] [PubMed] [Google Scholar]
- 55.Shin JT & Fishman MC 2002. From zebrafish to human: modular medical models. Annu. Rev. Genomics Hum. Genet 3: 311–340. [DOI] [PubMed] [Google Scholar]
- 56.Langheinrich U, Vacun G & Wagner T 2003. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol. Appl. Pharmacol 193: 370–382. [DOI] [PubMed] [Google Scholar]
- 57.Milan DJ, Peterson TA, Ruskin JN, et al. 2003. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 107: 1355–1358. [DOI] [PubMed] [Google Scholar]
- 58.MacRae CA & Fishman MC 2002. Zebrafish: the complete cardiovascular compendium. Cold Spring Harb. Symp. Quant. Biol 67: 301–307. [DOI] [PubMed] [Google Scholar]
- 59.MacRae CA & Peterson RT 2003. Zebrafish-based small molecule discovery. Chem. Biol 10: 901–908. [DOI] [PubMed] [Google Scholar]
- 60.Ton C, Stamatiou D, Dzau VJ & Liew CC 2002. Construction of a zebrafish cDNA microarray: gene expression profiling of the zebrafish during development. Biochem. Biophys. Res. Commun 296: 1134–1142. [DOI] [PubMed] [Google Scholar]
- 61.Stickney HL, Schmutz J, Woods IG, et al. 2002. Rapid mapping of zebrafish mutations with SNPs and oligonucleotide microarrays. Genome Res 12: 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nasevicius A & Ekker SC 2000. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet 26: 216–220. [DOI] [PubMed] [Google Scholar]
- 63.Wienholds E, Schulte-Merker S, Walderich B & Plasterk RH 2002. Target-selected inactivation of the zebrafish rag1 gene. Science 297: 99–102. [DOI] [PubMed] [Google Scholar]
- 64.Meng X, Noyes MB, Zhu LJ, et al. 2008. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat. Biotechnol 26: 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foley JE, Maeder ML, Pearlberg J, et al. 2009. Targeted mutagenesis in zebrafish using customized zinc-finger nucleases. Nat. Protoc 4: 1855–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moon HS, Jacobson EM, Khersonsky SM, et al. 2002. A novel microtubule destabilizing entity from orthogonal synthesis of triazine library and zebrafish embryo screening. J. Am. Chem. Soc 124: 11608–11609. [DOI] [PubMed] [Google Scholar]
- 67.Khersonsky SM, Jung DW, Kang TW, et al. 2003. Facilitated forward chemical genetics using a tagged triazine library and zebrafish embryo screening. J. Am. Chem. Soc 125: 11804–11805. [DOI] [PubMed] [Google Scholar]
- 68.Peterson RT, Link BA, Dowling JE & Schreiber SL 2000. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc. Natl. Acad. Sci. U.S.A 97: 12965–12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peterson RT, Mably JD, Chen JN & Fishman MC 2001. Convergence of distinct pathways to heart patterning revealed by the small molecule concentramide and the mutation heart-and-soul. Curr. Biol 11: 1481–1491. [DOI] [PubMed] [Google Scholar]
- 70.Spring DR, Krishnan S, Blackwell HE & Schreiber SL 2002. Diversity-oriented synthesis of biaryl-containing medium rings using a one bead/one stock solution platform. J. Am. Chem. Soc 124: 1354–1363. [DOI] [PubMed] [Google Scholar]
- 71.Sternson SM, Louca JB, Wong JC & Schreiber SL 2001. Split–pool synthesis of 1,3-dioxanes leading to arrayed stock solutions of single compounds sufficient for multiple phenotypic and protein-binding assays. J. Am. Chem. Soc 123: 1740–1747. [DOI] [PubMed] [Google Scholar]
- 72.Peterson RT, Shaw SY, Peterson TA, et al. 2004. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat. Biotechnol 22: 595–599. [DOI] [PubMed] [Google Scholar]
- 73.Weinstein BM, Stemple DL, Driever W & Fishman MC 1995. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat. Med. 1: 1143–1147. [DOI] [PubMed] [Google Scholar]
- 74.Burns CG, Milan DJ, Grande EJ, et al. 2005. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat. Chem. Biol 1: 263–264. [DOI] [PubMed] [Google Scholar]
- 75.Kokel D, Bryan J, Laggner C, White R, et al. 2010. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol 6: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kerger H, Dodidou P, Passani-Kruppa D, et al. 2005. Excessive methaemoglobinaemia and multi-organ failure following 4-DMAP antidote therapy. Resuscitation 66: 231–235. [DOI] [PubMed] [Google Scholar]
- 77.Mousa HM & Davis RH 1991. Alternative Sulphur donors for detoxification of cyanide in the chicken. Comp. Biochem. Physiol. C 99: 309–315. [DOI] [PubMed] [Google Scholar]
- 78.Yu PB, Hong CC, Sachidanandan C, et al. 2008. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol 4: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cuny GD, Yu PB, Laha JK, et al. 2008. Structure–activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg. Med. Chem. Lett 18: 4388–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ren B, Deng Y, Mukhopadhyay A, et al. 2010. ERK1/2-Akt1crosstalk regulates arteriogenesis in mice and zebrafish. J. Clin. Invest 120: 1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parng C, Seng WL, Semino C & McGrath P 2002. Zebrafish: a preclinical model for drug screening. Assay Drug Dev. Technol 1: 41–48. [DOI] [PubMed] [Google Scholar]
- 82.Perkins R, Fang H, Tong W & Welsh WJ 2003. Quantitative structure–activity relationship methods: perspectives on drug discovery and toxicology. Environ. Toxicol. Chem 22: 1666–1679. [DOI] [PubMed] [Google Scholar]
- 83.Tong W, Welsh WJ, Shi L, et al. 2003. Structure–activity relationship approaches and applications. Environ. Toxicol. Chem 22: 1680–1695. [DOI] [PubMed] [Google Scholar]
- 84.Shin JT, Pomerantsev EV, Mably JD & MacRae CA 2010. High-resolution cardiovascular function confirms functional orthology of myocardial contractility pathways in zebrafish. Physiol. Genomics 42: 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Panakova D, Werdich AA & Macrae CA 2010. Wnt11 patterns a myocardial electrical gradient through regulation of the L-type Ca2+ channel. Nature 466: 874–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hentschel DM, Park KM, Cilenti L, et al. 2005. Acute renal failure in zebrafish: a novel system to study a complex disease. Am. J. Physiol. Renal Physiol 288: F923–F929. [DOI] [PubMed] [Google Scholar]
- 87.Dunbar SA 2006. Applications of Luminex xMAP technology for rapid, high-throughput multiplexed nucleic acid detection. Clin. Chim. Acta 363: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rochfort S 2005. Metabolomics reviewed: a new “omics” platform technology for systems biology and implications for natural products research. J. Nat. Prod 68: 1813–1820. [DOI] [PubMed] [Google Scholar]
- 89.Weckwerth W & Morgenthal K 2005. Metabolomics: from pattern recognition to biological interpretation. Drug Discov. Today 10: 1551–1558. [DOI] [PubMed] [Google Scholar]
- 90.Sabatine MS, Liu E, Morrow DA, et al. 2005. Metabolomic identification of novel biomarkers of myocardial ischemia. Circulation 112: 3868–3875. [DOI] [PubMed] [Google Scholar]
- 91.Marton MJ, DeRisi JL, Bennett HA, et al. 1998. Drug target validation and identification of secondary drug target effects using DNA microarrays. Nat. Med 4: 1293–1301. [DOI] [PubMed] [Google Scholar]